Abstract
Common symptoms associated with HIV disease and its management are often underrecognized and undertreated. A clinical decision support tool for symptom management was developed within the Veterans Health Administration electronic medical record (EMR), aiming at increasing provider awareness of and response to common HIV symptoms. Its feasibility was studied in March to May 2007 by implementing it within a weekly HIV clinic, comparing a 4-week intervention period with a 4-week control period. Fifty-six patients and their providers participated in the study. Patients' perceptions of providers' awareness of their symptoms, proportion of progress notes mentioning any symptom(s) and proportion of care plans mentioning any symptom(s) were measured. The clinical decision support tool used portable electronic “tablets” to elicit symptom information at the time of check-in, filtered, and organized that information into a concise and clinically relevant EMR note available at the point of care, and facilitated clinical responses to that information. It appeared to be well accepted by patients and providers and did not substantially impact workflow. Although this pilot study was not powered to detect effectiveness, 25 (93%) patients in the intervention group reported that their providers were very aware of their symptoms versuas 27 (75%) control patients (p = 0.07). The proportion of providers' notes listing symptoms was similar in both periods; however, there was a trend toward including a greater number of symptoms in intervention period progress notes. The symptom support tool seemed to be useful in clinical HIV care. The Veterans Health Administration EMR may be an effective “laboratory” for developing and testing decision supports.
Introduction
Many of the symptoms related to HIV disease, its complications, and/or its management (e.g., fatigue, pain, diarrhea, sleep disturbances) are underrecognized and therefore undertreated in many care settings.1–5 Although antiretroviral therapy (ART) has greatly increased life expectancy, it may precipitate side effects that substantially decrease quality of life1 and may create a barrier to the high adherence levels necessary for maximum benefit.2 Survey instruments that detect symptoms common in HIV care have been developed to facilitate effective symptom management6–8 but those instruments have not been regularly incorporated into clinical practice.
We postulated that providers underrecognize and undertreat common symptoms because of the substantial time burden required to ask about the many individual symptoms, and because of providers' lack of comfort regarding appropriate management strategies. At the same time, we observed that the growing sophistication of clinical decision support tools may alleviate such barriers,9–12 and that the advanced electronic medical record (EMR) system of the Veterans Health Administration (VHA) may serve as a “laboratory” to test the feasibility of new types of clinical decision supports. With this in mind, we constructed an EMR-based clinical decision support tool to increase providers' awareness of and responses to common symptoms, and tested the feasibility of incorporating our tool into routine care.
Methods
We sought to construct a clinical decision support tool that would elicit information about symptoms at the time of check-in for a routine clinic visit, organize that information to emphasize what is most useful for clinical care, present that information in an easy-to-use form at the point-of-care, and recommend clinical responses based on that information. We chose these design factors because they encompass a broad range of information management necessary for clinical care. In addition, many of those design factors have been shown to help integrate computerized systems into clinical workflow.9 Because our ultimate objective was to improve symptom management in HIV care, we refer to our decision support tool as the Tool to Enhance Management of Symptoms (TEMS). Because clinical guidelines suggest assessing and reinforcing adherence at each visit13 and because adherence rates may be related to the prevalence of side effects, we augmented the symptom information with a briefer query regarding medication adherence.
Clinical setting
We aimed to implement our TEMS within a weekly half-day HIV clinic at our urban Veterans Affairs (VA) Medical Center. Approximately 30 patients are seen in a typical clinic session, which is staffed by two to four attending physicians (from General Internal Medicine and Infectious Diseases), one physician assistant, one clinic coordinator, and several rotating trainees (fellows and residents).
Design of clinical decision support tool
We sought to base recommended response strategies for symptoms on evidence-based guidelines when available, and to supplement those with expert opinion from our senior clinic personnel. The site principal investigator (R.B.), along with the clinic director and infectious diseases trainee, constructed response strategies based on their expert opinion, in conjunction with HIV textbooks,14,15 recent Department of Health and Human Services guidelines,13 well-regarded HIV care websites,16–18 and other sources. The informatics infrastructure underlying the TEMS was developed by a senior programmer having 14 years of experience with VHA data systems (F.L.), working in conjunction with the Chief Information Officer of the Connecticut VA Healthcare System (J.E.) and the director of the Informatics Fellowship program at our institution (C.B.). The senior programmer required approximately 200 hours of programming time to implement the intervention.
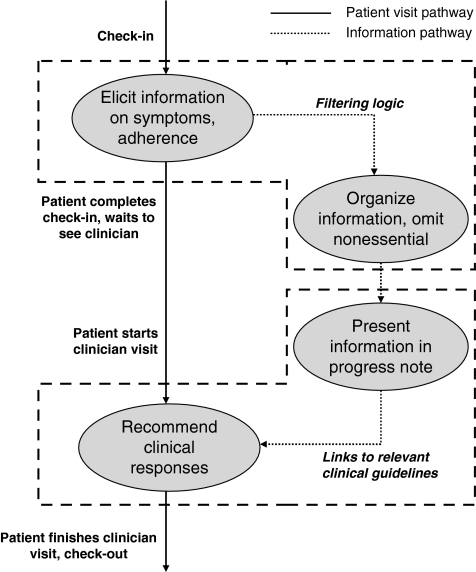
We describe our design aims by discussing each of the four main design factors that we sought to incorporate into TEMS: elicitation, organization, presentation, and recommendation (Fig. 1 and Appendix).
