Summary
Immunoglobulin D (IgD) has remained a mysterious antibody class for almost half a century. IgD was initially thought to be a recently evolved Ig isotype expressed only by some mammalian species, but recent discoveries in fishes and amphibians demonstrate that IgD was present in the ancestor of all jawed vertebrates and has important immunological functions. The structure of IgD has been very dynamic throughout evolution. Mammals can express IgD through alternative splicing and class switch recombination (CSR). Active cell-dependent and T-cell-independent IgM-to-IgD class switching takes place in a unique subset of human B cells from the upper aerodigestive mucosa, which provides a layer of mucosal protection by interacting with many pathogens and their virulence factors. Circulating IgD can bind to myeloid cells such as basophils and induce antimicrobial, inflammatory, and B-cell-stimulating factors upon cross-linking, which contributes to immune surveillance but also inflammation and tissue damage when this pathway is overactivated under pathological conditions. Recent research shows that IgD is an important immunomodulator that orchestrates an ancestral surveillance system at the interface between immunity and inflammation.
Keywords: IgD, class switch recombination, basophil, antimicrobial peptide, autoinflammatory syndromes
Introduction
Immunoglobulin D (IgD) has remained an enigmatic antibody class since its discovery almost 50 years ago. Because of its spotty presence in mammals and absence in birds, IgD was initially thought to be a recently evolved Ig isotype. Recent discoveries of IgD in more ancient vertebrates, such as fishes and amphibians, demonstrate that IgD was present in the ancestor of all jawed vertebrates and arose together with IgM at the time of the emergence of the adaptive immune system, approximately 500 million years ago. While IgM remains stable over evolutionary time, IgD shows greater structural plasticity and can be predominantly expressed as a transmembrane or secreted molecule in a species-specific manner. One possible interpretation is that IgD has been preserved as a structurally flexible locus to complement the functions of IgM.
IgM and IgD are the first antibody isotypes expressed during B-cell ontogeny. After leaving the bone marrow to colonize secondary lymphoid organs, B cells acquire surface IgD of the same specificity as surface IgM through alternative splicing of a pre-messenger RNA comprising V(D)J and both heavy chain constant μ (Cμ) and Cδ exons. After encountering antigen in secondary lymphoid organs, mature B cells transcriptionally downregulate surface IgD and undergo somatic hypermutation (SHM) and class switch DNA recombination (CSR) to further diversify their Ig gene repertoire. Some B cells class switch from IgM to IgD, at least in humans, suggesting that IgD confers some functional advantage over IgM. Here we review the literature on the evolution of IgD in jawed vertebrates and focus the discussion on the major problems that have surrounded IgD since its discovery, including the regulation of IgD expression and secretion, the immunological function of secreted IgD, and the putative role of secreted IgD in various diseases. The B-cell-activating and signaling properties of transmembrane IgD will not be discussed in detail, because IgD seems to share most of these functions with IgM.
Discovery and evolution of IgD
Discovery of IgD
It has been almost half a century since IgD was discovered as a novel Ig class, first in humans. In 1964, physicians David Rowe and John Fahey (1–2), while studying the disease multiple myeloma, identified and characterized an unusual myeloma protein with electrophoretic and metabolic properties distinct from the known Ig classes at that time, namely IgM, IgG, and IgA. The myeloma protein displayed no reactivity to the anti-sera against IgM, IgG, or IgA. In addition, an antigenically related form of this protein was detected at low concentrations in the serum of healthy subjects. It did not possess the antigenic determinants characteristic of IgM, IgG, or IgA, and thus did not appear to be a subclass of any of these three Ig classes. The evidence collectively suggested that this myeloma protein represented a member of a novel Ig class rather than an abnormal product of the malignant myeloma cells. They subsequently named this new Ig class IgD. Rowe and Fahey stated in their original paper on the discovery of IgD (2) that the reason why they named the new Ig class IgD was ‘because of the evidence that the distinctive properties lie in the heavy polypeptide chains and the properties are sufficient to clearly differentiate these proteins from IgG, IgA, and IgM classes, or from known subclasses of these groups.’ However, based on some anecdotal communication, the selection of the letter ‘D’ was indeed a decision arrived upon with few other choices (3). IgA, IgM, and IgG had already been designated at that time. When Rowe and Fahey were looking for a name for their newly discovered antibody class, their first choice was IgB or β-globulin, but at that time it was expected that the murine Igs would be called β-globulin even though this never eventuated. The Roman letter ‘C’ has no Greek equivalent. The ‘E’ reactive antibody, later termed IgE, had traditionally been associated with allergy. Thus, Fahey and Rowe were left with little choice but to name the new class of antibody IgD. Interestingly, ‘IgD’ is probably a name that cannot be more appropriate for this Ig class, because it is not only distinctive and to be differentiated from other Ig classes but also displays considerable levels of diversity in both structure and abundance both in a single individual and among different individuals, and is probably the most evolutionarily dynamic Ig class among all vertebrate Igs.
Studies of IgD in other species in the late 1970s and early 1980s identified IgD only in primates, rodents, and selected species of mammals, including dog, mouse, rat, rabbit, and guinea pig, whereas it was undetectable in other mammals, such as swine and birds (4–8). The discordance of the spotty presence of IgD with the animal phylogeny and evolution had led to the general view that IgD was a recently evolved Ig class selected for certain species-specific functions in the respective host. With the increasing availability of animal genome sequences and the rapid developments of gene identification tools, the past 20 years have witnessed a series of discoveries of IgD or its homologs and orthologs in many more mammalian species, including those where IgD was thought to be absent, such as cattle, sheep, and pig (9–10). More importantly, IgD and its homologs and orthologs have recently been found in a wide spectrum of species that are evolutionarily much more ancient, such as cartilaginous fishes, bony fishes, amphibians, and reptiles, with the exception of birds (9–22). The most primitive form of these species, i.e. the cartilaginous fishes, appeared on earth as many as some 470 million years ago, when jawed vertebrates first evolved and adaptive immune systems first appeared. These findings demonstrated that IgD is indeed an ancient Ig class selected throughout evolution just like IgM and suggested that IgD or its homologs and orthologs are probably present in not only all extant mammals but also all jawed vertebrates (perhaps except birds). This striking evolutionary perpetuation clearly pinpoints to some important functions of IgD associated with survival advantages of the host and has rekindled much research interest in demystifying the functions of this enigmatic Ig class.
