Abstract
Background
The incidence of thyroid cancer is four to five times higher in women than in men, suggesting a role for estrogen (E2) in the pathogenesis of thyroid proliferative disease (TPD) that comprises cancer and goiter. The objective of this study was to investigate the antiestrogenic activity of 3,3′-diindolylmethane (DIM), a bioactive compound derived from cruciferous vegetables, in patients with TPD.
Methods
In this limited phase I clinical trial study, patients found to have TPD were administered 300 mg of DIM per day for 14 days. Patients subsequently underwent a total or partial thyroidectomy, and tissue, urine, and serum samples were collected. Pre- and post-DIM serum and urine samples were analyzed for DIM levels as well as estrogen metabolites. DIM levels were also determined in thyroid tissue samples.
Results
DIM was detectable in thyroid tissue, serum, and urine of patients after 14 days of supplementation. Urine analyses revealed that DIM modulated estrogen metabolism in patients with TPD. There was an increase in the ratio of 2-hydroxyestrones (C-2) to 16α-hydroxyestrone (C-16), consistent with antiestrogenic activity that results in more of C-2 product compared with C-16.
Conclusion
Our data suggest that DIM enhances estrogen metabolism in TPD patients and can potentially serve as an antiestrogenic dietary supplement to help reduce the risk of developing TPD. The fact that DIM is detected in thyroid tissue implicates that it can manifest its antiestrogenic activity in situ to modulate TPD.
Introduction
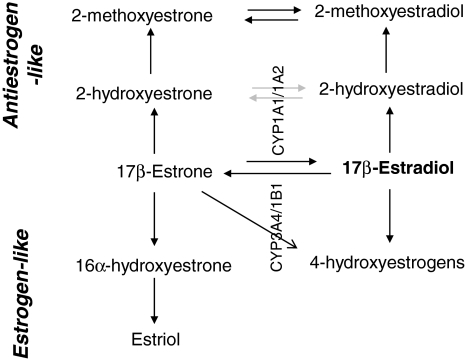
Estrogens are a group of three biochemically distinct hormones, estradiol (E2), estrone (E1), and estriol (E3), with 17β-estradiol being the active form. The mitotic activity of estradiol in estrogen responsive tissue such as breast has resulted in development of several antiestrogenic compounds as preventive or therapeutic agents. Premenopausal women naturally produce hundreds of micrograms of estrogen daily, which is enzymatically converted to specific estrogen metabolites such as 2-hydroxyestrogens (2-OHE's), 2-methoxyestrogens, 16α-hydroxyestrone (16-OHE1), and 4-hydroxyestrogens (4-OHE's) (1). Two estrogen metabolites, in particular, 16-OHE1 and 2-OHE, have contrasting cellular activities with 16-OHE1 being an estrogen agonist, whereas 2-OHE is an estrogen antagonist. Some other estrogen metabolites, 4-OHE1 and 4-OHE2, are also estrogen agonists but their relative concentrations are too low to be of physiological significance. Several studies using cell culture models have demonstrated the proliferative effects of 16-OHE1 and the antiproliferative effects of 2-OHE (1–4). The relative concentration of 2-OHE:16-OHE1 can increase or decrease the risk of developing hormone responsive cancers (breast and cervical) (1,2,5–9) (Fig. 1) and is used as a predictive biomarker for these cancers (10) but has not been evaluated in response to chemopreventive agents in thyroid proliferative diseases (TPDs).
FIG. 1.
Estradiol metabolism schema. 2-hydroxyestrone and 16-alphahydroxyestrone are the major metabolites in estrogen metabolism.
According to American Thyroid Association, women are four to five times more prone to developing thyroid disorders than men, with pregnancy and early menopause increasing the risk (11), suggesting the significance of hormonal factors such as estrogen in the etiology and pathogenesis of TPD. Some studies from literature, including a recent study from our laboratory, provide evidence that thyroid cells express functional estrogen receptor and are estrogen responsive (12–14). Moreover, in 2006, Chan et al. demonstrated that estrogen metabolism in patients with TPD is distinct from matched controls, with a similar low urinary 2-OHE:16-OHE1 ratio observed among TPD patients as other hormone related cancers (15). The fact that thyroid cancer cells are estrogen responsive and that TPD patients metabolize estrogen, leading to pro-mitogenic conditions, opens up a new area for the use of established therapies or discovery of novel therapies targeted at increasing the 2-OHE:16-OHE1 ratio as a desired outcome resulting from treatment of anticancer agents. In recent years, natural compounds found in diet, such as indoles, have been shown to possess antiestrogenic activity (16). It is the goal of our chemoprevention program to examine the preclinical and clinical efficacies of that compound.
