Summary
Enteroviruses, including coxsackievirus B (CVB) and poliovirus (PV), can access the CNS through the blood brain barrier (BBB) endothelium to cause aseptic meningitis. To identify cellular components required for CVB and PV infection of human brain microvascular endothelial cells, an in vitro BBB model, we performed comparative RNAi screens and identified 117 genes that influenced infection. Whereas a large proportion of genes whose depletion enhanced infection (17 of 22) were broadly anti-enteroviral, only 46 of the 95 genes whose depletion inhibited infection were required by both CVB and PV and included components of cell signaling pathways such as adenylate cyclases. Downregulation of genes including Rab GTPases, Src tyrosine kinases, and tyrosine phosphatases, displayed specificity in their requirement for either CVB or PV infection. These findings highlight the pathways hijacked by enteroviruses for entry and replication in the BBB endothelium, a specialized and clinically relevant cell type for these viruses.
Introduction
Many viruses exhibit specific tropism for polarized epithelia and/or endothelia to promote host invasion and/or facilitate spread. Despite this, little is known regarding the host cell molecules that facilitate infection of polarized cells, and whether these molecules are specific to polarized cells or are specific between the epithelium and endothelium. Enteroviruses, which belong to the Picornaviridae family, are a large family of non-enveloped single stranded RNA viruses of ~8kb in length (Pallansch and Roos, 2001). Enteroviruses generally utilize some form of endocytosis to gain entry into the host cell cytoplasm and once internalized, undergo uncoating and subsequent translation of the single open reading frame of the incoming viral RNA via an internal ribosome entry site. Picornaviruses remodel cellular organelles and block host cell translation to allow for high levels of viral replication. The structural proteins assemble with the viral genomic RNA and exit the cell via the destruction of the host cell membranes. These viruses initiate infection in the highly polarized intestinal epithelium before spreading to a variety of polarized and non-polarized cell types during the course of an infection. Secondary sites of infection can include the spinal cord and brain, the heart, or the skin and play an important role in the pathogenic outcome of infection (Morens, 1995).
Our previous studies have established that two enteroviruses, coxsackievirus B (CVB) and poliovirus (PV), enter polarized cells by endocytic mechanisms that require activation of specific intracellular signaling molecules (Coyne and Bergelson, 2006; Coyne et al., 2007a; Coyne et al., 2007b). These studies have highlighted the specificity of intracellular signals required for virus entry—whereas CVB induces a cascade of tyrosine kinase-mediated signals to enter the epithelium (Coyne and Bergelson, 2006), PV entry into polarized endothelia requires the activation of tyrosine phosphatases (Coyne et al., 2007a). Although these studies highlight the divergent signaling molecules that facilitate CVB and PV entry into polarized cells, it remains unclear if there are common host factors that mediate infection by these related viruses in the same cell type.
The use of RNA interference (RNAi) screens have extended our knowledge of the complex interplay between a virus and host and have implicated a wide variety of cellular factors required for infection of a number of viruses (Brass et al., 2008; Cherry et al., 2006; Hao et al., 2008; Krishnan et al., 2008; Pelkmans et al., 2005; Sessions et al., 2009; Tai et al., 2009). Although these studies identified host factors required for viral infection, they were conducted in non-polarized cell types that may not mimic the in vivo cell types targeted by these pathogens. As enteroviruses are commonly associated with neurological disease and are the major etiological agents associated with aseptic meningitis in adults and children (Morens, 1995), understanding the host factors required for infection of the blood-brain barrier (BBB), a polarized endothelium, will allow us to dissect the host cell factors that may regulate enterovirus infection of the CNS.
To overcome these limitations to our understanding of enterovirus infection of the polarized endothelium, we performed RNAi screens using the druggable genome library (~5500 genes) in human brain microvascular endothelial cells (HBMEC), an immortalized cell line that replicates many of the functional and morphologic characteristics of the BBB (Stins et al., 2001), to identify factors that impact enterovirus infection. We performed the screen using both CVB and PV to identify the host factors co-opted by each virus and to determine if there are commonalites to the host factor requirements. This study sheds light on genes in polarized endothelial cells that control enterovirus infection and highlights many host cell factors that mediate infection of CVB and PV in this specialized cell type.
Results
RNAi screening in HBMEC
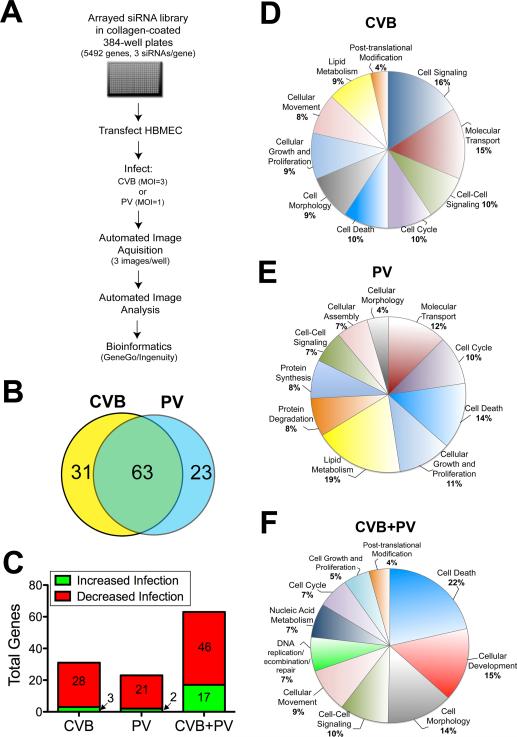
To study the early steps in the infection cycle, we developed a single-round high-content assay (schematic, Figure 1A). To this end, the Ambion Druggable Genome™ library (5492 genes) containing pooled siRNAs (3 siRNAs/gene) was arrayed in collagen-coated 384-well plates, reverse transfected into HBMEC, and 48 hrs post-transfection infected with either CVB (3 PFU/cell) or PV (1 PFU/cell). The cells were fixed and stained for the viral VP1 antigen (an enterovirus capsid protein) 14 hrs post-infection (p.i.) and counterstained for nuclei. Automated microscopy and image analysis was used to calculate the total number of cells per well and the percentage of infected cells. These metrics were used to identify candidate genes that modulated infection by a robust Z score of >2 or <-2 (which approximates a >2 standard deviation difference from the mean; >50% difference in virus infection) in duplicate screens (p<0.001; Figure S1Ai, ii). Toxic siRNAs were excluded based upon decreased cell viability as measured by a robust Z score <-2 in duplicate screens (>30% decrease in cell number, Figure S1Aiii). As a positive control, siRNAs against coxsackievirus and adenovirus Receptor (CAR) (Coyne and Bergelson, 2006) or poliovirus receptor (PVR) (Coyne et al., 2007a) were included in three wells of each plate and were positively identified (Figure S1Aiv). The library contained both CAR and PVR siRNAs, which were also positively identified in the screen (Figure S1Aiv).