Evolution and diversity of IgD
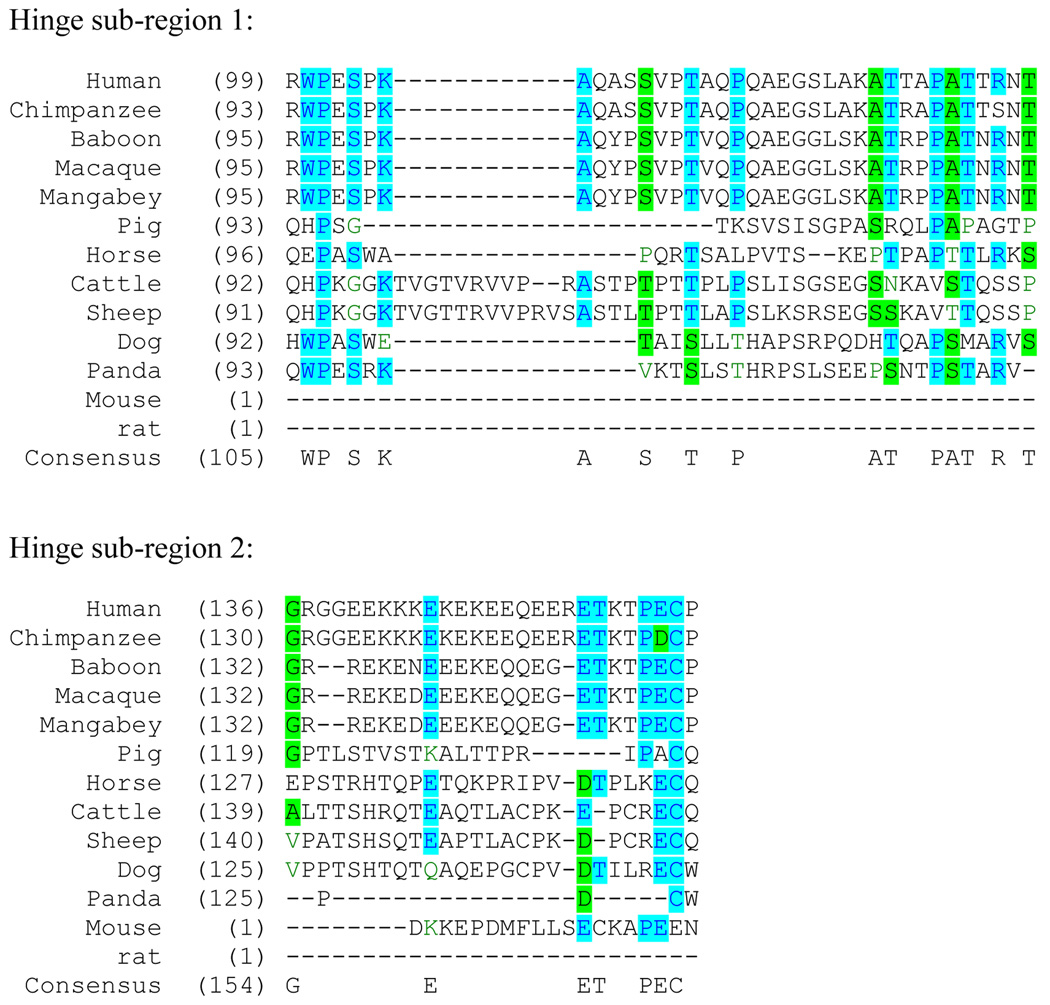
Although as primordial as IgM, IgD in various vertebrate species show much greater plasticity in terms of both the number of C domains and the types of splice variants found, especially in fish. The variability in the numbers of C domains in fish IgW and IgNAR, which are related to mammalian IgD, was frequently caused by various intragenic duplications and alternative splicing. In cartilaginous fish, two of the C domains were derived approximately 250 million years ago by a tandem duplication event (23), and in some bony fish there was a three-domain duplication (11). Within teleost fish, the number of C exons is different in various species (16), and the secreted and transmembrane forms are encoded by different loci in the channel catfish (21). In addition, in teleosts the Cμ1 domain exon is spliced into the IgD transcript (15, 24), a unique way to express IgD in fish. In xenopus, there was a recent two-domain tandem duplication event (12). Xenopus IgD contains eight Cδ domains per chain and represents an intermediate form between fish IgD and mammalian IgD. Splicing of the Cμ1 exon onto the IgD sequences is not observed in xenopus. Rather, the expression of IgD is similar to that in mammals, where rearranged V(D)J sequences are joined directly to the Cδ sequence. In the reptile leopard gecko (Eublepharis macularius), two IgD genes have been discovered (13). The first IgD is made up of 11 Cδ domains without evidence of recent intragenic duplications of exons. The second IgD has seven C domains, with the first four and the seventh generated by a recent partial duplication of the domains of the first IgD gene, and the other two (fifth and sixth) arising from exon shuffling of domains 3 and 4 of an IgA-like molecule. In the green anole lizard (Anolis carolinensis), one IgD gene was identified to contain 11 Cδ domains, but only the first four Cδ domains are used in the expression of membrane-bound form of IgD (14). IgD has also been detected at mRNA level in the Chinese soft shell turtle (Pelodiscus sinensis) and is predicted to be a non-transmembrane protein with six Cδ domains (25). In mammals, structural diversity of IgD is also widely present. Rodent IgD differs from human IgD by the absence of the Cδ2 domain. In artiodactyl species including pig, sheep, cow, and horse, in spite of the overall similarity of domain structure to human IgD, the Cδ1 exon has been deleted and replaced with a duplicated Cμ1 or Cμ1-like exon (20, 26–27). The hinge region of mammalian IgD is of great diversity in terms of both length and amino acid composition (Fig. 1). Rodent IgD hinge region is encoded by one exon, while in ungulates the IgD hinge regions are encoded by two exons. Ungulate IgD hinge regions, such as those of sheep, cow, pig, and horse, however, are structurally distinct from each other (20). Dog IgD hinge region is related more closely to that of ungulates and is distinct to the hinge regions of primate IgD by the absence of a highly charged C-terminal region. Primate IgD hinge regions are highly conserved, all of which contain an N-terminal sub-region rich in alanine and threonine and a C-terminal sub-region rich in charged amino acids including lysine, glutamate, and arginine.
Fig. 1. Protein sequence alignment of mammalian IgD heavy chain hinge region.
Sequences, with accession numbers in brackets, were downloaded from NCBI GenBank: human (P01880.2), chimpanzee (AAB89456.1), baboon (ABB89458.1), macaque (ABB89463.1), mangabey (ABB89466.1), pig (AAN03672.1), horse (AAU09794.1), cattle (AAN03673.1), sheep (AAN03671.1), dog (ABB89467.1), giant panda (AAX73311.1), mouse (P01881.2), and rat (P01883.1). Sequences were aligned using VectorNTI (Invitrogen Corporation). Hinge regions are divided into two sub-regions, with the second sub-region containing the conserved stretch of charged amino acids in primates. Hinge region boundary is based on the peptides encoded by the two hinge exons of human IgD.