FIG. 1.
Schematic diagram of design factors for Tool to Enhance Management of Symptoms (TEMS). These factors encompass a wide spectrum of information management necessary for clinical care, and are synchronized with the workflow of a typical clinic visit. Information is elicited at the same time that vital signs are measured, and the computer-generated progress note can be viewed at the same time that other patient information is used for decision making.
Elicitation. We strove to elicit information in a manner that would minimize respondent burden, would not interrupt the clinical workflow, and would direct information transfer into the EMR without manual transcription. For those reasons, we sought to identify surveys of HIV symptoms that were validated yet comparatively brief, and could be administered by using portable devices that could interface with the EMR.
Among several HIV symptom indices that we considered as candidates for inclusion in TEMS, only 2 have been validated in the ART era.6–8 We chose the 20-item HIV Symptom Index6 because it has been widely used in clinical studies of HIV/AIDS, including Adult AIDS Clinical Trials Group (AACTG) studies and the Veterans Aging Cohort Study (VACS).19 An example of an item in the index is, “How much have you been bothered by fatigue or loss of energy?” with possible responses, “I do not have this symptom.” “I have it and it doesn't bother me.” “I have it and it bothers me a little.” “I have it and it bothers me.” and “I have it and it bothers me a lot.”
With the intent of minimizing additional respondent burden, we chose to gather information on medication adherence by using a single question, which we based on a patient adherence instrument developed by the Outcomes Committee of the AACTG.20 The question asks, “When was the last time you missed one or more doses of your HIV medications?” with possible responses of “today,” “yesterday,” “within the last week,” “within the last month,” and “more than 1 month ago.”
Organization. We strove to organize the information to deemphasize any that was not clinically important or actionable, as evidence suggests that providers often ignore clinical decision support information that is insufficiently specific.12 We sought to use two distinct “filters” on the survey information, the first excluding information pertaining to symptoms that were not sufficiently bothersome to prioritize, and the second limiting the detail of information when a patient appeared to endorse a great number of unrelated symptoms simultaneously (such patients would be unlikely to respond to any 1 or 2 interventions to alleviate symptoms, and may have a more global problem such as depression).
Presentation. We strove to ensure that symptom information was presented through an interface that was simple and clear and had the capacity to be linked to response strategies. For that reason, we collected the symptom information on a “tablet” thin-client computer and aimed to present the information within an EMR progress note that could be accessed during the patient encounter. We aimed to construct flexible templates for notes (i.e., notes that could have alternative structures depending upon the level of information detail), so that notes could generally remain as brief as possible yet “telescope” into longer notes when it was essential to communicate additional information.
Recommendation. We sought to provide clinical recommendations by developing an interface that would anticipate clinicians' information needs and address them rapidly, in real time.12 Our goals were (1) to fit into the user's workflow12 (e.g., allowing the clinician to place an order to address the symptom while viewing the note that alerted her to that symptom); (2) to minimize medical knowledge barriers (e.g., permitting the clinician to retrieve the preferred approach for addressing a symptom in case she does not already know it); and (3) to minimize institution-specific knowledge barriers (e.g., allowing the clinician to learn how a particular drug or laboratory test is listed in the EMR order menu in case she does not already know it). Consequently, we sought to embed hyperlinks in the note to clinical and diagnostic algorithms, experts' recommendations, and relevant orders (including medications, tests, and consultations).
Study design
Four clinic sessions comprised the control phase, and were followed by four clinic sessions comprising the intervention phase. During the control phase (March 2007), TEMS was not activated and therefore participants did not complete the HIV symptom survey. During the intervention phase (mid-April through mid-May 2007), TEMS was activated, and all participants were asked to complete the HIV symptom survey. Therefore, only in the intervention phase did providers receive the computer-generated progress notes and have access to the other functionalities of TEMS. In both the control phase and the intervention phase, all participants received a one-item postvisit survey in which they were queried about their perception of their providers' level of awareness about their symptoms. The item asked, “How aware of your symptoms do you think your health care provider was?” with possible responses, “not at all aware,” “a little bit aware,” “somewhat aware,” “quite a bit aware,” and “very much aware.”21,22
To maximize the generalizability of the study, we did not impose any inclusion criteria other than having a clinic appointment during the period of the study (March 2007–May 2007), having HIV infection, and providing informed consent. Patients were excluded only if they were making their first visit to the clinic because a thorough symptom review would likely be performed as part of routine care at initial visits. Each individual could participate in each phase of the study no more than once (i.e., a patient could participate once in both phases of the study, but could not participate in either phase twice); however, we did not require that patients who participated in one phase also participate in the other phase. This study was approved by the Institutional Review Board at the Connecticut VA Healthcare System and the Human Investigations Committee at Yale University. The sponsor did not have any role in the collection, analysis, or interpretation of data; in the decision to submit study results for publication; or in the drafting or revision of the manuscript.