Figure 1. RNAi screening.
(A) Schematic of screening strategy. (B) Venn diagram highlighting the degree of overlap between hits validated in CVB and PV screens. (C) Distribution of genes identified whose downregulation increased (green) or decreased (red) virus replication and were either specific for CVB or PV or were involved in regulating both viruses. (D, E, F) Pie charts showing the frequency of functional groups (curated by Ingenuity Pathway Analysis) from CVB and PV screens. Shown in (D, E) are pathways curated from genes with specific effects on either CVB (D) or PV (E) replication. Shown in (F) are pathways curated from genes whose downregulation modulated both CVB and PV infection. Categories that are overrepresented (p < 0.001) are shown. See also Figures S1A-E and Tables S1A-D.
We identified three classes of factors by RNAi screening—those that specifically modulated CVB infection or PV infection and those that impacted both viruses. Amongst these genes were 31 positive and 144 negative regulators of infection for CVB infection and 65 positive and 155 negative regulators for PV infection (Table S1A). To confirm the hits identified in our primary screen, we screened three unique and independent siRNAs targeting each gene (Qiagen) identified in the primary screen (310 total genes). We identified hits as those genes in which at least one additional siRNA displayed a significant impact on infection (z-score +/- 1.5, change in infection of >30%, p<0.009). Of the 117 genes that we validated (a confirmation rate of 38%), 100 had multiple siRNAs that significantly affected infection while in only 17 cases did only 1 Qiagen siRNA impact infection (Table S1B). [A complete list of genes that were not verified by secondary screening can be found in Table S1C].
Using this screening strategy, we validated genes in the three classes—those that specifically modulated CVB infection (31 genes) or PV infection (23 genes) and those that were required by both viruses (63 genes) (Figure 1B, Table S1B). Of the genes identified that were involved in regulating infection of both viruses, 46 genes promoted infection while 17 functioned in an antiviral capacity (Figure 1C). In contrast, genes that were specific to either CVB or PV were largely found to be required for promoting rather than restricting infection (Figure 1C). There was a substantial overlap in the genes that were required by both viruses (p<10-9), suggesting that these two related enteroviruses use many of the same factors to replicate in HBMEC.
Molecular function analysis revealed that the genes required for CVB infection were enriched in the following pathways: cell signaling, molecular transport, cell-cell signaling and interaction, cell cycle, cell death, among others (Figure 1D, Figure S1Bi). Genes involved in regulating PV infection were enriched in lipid metabolism, cell death, cell growth and proliferation, cell cycle, among others (Figure 1E, Figure S1Bii). Genes that were involved in regulating infection by both viruses were enriched in cell death, cellular development, cell morphology, cell-cell signaling and interaction, cellular movement, among others (Figure 1F, Figure S1Biii).
Protein network analysis revealed interactions between genes identified as regulators of CVB and PV infection in cell death and cell signaling pathways and cell growth and development, respectively (Figure S1C, S1D). Pathway analysis also revealed an enrichment of genes associated with the regulation of intracellular calcium (Cai2+) signaling or which depend on alterations in Cai2+ for their activation and/or function specifically involved in the regulation of CVB infection (Figure S1E).
Furthermore, microarray analysis of a previous study using HBMEC revealed that 68 of these genes were expressed as monitored using an Affimetrix platform (Tripathi et al., 2009) (Table S1B).
Akt and MAPK Family Members Restrict Enterovirus Replication in HBMEC
We identified 22 genes whose depletion upregulated infection of CVB, PV, or both. Of these genes, 17 were broadly antiviral against both CVB and PV. Because of the high degree of overlap between immune-related factors involved in regulating CVB and PV infection, we characterized a subset of these potential innate immune candidates that restricted both viruses. Amongst these candidates, we identified Akt1 and Akt2, two of the three family members of the Akt family as genes whose knockdown of multiple independent validated siRNAs led to a significant enhancement of CVB and PV infection in HBMEC (Figure 2A, Table S1B). We further studied the role of these Akt candidates using a number of different approaches. First, we found that overexpression of a dominant-negative mutant of Akt1 (Akt1K179M) or Akt2 (Akt2K181M) increased enteroviral infection (Figure 2B). Next, we used pharmacological inactivation of Akt using the Akt1/Akt2 inhibitor SH-6 and found that this led to increased enteroviral infection (not shown). To determine if the requirement for Akt in antiviral defense occurs via the canonical Akt-mTOR signaling pathway we treated cells with rapamycin, an inhibitor of TOR and found that indeed infection was increased under these conditions (Figure 2B). Furthermore, we found that rapamycin and Akt2 siRNA also significantly enhanced CVB replication in primary HBMEC, indicating these results are not a consequence of cell immortalization (Figure S2Ai, ii).
Figure 2. Akts, MAPKs restrict CVB and PV infection.
(A) Enhanced replication in HBMEC transfected with Akt1 and Akt2, but not Akt3, siRNAs compared to an siRNA that had no effect on viral replication. (B) The percentage of infected CVB- or PV-infected HBMEC (normalized to no inhibitor or wild-type controls) in cells transfected with either dominant-negative Akt1 or Akt2, or treated with rapamycin. (C) Enhanced replication in HBMEC transfected with MAP3K4, MAP3K1 and p21 Ras GAP siRNAs compared to an siRNA which had no effect on viral replication. (D) Shown are the percentage of CVB- or PV-infected cells (normalized to no inhibitor or wild-type controls) in HBMEC transfected with either dominant-negative MAP3K4 or treated with FR180204. E) Enhanced replication in HBMEC transfected with IRAK1 and TLR8, but not TLR7, siRNAs compared to an siRNA which had no effect on viral replication. (F) Shown are the percentage of CVB- or PV-infected cells (normalized to control plasmid (pcDNA)) in HBMEC transfected with either dominant-negative TLR8 (TLR8ΔTIR), IRAK1 (IRAK1DN), or MyD88 (MyD88DN) For (B), (D), and (F), data are represented as mean ± SD; *p < 0.05. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. See also Figures S2A-B and Table S2.