One possible interpretation of the presence of this striking diversity of IgD throughout evolution is that IgD has been selected as a structurally flexible locus to back up and complement the functions of IgM. The presence of IgD may ensure the preservation of essential immune functions in case of IgM defects, and the structure flexibility of IgD may provide additional immune functions in a species-specific manner.
Distribution of IgD in human
The distribution of IgD in species other than human and mouse, especially in non-mammals, is poorly characterized. This is at least in part due to the unavailability of antibodies against IgD and the low abundance of IgD in those species. This section therefore focuses on the discussion of the distribution of IgD in human.
Soluble IgD
Secreted IgD has been detected in various human body compartments using different techniques. The first measurement was probably done by Rowe and Fahey (2) when they discovered IgD. Using quantitative gel diffusion, they measured serum IgD concentrations of 72 individuals of different sexes and ages. The median was 30 µg/ml. Some prominent and rather unique features of IgD were observed. Serum IgD concentrations spanned a very wide range, from barely detectable to more than 400 µg/ml. The values were not distributed in a Gaussian manner, in contrast to the log Gaussian distribution of serum IgG, IgM, and IgA concentrations observed by many other studies (28–34). There was also no correlation of serum IgD level with age or sex. In addition, IgD was electrophoretically heterogeneous, indicating differences in molecular weight of IgD molecules found in serum of a given individual and among different individuals (35–38). Other studies carried out later with more sensitive techniques, such as radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), nephelometry, and two-dimensional gel electrophoresis, confirmed the findings of Rowe and Fahey on the heterogeneity of structure and abundance of IgD. The median serum IgD concentrations generally range from 20–50 µg/ml, with considerable variation even among people of the same age and sex. No consistent correlation with sex or age was found. The normal serum range of IgD is wider than that of any other Ig isotype. Individuals can be high or low producers, and low producers can convert to high producers in the setting of certain infections and immune activation. Therefore, the large variability and lack of correlation with demographic parameters of serum IgD might be contributed by the distinct history of immunological exposure in different individuals. In addition to blood, IgD is also present in human nasal, lacrimal, salivary, mammary, bronchial, pancreatic, and cerebrospinal fluids (39–45), and in the amniotic fluid of pregnant women with concentrations progressively increasing during the first half of pregnancy (46). Only trace amounts of IgD are present in intestinal mucosal secretions (39, 43, 47–49). The distribution of soluble IgD largely correlates with the distribution of IgD-producing B cells. Intestinal mucosa, liver, peripheral lymph nodes, spleen, and bone marrow contain very few IgD-producing B cells, while tonsils, adenoids, salivary, and lachrymal glands, and nasal mucosa harbor abundant IgD-producing B cells (39, 43–45, 50–53). IgD-producing B cells can account for up to 20% of all Ig-secreting cells in human tonsils (53–55). The reason why they are rarely found in bone marrow and gut associated-lymphoid tissues (GALTs) in healthy individuals is probably because they are not normally generated there and they express a homing profile not in favor of the intestinal mucosa (α4β7int/lowCCR9lowCCR7highCD62Lhigh) (56). The numbers of IgD-producing B cells in the upper aerodigestive mucosa are drastically increased in patients with IgA deficiency (57–59).
Despite of the fact that IgD-producing B cells express abundant J chain (43, 45, 47, 51), IgD is generally recognized not to associate with J-chain or the secretory component (SC) and not to cross epithelium, placenta, or blood brain barrier (58). IgD found on the surface of epithelium and in mucosal secretions, in cerebrospinal fluid, as well as in the cord blood of some pregnant women (60–63) is thought to result from paracellular diffusion through cell junctions and production of IgD in the fetus. Nonetheless, evidence supporting the transepithelial and transplacental movements of IgD has been documented (64).
The metabolism of IgD was studied by Rogentine and colleagues (65) soon after its discovery. The half-life of serum IgD was approximately 2.8 days. Serum IgD concentration was largely determined by the rate of synthesis because of the strong correlation between the two parameters. The fractional catabolic rate (fraction of the intravascular pool catabolized per day) of IgD was similar to that of IgA and much higher than those of IgM and IgG, which indicates that IgD might be catabolized in similar ways as IgA but in very different ways from IgM and IgG. IgD is often viewed as a predominantly mucosal Ig isotype because of the preferential localization of IgD-producing B cells in the upper aerodigestive mucosa, such as tonsils, adenoids, and nasal mucosa, but the presence of IgD as well as IgD-producing B cells in circulation in addition to upper aerodigestive mucosa-associated lymphoid tissues (MALTs) indicates that IgD can potentially exert its immunological functions both systemically and in mucosal districts. In fact, intravascular IgD takes up 73% of total IgD in human, despite of its higher susceptibility to proteolysis, in contrast to IgA and IgG, of which only 40–50% is in the vascular compartment and are less susceptible to proteolysis (65). Therefore, this ancient Ig isotype may be equally important in monitoring the presence of systemic antigens as compared to mucosal antigens.
Cell-associated IgD
Cell-associated IgD includes transmembrane IgD, intracellular IgD, and secreted IgD bound to various cell types. Transmembrane IgD is expressed by mature naive B cells prior to antigenic stimulation and class switch recombination (CSR) and by IgD-producing B cells in the upper aerodigestive MALTs and peripheral blood of healthy individuals. B cells, in addition to expressing transmembrane IgD, can also bind secreted IgD (66). The studies of interaction of secreted IgD with T cells postulated a putative IgD receptor on a small fraction of T cells in human peripheral blood and mouse peripheral lymphoid organs, which binds to N-linked carbohydrate moieties of IgD (67–68). Crosslinking of IgD receptor on T cells was shown to protect T cells from apoptosis (69). It has also been shown that this IgD receptor could promote the formation of immune synapse between cognate T cells and naive B cells that express transmembrane IgD and thereby augment antigen presentation and antibody production (70–71). The expression of this receptor was detected on CD4+ T cells in mice within minutes after oligomeric or aggregated but not monomeric IgD injection into mouse and was inhibited by the administration of tyrosine kinase inhibitors (67). However, many data were not readily explainable. IgD receptor-expressing T cells did not immediately show an activated phenotype, such as the expression of CD25. Co-administration of T-cell-specific activating agents with oligomeric IgD did not enhance IgD receptor expression on T cells. The IgD receptor on T cells has not been identified. One reason that may have given rise to the many intriguing data is that this putative IgD receptor may not normally be expressed by T cells but rather be released by other cell types and binds to T cells after oligomeric IgD injection into mice. The release of the IgD receptor and its binding to T cells would be very rapid in response to oligomeric IgD injection, which may explain the rapid appearance of IgD receptor on T cells.