Outcomes
In a subset of participants, we assessed the acceptability of the system and the information provided to physicians by having a cognitive engineer (M.R.) review the entire process. By using a human factors approach, the cognitive engineer analyzed barriers to: (1) entering symptoms into the tablet personal computer; (2) transferring the symptoms electronically into the VHA EMR; and (3) having providers act on the symptom information. In addition, the study coordinator (K.M.) independently noted which barriers seemed to be most prevalent for participants.
Because this was a pilot study and its primary outcome was to assess the feasibility of TEMS, the study was not powered to detect clinically significant changes in the effectiveness of symptom recognition and/or management. However, this study did incorporate two prospectively defined effectiveness measures as secondary outcomes. First, we evaluated responses on the postvisit survey, comparing the proportion of intervention versus control participants who thought that their providers were “very aware” of their symptoms by using the χ2 test for proportions. Second, we performed chart reviews to assess the proportion of progress notes that included at least one symptom addressed by TEMS. The reviews were completed in a blinded fashion and in duplicate (C.N. and S.F.); the agreement between the blinded reviewers was high (κ score = 0.862 for having at least one symptom mentioned in progress note; κ score = 1.00 for having at least 1 symptom mentioned in the treatment plan). Because only a minority (N = 8) of patients participated in both the control and intervention phases and therefore could serve as their own control, each progress note during the intervention period was compared with the most recent progress note that preceded the intervention period, even if it pertained to a visit prior to the control phase.
Results
Of 60 clinic patients invited to participate, 56 (93%) agreed to participate, and 55 (98%) completed all parts of the study (1 patient left before completing the postvisit survey). Eight patients (14%) participated in both the intervention and control phases, 28 patients (50%) participated in the control phase only, and 20 patients (36%) participated in the intervention phase only (Table 1). The mean (standard deviation [SD]) age of the patients was 54.4 (9.5); 40 (73%) were minorities and 33 (59%) had hepatitis C coinfection. Approximately two thirds had plasma HIV RNA levels below 400 copies per milliliter, and the median CD4 count was approximately 400 cells per milliliter. Most (79%) reported last missing a dose of anti-retroviral medications more than 1 month ago, but a substantial minority (21%) reported more recent nonadherence (4% within the last week to month, 14% within the last day to week, and 4% on the day of the survey).
Table 1.
Baseline Characteristics of Study Participants
| Characteristics | Intervention group (n = 28) | Control group (n = 36) | All (n = 56)a |
|---|---|---|---|
| Mean (SD) age, years | 53.6 (11.2) | 55.7 (8.4) | 54.4 (9.5) |
| Race | |||
| Black, n (%) | 21 (75.0) | 24 (66.7) | 38 (67.9) |
| White, n (%) | 5 (17.9) | 10 (27.8) | 15 (26.8) |
| Hispanic, n (%) | 2 (7.1) | 2 (5.6) | 3 (5.4) |
| Male gender, n (%) | 27 (96.4) | 35 (97.2) | 55 (9.8) |
| CD4+ count (cells/mm3) | |||
| Mean (SD) | 410.7 (241.6) | 388.0 (218.5) | 409.5 (225.6) |
| Median | 410 | 383 | 394 |
| HIV RNA ≤400 copies/mL, n (%) | 18 (64.3) | 24 (66.7) | 38 (67.9) |
| Type of current ARV therapy | |||
| NNRTI-based regimen, n (%) | 4 (19.0) | 6 (19.3) | 10b (20.8) |
| PI-based regimen, n (%) | 2 (9.5) | 3 (9.7) | 3b (6.2) |
| Boosted PI-based regimen, n (%) | 13 (61.9) | 13 (41.9) | 24b (50.0) |
| Other, n (%) | 2 (9.5) | 9 (29.0) | 11b (22.9) |
| Coinfection with HCV, n (%) | 17 (60.7) | 22 (61.1) | 33 (58.9) |
Eight patients participated in both the intervention group and the control group.
Some patients were not taking any ARV medication (total of 8).
ARV, antiretroviral; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; HCV, hepatitis C virus; SD, standard deviation.
Feasibility
The vast majority of patients required fewer than 5 minutes to complete the survey, and none required more than 10 minutes. Approximately half of the patients were able to use the electronic tablet on their own, whereas the other half needed assistance. Based on observations made by the cognitive engineer, the most common reasons for needing assistance were inability to read the text because of small font; difficulty handling the stylus because of arthritis or other dexterity impairments; or difficulty understanding particular words (for example, asking what a “provider” was). Those factors were also independently endorsed as particularly important by our study coordinator. Although all patients completed the symptom survey, some patients were concerned about the confidentiality of their information, and others were concerned about its usefulness (“doctors never check the computer anyway”). Additionally, one patient with a superficial skin infection was concerned that the tablet and stylus may serve as a vector for communicable disease.