We also identified the MAPK pathway as a negative regulator of enterovirus replication as downregulation of both MAP3K4 [also known as MEKK4] and MAPK1 [also known as ERK1] by multiple independent validated siRNAs significantly increased CVB and PV infection in HBMEC (Figure 2C, Table S1B). We further studied the role of this pathway by expression of a kinase-defective mutant of MAP3K4 (MAP3K4K1361M) and by treatment of cells with a specific ERK1/2 inhibitor (FR180204), both of which enhanced CVB and PV infection (Figure 2F).
As our initial screen targeted a polarized cell type, we were interested in whether the factors identified in the primary screen that functioned to restrict enterovirus replication were specific to polarized cells or whether these genes would also be involved in restricting infection in a non-polarized cell type. To that end, we tested the role of Akt1, Akt2, MAP3K4 and MAPK1 using the same siRNAs used in our secondary screening in HBMEC. We found that knockdown of all four genes significantly increased CVB replication in U2OS cells (Figure S2Bi, Table S2). Furthermore, we found that pharmacological inhibitors of Akt (SH-6), ERKI/II (FR180204), and mTOR (rapamycin) as well as dominant-negative mutants of Akt2 and MAP3K4 also enhanced CVB replication in U2OS cells (Figure S2Bii). Taken together, these data show that Akt and MAPK signaling restricts enterovirus infection across cell types of both non-polarized and polarized origins and may provide insights into the signaling pathways that restrict enterovirus infection in non-immune cells.
TLR8 Restrict CVB and PV Replication in HBMEC
The induction of type I IFN signaling is essential for the restriction of enterovirus infections, as evidenced by enhanced CVB-induced lethality in type I IFN receptor (IFN-α/β R) null mice (Wessely et al., 2001), increased susceptibility to CVB infection in IFNβ-deficient mice (Deonarain et al., 2004), and increased lethality and CNS entry of PV in IFN-α/β R null mice (Lancaster and Pfeiffer, 2010). We identified toll like receptor 8 (TLR8) and its downstream signaling adapter interleukin-1 receptor-associated kinase 1 (IRAK1) with multiple independent siRNAs as antiviral host genes against CVB and PV in HBMEC (Figure 2E, Table S1B). In contrast, downregulation of TLR7, which is closely related to TLR8, had no effect (Figure 2E). To further study the role for TLR8 and IRAK1, we overexpressed dominant-negative mutants of TLR8 (TLR8ΔTIR), IRAK1 (IRAK1DN), or the TLR8 adaptor Myeloid differentiation primary response gene (88) (MyD88) and determined the effects of this expression on CVB and PV infection of HBMEC. Consistent with our screening results, we found that overexpression of dominant-negative mutants of TLR8, IRAK1, or MyD88 all led to an enhancement of CVB and PV replication in HBMEC (Figure 2F). Similar to our findings with Akts and MAPKs (Figure S2Bi), we also found that RNAi-mediated silencing of IRAK1 and TLR8 enhanced CVB replication in nonpolarized U2OS cells (Figure S2Biii and Table S2). Taken together, these data implicate TLR8 as playing a prominent role in the detection of enterovirus infections in the BBB and perhaps in other non-polarized cell types.
Adenylate Cyclases Mediate CVB and PV Infection of HBMEC
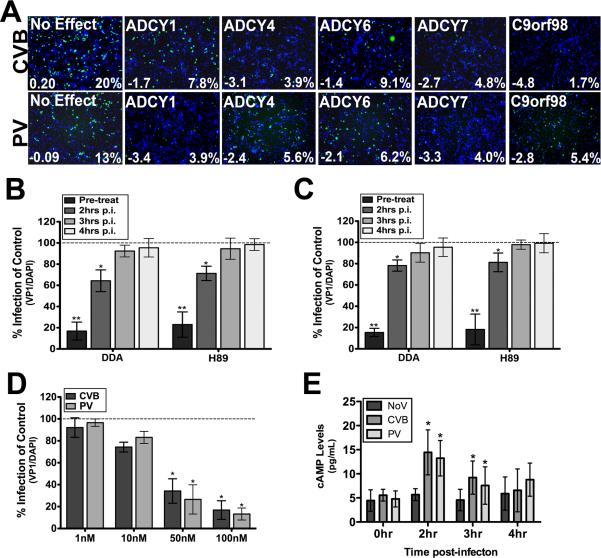
In contrast to the cellular factors described above that played antiviral roles against both viruses; we identified a number of factors whose depletion significantly impaired replication of both CVB and PV. Among these factors were several members of the adenylate cyclase (ADCY) family. ADCYs catalyze the conversion of ATP into cAMP, an important second messenger that regulates a diverse array of cellular process and often converges of cAMP dependent protein kinase (PKA) activation. ADCYs can be categorized into four groups (classified as A-D) that differ in their upstream modulators. We identified ADCY family members in groups A-C, including ADCY1, ADCY4, ADCY6, and ADCY7 with multiple independent siRNAs which were each required for infection by CVB and PV (Figure 3A, Table S2). We also identified a putative adenylate kinase-like protein (C9orf98) with multiple siRNAs, which was the strongest candidate gene in our primary CVB screen (Figure 3A, Table S1B). We used an ADCY inhibitor to confirm a role for ADCYs in facilitating CVB and PV replication and to dissect at which step ADCYs may be required for viral replication. Treatment of cells with the broad spectrum ADCY inhibitor 2,5-dideoxy-adenosine (DDA) led to a significant dose-dependent inhibition of both CVB and PV replication in HBMEC, consistent with our RNAi screen findings (Figure 3B, 3C, 3D). Mechanistically, the role of ADCYs seemed to occur early in infection as DDA lost its inhibitory effect when added at early time post-infection (2-3hrs p.i.) (Figure 3B, 3C).
Figure 3. Adenylate Cyclase are Required for CVB and PV Replication.