Dietary indoles present in cruciferous vegetables, such as broccoli and cauliflower, have been shown to possess chemopreventive and chemotherapeutic properties against a wide variety of cancers (16–19). One such dietary indole, Indole-3-carbinol (I3C), is the bioactive phytochemical and a presumed modulator of reduced cancer risk in areas with high cruciferous vegetable consumption (16–19). We were one of the first groups to recognize anticancer properties of I3C against hormonally dependent breast and prostate cancer (16,20,21). The major drawback with clinical use of I3C as an anticancer drug is its molecular instability. I3C readily dimerizes into 3,3′-diindolylmethane (DIM), which is an acid catalyzed stable compound and a presumed active chemopreventive agent.
Our preclinical thyroid cell culture models strongly implicated the role of estradiol (12) and indicate that DIM is an indirect modulator of estradiol-mediated mitotic activity. Based on these findings, we initiated a pilot clinical study to examine the effects of oral DIM supplementation in women with TPD. This study recruited seven patients and examined the effect of oral administration of 300 mg DIM/day for 14 days. At the end of 14-day DIM supplementation, the concentration of DIM was determined in thyroid tissues and urine and serum samples of obtained from same patients. A portion of the patient's urine and serum samples were collected and analyzed for the DIM's effect on estrogen metabolism by quantitating the levels of 2-OHE and 16-OHE1. Delineating the effect of DIM on estrogen metabolism can potentially allow for the development of DIM as a novel effective antiestrogenic compound for TPD and may have implications for new modalities in the management of thyroid disease.
Materials and Methods
Patient recruitment and DIM administration
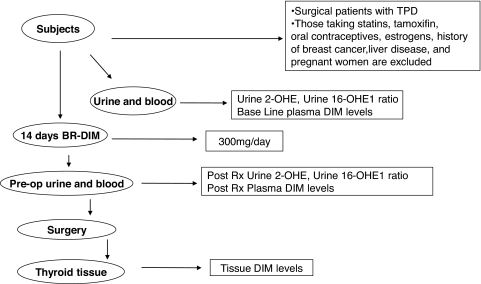
Patients were recruited at New York Eye and Ear Infirmary (New York, NY) and Westchester Medical Center (Valhalla, NY). The study protocol was approved by the Institutional Review Board committee at each institution and included informed consent by each patient. Seven female patients were recruited with varying TPDs as indicated in Table 1. These patients were already scheduled for a partial or total thyroidectomy. Blood and urine samples were collected 1 day before they were started on BioResponse® DIM tablets (Bioresponse, Boulder, CO) (300 mg/day) for 14 days. All patients were euthyroid with normal thyroid hormone levels (mean thyrotropin = 1.29 μIU/mL of serum; mean thyroxine = 1.12 nmol/L of serum). After 14 days, patients underwent total or partial thyroidectomies at which time excised thyroid tissues, urine, and serum were collected (Fig. 2). All pre- and post-DIM urine and serum samples were frozen at −80°C and thyroid tissues were snap-frozen in liquid nitrogen and stored at −80°C until the analyses were performed. Samples were evaluated for bioavailability of DIM and estrogen metabolite ratios and compared between pre- and post-DIM administration.
Table 1.
Clinical Features of Thyroid Proliferative Disease Patients Enrolled in Pilot Study
| Patient 1 | Left thyroidectomy | Multinodular goiter |
| Patient 2 | Right thyroidectomy | Follicular adenoma |
| Patient 3 | Left thyroidectomy | Multinodular goiter |
| Patient 4 | Total thyroidectomy | Invasive papillary carcinoma |
| Patient 5 | Right thyroidectomy | Follicular adenoma, |
| Patient 6 | Right thyroidectomy | Micro-papillary carcinoma |
| Patient 7 | Right thyroidectomy | Adenomatoid goiter |
FIG. 2.
Pilot study methodology schema. BR, bioresponse; DIM, 3,3′-diindolylmethane; TPD, thyroid proliferative diseases; 2-OHE, 2-hydroxyestrogen; 16-OHE1, 16α-hydroxyestrone.