While this pilot study did not include quantitative end points for provider feasibility, the cognitive engineer qualitatively assessed provider feasibility by reviewing the electronic medical records and meeting with the providers individually at the end of the study period. She noted that most of the physicians acted upon the new information in the computer-generated progress note (e.g., some physicians copied and pasted the information from the symptom index-generated note into their own progress note) and that the workflow was only slightly affected.
Patients seemed to be very comfortable using TEMS to endorse symptoms. Twelve of the 20 symptoms queried were endorsed by most participants (Table 2). However, because TEMS used “bothersome” as the minimum severity threshold for inclusion in the computer-generated progress note, most endorsed symptoms were not included in the notes. Indeed, participants were able to use the tool to discriminate among varying severities of symptoms; only 1 symptom (“pain, numbness, or tingling in hands or feet”) was rated as “bothersome” by most participants.
Table 2.
Endorsement of Symptoms by Participants in the Intervention Phase
| Any symptom (Score ≥1)a | Bothersome symptoms (Score ≥ 3)a | Of patients with bothersome symptoms, patients attributing them to HIV medications | |
|---|---|---|---|
| Fatigue or loss of energy, n (%) | 19 (67.9) | 9 (32.1) | 0 (0.0) |
| Fever, chills, or sweats, n (%) | 10 (35.7) | 5 (17.9) | 1 (20.0) |
| Feeling dizzy or lightheaded, n (%) | 10 (35.7) | 3 (10.7) | 0 (0.0) |
| Pain, numbness, or tingling in hands or feet, n (%) | 17 (60.7) | 14 (50.0)b | 1 (7.1) |
| Trouble remembering, n (%) | 18 (64.3) | 6 (21.4) | 0 (0.0) |
| Nausea or vomiting, n (%) | 4 (14.3) | 3 (10.7) | 0 (0.0) |
| Diarrhea or loose bowel movements, n (%) | 15 (53.6) | 3 (10.7) | 3 (100.0) |
| Sad, down, or depressed, n (%) | 13 (46.4) | 4 (14.3) | 1 (25.0) |
| Felt nervous or anxious, n (%) | 17 (60.7) | 6 (21.4) | 2 (33.3) |
| Skin problems,cn (%) | 15 (53.6) | 8 (28.6) | 1 (12.5) |
| Cough or trouble catching your breath, n (%) | 12 (42.9) | 6 (21.4) | 0 (0.0) |
| Headache, n (%) | 11 (39.3) | 2 (7.1) | 0 (0.0) |
| Loss of appetite or change in the taste of food, n (%) | 16 (57.1) | 6 (21.4) | 2 (33.3) |
| Bloating, pain, or gas in your stomach, n (%) | 14 (50.0) | 5 (17.9) | 1 (20.0) |
| Muscle aches or joint pain, n (%) | 16 (57.1) | 10 (35.7) | 0 (0.0) |
| Problems with having sex,dn (%) | 14 (50.0) | 9 (32.1) | 3 (33.3) |
| Changes in the way your body looks,en (%) | 11 (39.3) | 5 (17.9) | 1 (20.0) |
| Weight loss or wasting, n (%) | 14 (50.0) | 8 (28.6) | 2 (25.0) |
| Hair loss or changes in the way your hair looks, n (%) | 6 (21.4) | 2 (7.1) | 1 (50.0) |
The following scoring system was used:
score 0—“I do not have this symptom”; score 1—“I have this symptom and it doesn't bother me”; score 2—“I have this symptom and it bothers me a little”; score 3—“I have this symptom and it bothers me”; and score 4—“I have this symptom and it bothers me a lot.”
Only “pain, numbness, or tingling in hands or feet” was rated as bothersome or very bothersome by most patients.
Skin problems, such as rash, dryness, or itching.
Problems with having sex, such as loss of interest or lack of satisfaction.
Changes in the way your body looks, such as fat deposits or weight gain.
Effectiveness
In the intervention phase, 25 (93%) participants thought that their clinicians were “very aware” of their symptoms, whereas during the control phase, only 27 (75%) participants thought their clinicians were very aware (p = 0.07; Table 3).
Table 3.
Post-visit Survey: Patients' Perceptions of Providers' Awareness of their Symptoms
| Control group (n = 36) | Intervention group (n = 27)a | |
|---|---|---|
| Not at all aware, n (%) | 0 (0.0) | 0 (0.0) |
| A little bit aware, n (%) | 1 (2.8) | 0 (0.0) |
| Somewhat aware, n (%) | 3 (8.3) | 1 (3.7) |
| Quite a bit aware, n (%) | 5 (13.9) | 1 (3.7) |
| Very much aware, n (%) | 27 (75.0)b,c | 25 (92.6)b,c |
Only 27 of the 28 patients enrolled in the intervention phase completed the post-visit survey.
p = 0.07 based on differences between “very much aware” versus all other response categories.