Many myeloid cell types have been shown to be able to bind secreted IgD. In humans, circulating basophils as well as basophils in tonsils, although rare in healthy subjects, displayed abundant surface IgD (53). Circulating malignant mastocytomas also bound IgD on the cell surface (53). This binding pattern of IgD to basophils and mast cells was consistent with the specific binding of IgD to basophilic and mast cell lines in vitro (53, 72). Neutrophils and/or eosinophils showed no or low IgD binding under physiological conditions (53, 73–75) but can bind significant levels of IgD under certain pathological conditions, such as skin allergy and inflammation (76–77). Peripheral blood adherent monocytes have been shown to produce pro-inflammatory cytokines upon IgD treatment (78), but other studies showed that monocytes did not have significant IgD binding (75, 79).
The IgD paradox
Soon after the discovery of IgD, a preferential association of many IgD myeloma proteins with λ light chain was observed (80–86). This association was confirmed to be true also for secreted IgD found in healthy individuals (54–55, 81, 87). Evidence of this preferential association of secreted IgD with λ light chain also came from studies showing that concentrations of both serum IgD and secreted IgD induced in cell culture correlated well with λ light chain concentrations (88–90). While the ratio of κ to λ light chains in other transmembrane or secreted Ig classes are approximately 2:1, the preference for λ light chain in secreted IgD can be as high as 60% to 90%. Transmembrane IgD, in contrast, predominantly contains κ light chain. This biased preference of secreted IgD for λ light chain and of transmembrane IgD for κ light chain observed more than 30 years ago is still not understood and has been termed the ‘IgD paradox’.
It has been hypothesized that the biased λ light chain association with secreted IgD results from receptor editing in the precursors of IgD+IgM− B cells in bone marrow or receptor revision in class switched IgD+IgM− B cells in the germinal center environment (55). Receptor editing is a process through which B-cell progenitors change the Ig light chain in their BCR in bone marrow in order to limit self-reactivity and is achieved by consecutive rearrangements of Vκ and Jκ gene segments at the κ locus and subsequently rearrangements of Vλ and Jλ gene segments at the λ locus; the latter often occurs after rearrangement of the noncoding combining sequence (RS) element with either a Vκ segment or a recombination signal sequence in the intronic region (IRS) of the Igκ locus, leading to the inactivation of the Igκ locus (RS combination) (91–92). Receptor revision results from secondary Ig gene rearrangement at the Ig light chain loci in the germinal center environment elicited by unfavorable somatic mutations that cause loss of Ig expression or disturb pairing of Ig heavy and light chains. In both cases, the usage of the λ light chain is expected to be increased. However, a recent study (93) found no evidence of receptor revision in class-switched IgD multiple myeloma cells, arguing against receptor revision or receptor editing as the underlying mechanism of the IgD paradox. Interestingly, the development of λ+ B cells, but not receptor editing, has now been found to be dependent on NF-κB signals (94). Therefore, it is possible that IgD+IgM− B cells predominantly develop from a precursor population that relied on NF-κB signals in bone marrow.
Expression of IgD
Vertebrates have evolved two major strategies to express Igs, alternative RNA splicing and CSR. In fish, alternative splicing is used to express multiple forms of IgD, while in other higher vertebrates, the expression of IgD utilizes both strategies.
Expression of IgD by alternative splicing
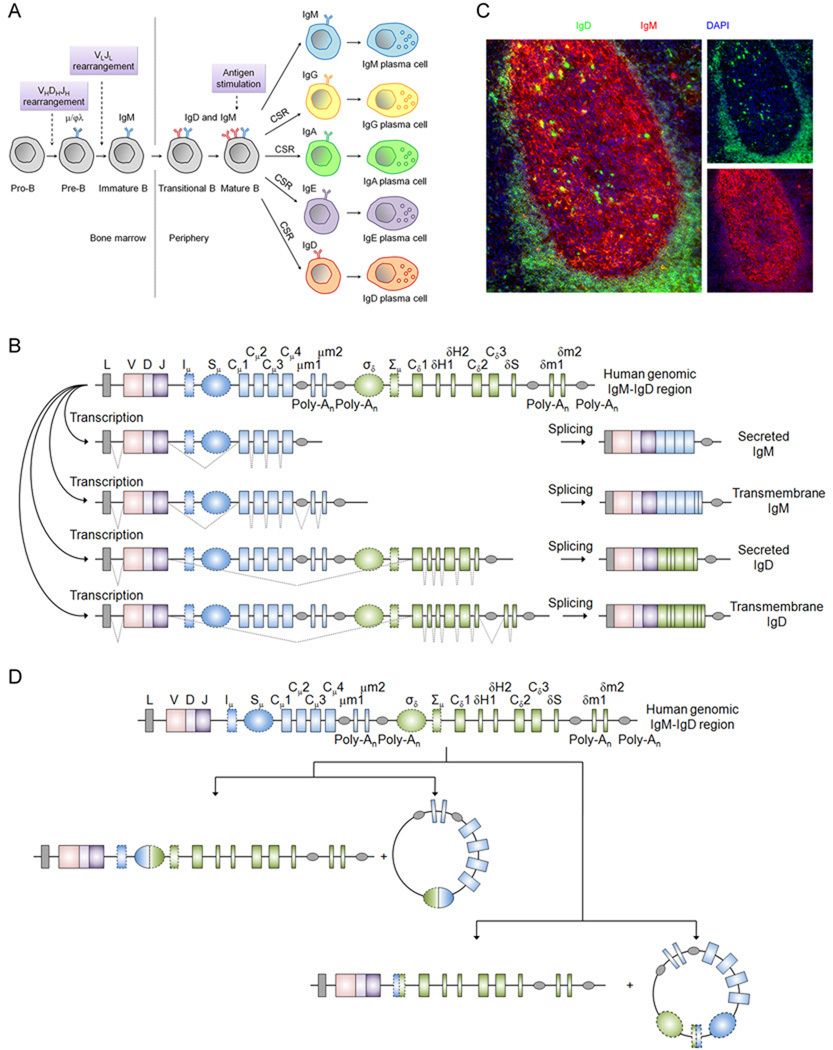
Bony fishes use alternative splicing as the strategy to produce IgD by splicing the Cμ1 to numerous duplicated Cδ exons (15, 21, 95–96). In amphibians, reptiles, and mammals, the Cδ gene is positioned immediately downstream of the Cμ gene in the same transcriptional unit, allowing these two primordial Ig isotypes to be coordinately regulated at the transcriptional level. In early stages of B-cell development prior to the mature B-cell stage, only IgM is expressed. The expression of IgD first starts when the B cell leaves the bone marrow to populate secondary lymphoid organs. Mature B cells of these species co-express IgM and IgD on the cell surface (Fig. 2A). IgM and IgD are generated by alternative splicing of a long primary mRNA transcript containing the rearranged VDJ exons and the Cμ and Cδ exons. The recombined VDJ exons are spliced to the first Cμ exon to generate IgM, or to the first Cδ exon to generate IgD (Fig. 2B). In addition, alternative splicing also determines if a cell expresses the transmembrane or secreted form of these two isotypes.