Determination of levels of DIM in biological samples
DIM was extracted and quantitated from thyroid tissue, urine, and serum samples as described by Sepkovic et al. (22). Briefly, for measurement of levels of DIM in thyroid tissues, tissues were homogenized and incubated with sodium acetate buffer (pH 4.8) and glucuronidase (110,200 units/mL; Sigma, St. Louis, MO). Similarly, for determination of levels of DIM in urine and serum samples, 1 mL of sample was diluted with sodium acetate and glucuronidase buffer. The solutions were then incubated at 40°C for 24 hours. The internal standard, 4,4-dichlorodiindolylomethane (dichloro-DIM, generously provided by Dr. Stephen Safe), was then added, and the samples were extracted with chloroform. 1:4 of dry pyridine and N,O-bis (trimethylsilyl) trifluoroacetamide, catalyzed with 1% trimethyl-chlorosilane (Pierce Chemical, Rockford, IL), were added, and the samples were heated to 100°C for 1 hour. One microliter of each sample was injected into gas chromatography–mass spectrometry without further treatment. Gas chromatography–mass spectrometry conditions were identical as described by Sepkovic et al. (22). DIM concentrations in urine were normalized using creatinine levels. Urine creatinine was determined spectrophotometrically using a Beckman Creatinine Analyzer II. After the ELISA was performed, the DIM concentrations were normalized per milligram of creatinine in urine samples.
Examination of estrogen metabolites levels in urine
Analyses of 2-OHE and 16-OHE1 were performed using a competitive solid-phase enzyme immunoassay (IMMUNA CARE Corporation, Bethlehem, PA). Each urine sample was also assayed for creatinine levels to normalize the absolute values of 2-OHE and 16-OHE1 using a Beckman Creatinine Analyzer II. The estrogen metabolites in urine samples were normalized per milligram of creatinine.
Statistical calculation
Statistical significance was determined using the paired Student's t-test with a probability (p-value) ≤0.05 used to reject the null hypothesis.
Results
A total of seven patients were recruited for the study; three patients had multinodular goiter, two patients had follicular adenoma, and two patients had papillary carcinoma. All the patients were between ages of 39–56 years (mean age 46.7 years), with three patients being premenopausal and four patients postmenopausal. Body mass index of these patients was 25.7–35.3 kg/m2 (mean 30.6 kg/m2). None of these patients were taking oral contraceptives, tamoxifen, estrogens, or statins; had any history of breast cancer, severe systemic diseases, or liver diseases; or were pregnant or breast feeding. Blood and urine samples were collected 1 day before the patients were started a DIM regimen. DIM tablets (75 mg × 4 per day) were given to patients for 14 days after which patients underwent partial thyroidectomy except patient 4, who underwent total thyroidectomy as described in Table 1. At day 14, thyroid tissue and serum was collected to determine the concentration of DIM. Urine was also collected to determine the concentration of DIM as well as for analysis of estrogen metabolites (Fig. 2).
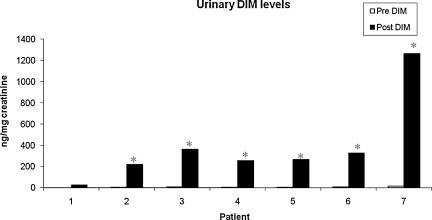
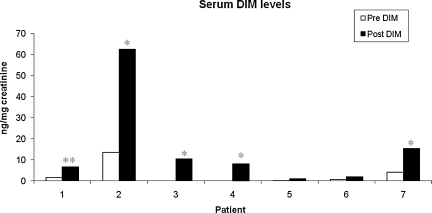
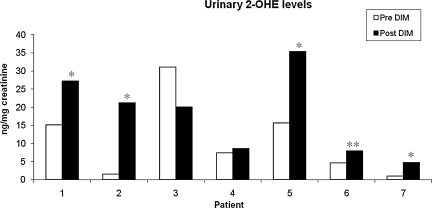
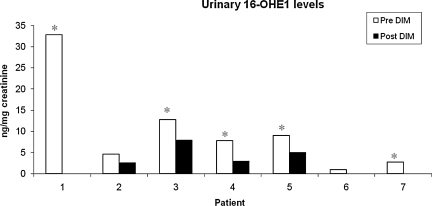
To determine the effect of DIM on estrogen metabolism, we first evaluated levels of DIM in urine samples of patient's pre- and post-DIM administration. After 14 days of DIM administration, the levels of DIM were significantly increased in urine samples for all seven patients (Fig. 3). The mean increase in urine DIM levels was 383.5 ng/mg of creatinine. Further, we found that the levels of DIM in serum were also increased significantly after DIM administration (Fig. 4) with a mean increase of 12.32 ng/mg of creatinine. We also observed that bioresponse-DIM was taken up by patient's thyroid tissues with a mean concentration 40.67 ng of DIM/g of thyroid tissue (range = 0.8–128.7 ng/g of thyroid tissue) after 14 days (Table 2). Further, our results indicate an increase in the 2-OHE level for six out of seven patients (Fig. 5), with a mean increase of 6.98 ng/mg creatinine. A decrease in the 16-OHE1 level was observed for all seven patients (Fig. 6), with a mean decrease of 7.47 ng/mg of creatinine (range 0–7.9 ng/mg of creatinine). These studies were undertaken to define the systemic activity of DIM and its stability in biological fluids as well as to define estrogen metabolic byproduct(s) as a biological surrogate intermediate biomarker. This intermediate surrogate bio-marker could serve to detect the ability of DIM and/or patient compliance.