Excluding patients who participated in both phases, 90% of intervention patients' physicians were thought to be “very much aware” vs. 75% of control subjects' physicians (p = 0.02).
Additionally, although the proportion of providers' notes listing symptoms was identical in the control and intervention phases (Table 4), there was a trend toward including a greater number of symptoms in intervention phase progress notes (mean [SD] number of symptoms mentioned in the progress note but not in the problem list: 3.6 [3.2] in the intervention phase versus 2.7 [2.3] in control phase, p = 0.07; mean [SD] number of symptoms mentioned in the problem list: 1.9 [1.5] in the intervention phase versus 1.6 [1.3] in control phase, p = 0.22).
Table 4.
Symptoms Mentioned in Progress Notes and Treatment Plansa
| Control Phaseb (n = 28) | Intervention Phaseb (n = 28) | |
|---|---|---|
| Progress notes mentioning symptoms, n (%) | 22 (78.6) | 22 (78.6) |
| Number of symptoms mentioned: | ||
| Mean (SD) | 2.7 (2.3) | 3.6 (3.2) |
| Median | 2 | 3 |
| Treatment plans listing symptoms, n (%) | 23 (82.1) | 23 (82.1) |
| Number of symptoms listed: | ||
| Mean (SD) | 1.6 (1.3) | 1.9 (1.5) |
| Median | 2 | 2 |
Progress notes were defined exclusive of treatment plans.
Because not all patients in the intervention phase also participated in the control phase, we used their most recent prior visit as a surrogate.
SD, standard deviation.
Discussion
We have developed a clinical decision support tool (TEMS) that focuses on symptom management in HIV care. TEMS encompasses a wide breadth of information management in clinical care, from eliciting information through recommending clinical approaches to that information. Its design is particularly noteworthy because it processes and filters elicited information in order to emphasize that which is most clinically relevant, and therefore minimizes additional time burden on clinicians. In that respect, TEMS is innovative because informatics interventions generally do not place a great emphasis on minimizing demands on clinicians' time and attention, and therefore are at risk of inducing “alert fatigue” and subsequent reductions in effectiveness.
Our pilot study suggests that TEMS was accepted by clinicians and did not substantially impede workflow, and therefore it was successful in this initial feasibility test. We have learned that TEMS acceptance by patients could be improved by increasing font size, increasing stylus size, simplifying language, reassuring patients about confidentiality, and cleaning the keyboard/stylus with disinfectant in between uses. Nevertheless, available resources did not allow us to assess many important aspects of its feasibility. Future work should better evaluate the impact of TEMS on providers by measuring the additional time required for clinicians to view notes and to respond to them, assessing more precisely patient time requirements and staffing requirements generated by TEMS, and surveying providers regarding their satisfaction with the tool. Additionally, the favorable trends that were observed regarding effectiveness (i.e., perception of providers' symptom awareness) need to be confirmed in future studies having greater statistical power.
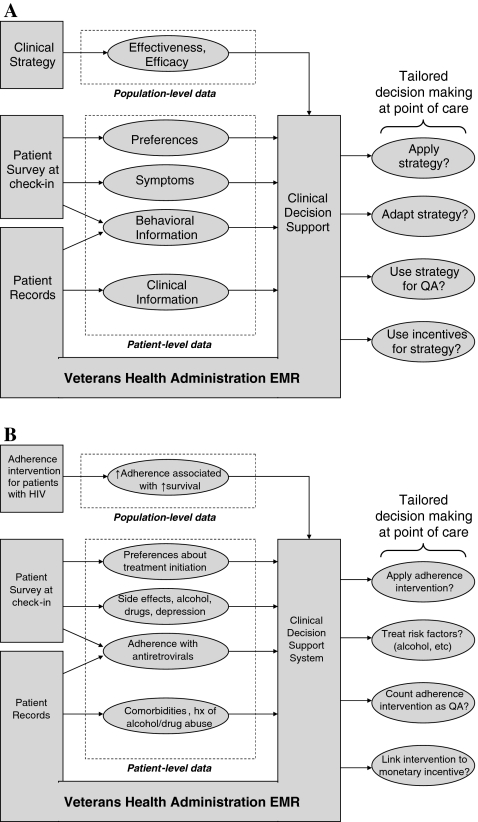
There are many possible ways to use clinical decision support systems to individualize care in the VHA, as EMR information may be combined with self-reported patient information in a wide range of domains (i.e., preferences, symptoms, behaviors, clinical data), and the resulting information may be used to tailor clinical strategies for individual patients (Fig. 2A). For example, an expanded version of TEMS could be developed with the aim of improving adherence to ART (Fig. 2B) by combining pharmacy refill data with symptom survey data to identify patients with probable adherence difficulties, and to individualize approaches to improve adherence based on relevant behavioral risk factors, for example, depression,2,23,24 alcohol abuse,25–28 or other substance abuse.29–32
FIG. 2.