Fig. 2. Expression of human IgD by alternative splicing and CSR.
(A) Ig isotype expression during human B-cell development. Pre-B cells and immature B cells developing in the bone marrow express μ heavy chain associated with either surrogate light chains VpreB and Vλ5 before light chain rearrangement or rearranged L chain after L chain rearrangement. Upon exiting the bone marrow, transitional and mature B cells co-express IgM and IgD through alternative splicing. IgD is expressed at a higher level on the cell surface than IgM. After antigenic stimulation, most mature B cells rapidly downregulate IgD expression and undergo class switch recombination (CSR) to express either IgG, IgA, or IgE in response to the antigenic signals encountered. Some human B cells also undergo CSR to IgD. Class-switched B cells can further differentiate into plasmablasts or plasma cells (PCs) that secrete the respective Ig isotype. Some mature B cells can downregulate IgD and develop into IgM-secreting PCs perhaps either without undergoing CSR or by undergoing ‘silent CSR’ involving intra-Sμ region DNA recombination (Chen and Cerutti, unpublished data). (B) Expression of IgD and IgM by alternative splicing in mature B cell. Exons encoding the rearranged VDJ region and the various domains (including the membrane and secreted portions) of IgM and IgD are shown in boxes. Dotted gray lines show the various splicing configurations of primary transcripts to yield secreted and transmembrane forms of IgM and IgD. (C) IgD and IgM expression in follicular mantle and germinal center B cells. A lymphoid follicle in human tonsil is shown. Mature B cells located in the follicular mantle zone prior to antigen encounter express more IgD (green) than IgM (red) on the surface due to the higher stability and translation efficiency of δ mRNA than μ mRNA. After antigenic stimulation, they enter the germinal center, where IgD is rapidly downregulated at transcriptional level and IgM is transcriptionally upregulated and the half-life of μ mRNA is significantly prolonged, as evidenced by the drastic reduction of IgD and increase in IgM in the germinal center. Cells that stain strongly with IgD in the germinal center are class switched IgD-secreting cells. Some IgM staining in the germinal center is contributed by follicular dendritic cells that capture IgM-containing immune complexes. DAPI (blue) stains cell nuclei. Original magnification, ×40. (D) Expression of IgD by CSR. Schematic representation of Cμ-to-Cδ CSR in human, which has been found to occur between Sμ and σδ regions (left) and between Iμ and Σμ regions (right) in normal and malignant B cells. The intervening DNA is looped out after CSR and forms a switch circle.
The alternative splicing process is tightly regulated during B-cell development. However, the mechanism of regulation is poorly understood. The transcription ratio of μ and δ exons varies in different types of B cells and B-cell lines (97) and does not necessarily reflect the abundance of membrane protein of either isotype. For example, in mature resting follicular mantle B cells, more μ mRNA than δ mRNA is transcribed, but IgD is expressed at higher densities on the surface than IgM. This has been attributed to the higher stability and better translation efficiency of δ mRNA than μ mRNA and the higher turnover rate of transmembrane IgM protein (98–99). When activated by antigen, μ exon transcription is preferentially induced and the half-life of μ mRNA is significantly increased. In contrast, δ exon transcription and IgD expression are significantly repressed. The result is the drastic downregulation of IgD expression by antigen-activated B cells, which explains the low level of IgD found in germinal centers of lymphoid tissues, such as lymph nodes, spleen, and tonsils (Fig. 2C). Studies have shown the existence of a specific region between the μ and δ genes that attenuates the transcription complex 5′ to the δ exons and forces the preferential production of mRNA for membrane IgM. Binding of lineage-specific regulatory proteins to this region may alter the ratio of processing of the primary mRNA transcripts (100–101). In terms of the regulation of alternative splicing between transmembrane and secreted forms, limited information is known for IgM. Peterson and Perry (102) have suggested that there is competition between the tails of Cμ3 exon and Cμ2 exon for the cleavage and polyadenylation factors and that the biases in this competition change as the B cell matures. Indeed, these two sites compete for limiting amounts of a factor, CstF-64, that directs the cutting enzymes to the 3′ splice site (103). The site for the transmembrane form of the μ chain is more efficient at binding. Hence, at the low amounts of CstF present in the B cell, only the transmembrane form of IgM is made. However, when the B cell differentiates into a plasma cell, more CstF-64 is produced, and both sites are utilized. The developing plasma cell synthesizes both transmembrane and secreted forms of IgM. Whether such a mechanism is also involved in the regulation of alternative splicing of transmembrane versus secreted forms of IgD is unknown.
Expression of IgD by CSR
In contrast to other Ig isotypes, there is no canonical switch region 5′ to the Cδ gene in mammals. Only rudimentary switch sequences are present in human and mouse. Consequently, CSR from μ to δ is traditionally considered a very rare event. Study of human normal tonsillar and leukemic B cells revealed that a region called σδ located in the intron between Cμ and Cδ exons and containing G-rich pentameric repeats is able to serve as an actual switch region to mediate CSR with Sμ and result in the deletion of Cμ (55, 104). Study of human and murine myeloma and hybridoma cells also demonstrated recombinations involving two regions that contain homologous sequences, namely Iμ and Σμ located 5′ and 3′ to the Cμ gene, respectively, followed by the deletion of Cμ (9, 105) (Fig. 2D). Therefore, B cells that switch to IgD and are capable of secreting large amounts of IgD do exist in normal individuals. Indeed, they are quite abundant in the upper aerodigestive MALTs, such as tonsils, adenoids, and nasal mucosa, as discussed earlier, which corresponds to the abundant IgD found in these tissues.
