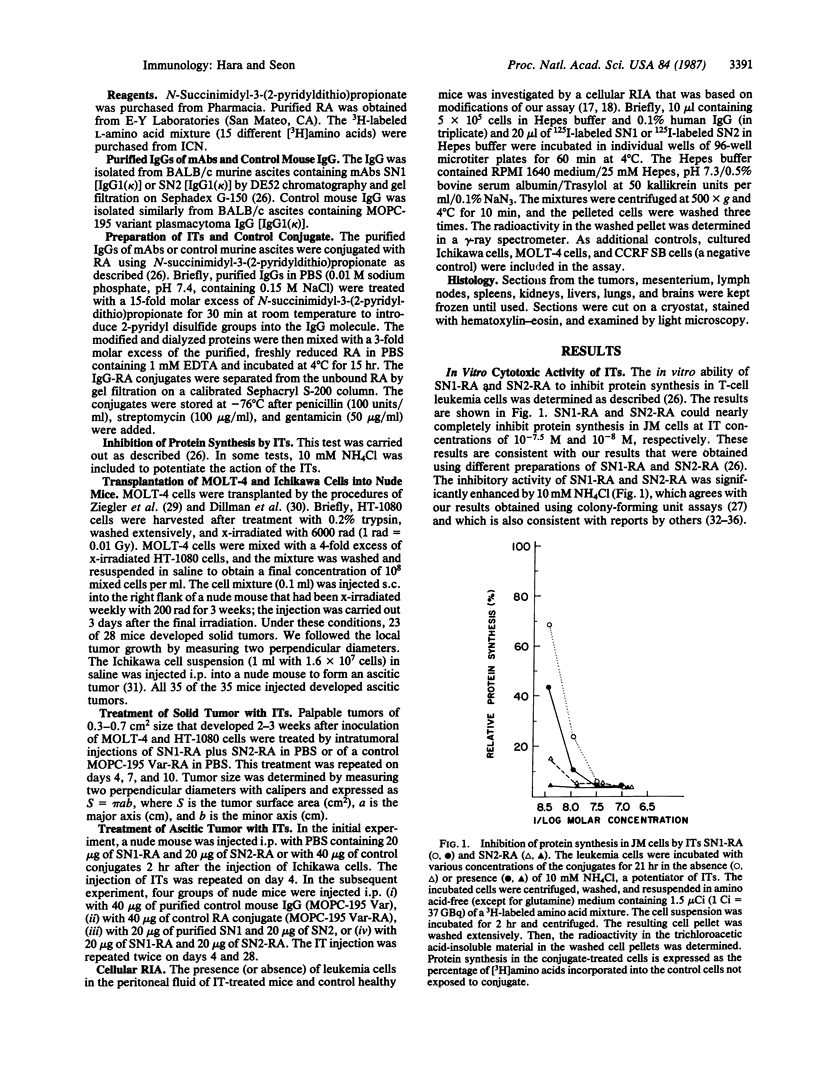
Abstract
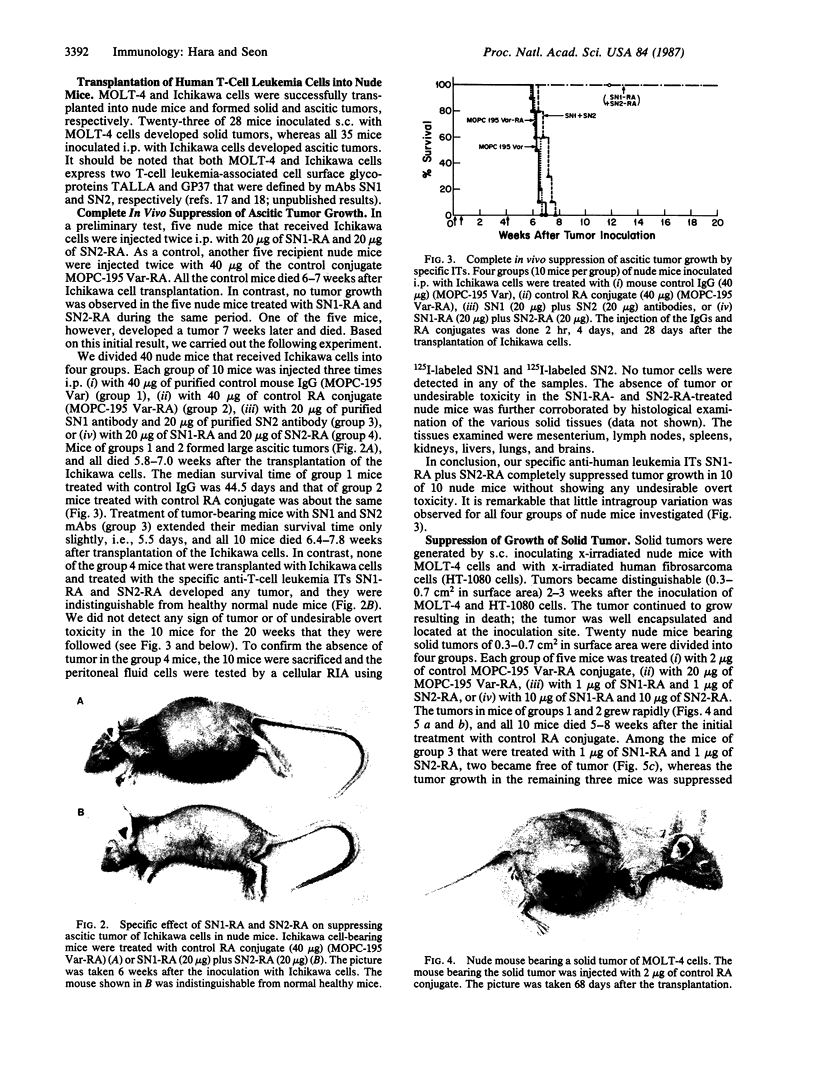
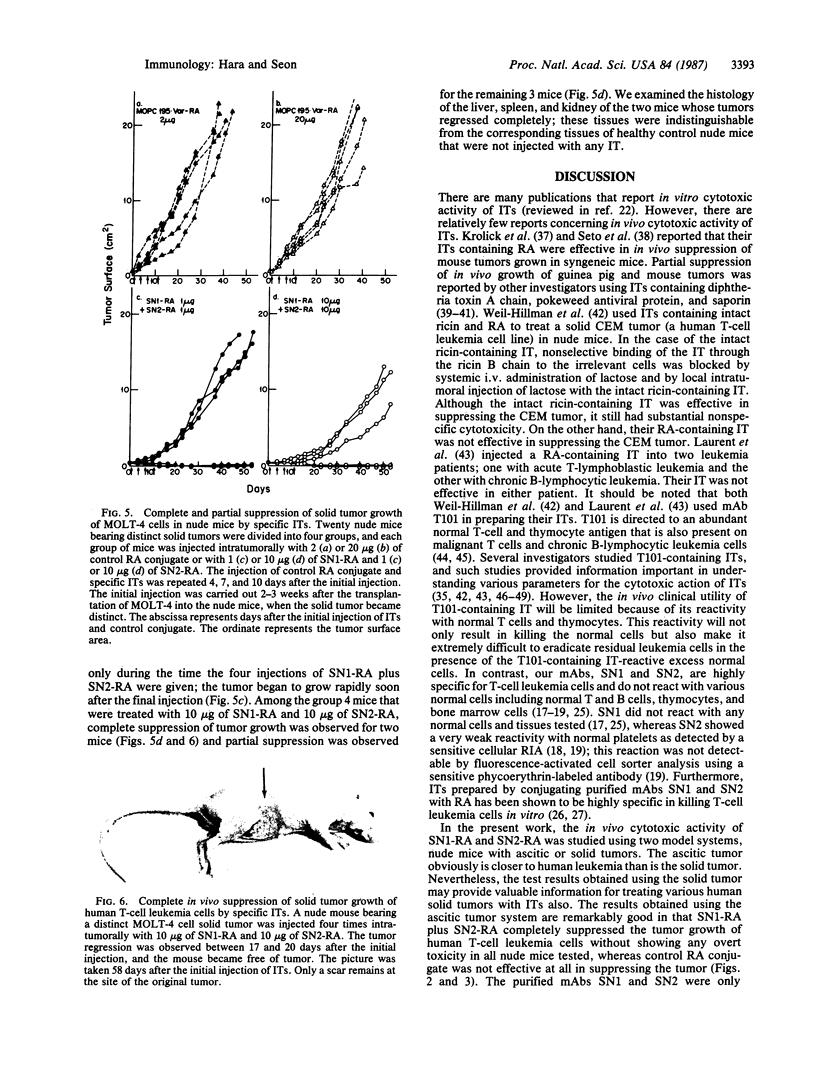
In this study, immunotoxins containing monoclonal anti-human T-cell leukemia antibodies are shown to be capable of completely suppressing the tumor growth of human T-cell leukemia cells in vivo without any overt undesirable toxicity. These immunotoxins were prepared by conjugating ricin A chain (RA) with our monoclonal antibodies, SN1 and SN2, directed specifically to the human T-cell leukemia cell surface antigens TALLA and GP37, respectively. We have shown that these monoclonal antibodies are highly specific for human T-cell leukemia cells and do not react with various normal cells including normal T and B cells, thymocytes, and bone marrow cells. Ascitic and solid human T-cell leukemia cell tumors were generated in nude mice. The ascitic tumor was generated by transplanting Ichikawa cells (a human T-cell leukemia cell line) i.p. into nude mice, whereas the solid tumor was generated by transplanting s.c. MOLT-4 cells (a human T-cell leukemia cell line) and x-irradiated human fibrosarcoma cells into x-irradiated nude mice. To investigate the efficacy of specific immunotoxins in suppressing the in vivo growth of the ascitic tumor, we divided 40 nude mice that were injected with Ichikawa cells into four groups. Each group of 10 mice was injected with one of the following mixtures: 40 micrograms of purified control mouse IgG [IgG1(kappa)] (group 1), 40 micrograms of control RA conjugate (group 2), 20 micrograms of purified SN1 antibody [IgG1(kappa)] and 20 micrograms of purified SN2 antibody [IgG1(kappa)] (group 3), or 20 micrograms of SN1-RA and 20 micrograms of SN2-RA (group 4). Mice in groups 1 and 2 formed large ascitic tumors, and died 5.8-7.0 weeks after the transplantation. Group 3 mice also formed large ascitic tumors and died 6.4-7.8 weeks after the transplantation. However, none of the mice in group 4 that were treated with SN1-RA and SN2-RA showed any signs of a tumor or undesirable toxic effects for the 20 weeks that they were followed after the transplantation; these mice were indistinguishable from healthy control nude mice that were not injected with Ichikawa cells. Treatment with SN1-RA plus SN2-RA completely suppressed solid tumor growth in 4 of 10 nude mice carrying solid tumors and partially suppressed the tumor growth in the remaining 6 nude mice. These results strongly suggest that SN1-RA and SN2-RA may be useful for clinical treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard M. I., Foon K. A., Oeltmann T. N., Key M. E., Hwang K. M., Clarke G. C., Christensen W. L., Hoyer L. C., Hanna M. G., Jr, Oldham R. K. Guinea pig line 10 hepatocarcinoma model: characterization of monoclonal antibody and in vivo effect of unconjugated antibody and antibody conjugated to diphtheria toxin A chain. Cancer Res. 1983 Sep;43(9):4420–4428. [PubMed] [Google Scholar]
- Casellas P., Brown J. P., Gros O., Gros P., Hellström I., Jansen F. K., Poncelet P., Roncucci R., Vidal H., Hellström K. E. Human melanoma cells can be killed in vitro by an immunotoxin specific for melanoma-associated antigen p97. Int J Cancer. 1982 Oct 15;30(4):437–443. doi: 10.1002/ijc.2910300410. [DOI] [PubMed] [Google Scholar]
- Dillman R. O., Johnson D. E., Shawler D. L., Halpern S. E., Leonard J. E., Hagan P. L. Athymic mouse model of a human T-cell tumor. Cancer Res. 1985 Nov;45(11 Pt 2):5632–5636. [PubMed] [Google Scholar]
- Douay L., Gorin N. C., Lopez M., Casellas P., Liance M. C., Jansen F. K., Voisin G. A., Baillou C., Laporte J. P., Najman A. Evidence for absence of toxicity of T101 immunotoxin on human hematopoietic progenitor cells prior to bone marrow transplantation. Cancer Res. 1985 Jan;45(1):438–441. [PubMed] [Google Scholar]
- Eiklid K., Olsnes S., Pihl A. Entry of lethal doses of abrin, ricin and modeccin into the cytosol of HeLa cells. Exp Cell Res. 1980 Apr;126(2):321–326. doi: 10.1016/0014-4827(80)90270-0. [DOI] [PubMed] [Google Scholar]
- Haruta Y., Seon B. K. Distinct human leukemia-associated cell surface glycoprotein GP160 defined by monoclonal antibody SN6. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7898–7902. doi: 10.1073/pnas.83.20.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn D., Koprowski H. IgG2a monoclonal antibodies inhibit human tumor growth through interaction with effector cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4761–4765. doi: 10.1073/pnas.79.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F. K., Blythman H. E., Carrière D., Casellas P., Gros O., Gros P., Laurent J. C., Paolucci F., Pau B., Poncelet P. Immunotoxins: hybrid molecules combining high specificity and potent cytotoxicity. Immunol Rev. 1982;62:185–216. doi: 10.1111/j.1600-065x.1982.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Siliciano R. F., Shin H. S. Suppression of antibody-sensitized tumor cells by macrophages: insufficient supply or activation of macrophages within large tumors. J Immunol. 1979 Feb;122(2):379–382. [PubMed] [Google Scholar]