General framework for using clinical decision support tools within the Veterans Health Administration electronic medical record (EMR) system to individualize care. The clinical decision support tool described in this report is only one example of a nearly infinite variety of ways in which the EMR may be used to adapt clinical strategies to the preferences, symptoms, behaviors, and clinical histories of individual patients (A). For example, a more comprehensive version of the clinical decision support tool described in this report could aim to improve medication adherence based on patient-level behavioral risk factors, symptoms, and comorbidities (B). EMR, electronic medical record; QA, quality assurance; ↑ increased; Hx, history.
TEMS has important limitations, some of which were not anticipated during its design. Even though it uses portable electronic tablets that have the capacity to transmit information wirelessly, evolving VA security standards have forced us to use hardwired network connections, thereby removing some of the tablet's flexibility. We envisioned a wide network of hyperlinks to help clinicians respond to symptom information, but constructing those links was not possible within the time and budgetary constraints of this pilot project. More generally, clinical decision support tools require regular updates of clinical knowledge and technical support in order to impact care in a sustainable manner.10–12 Nonetheless, we have accomplished our main objectives and have designed a clinical decision support tool having a structure that can be generalized to other diseases and clinical management questions. Because informatics expertise and EMRs are becoming increasingly sophisticated, we may be entering a promising era for clinical decision support tools that aim to individualize care.
Appendix. Detailed Description of Incorporating Design Factors into TEMS
This appendix discusses in more detail how we incorporated each of the four main design factors into TEMS: elicitation, organization, presentation, and recommendation.
Elicitation
TEMS collects information on a portable electronic “tablet” (Panasonic model CF-08) that enables respondents to answer questions by using a hand-held stylus similar to a pen, or alternatively by touching the screen with a finger. After a patient has his or her vital signs measured, the medical assistant registers the patient in the tablet-based survey application and remains with the patient in order to help with any technical difficulties that may arise as the patient uses the tablet. The tablet queries patients about their symptoms by using the 20-question HIV Symptom Index. Because the HIV Symptom Index does not fit onto 1 screen, patients must proceed through 5 screens in order to answer all questions. The implementation was programmed so that respondents are not able to “skip” a question.
We developed TEMS in conjunction with the clinicians who would implement it, and strove to incorporate their suggestions. Clinicians thought that the usefulness of symptom information would be greatly enhanced if the duration of each symptom could be reported, along with patients' judgment about whether that symptom was due to a medication side effect. For that reason, we augmented the HIV Symptom Index by asking additional questions regarding duration of symptoms (“How long has this symptom bothered you?” with possible responses of “less than 1 week,” “between 1 week and 1 month,” “between 1 month and 1 year,” and “longer than 1 year”) and attributability of symptoms to medications using the 1-question item adapted from the AACTG questionnaire (“Do you think that this symptom is caused by drugs that you take to treat your HIV infection?” with possible responses of “yes,” “unsure,” and “no”). Also, in response to ideas from providers, we ensured that each patient's vital signs would be included with the symptom information. To avoid errors that might result from manual entry, TEMS extracts vital sign information automatically from the electronic medical record (EMR).
We had hoped to use the wireless capacities of the tablet to transmit symptom information into the EMR in order to minimize staff burden (i.e., they would have the flexibility to elicit symptom information at a variety of places and times). However, shortly before beta-testing TEMS, the VHA issued an embargo of wireless data-encoding algorithms. Therefore, we beta-tested TEMS by using a wired connection to transmit the data, and will try wireless transmission pending the approval of a VHA-compliant data-coding algorithm.
Organization
We maximized the specificity of information to be presented to the provider by using two distinct information filters, each of which reduces the level of information detail when that information is unlikely to lead to an effective clinical response. The first filter excludes symptom information when a symptom is sufficiently minor so that it is unlikely to warrant a clinical response (i.e., if the symptom is not bothersome or very bothersome). The second filter reduces the detail of symptom information when a patient endorses multiple unrelated symptoms, because clinical approaches directed at the various symptoms themselves may be less effective than clinical approaches geared to a latent underlying condition (e.g., depression). Thus, we defined a specifiable “threshold” for the number of symptoms: if a patient endorsed more than the threshold number of symptoms, then the note informed the provider about which symptoms were endorsed but did not include duration and attributability; if a patient endorsed the threshold number of symptoms or fewer, then the note included the full detail of symptom duration and attributability. We have currently set the threshold at three symptoms, but we have not yet determined the optimal threshold value.