Similar to the regulation of alternative splicing, the regulation of IgD CSR and production are not well understood. Genotypic analysis has obtained evidence of the oligoclonal nature of tonsillar IgD-secreting B cells and their generation in superantigen-driven immune responses (106). Germinal center IgD+IgM−CD38+ B cells in tonsils could differentiate into IgD-secreting cells in the presence of the combined stimulation of CD40 ligand (CD40L) (or CD154), IL-2, and IL-10. However, these germinal center-derived precursor cells were probably already switched to IgD before stimulation, because they lacked the expression of IgM. It was also clear that stimulation through CD40 is dispensable in the induction of IgD secretion (9). IL-2 and IL-10 thus contribute to the differentiation of IgD class-switched B cells and their secretion of IgD in vitro. IgD can be spontaneously secreted by cultured peripheral blood and tonsil mononuclear cells, and the secretion was markedly increased by anti-CD40 stimulation in the presence of T-helper 2 (Th2) cytokines such as IL-4 and IL-10. However, the spontaneous and Th2 cytokine-induced IgD secretion was only observed in certain healthy donors. Furthermore, as no assessment of CSR was performed and mixed populations of unswitched and IgD class-switched B cells were present in the culture, it was not possible to delineate whether anti-CD40 and Th2 cytokines induced B cells to undergo IgD CSR and differentiation into IgD-secreting cells or simply increased IgD secretion by unswitched or IgD class-switched B cells. The signals for IgD CSR and secretion in vivo are further obscured by the frequently increased IgD levels in patients with HIGMs. These patients have elevated serum IgM but reduced IgG, IgA, and IgE due to defects in various components of the CSR machinery. The increase in IgD may be a result of the compensatory response from IgM+IgD+ B cells due to the absence of other switched isotypes rather than an indication of the inhibitory effect of the CSR machinery on IgD CSR and secretion in vivo. Therefore, the delineation of the signals that drive Cμ-to-Cδ CSR and the secretion of IgD requires an experimental strategy that directly assesses Cμ-to-Cδ CSR and differentiation of IgD-producing cells and simultaneously correlates those with the secretion of IgD. Using unswitched IgD+IgM+ B cells purified from human peripheral blood, we found that B-cell activation factor of the tumor necrosis factor family (BAFF), also known as B lymphocyte stimulator (BLyS), in combination with IL-15 and IL-21or IL-2 and IL-21 could simultaneously induce CSR to IgD, development of IgD+IgM− B cells, as well as IgD secretion in vitro. A proliferation-inducing ligand (APRIL) in combination with IL-15 and IL-21 or IL-2 and IL-21 was also able to do so (53). The number of circulating IgD class-switched B cells is reduced in patients with defects in the CD40L–CD40 and BAFF/APRIL-transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) signaling pathways, suggesting the involvement of both T-cell-dependent and T-cell-independent pathways for IgD CSR in vivo. The localization of IgD class-switched B cells both inside germinal centers and outside follicles also seems to support the notion that multiple pathways participate in IgD CSR in vivo. Activation-induced cytidine deaminase (AID), a DNA-editing enzyme essential for CSR to other Ig isotypes, was required for CSR to IgD both in vivo and in vitro.
Biochemical properties of IgD and functional implications
IgD is a monomer made up two identical heavy and light chains organized into variable and constant domains, similar to IgG. Human IgD has three Cδ domains. The structure of Cδ1 and Cδ2 domains is similar to that of C domains of other Ig isotypes. Cδ3, in contrast, is unique by the absence of several proline residues (which usually introduce turns in polypeptide backbones) and by the presence of two N-linked glycosylations. The overall conformation of Cδ3 domain is thus different from that of the C domains of other isotypes. Several studies suggest that these structural features and the resulting configuration of the molecule participate in specific biological properties of IgD (37, 107–109). An additional N-linked glycosylation in the Cδ2 domain close to the inter-H chain disulfide bond was found to be essential for the folding and secretion of the intact IgD molecule (110). This glycan is usually in the high-mannose form and is buried within the folded IgD molecule.
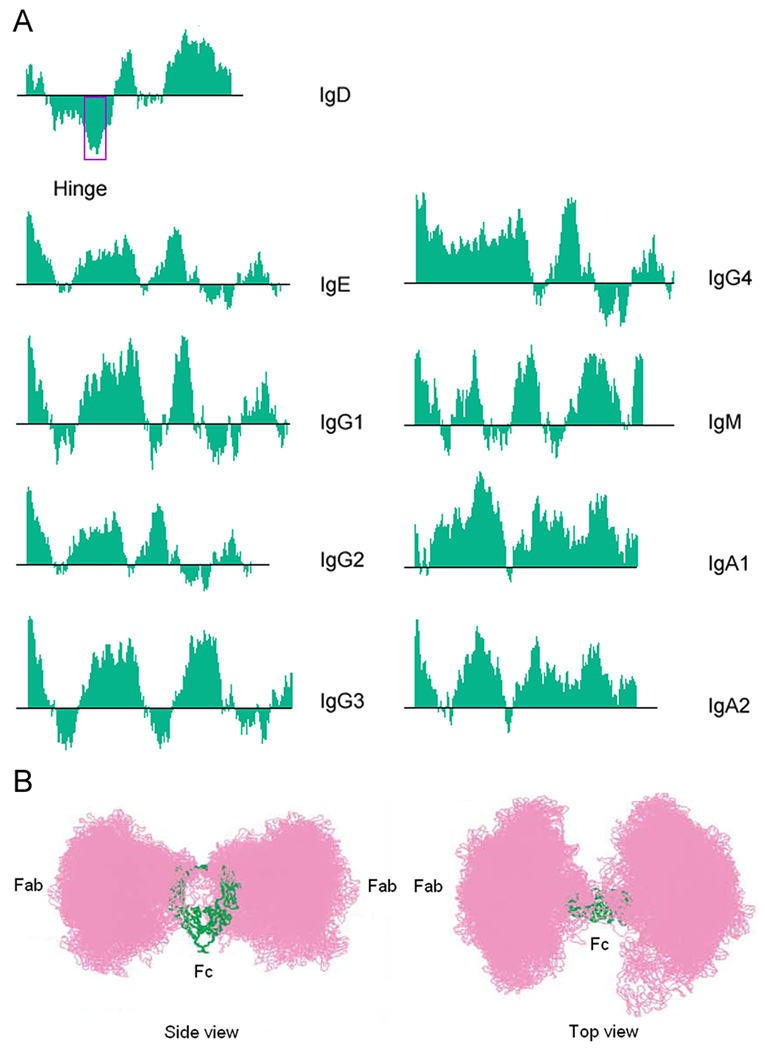
Mammalian IgD has a hinge made up of 64 amino acids, longer than the hinge of any other Ig isotype. It includes two sub-regions encoded by two exons respectively (Fig. 1). The first sub-region is alanine- and threonine-rich and is densely decorated with multiple (up to seven) O-linked glycosylations that contain N-acetylgalactosamine moieties in the attached glycans, a unique structure only found in IgA1 and IgD (9, 37, 108, 110–114). Together with the N-linked glycans, they can contribute up to 15% of the molecular weight of an IgD molecule. This high carbohydrate content and the differential degrees of glycosylation in different IgD preparations had in fact led to discrepancies in the findings of several studies in the 1970s and caused confusion as to whether the δ chain had three or four constant domains (115). The second sub-region has a highly charged and hydrophilic stretch of amino acids, a feature unique to IgD (Fig. 3A). Such a high degree of hydrophilicity in the IgD hinge results from the presence of abundant charged amino acid residues, especially lysine (K), glutamate (E), serine (S), and threonine (T). The sequence of this sub-region might explain the very high sensitivity of IgD to proteolytic enzymes, as studies of IgD myeloma proteins showed the presence of sites of ‘spontaneous’ proteolysis in this glutamate- and lysine-rich sub-region (116–117). However, it was possible that the IgD preparations in those studies were contaminated with plasmin, as IgD purified from serum by a two-step procedure of ammonium sulfate precipitation and gel filtration was stable for extended periods (118). Sites susceptible to proteolysis have also been found in the Cδ1 domain and in the lysine- and arginine-rich C-terminal portion of the Cδ3 domain. Noteworthy, IgD is known to display significant heterogeneity in both size and charge not only in healthy individuals but also in myeloma patients that have mostly monoclonal IgD (35, 37–38), highlighting the susceptibility of IgD to multiple post-translational modifications and the complexity of the regulation of its metabolism and function.