- Johnson W. J., Steplewski Z., Matthews T. J., Hamilton T. A., Koprowski H., Adams D. O. Cytolytic interactions between murine macrophages, tumor cells, and monoclonal antibodies: characterization of lytic conditions and requirements for effector activation. J Immunol. 1986 Jun 15;136(12):4704–4713. [PubMed] [Google Scholar]
- Kernan N. A., Knowles R. W., Burns M. J., Broxmeyer H. E., Lu L., Lee H. M., Kawahata R. T., Scannon P. J., Dupont B. Specific inhibition of in vitro lymphocyte transformation by an anti-pan T cell (gp67) ricin A chain immunotoxin. J Immunol. 1984 Jul;133(1):137–146. [PubMed] [Google Scholar]
- Kipps T. J., Parham P., Punt J., Herzenberg L. A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985 Jan 1;161(1):1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch M. E., Hammerling U. Immunotherapy of murine leukemias by monoclonal antibody. I. Effect of passively administered antibody on growth of transplanted tumor cells. J Immunol. 1981 Aug;127(2):805–810. [PubMed] [Google Scholar]
- Krolick K. A., Uhr J. W., Slavin S., Vitetta E. S. In vivo therapy of a murine B cell tumor (BCL1) using antibody-ricin A chain immunotoxins. J Exp Med. 1982 Jun 1;155(6):1797–1809. doi: 10.1084/jem.155.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Schlick E., Waldmann T. A., Vitetta E. S., Greene W. C. Selective killing of human T-lymphotropic virus-I infected leukemic T-cells by monoclonal anti-interleukin 2 receptor antibody-ricin A chain conjugates: potentiation by ammonium chloride and monensin. Cancer Res. 1986 Jul;46(7):3295–3298. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laurent G., Pris J., Farcet J. P., Carayon P., Blythman H., Casellas P., Poncelet P., Jansen F. K. Effects of therapy with T101 ricin A-chain immunotoxin in two leukemia patients. Blood. 1986 Jun;67(6):1680–1687. [PubMed] [Google Scholar]
- Leonard J. E., Taetle R., To D., Rhyner K. Preclinical studies on the use of selective antibody-ricin conjugates in autologous bone marrow transplantation. Blood. 1985 May;65(5):1149–1157. [PubMed] [Google Scholar]
- Leonard J. E., Wang Q. C., Kaplan N. O., Royston I. Kinetics of protein synthesis inactivation in human T-lymphocytes by selective monoclonal antibody-ricin conjugates. Cancer Res. 1985 Nov;45(11 Pt 1):5263–5269. [PubMed] [Google Scholar]
- Levy R., Miller R. A. Tumor therapy with monoclonal antibodies. Fed Proc. 1983 Jun;42(9):2650–2656. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Vitetta E. S. A ricin A chain-containing immunotoxin that kills human T lymphocytes in vitro. Blood. 1985 Oct;66(4):908–912. [PubMed] [Google Scholar]