FIG. 3.
Levels of DIM in urine samples. DIM (300 mg/day) was orally administrated for 14 days and urine samples were collected pre- and post-DIM administration. White bars are pre-DIM levels and black bars are post-DIM levels. Data expressed as DIM ng/mg of creatinine. The asterisk denotes statistically significant increase (*p < 0.05) in the post-DIM urine samples as compared with pre-DIM urine samples.
FIG. 4.
Levels of DIM in serum samples. DIM (300 mg/day) was orally administrated for 14 days and serum was collected pre- and post-DIM administration. White bars are pre-DIM levels and black bars are post-DIM levels. Data expressed as DIM ng/mg of creatinine. Single asterisk (*p < 0.05) and double asterisk (**p < 0.01) denote statistically significant increase in the in the post-DIM serum samples as compared with pre-DIM serum samples.
Table 2.
Concentration of 3,3′-Diindolylmethane in Thyroid Tissue of Thyroid Proliferative Disease Patients
| Patient 1 | 120 ng/g |
| Patient 2 | 3.2 ng/g |
| Patient 3 | 128.7 ng/g |
| Patient 4 | 2.2 ng/g |
| Patient 5 | 0.8 ng/g |
| Patient 6 | 15.0 ng/g |
| Patient 7 | 14.8 ng/g |
FIG. 5.
Levels of urinary 2-OHE. An increasing trend in 2-OHE was observed in urine samples of all patients except patient 3 post-DIM administration. White bars are pre-DIM levels and black bars are post-DIM levels. Data expressed as 2-OHE ng/mg of creatinine. Single asterisk (*p < 0.05) and double asterisk (**p < 0.01) denote statistically significant increase in the in the post-DIM samples as compared with pre-DIM urinary 2-OHE samples.
FIG. 6.
Levels of urinary 16-OHE1. 16-OHE1 concentrations were decreased in urine samples of all patients post-DIM administration. White bars are pre-DIM levels and black bars are post-DIM levels. Data expressed as 16-OHE1 ng/mg of creatinine. The asterisk denotes statistically significant decrease (*p < 0.05) in the post-DIM samples as compared with pre-DIM urinary 16-OHE1 samples.
Discussion
Diet has long been considered of prime importance in its inverse association with cancer risk with consumption of dietary products, such as tomatoes, soy, and cruciferous vegetables. Specifically, cruciferous vegetables contain glucobracissin, which is converted to I3C and ultimately acid-catalyzed to an I3C dimer (better known as DIM). Many in vitro and in vivo studies have demonstrated the chemotherapeutic and chemopreventive activities of DIM against several cancers, such as breast cancer, by counteracting the adverse effects of estrogen. Currently, the precise cellular and biochemical mechanism by which DIM exerts its chemotherapeutic properties remains to be fully elucidated but based on available literature, DIM interferes with signal transduction pathways. In breast and prostate cancers, DIM has been shown to induce a dose dependent apoptosis by inhibiting AKT kinase and IKK-mediated phosphorylation, thus leading to decreased cell growth and proliferation (23,24). One target of DIM, which has been observed in various studies, is modulating estrogen metabolism.