Presentation
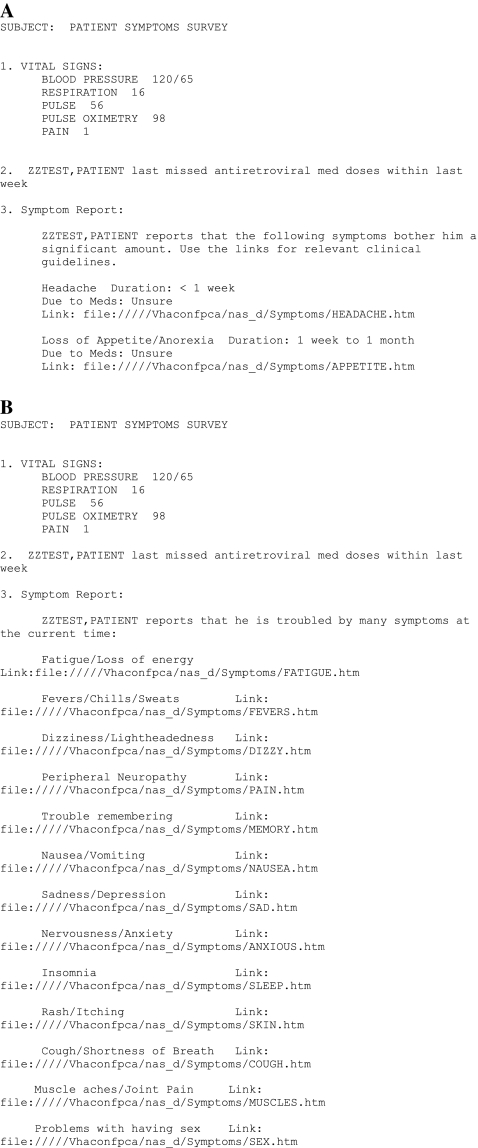
TEMS generates progress notes by using one of two templates (Fig. 1). If a patient endorses a number of symptoms that falls below the numerical threshold, then the note gives full detail about each symptom. Appendix Figure 1A shows the note that was generated for a patient who endorsed two symptoms (headache, which has lasted less than 1 week, with the patient being unsure of its relationship to medications; and loss of appetite, which has lasted for 1 week to 1 month, attributed by the patient to his HIV medications). However, if a patient endorses a number of symptoms that exceeds the specified threshold, then the note limits the level of detail. Appendix Figure 1B shows the note that was generated when a patient endorsed 13 separate symptoms. Although the patient reported severity and symptom attribution, that information is not represented in the note. Both note formats contain the result of the one-item adherence query and the vital signs.
In our original concept of the tool, we had planned for physicians to see a “pop-up” alert on their computer screen when the computer-generated progress note was created (we thought that this would be desirable because many physicians review the patient record before patients enter the examination room). However, the pop-up presented an unforeseen programming burden because of technical features of the VHA's EMR architecture, and therefore we had to substitute a low-tech approach in which patients carried a bright yellow “alert” card into their examination room, serving as a prompt for the physician to look at the results of the symptom index.
Recommendation
Each symptom in each progress note is linked to a file that recommends a corresponding clinical response strategy (Fig. 2), either diagnostic considerations or symptom treatments. Because diagnostic considerations are often distinct for individuals with HIV, TEMS emphasizes those considerations that are particularly applicable to individuals with HIV infection. Additionally, because HIV-infected individuals who are severely immunosuppressed have particular diagnostic considerations (i.e., opportunistic infections, etc.), TEMS stratifies strategies by CD4 count when indicated (<200 cells per milliliter versus ≥200 cells per milliliter). When diagnoses would be facilitated by using another screening instrument (e.g., the Alcohol Use Disorders Identification Test to screen for hazardous alcohol consumption), that instrument along with its scoring algorithm is included in the response strategy.
To mitigate barriers to provider adherence, each recommendation is expressed by using the terminology and care options particular to our institution (e.g., rather than recommending “refer for alcohol treatment,” TEMS will recommend “Substance Abuse Clinic consult”), and we designed our therapeutic strategies to include only generic drugs that were on formulary. The full text of all response strategies is available at www.vacohort.org.
We had intended to link each symptom in the computer-generated progress note to its response strategy by means of a hyper-link, but technical barriers discouraged such an approach. As an alternative, we placed a Uniform Resource Locator (URL) next to each symptom so that the URL could be copied and pasted into a Microsoft Word document and “pointed” to a document that contains the corresponding response strategy. Similarly, we intended to embed in each response strategy hyper-links to relevant orders, but that also proved to be prohibitively burdensome. As an alternative, we carefully edited each response strategy to ensure concordance between an order's specification in our response strategy and its representation in the EMR.
APPENDIX FIG. 1.
Examples of computer-generated progress notes. The template for the note varies depending upon the number of symptoms endorsed, in order to maximize conciseness and clinical specificity. Figure 1A shows a note that is generated for a hypothetical patient, “ZZTEST, PATIENT,” when few symptoms are endorsed. It contains comparatively detailed information about each symptom. Figure 1B shows a note that is generated when many symptoms are endorsed. It contains comparatively limited information about each symptom. Both notes contain information on vital signs and adherence as well as links to response strategies for each symptom.
APPENDIX FIG. 2.