- Negoro S., Seon B. K. Several new monoclonal antibodies directed to human T-cell leukemia antigens. Cancer Res. 1982 Oct;42(10):4259–4262. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Chimeric toxins. Pharmacol Ther. 1981;15(3):355–381. doi: 10.1016/0163-7258(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Cell-mediated lysis of tumor targets directed by murine monoclonal antibodies of IgM and all IgG isotypes. J Immunol. 1983 Aug;131(2):1028–1031. [PubMed] [Google Scholar]
- Ramakrishnan S., Houston L. L. Prevention of growth of leukemia cells in mice by monoclonal antibodies directed against Thy 1.1 antigen disulfide linked to two ribosomal inhibitors:pokeweed antiviral protein or ricin A chain. Cancer Res. 1984 Apr;44(4):1398–1404. [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Schlossman S. F. Modulation of human acute lymphoblastic leukemia antigen induced by monoclonal antibody in vitro. J Immunol. 1980 Oct;125(4):1506–1514. [PubMed] [Google Scholar]
- Ritz J., Schlossman S. F. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982 Jan;59(1):1–11. [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Seon B. K., Fukukawa T., Jackson A. L., Chervinsky D., Tebbi C. K., Freeman A. I., Matsuzaki H. Human T-cell leukemia-associated cell surface glycoprotein GP37: studies with three monoclonal antibodies and a rabbit antiserum. Mol Immunol. 1986 Jun;23(6):569–580. doi: 10.1016/0161-5890(86)90093-3. [DOI] [PubMed] [Google Scholar]
- Seon B. K., Negoro S., Barcos M. P. Monoclonal antibody that defines a unique human T-cell leukemia antigen. Proc Natl Acad Sci U S A. 1983 Feb;80(3):845–849. doi: 10.1073/pnas.80.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seon B. K., Negoro S., Barcos M. P., Tebbi C. K., Chervinsky D., Fukukawa T. Monoclonal antibody SN2 defining a human T cell leukemia-associated cell surface glycoprotein. J Immunol. 1984 Apr;132(4):2089–2095. [PubMed] [Google Scholar]
- Seon B. K., Negoro S., Minowada J., Yoshizaki K. Human T cell leukemia antigens on the cell membranes: purification molecular characterization, and preparation of specific antisera. J Immunol. 1981 Dec;127(6):2580–2588. [PubMed] [Google Scholar]
- Seon B. K. Specific killing of human T-leukemia cells by immunotoxins prepared with ricin A chain and monoclonal anti-human T-cell leukemia antibodies. Cancer Res. 1984 Jan;44(1):259–264. [PubMed] [Google Scholar]