Estrogen is irreversibly converted into estrogen metabolites such as 2-hydroxyestrone (2-OHE1) and 16-OHE1, which have stronger (16-OHE1) or weaker (2-OHE1) estrogenic activity, and their relative concentration can increase or decrease the risk of one developing estrogen responsive cancers. In one study by Dalessandri et al., it was also observed that DIM increased the levels of 2-OHE in postmenopausal women with a history of breast cancer (25) resulting in an overall increase in 2-OHE/16-OHE1 ratio, thus favoring antiproliferative conditions. The average 2/16-OHE1 ratio is ∼2.27 for normal women and ratios <2.0 are observed to have an increased risk for developing estrogen-sensitive cancers, such as breast and cervical cancers. Five patients in our pilot study had an initial pre-DIM-2/16-OHE1 ratio of <2. We observed that after completing their DIM regimen, all patients had a significant increase (>2) in the overall urinary 2/16-OHE1 ratio, placing them outside the zone of predisposition for developing estrogen sensitive cancers. These results suggest that DIM modulates estrogen metabolism, thus generating metabolites with antiestrogenic activity. An increased 2/16-OHE1 ratio favors a decrease in the long-term risk for developing cancer and thus has potential to be associated with the chemopreventive activity of DIM in TPD (5–9,26).
In our study, a 14-day administration of DIM did not result in any measurable or noticeable toxicity as monitored by hematologic and liver toxicity tests such as aspartate aminotransferase/alanine aminotransferase ratio. We also detected DIM levels in the patient's thyroid tissue, serum, and urine, indicative of DIM's stability in biological samples. Our study is supported by a human study conducted by Zeligs et al. in 2002 (27), whereby they discovered that 150 mg of DIM increases the ratio of 2-hydroxyestrone to 16-OHE1 by 76% in benign or precancerous conditions. Three hundred mg of DIM increased this ratio by 170%. The concentration of DIM used in our study (300 mg DIM/day) corresponds to ∼4–5 mg/kg/day dose for an average female (∼65 kg) and is well below the 450 mg/kg/day dose of DIM where toxic responses were observed in Beagle dogs in a National Cancer Institute-sponsored study (28).
In TPD, estrogen metabolism as a surrogate marker of anticancer activity has not been studied, and hence data presented in this communication along with extensive in vitro studies conducted by us and others indicate that E2-ER interaction and estrogen metabolism is modulated by DIM. This is the first study to show sequestering of administered DIM in thyroid tissues and a favorable anticancer ratio of estrogen metabolites observed in biological fluids after DIM administration. This pilot study opens up new avenues of estrogen metabolism-based analysis of surrogate intermediate biomarkers. A further larger study will be required to assess its efficacy as a therapeutic adjuvant or preventive additive in TPD, plans for which are underway both at New York Medical College and at the New York Eye and Ear Infirmary under an NIH sponsored study.
Acknowledgments
The studies were supported by grants from National Cancer Institute 1R01-CA131946 and clinical funding from New York Eye and Ear Infirmary (New York, NY). We also thank Dr. Michael Zeligs (Bioresponse) for providing DIM.
Disclosure Statement
The authors declare that no competing financial interest exists.
References
- 1.Lord RS. Bongiovanni B. Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7:112–129. [PubMed] [Google Scholar]
- 2.Meilahn EN. De Stavola B. Allen DS. Fentiman I. Bradlow HL. Sepkovic DW. Kuller LH. Do urinary estrogen metabolites predict breast cancer? Follow up of the Guernsey III cohort. Br J Cancer. 1998;78:1250–1255. doi: 10.1038/bjc.1998.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider J. Huh MM. Bradlow HL. Fishman J. Antiestrogen action of 2-hydroxyestrone on MCF-7 human breast cancer cells. J Biol Chem. 1984;259:4840–4845. [PubMed] [Google Scholar]
- 4.Telang NT. Suto A. Wong GY. Osborne MP. Bradlow HL. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992;84:634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 5.Ho GH. Luo XW. Ji CY. Foo SC. Ng EH. Urinary 2/16alpha-hydroxyestorne ratio: correlation with serum insulin-like growth factor binding protein-3 and a potential biomarker of breast cancer risk. Ann Acad Med Singap. 1998;27:294–299. [PubMed] [Google Scholar]
- 6.Zheng W. Dunning L. Jin F. Holtzman J. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomark Prev. 1997;6:505–509. [PubMed] [Google Scholar]