(A) Decreased CVB and PV replication in HBMEC transfected with adenylate cyclase 1 (ADCY1), ADCY4, ADCY6, ADCY7, and C9orf98 siRNAs compared to an siRNA which had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (B, C) CVB (B) and PV (C) infection of HBMEC is diminished when cells are pre-treated with DDA or H89, but not when either inhibitor is added at >2hrs post-infection (p.i.). (D), Dose-dependent inhibition of CVB replication in HBMEC treated with the indicated concentration of DDA. (E), Elevated cAMP levels in HBMEC infected with CVB or PV for the indicated times. For (B-E), data are shown as mean ± SD; *p < 0.05, **p<0.001.
If ADCYs play a role mediating enterovirus infection, then levels of cAMP should increase during the course of infection. Indeed, we found that cAMP levels increased significantly in HBMEC infected with CVB or PV within 2hr p.i. [which corresponds to the time that ADCY activity is required (Figure 3E)]. Since PKA is a major downstream effector of ADCYs and is dependent upon cAMP for its activation, we tested whether PKA was important for enteroviral infection. We found that CVB and PV infection were attenuated when cells were treated with H89, a potent selective inhibitor of PKA (Figure 3B, 3C). Together, these data implicate a prominent role for ADCY-dependent cAMP generation in enterovirus infection of HBMEC, and likely at an early step in the replication cycle.
cAMP Signaling Mediates CREB-dependent Transcription During CVB and PV Infection of HBMEC
As we observed a role for ADCYs, cAMP and PKA signaling in CVB and PV infection of HBMEC, we next determined the mechanism(s) by which these signaling events regulated infection. cAMP-dependent activation of PKA has been shown to result in activation of gene transcription via the transcription factor cAMP-response element (CRE)-binding protein (CREB). CREB is directly phosphorylated by PKA at Serine133 (pSer133) and once activated, binds to the nuclear factor CREB binding protein (CREBBP), or a closely related protein p300, to induce gene transcription. Pathway analysis revealed several components of CREB-dependent transcription as being required for CVB and PV infection of HBMEB (p<10-5) (not shown). These included CREBBP, RNA polymerase II (POLR2K), and the transcription factor STAT1 (Figure 4A, Table S1B).
Figure 4. CREB-dependent transcription is involved in CVB and PV replication in HBMEC.
(A), Decreased CVB and PV replication in HBMEC transfected with CREBBP, RNA polymerase II (POLR2K), or STAT1 siRNAs compared to an siRNA which had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (B), Activation of CRE-dependent transcription as assessed by luciferase assay in CVB or PV-infected HBMEC either without inhibitor (NoI) or in the presence of DDA. Data are presented as mean ± SD; *p < 0.05 and are displayed as fold change over uninfected (NoV) controls. (C), Immunofluoresence microscopy for total CREB (magenta), activated pS133 CREB (green), VP1 (red), and DAPI-stained nuclei (blue) in HBMEC infected with CVB (left) or PV (right) for the indicated times. (D, E), Quantification of nuclear localized pSer133-CREB (D) and total CREB (E) in uninfected (NoV) cells or in cells infected with CVB or PV for the indicated times. For (B) (D), and (E), data are shown as mean ± SD; *p < 0.05. See also Figure S3.
We found that CVB and PV infection of HBMEC induced significant enhancement of CRE-mediated gene transcription as assessed by CRE-luciferase reporter assays (Figure 4B). This enhancement required the activity of ADCYs as treatment of cells with the ADCY inhibitor DDA inhibited CVB- and PV-induced CRE activation (Figure 4B). We also found CVB- and PV-induced CRE activation in human embryonic kidney cells (HEK293) (Figure S3).
Furthermore, using immunofluorescence microscopy with an antibody specific to activated (pSer133) CREB, we observed significant increases in CREB activation in CVB- and PV-infected HBMEC (Figure 4C). Increased nuclear staining for pSer133 CREB was evident within 2hrs p.i. in both CVB- and PV-infected HBMEC and persisted until ~3hrs p.i. (Figure 4C, 4D). This is prior to the appearance of newly replicated viral RNA or protein (not shown). However, at late stages of viral replication (>5hrs p.i.), there was a marked decrease in pSer133 CREB and a corresponding decrease in overall CREB levels (Figure 4C, 4D, 4E). These data indicate that ADCY-dependent cAMP generation induces CREB-mediated gene transcription to facilitate enterovirus replication and that there is temporal control as the system is shut-off during later times post infection.
Endosomal Trafficking of Internalized CVB and PV Particles is Regulated by LMTK2
Another common factor required for CVB and PV infection of HBMEC and identified by our RNAi screening (with multiple independent siRNAs) was lemur tyrosine kinase 2 (LMTK2) (Figure 5A, Table S1B). While microarray data suggested that LMTK2 was not expressed in HBMECs, we detected protein expression by immunoblot that was reduced upon siRNA-mediated knock down (Figure 5D, Table S1B). LMTK2 has been shown to associate with the actin-based motor protein myosin IV and regulate endosomal trafficking events (Chibalina et al., 2007; Inoue et al., 2008). Because of the association between LMTK2 and vesicular trafficking, we investigated whether this kinase might facilitate the cytoplasmic trafficking of CVB and PV upon their internalization into HBMEC.
Figure 5. LMTK2 Regulated Endosomal Trafficking of CVB and PV.
(A), Decreased CVB and PV replication in HBMEC transfected with LMTK2 siRNA compared to an siRNA which had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (B), Immunofluorescence microscopy for Rab5 GTPase (top) or early endosome antigen-1 (EAA1) (bottom) in HBMEC transfected with either a control (scrambled, Con) or LMTK2 siRNA. (C), Immunofluorescence microscopy for CVB (top) or PV (bottom) at the indicated times in cells transfected with either a control (scrambled, Con) or LMTK2 siRNA. (D), Immunoblot for LMTK2 in HBMEC transfected with either control (siCon) or LMTK2 (siLMTK2) siRNAs (GAPDH is included as a loading control). (E), CVB and PV replication in either HBMEC or Caco-2 cells transfected with controls or LMTK2 siRNAs. Data are presented as mean ± SD; *p < 0.05, and are normalized to control siRNA-transfected cells.