Fig. 3. Human IgD hinge region contains a sequence resembling that of consensus ligand of heparin.
(A) Hydrophobicity indices of human Ig isotypes at pH 3.4 determined in silico using the VectorNTI software. The region of high hydrophilicity in IgD is demarcated by a rectangle. Sequences of these proteins were downloaded from NCBI GenBank, with GenBank ID in brackets: IgD (P01880.2), IgE (P01854.1), IgG1 (AAH73782), IgG2 (AAH62335), IgG3 (AAH33178), IgG4 (AAh25985), IgM, (AAH89412), IgA1 (AAH87841), IgA2 (AAH73765). (B) Three-dimensional motions of the IgD Fab parts around the Fc part. The Fab parts are colored in pink and the Fc part is colored in green. The length and the semi-extended flexible conformation of the IgD hinge allow the Fab parts to swivel around the Fc part in three dimensions in solution. This motion gives the Fab parts extraordinary flexibility in capturing antigens, as well as renders the Fc part shielded by the Fab parts in solution. The Protein Data Bank (PDB) ID of the best-fit IgD structure predicted in this study is 1ZVO. Modified from (119) with permission. [Permission from Elsevier to use the figure shown in Fig. 3B, modified from (119), can be found at https://s100.copyright.com/CustomerAdmin/PLF.jsp?lID=2010031_1268678952456.]
The average structure of a myeloma IgD protein in solution has been predicted using constrained X-ray scattering (119), which revealed some unique features of IgD. The hinge of IgD adopts a semi-extended α-helical conformation in solution. The entire IgD molecule adopts an overall flexible T shape rather than Y shape as predicted for other Ig isotypes. The two Fab fragments are located primarily on the two sides of the Fc fragments and can swivel about the Fc fragments owing to the flexibility of the semi-extended hinge. The Fc fragments are thus shielded by the Fab fragments (Fig. 3B). There are two major conclusions based on this structure model: (i) the antigen-binding part of the IgD molecule is very flexible, and (ii) the Fc part of the IgD molecule is usually concealed by the motion of the Fab part in solution. These two conclusions have several important functional implications. (i) The co-expression of IgM and IgD as B-cell antigen receptors may optimize the recognition of antigens at different antigen concentrations (120). IgM facilitates antigen binding at high concentrations, while IgD facilitates antigen binding at lower concentrations owing to its reach and flexibility. (ii) The Fab part of IgD may mask large regions of the surface of the Fc part in solution, which might provide a mechanism to regulate the binding of IgD to its potential receptor(s). The interaction of IgD with its receptor(s) might only be possible when the motion of the Fab part is somewhat constrained by binding to antigen or certain auxiliary molecules, or by proteolytic cleavage of the Fab part. Heavy O-glycosylation in the first sub-region of the hinge may increase the rigidity of the hinge and limit the movement of the Fab parts, thus facilitating the exposure of the Fc part and its interaction with potential receptor(s); it may also restrict the access of proteolytic enzymes to the second sub-region of the hinge. All these factors potentially contribute to the complexity of the regulation of IgD’s function.
Immunological functions of IgD
Transmembrane and GPI-anchored IgD as B-cell antigen receptors
IgD is found as an antigen receptor on the surface of the majority of human (121–123) and murine B cells prior to antigenic stimulation and CSR (124–125). The advantage of having both IgD and IgM with the same antigen specificity on the same cell is still not clear. Initially, it was proposed that the two receptor classes deliver different signals to the B cell (126–128). However, contradictory results were obtained. Engagement of IgM on immature B cells in vivo and in IgM-transfected cells in vitro results in apoptosis, whereas engagement of IgD failed to do so (129–130). Reverse results were obtained in another study, which showed that a minimal stimulation with anti-IgD antibodies but not with anti-IgM antibodies induced apoptosis of mature resting B cells (131). Reasons for the discrepancies in the results might be that BCR signaling depends on the developmental stage of the B cell, the type of B cell line used, as well as the receptor density on the cell surface (132–133). Experiments involving transgenic mice showed that IgD could fully substitute the function of IgM in early B-cell development (134), and BCR of one class could largely compensate for the loss of the other because mice deficient for either the IgM or IgD heavy chain showed only mild phenotypes (135–137). Mice deficient in IgD did not show apparent abnormality in B-cell development and function, although the antibody response was slightly delayed as compared to wildtype mice (136–137). IgD-deficient mice had 30–50% reduction in the number of peripheral mature B cells, pointing to a role of IgD in B-cell homeostasis (136–137).
In terms of signaling, transmembrane IgM and transmembrane IgD are associated with different signaling molecules in human B cells (9). The cytoplasmic parts of IgM and IgD are identical, consisting of only three amino acids. The association of different signaling molecules may lead to different functional outcomes when the B cell is stimulated through IgM as compared to through IgD or through both IgM and IgD. Geisberger and colleagues (138) proposed that transmembrane IgD modulates the function of transmembrane IgM by increasing the threshold of IgM signaling required for inducing cell activation, anergy, or cell death; therefore, IgD delivers a negative or tolerogenic signal. This model is supported by several studies using human and mouse B cells (139–142). Loset and colleagues(120), however, have proposed another model that IgD and IgM are optimal for antigen capturing at different concentrations of the antigen. This model is supported by the solved crystal structure of IgD showing that IgD is a T-shaped molecule with flexible F(ab′)2 domains owing to the long hinge region (119). IgD can also be post-translationally processed and linked to the membrane lipids via a GPI anchor (143), initiating different signaling cascades from the transmembrane IgD molecule. The GPI-anchored IgD selectively activates cyclic adenosine monophosphate (cAMP)-dependent signaling pathways (144), which synergizes with transmembrane IgM and transmembrane IgD in initiating Ca2+-dependent signaling.