Example of a recommendation corresponding to a particular symptom (“trouble remembering”). The recommendation contains diagnostic considerations as well as guidelines for symptomatic treatment. Recommendations were developed in conjunction with senior clinic staff. Screening instruments that are cited by recommendations (i.e., the Primary Care Depression Screen and the Alcohol Use Disorder Identification Test) are not reproduced here but are included in the recommendation along with scoring algorithms. Abbreviations are used when they are generally known by clinic practitioners.
Acknowledgments
This work was funded by Department of Veterans Affairs, Health Services Research and Development grant PCC 05-069-1, Implementing Symptoms Assessment into Clinical HIV Care.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Braithwaite RS. Goulet J. Kudel I. Tsevat J. Justice AC. Quantifying the decrement in utility from perceived side effects of combination antiretroviral therapies in patients with HIV. Value Health. 2008;11:975–979. doi: 10.1111/j.1524-4733.2007.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson DL. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Justice AC. Rabeneck L. Hays RD. Wu AW. Bozzette SA. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: A comparison with self-reported symptoms. Outcomes Committee of the AIDS Clinical Trials Group. J Acquir Immune Defic Syndr. 1999;21:126–133. [PubMed] [Google Scholar]
- 4.Justice AC. Chang CH. Rabeneck L. Zackin R. Clinical importance of provider-reported HIV symptoms compared with patient-report. Med Care. 2001;39:397408. doi: 10.1097/00005650-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine A. Larue F. Lassauniere JM. Physicians' recognition of the symptoms experienced by HIV patients: How reliable? J Pain Symptom Manage. 1999;18:263–270. doi: 10.1016/s0885-3924(99)00078-0. [DOI] [PubMed] [Google Scholar]
- 6.Justice AC. Holmes W. Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 7.Holzemer WL. Hudson A. Kirksey KM. Hamilton MJ. Bakken S. The revised Sign and Symptom Check-List for HIV (SSC-HIVrev) J Assoc Nurses AIDS Care. 2001;12:60–70. doi: 10.1016/s1055-3290(06)60263-x. [DOI] [PubMed] [Google Scholar]
- 8.Holzemer WL. Henry SB. Nokes KM, et al. Validation of the Sign and Symptom Check-List for Persons with HIV Disease (SSC-HIV) J Adv Nurs. 1999;30:1041–1049. doi: 10.1046/j.1365-2648.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman RN. Liaw Y. Brandt CA. Corb GJ. Computer-based guideline implementation systems: A systematic review of functionality and effectiveness. J Am Med Inform Assoc. 1999;6:104–114. doi: 10.1136/jamia.1999.0060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim I. Gorman P. Greenes RA, et al. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8:527–534. doi: 10.1136/jamia.2001.0080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates DW. Kuperman GJ. Wang S, et al. Ten commandments for effective clinical decision support: Making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10:523–530. doi: 10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osheroff JA. Teigh JM. Middleton B. Steen EB. Wright A. Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14:141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. The Panel on Clinical Practices for the Treatment of HIV Infection convened by the Department of Health and Human Services. Oct 10, 2006. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Nov;2006 ]. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 14.Wormser G. AIDS and Other Manifestations of HIV Infection. Fourth. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 15.Sande M. Eliopoulos G. Moellering R, et al. The Sanford Guide to HIV/AIDS Therapy. Antimicrobial Therapy, Inc. 2006.
- 16.Currently Approved Drugs for HIV: A Comparative Chart. www.aidsmeds.com/lessons/drugchart.htm. [Dec;2008 ]. www.aidsmeds.com/lessons/drugchart.htm
- 17.HIV Drug Interactions. www.hiv-druginteractions.org. [Dec;2008 ]. www.hiv-druginteractions.org
- 18.HIV InSite. University of California; San Francisco: [Dec;2008 ]. [Google Scholar]
- 19.Justice AC. Dombrowski E. Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesney MA. Ickovics JR. Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 21.Detmar SB. Aaronson NK. Quality of life assessment in daily clinical oncology practice: A feasibility study. Eur J Cancer. 1998;34:1181–1186. doi: 10.1016/s0959-8049(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 22.Detmar SB. Muller MJ. Schomagel JH. Wever LD. Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 23.Tucker JS. Burnam MA. Sherbourne CD. Kung FY. Gifford AL. Substance use and mental health correlates of non-adherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 24.Kleeberger CA. Buechner J. Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18:683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 25.Cook RL. Sereika SM. Hunt SC. Woodward WC. Erlen JA. Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samet JH. Horton NJ. Meli S. Freedberg KA. Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 27.Braithwaite RS. McGinnis KA. Conigliaro J, et al. The temporal association between alcohol consumption and adherence in a cohort of HIV+ and matched HIV− patients. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 28.Braithwaite RS. Conigliaro J. Roberts MS, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas GM. Gebo KA. Chaisson RE. Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 30.Golin CE. Liu H. Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker JS. Burnam MA. Sherbourne CD. Kung FY. Gifford AL. Substance use and mental health correlates of non-adherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 32.Kleeberger CA. Buechner J. Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18:683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 33.Lucas GM. Mullen A. Weidle PJ. Hader S. McCaul ME. Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among comparison groups. Clin Infect Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]