Consistent with its role in vesicular trafficking, we found that RNAi-mediated silencing of LMTK2 expression altered the morphology of endosomal compartments within HBMEC as assessed by immunoflorescence microscopy for early endosome antigen-1 (EEA1) and Rab5 GTPase (Figure 5B, 5D). Moreover, whereas internalized CVB and PV particles reached a perinuclear compartment within 60min (CVB) or 2.5hrs (PV) p.i. in HBMEC transfected with a control siRNA, we found that LMTK2 silencing induced the appearance of mislocalized CVB- and PV-containing vesicles within the cytoplasm that failed to traffic to a perinuclear compartment (Figure 5C, 5D). In contrast, we found that LMTK2 siRNA had no effect on CVB or PV infection of polarized intestinal Caco-2 cells, indicating that its function in intracellular trafficking of internalized CVB and PV particles may be specific to polarized endothelium (Figure 5E).
Unique Rab GTPases Regulate CVB and PV Trafficking in HBMEC
In addition to the genes that facilitated infection of both CVB and PV, we also identified genes that specifically facilitated CVB or PV infection. This included the Rab GTPase Rab17 as a specific regulator of CVB, but not PV, infection (Figure 6A, Table S1B). Rab GTPases are important regulators of vesicular trafficking and often display specificity with regard to their endosomal localization and functioning (Zerial and McBride, 2001). Rab17 is a polarized cell-specific Rab GTPase family member known to regulate apical vesicular trafficking in polarized cells (Hunziker and Peters, 1998; Lutcke et al., 1993). While microarray analysis suggested that Rab17 was not expressed in HBMEC, we found that it is expressed at low levels (Table S1B, not shown). To verify that the phenotype was due to depletion of Rab17 and not an off-target effect, we generated a Rab17 allele that was resistant to siRNA-mediated silencing (siRes). We found that indeed we could rescue the defect in infection mediated by siRNA against Rab17 by expression of the resistant Rab17 allele (Figure 6B).
Figure 6. Unique Rab GTPases Regulate CVB and PV Infection.
(A), CVB and PV replication are differentially impacted in HBMEC transfected with Rab17 siRNA Rab34 siRNA or an siRNA that had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (B), HBMEC expressing dominant-negative or wild-type forms of Rab5, Rab17, or Rab34 were infected with CVB or PV. The graph shows the percent of transfected cells expressing VP1 (mean ±SD for ~2000 cells; *p < 0.05) normalized to wild-type transfected controls. (C) Immunofluorescence microscopy for CVB in HBMEC transfected with wild-type EGFP-Rab17 at 60min p.i. (D), HBMEC transfected with control or Rab17 siRNAs and co-transfected with either wild-type or siRNA-resistant (siRes) EGFP-fused Rab17. Cells were infected with CVB (48hrs post-transfection) for 8hrs and the extent of viral replication measured by immunofluorscence microscopy (VP1/DAPI). In parallel, lysates were collected and immunoblotted for GFP (top) or GAPDH (bottom). (E), HBMEC or Caco-2 cells expressing dominant-negative or wild-type forms of Rab5, Rab17, or Rab34 were infected with CVB. The graph shows the number of transfected cells expressing VP1 (mean ±SD for ~2000 cells; *p < 0.05) normalized to wild-type transfected controls. See also Table S3.
We further explored a role for Rab17 in CVB infection of HBMEC by expressing either a dominant-negative (Rab17N132I) or a constitutively active (Rab17Q77L) mutant of Rab17. Both mutants significantly reduced CVB infection of HBMEC, whereas PV infection was unaffected by either (Figure 6C and not shown). Furthermore, we found that internalized CVB particles colocalized with Rab17 (Figure 6D). The role of Rab17 in CVB infection appears to be specific for HBMEC as neither Rab17N132I nor Rab17Q77L mutants had any effect on CVB infection of Caco-2 cells (Figure 6E and not shown).
Since Rab17 had no affect on PV infection, we reasoned that there might be another Rab responsible for its entry and/or trafficking. Therefore, we reanalyzed our primary RNAi screen data and found that Rab34 was the only other Rab to have a significant effect on PV infection [and only did so with PV with a Z-score of -1.84, thus missing our cut-off (Table S3 contains a list of z-scoes of all Rab GTPases contained in our primary screen)]. Rab34 has been identified as playing a role in macropinocytosis as well as trafficking within the Golgi (Goldenberg et al., 2007; Sun et al., 2003). Therefore, we tested whether Rab34 played a role in PV or CVB infection using secondary siRNAs and indeed observed an effect on PV but not CVB infection (Figure 6A). Furthermore, we found that overexpression of a dominant-negative mutant of Rab34 (Rab34T66N) significantly impaired PV replication while having no effect on CVB infection of HBMEC (Figure 6B). As a control, we also tested whether a dominant-negative form of Rab 5 GTPase, which was not identified in our RNAi screen, had any effect on CVB or PV infection (Figure 6A). Consistent with our screen data, we found that dominant-negative Rab5 (Rab5S34N) had no effect on CVB or PV infection of HBMEC (Figure 6B). Thus, while Rab5 and Rab34 GTPases are required for uptake of CVB into Caco-2 cells (Coyne et al., 2007b), they are dispensable for CVB infection of HBMEC (Figure 6E). These findings implicate vesicular trafficking mediated by specific RabGTPases as cell-type and enterovirus-specific regulators of entry.
Unique Tyrosine Kinases and Phosphatases Control CVB and PV Infection of HBMEC
One important consequence of receptor binding by viruses is the activation of intracellular signaling molecules that regulate entry. Our previous studies established a role for the Src family non-receptor kinase (SFK) member Fyn in mediating CVB entry into Caco-2 cells (Coyne and Bergelson, 2006). Although the RNAi library used in our screening included siRNAs targeting all 9 SFKs (including Fyn), Yes kinase was the only SFK member identified as being required for infection by CVB in HBMEC (Figure 7A, Table S1b). Interestingly, PV infection of HBMEC was also independent of Yes kinase (and Fyn) but instead required the expression of Lyn kinase (Figure 7A, Table S1B).
Figure 7. Tyrosine kinases and Phosphatases Regulate CVB and PV Infection.