- Seto M., Takahashi T., Nakamura S., Matsudaira Y., Nishizuka Y. In vivo antitumor effects of monoclonal antibodies with different immunoglobulin classes. Cancer Res. 1983 Oct;43(10):4768–4773. [PubMed] [Google Scholar]
- Seto M., Umemoto N., Saito M., Masuho Y., Hara T., Takahashi T. Monoclonal anti-MM46 antibody:ricin A chain conjugate: in vitro and in vivo antitumor activity. Cancer Res. 1982 Dec;42(12):5209–5215. [PubMed] [Google Scholar]
- Shouval D., Shafritz D. A., Zurawski V. R., Jr, Isselbacher K. J., Wands J. R. Immunotherapy in nude mice of human hepatoma using monoclonal antibodies against hepatitis B virus. Nature. 1982 Aug 5;298(5874):567–569. doi: 10.1038/298567a0. [DOI] [PubMed] [Google Scholar]
- Stong R. C., Uckun F., Youle R. J., Kersey J. H., Vallera D. A. Use of multiple T cell-directed intact ricin immunotoxins for autologous bone marrow transplantation. Blood. 1985 Sep;66(3):627–635. [PubMed] [Google Scholar]
- Tebbi C. K., Chervinsky D., Seon B. K. Effect of specific anti-human leukaemia immunotoxins on the colony formation by human haematopoietic progenitors and by human leukaemia cells. Clin Exp Immunol. 1985 Jun;60(3):496–500. [PMC free article] [PubMed] [Google Scholar]
- Thorpe P. E., Brown A. N., Bremner J. A., Jr, Foxwell B. M., Stirpe F. An immunotoxin composed of monoclonal anti-Thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. J Natl Cancer Inst. 1985 Jul;75(1):151–159. [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunotoxins. Annu Rev Immunol. 1985;3:197–212. doi: 10.1146/annurev.iy.03.040185.001213. [DOI] [PubMed] [Google Scholar]
- Weil-Hillman G., Runge W., Jansen F. K., Vallera D. A. Cytotoxic effect of anti-Mr 67,000 protein immunotoxins on human tumors in a nude mouse model. Cancer Res. 1985 Mar;45(3):1328–1336. [PubMed] [Google Scholar]
- Wormsley S. B., Collins M. L., Royston I. Comparative density of the human T-cell antigen T65 on normal peripheral blood T cells and chronic lymphocytic leukemia cells. Blood. 1981 Apr;57(4):657–662. [PubMed] [Google Scholar]
- Yamaizumi M., Mekada E., Uchida T., Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978 Sep;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Hakomori S. I. Therapy of mouse lymphoma with monoclonal antibodies to glycolipid: selection of low antigenic variants in vivo. Science. 1981 Jan 30;211(4481):487–489. doi: 10.1126/science.7455688. [DOI] [PubMed] [Google Scholar]
- Ziegler H. W., Frizzera G., Bach F. H. Successful transplantation of a human leukemia cell line into nude mice: conditions optimizing graft acceptance. J Natl Cancer Inst. 1982 Jan;68(1):15–18. [PubMed] [Google Scholar]