- 7.Ursin G. London S. Stanczk FZ. Gentzschein E. Paganini-Hill A. Ross RK. Pike MC. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:1067–1072. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 8.Muti P. Bradlow HL. Micheli A. Krogh V. Freudenheim JL. Schunemann HJ. Stanulla M. Yang J. Sepkovic DW. Trevisan M. Berrino F. Metabolism and risk of breast cancer: a prospective analysis of 2:16 hydroxyestrone ratio and risk of breast cancer in premenopausal and postmenopausal women. Cancer Epidemiol. 2000;11:635–640. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Pasagian-Macauley A. Meilahan EN. Bradlow HL. Sepkovic DW. Buhari AM. Simkin-Silverman L. Wing RR. Kuller LH. Urinary markers of estrogen metabolism in premenopausal women. Steroids. 1996;61:461–467. doi: 10.1016/0039-128x(96)00089-x. [DOI] [PubMed] [Google Scholar]
- 10.Telang NT. Katdare M. Bradlow HL. Osborne MP. Estradiol metabolism: an endocrine biomarker for modulation of human mammary carcinogenesis. Environ Health Perspect. 1997;105:559–564. doi: 10.1289/ehp.97105s3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemal A. Siegel R. Ward E. Hao Y. Xu J. Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 12.Rajoria S. Suriano R. Shanmugam A. Wilson YL. Schantz SP. Geliebter J. Tiwari RK. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manole D. Schildknecht B. Gosnell B. Adams E. Derwahl M. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology. 1999;140:5705–5711. doi: 10.1210/endo.140.12.7197. [DOI] [PubMed] [Google Scholar]
- 14.Manole D. Schildknecht B. Gosnell B. Adams E. Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86:1072–1077. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- 15.Chan EK. Sepkovic DW. Yoo Bowne HJ. Yu GP. Schantz SP. A hormonal association between estrogen metabolism and proliferative thyroid disease. Otolaryngol Head Neck Surg. 2006;134:893–900. doi: 10.1016/j.otohns.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Ashok BT. Chen YG. Liu X. Garikapaty VP. Seplowitz R. Tschorn J. Roy K. Mittelman A. Tiwari RK. Multiple molecular targets of indole-3-carbinol, a chemopreventive anti-estrogen in breast cancer. Eur J Cancer Prev. 2002;2:S86–S93. [PubMed] [Google Scholar]
- 17.Bradlow HL. Sepkovic DW. Diet and breast cancer. Ann NY Acad Sci. 2002;963:247–267. doi: 10.1111/j.1749-6632.2002.tb04117.x. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar FH. Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bode AM. Dong Z. Cancer prevention research—then and now. Nat Rev Cancer. 2009;9:508–516. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garikapaty VP. Ashok BT. Chen YG. Mittelman A. Iatropoulos M. Tiwari RK. Anti-carcinogenic and anti-metastatic properties of indole-3-carbinol in prostate cancer. Oncol Rep. 2005;13:89–93. [PubMed] [Google Scholar]
- 21.Tiwari RK. Guo L. Bradlow HL. Telang NT. Osborne MP. Selective responsiveness of human breast cancer cells to indole-3-carbinol, a chemopreventive agent. J Natl Cancer Inst. 1994;86:126–131. doi: 10.1093/jnci/86.2.126. [DOI] [PubMed] [Google Scholar]
- 22.Sepkovic DW. Bradlow HL. Bell M. Quantitative determination of 3,3′-diindolylmethane in the urine of individuals receiving indole-3-carbinol. Nutr Cancer. 2002;41:57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 23.Li Y. Wang Z. Kong D. Murthy S. Dou QP. Sheng S. Reddy GP. Sarkar FH. Regulation of FOXO3a/β-catenin/GSK-3β signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 24.Rahman KM. Ali S. Aboukameel A. Sarkar SH. Wang Z. Philip PA. Sakr WA. Raz A. Inactivation of NF-κB by 3,3′-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther. 2007;6:2757–2765. doi: 10.1158/1535-7163.MCT-07-0336. [DOI] [PubMed] [Google Scholar]
- 25.Dalessandri KM. Firestone GL. Fitch MD. Bradlow HL. Bjeldanes LF. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 26.Lotinun S. Westerlind KC. Turner RT. Tissue-selective effects of continuous release of 2-hydroxyestrone and 16alpha-hydroxyestrone on bone, uterus and mammary gland in ovariectomized growing rats. J Endocrinol. 2001;170:165–174. doi: 10.1677/joe.0.1700165. [DOI] [PubMed] [Google Scholar]
- 27.Zeligs MA. Sepkovic DW. Manrique C. Macsalka M. Williams DE. Bradlow HL. Absorption-enhanced 3,3′-diindolylmethane: human use in HPV-relaed, benign and pre-cancerous conditions. Proc Am Assoc Cancer Res. 2002;43 3198 (abstract). [Google Scholar]
- 28.Krishnaraj R. Morrissey RL. Crowell J. Lyubimov AV 2004 A four-week oral toxicity study of 3,3′-diindolylmethane in dogs. Proc Am Assoc Cancer Res. 45 735 (abstract). [Google Scholar]