(A) CVB infection of HBMEC is inhibited by downregulation of Yes kinase (but not Lyn kinase) whereas PV infection requires Lyn, but not Yes kinase. Shown are HBMEC transfected with Yes or Lyn siRNAs compared to an siRNA which had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (B) HBMEC were transfected with dominant negative mutants of Yes (K308M) or Lyn (Y397F) kinases and infected with CVB or PV. Dashed line indicates the infection level of wild-type-transfected cells (shown: mean ±SD; *p < 0.05). (C) In vitro kinase measurement of HBMEC infected with CVB or PV. Src kinase activity was measured by phosphorylation of Src substrate peptide. Data are shown as mean±SD; *p < 0.05). (D) Shown are HBMEC transfected with PTPN2, PTPRF (LAR), or PTPN18 siRNAs compared to an siRNA which had no effect on viral replication. Shown in white text are the z-score (bottom left) and percent infection (bottom right) from the field displayed. Green, VP1 and blue, DAPI. (E) HBMEC were either pre-treated with phosphatase inhibitor IV or inhibitor was added at 3hrs p.i. with CVB or PV. Shown is the level of CVB or PV infection (mean ± SD; *p < 0.05), normalized to no inhibitor (control) cells. (F), In vitro phosphatase assay in HBMEC infected with CVB or PV at the indicated times (shown: mean ±SD; *p < 0.05).
We further explored the role for these SFKs in mediating CVB and PV infection by using dominant negative kinases. We found that expression of dominant-negative Yes kinase (YesK305M) led to a significant inhibition of CVB infection of HBMEC while having no effect on PV (Figure 7B). Likewise, expression of dominant-negative Lyn kinase (LynY397F) led to a significant inhibition of PV infection in HBMEC while having no effect on CVB (Figure 7B). These results confirm a specific role for both Yes and Lyn kinases in the infection of HBMEC by CVB and PV, respectively. We next assayed the kinetics of CVB-and PV-induced SFK activation. We found that CVB induced the rapid activation (<15min p.i.) of SFKs in HBMEC (Figure 7C). In contrast, PV infection did not lead to a significant induction of SFK activation (Figure 7C). Our previous studies have shown that PV entry into HBMEC occurs via a SFK independent mechanism and instead requires the activity of tyrosine phosphatases (Coyne et al., 2007a). Therefore, Lyn kinase likely plays a role in post-entry events associated with PV infection.
Tyrosine phosphatases function to tightly regulate a variety of cellular processes in coordination with tyrosine kinase signaling. In addition to the SFKs mentioned above, we identified a number of protein tyrosine phosphatases (PTPs) by RNAi screening that were involved in both CVB and PV infection, or specifically regulated infection by either virus with multiple siRNAS (Figure 7D, Table S1B). Whereas non-receptor PTPN2 was identified as playing a role in CVB and PV replication, receptor PTPRF (LAR) was involved in CVB infection and non-receptor PTPN18 was specifically required for PV infection of HBMEC (Figure 7D). We studied the role for PTPs in regulating CVB and PV infection of HBMEC by treating cells with a pan-PTP pharmacological inhibitor. We found that replication of both CVB and PV was diminished in cells treated with PTP inhibitor only when this inhibitor was added early in infection (Figure 7E), indicating that they may function at or near the time of virus entry. Consistent with this, we found robust activation of PTPs coincident with CVB and PV entry into HBMEC as assessed by in vitro PTP activation assays (Figure 7F). These data implicate a role for PTPs in addition to SFKs as important regulators of enterovirus infection in the endothelium.
Discussion
Enterovirus infections are commonly associated with neurological disease. By performing a druggable genome RNAi screen in a physiologically relevant cell type, we have identified a number of host cell factors that facilitate infection of the endothelium comprising the BBB, a major component of host defense in the CNS. By performing a comparative screen with two enteroviruses that are both associated with CNS-related pathology, we identified two classes of genes—those that regulated infection by both viruses versus those that were required in a virus-specific context. These findings highlight the complexity associated with the cell signaling pathways hijacked by enteroviruses to facilitate their entry and/or replication in the endothelium and point to a role for several classes of molecules whose function in enterovirus infections were unknown.
Since off-target effects are a major issue in interpreting gene-lists from RNAi screens we took a very conservative approach. First, we used a stringent cut-off in the primary screen (p<0.001). Second, rather than deconvoluting the original pools used for screening we ordered independent siRNAs from another company. This should remove any biases associated with siRNA prediction programs or peculiarities associated with synthesis. The ‘reagent redundancy’ argument states that the more siRNAs that have the same phenotype the more likely the phenotype is on-target (Echeverri and Perrimon, 2006). By these criteria, 100 genes validated with at least 2 of the 3 secondary siRNAs and 17 had 1 additional Qiagen siRNA validate (none of these had seed matches with the original pools) in addition to the original primary screen pool. Therefore, we had a validation rate of 38% for ≥2 siRNAs which is similar to other published screens (Brass et al., 2008). Furthermore, of the 930 secondary siRNAs we screened, 62 were validated by Qiagen to knock-down the gene-of-interest. In fact, 77% of these ‘validated knockdown’ siRNAs in our secondary screen scored as positives while only 22% of the ‘unvalidated’ ones did suggesting that we are likely missing some genes (false negatives) due to poor knock down (Table S1b, S1C). Since off-target effects are difficult to predict, each gene must be further characterized to be certain that the RNAi phenotypes are due to the gene-of-interest and not due to the targeting of another gene. To this end, for the 19 candidate genes discussed in more detail (Figures 2-7) we used orthogonal assays (Table S4). We combined pharmacological approaches with dominant negative mutants, cDNA rescue and activity assays to show that for each gene we have multiple independent lines of evidence that they are the bona fide targets that play a role in infection. Interestingly, three quarters of these validated genes impacted infection of both viruses, suggesting that the restrictive mechanisms at play in these cells are pan-enteroviral. These included members of the Akt family, which have been implicated in the regulation of CVB replication of nonpolarized cells through a pathway that might involve host cell apoptosis (Esfandiarei et al., 2004). Further studies are necessary to clarify the mechanism by which Akt signaling is antiviral.
In addition to Akt family members, we identified components of the MAPK signaling pathway as playing roles in restricting both CVB and PV Infection. CVB infection has been previously linked to biphasic ERK1/2 activation and in the cleavage of the upstream regulator of ERK, p21RasGTPase-activating protein (p21 RasGAP) in HeLa cells (Huber et al., 1999), and enhanced ERK1/2 activation has been detected in cardiac tissue from CVB-infected mice (Opavsky et al., 2002). Interestingly, we also identified p21 RasGAP as a gene whose downregulation enhanced CVB and PV infection in HBMEC (Figure 2D). Our findings thus support a model whereby increased ERK1/2 activation in CVB-infected cells may promote a robust antiviral immune response.
We also identified TLR8 as an important regulator in the control of CVB and PV replication in HBMEC. TLR8 serves as a cellular sensor for foreign ssRNA and is localized to endosomal membranes (Heil et al., 2003; Heil et al., 2004). A role for TLR8 in the detection of CVB and PV replication in HBMEC is supported by our identification of IRAK1, which serves a key role in TLR8-mediated signal propagation (Uematsu et al., 2005). TLR8 has also been shown to play an important role in neuronal functioning and innate immune signaling in the brain (Ma et al., 2006). Furthermore, our findings are consistent with a previous study suggesting that CVB RNA might be detected by TLR8 within endosomal compartments in cardiac tissue (Triantafilou et al., 2005). Our identification of TLR8 and associated signaling components suggests that this pathway plays an important role in limiting CVB and PV replication within the BBB.
We also identified 46 genes that were required for infection of both CVB and PV. This included a pathway whereby infection induced ADCY-dependent cAMP generation and subsequent CREB-mediated transcription to facilitate CVB and PV replication. Our data indicate that ADCY-dependent cAMP generation occurs within 2hrs p.i. (Figure 3D) and correlates with the activation of CREB (Figure 4C). As DDA loses its inhibitory effects early in infection (2-3 hrs p.i.), these data support a role for CREB-mediated transcription early in infection. Recently, a role for CREB-mediated upregulation of an innate immune-associated microRNA (miR-132) has been shown to facilitate Kaposi's sarcoma-associated herpesvirus infection of lymphatic endothelial cells (Lagos et al.). Our data might support a role for the early induction of CREB-generated microRNAs in the regulation of CVB and PV infection in the BBB.
While these data suggest a transcriptional response that facilitates infection it is somewhat surprising since it has been established that enteroviruses shut down host cellular transcription machinery during infection. These viruses accomplish this by expressing a virally-encoded enterovirus protease 3Cpro that cleaves several transcription factors including CREB (Yalamanchili et al., 1997) at late stages of infection (>3-4hrs p.i.). The reduction in CREB levels has been shown in CVB-infected cardiac tissue in vivo (Yang et al., 1999) and in PV-infected cells in culture (Kliewer et al., 1990; Yalamanchili et al., 1997). Consistent with this, we found that at later time points during infection of HBMEC by CVB or PV CREB levels were reduced. Altogether, our screen identified a role for this signaling pathway in facilitating enterovirus infection.
Following endocytosis, virus-containing vesicles must navigate through a complex network of endosomal compartments in order to gain access to deeper sites within the cytoplasm. In polarized cells, the endosomal trafficking system is under tight regulation by a number of cellular components and perturbations of these components may prevent viruses from accessing sites of uncoating, thus trapping viral particles at various stages of the endosomal pathway. We identified several host factors involved in the regulation of CVB and PV endosomal trafficking in HBMEC. LMTK2, regulated the trafficking of both CVB and PV, while Rab17 was specifically required for CVB trafficking. Both genes were only required in HBMEC as LMTK2 and Rab17 were dispensable in polarized epithelial Caco-2 cells. These results suggest that CVB and PV entry into HBMEC occurs by distinct mechanism from that in Caco-2 cells and indicates that viral entry into polarized cell types is a complex process that likely requires specialized host factors.
Previously, we found that CVB and PV entry into polarized cells requires the specific activation of tyrosine kinases and phosphatases (Coyne and Bergelson, 2006; Coyne et al., 2007a; Coyne et al., 2007b). At a minimum, these signals are essential for the reorganization of the cortical actin network, modulation of tight junction barrier function, and stimulation of viral endocytosis. Our RNAi screening results indicate that Src family kinases play an important role in mediating CVB and PV infection of HBMEC utilizing distinct members of the SFK family (Yes kinase for CVB and Lyn kinase for PV) to promote their infection. In addition, our screening results highlight the role of protein tyrosine phosphatases in regulating infection of CVB and PV as we identified both common (PTPN2) and unique genes (PTPRF, PTPN18) that regulated infection. The specificity of the kinases and phosphatases involved in infection likely arise from differences in the signals initiated by receptor binding and highlights the unique intracellular signaling molecules involved in regulating viral infections, even within the same family of viruses.
Within the gene-set identified as required for the infection of CVB, there was an enrichment of factors involved in the regulation of intracellular Ca2+ (Cai2+) signaling and factors that directly depend on elevated Cai2+ levels for their activation (Figure S5). We identified components involved in multiple stages of Cai2+ signaling including genes associated with the release of Ca2+ from ER-derived stores (ITPR1), Ca2+-dependent cellular components (CAPN2, CAPN13), and the return of Cai2+ to resting basal levels (ATP2B1). Recently, we found that CVB requires the induction of Cai2+ to facilitate its entry into HBMEC (Bozym et al., 2010). The additional genes identified in the current study expand our understanding of the host factors involved in this calcium-dependent step in the virus lifecycle.
Polarized cell layers are common sites of virus entry. In particular, enteroviruses are adept at infecting polarized cells and have therefore developed efficient strategies to exploit the epithelial and endothelial barrier. The molecules identified in this study may represent therapeutic targets of the endothelium toward a class of pathogens for which there are no effective therapeutics.
Experimental Procedures
Cells and viruses
HBMEC and Caco-2 cells were cultured as described previously (Coyne and Bergelson, 2006; Coyne et al., 2007a). Primary HBMEC were isolated and cultured as described (Stins et al., 2001). All experiments were performed with CVB3-RD or PV Sabin 2, as described. Experiments measuring productive virus infection were performed with 3 PFU/cell (CVB) or 1 PFU/cell (PV) for approximately 14 hrs. For virus entry assays, both CVB and PV were used at 50-100 PFU/cell.
Antibodies
Mouse anti-enterovirus VP1 (Ncl-Entero) was obtained from Novocastra Laboratories (New Castle upon Tyne, UK). Goat anti-EEA1 and rabbit anti-LMTK2 and GAPDH were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-Rab5 anti-Akt2, and CREB pSer133 and mouse anti-CREB were from Cell Signaling Technologies. Alexa fluor-conjugated secondary antibodies were from Invitrogen (Carlsbad, CA).
High-throughput RNAi screening
Pooled siRNAs (3 sites/target, 25nM final concentration; Ambion Silencer Library catalog number 81843, set ID 81990) or single siRNAs (3/gene, 25nM final concentration, Qiagen) spotted in collagen-coated 384-well plates were complexed with HiPerfect (0.5μL/well in 9.5μL OptiMem). For a full list of siRNA sequences of validated genes, see Table S7. Following complexing, HBMEC [4000 cells/well, cultured as described (Coyne et al., 2007a; Stins et al., 2001)] were added to each well 48 hrs later infected with either Coxsackievirus B3 (3 PFU/cell) or Poliovirus Sabin 2 (1 PFU/cell) for 14 hrs (Coyne and Bergelson, 2006; Coyne et al., 2007a). Cells were then fixed with methanol/acetone (3:1), washed, incubated with monoclonal anti-VP1, washed, and incubated with Alexa Fluor 488-conjugated secondary antibody in PBS containing 4′,6-diamidino-2-phenylindole (DAPI). All solutions were added using an automated liquid handling (Well-mate, Thermo Fisher) to limit well-to-well variability.
High Content Image Capturing and Data Analysis
Images were captured (3 sites/well) at 10X using an ImageXpressMicro Microscope (Molecular Devices). Automated image analysis (MetaXpress) was used to calculate the number of cells (Dapi+) and the number of infected cells (VP1+). These values were used to calculate the median and interquartile range (on log transformed data), which were then used to calculate robust Z scores. [The median of each plate in the screen was used to calculate baseline]. Hits were identified as those wells that exhibited a change in infection by the indicated standard deviations from calculated Z scores within each plate.
Supplementary Material
Acknowledgements
We are grateful to those who have generously shared reagents. We thank the Penn Cell Based Screening Core for technical assistance, and Dr. Robert Doms for careful review of the manuscript. This work was supported by grants from the NIH [R01AI081759 (CBC) and R01AI074951, U54AI057168 (SC)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bozym RA, Morosky SA, Kim KS, Cherry S, Coyne CB. Release of intracellular calcium stores facilitates coxsackievirus entry into polarized endothelial cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, Perrimon N. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2006;2:e102. doi: 10.1371/journal.ppat.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalina MV, Seaman MN, Miller CC, Kendrick-Jones J, Buss F. Myosin VI and its interacting protein LMTK2 regulate tubule formation and transport to the endocytic recycling compartment. J Cell Sci. 2007;120:4278–4288. doi: 10.1242/jcs.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Kim KS, Bergelson JM. Poliovirus entry into human brain microvascular cells requires receptor-induced activation of SHP-2. Embo J. 2007a;26:4016–4028. doi: 10.1038/sj.emboj.7601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007b;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonarain R, Cerullo D, Fuse K, Liu PP, Fish EN. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation. 2004;110:3540–3543. doi: 10.1161/01.CIR.0000136824.73458.20. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Perrimon N. High-throughput RNAi screening in cultured cells: a user's guide. Nat Rev Genet. 2006;7:373–384. doi: 10.1038/nrg1836. [DOI] [PubMed] [Google Scholar]
- Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol. 2004;78:4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- Goldenberg NM, Grinstein S, Silverman M. Golgi-bound Rab34 is a novel member of the secretory pathway. Mol Biol Cell. 2007;18:4762–4771. doi: 10.1091/mbc.E06-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–2997. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Huber M, Watson KA, Selinka HC, Carthy CM, Klingel K, McManus BM, Kandolf R. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J Virol. 1999;73:3587–3594. doi: 10.1128/jvi.73.5.3587-3594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kon T, Ohkura R, Yamakawa H, Ohara O, Yokota J, Sutoh K. BREK/LMTK2 is a myosin VI-binding protein involved in endosomal membrane trafficking. Genes Cells. 2008;13:483–495. doi: 10.1111/j.1365-2443.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- Jackson WT, Giddings TH, Jr., Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji WT, Liu HJ. PI3K-Akt signaling and viral infection. Recent Pat Biotechnol. 2008;2:218–226. doi: 10.2174/187220808786241042. [DOI] [PubMed] [Google Scholar]
- Kliewer S, Muchardt C, Gaynor R, Dasgupta A. Loss of a phosphorylated form of transcription factor CREB/ATF in poliovirus-infected cells. J Virol. 1990;64:4507–4515. doi: 10.1128/jvi.64.9.4507-4515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, et al. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 12:513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Lancaster KZ, Pfeiffer JK. Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke A, Jansson S, Parton RG, Chavrier P, Valencia A, Huber LA, Lehtonen E, Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J Cell Biol. 1993;121:553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M.a.P., M. Human Enterovirus Infections. American Society for Microbiology; Washington, D.C.: 1995. [Google Scholar]
- Opavsky MA, Martino T, Rabinovitch M, Penninger J, Richardson C, Petric M, Trinidad C, Butcher L, Chan J, Liu PP. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J Clin Invest. 2002;109:1561–1569. doi: 10.1172/JCI13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallansch M, Roos R. Enteroviruses: Polioviruses, Coxsackieviruses, Echoviruses, and Newer Enteroviruses. In: Knipe D, Howley P, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 723–775. [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins MF, Badger J, Kim KS. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- Sun P, Yamamoto H, Suetsugu S, Miki H, Takenawa T, Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–4071. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou K, Orthopoulos G, Vakakis E, Ahmed MA, Golenbock DT, Lepper PM, Triantafilou M. Human cardiac inflammatory responses triggered by Coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell Microbiol. 2005;7:1117–1126. doi: 10.1111/j.1462-5822.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ., Jr. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–4252. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation. 2001;103:756–761. doi: 10.1161/01.cir.103.5.756. [DOI] [PubMed] [Google Scholar]
- Yalamanchili P, Datta U, Dasgupta A. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J Virol. 1997;71:1220–1226. doi: 10.1128/jvi.71.2.1220-1226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Yu J, Luo Z, Carthy CM, Wilson JE, Liu Z, McManus BM. Viral myocarditis: identification of five differentially expressed genes in coxsackievirus B3-infected mouse heart. Circ Res. 1999;84:704–712. doi: 10.1161/01.res.84.6.704. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.