Secreted IgD as an immunomodulator in immunity and inflammation
The function of secreted IgD is yet another mystery of IgD and has been a longstanding puzzle in immunology. IgD secreted by IgD class-switched B cells was shown to be frequently autoreactive (145). Secreted IgD can bind to many pathogenic microorganisms and their products, many of which are virulence factors used in pathogenesis, such as rubella virus, measles virus, diphtheria toxin, Escherichia coli, streptococci streptolysin O, Moraxella catarrhalis and Haemophilus influenzae adhesin MID (also called Hag) (53, 64, 146–152). The fact that many of the above-mentioned microorganisms are pathogens found in the same anatomic location where IgD is abundant, i.e. the upper aerodigestive MALTs, strongly supports the notion that IgD has protective functions against these pathogens perhaps by contributing to immune exclusion or neutralization. Further evidence supporting this notion are the markedly increased numbers of IgD-producing B cells in these locations in IgA deficiency (59) and frequently increased serum IgD levels during various infections (153). Studies of immunosenescence also lend credence to this notion, as there is an age-dependent drop of serum IgD concentration, which correlates with a gradual decline of immunity in older people (154).
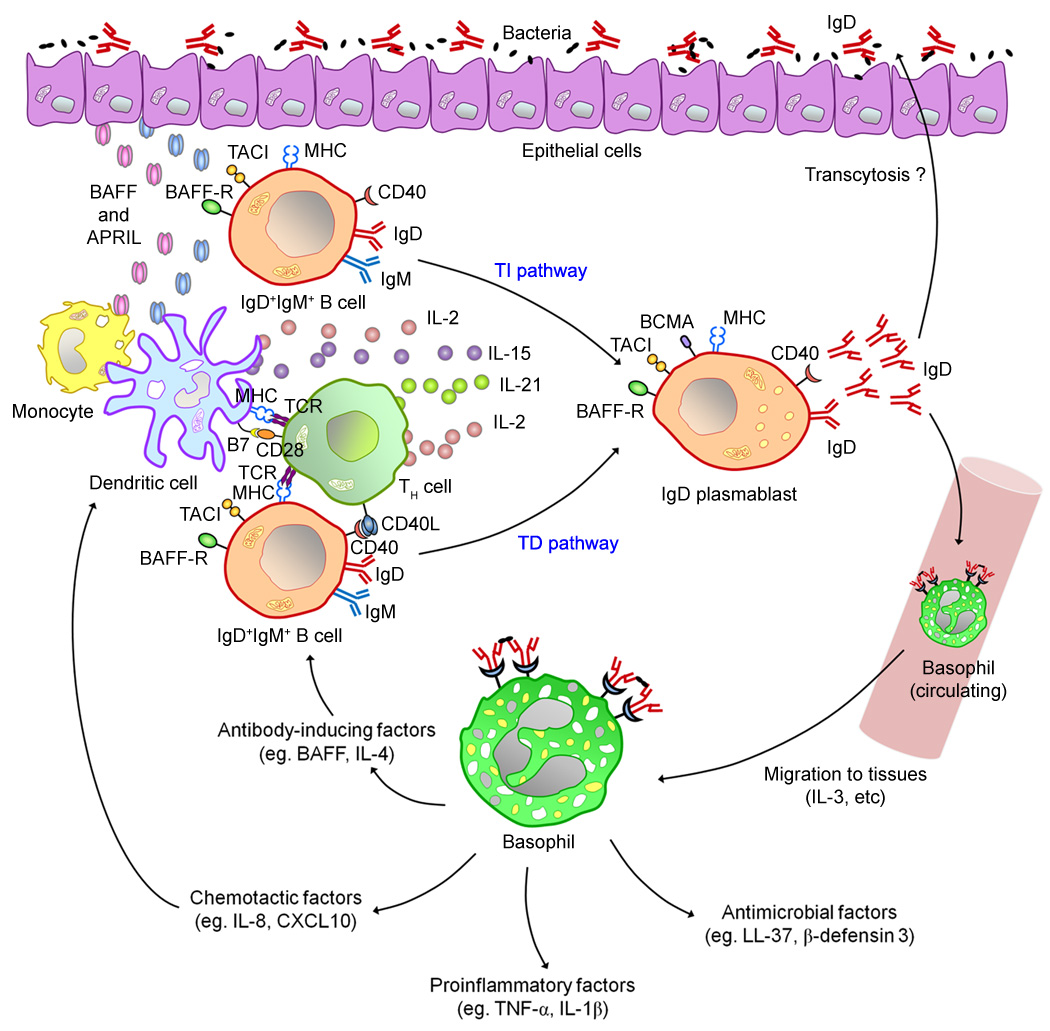
A careful scrutiny of the old literature reveals many intriguing connections of secreted IgD with myeloid cells. Early studies showed that granulocytes, such as neutrophils and eosinophils, could bind significant levels of IgD under certain pathological conditions, such as skin allergy and inflammation (76–77), although they bound little IgD under physiological conditions (73–75). IgD was the predominant Ig class present on neutrophils, probably together with complement, in patients with allergic contact dermatitis (76). IgD on the surface of neutrophils seemed to inhibit the release of oxygen free radicals, in contrast to IgG and IgA, which stimulated the release of oxygen free radicals (155). More recently, peripheral blood adherent monocytes were shown to produce pro-inflammatory cytokines including tumor necrosis factor (TNF), IL-1β, and IL-1 receptor antagonist (IL-1RA) upon incubation with IgD purified from serum, and this effect was not due to endotoxin contamination present in the IgD preparation (78). Confounding findings do exist in the literature regarding the binding of IgD to monocytes, neutrophils, and eosinophils. Several studies employing different methodologies showed no binding of IgD to monocytes, neutrophils, and eosinophils (73–75). Remarkably, Sjoberg isolated lymphocytes from peripheral blood using Ficoll-Isopaque gradient (density 1.076 – 1.078 g/mL) (156–157) and showed the presence of IgD receptor on T lymphocytes as well as many more non-T lymphocytes that were not monocytes (79). Based on what we now know about the composition of peripheral blood leukocytes isolated by Ficoll-Isopaque gradient, which include T cells, B cells, NK cells, monocytes, and basophils, the cells seen by Sjoberg to express IgD receptor could be basophils. Many studies have showed the presence of IgD on the surface of basophils and basophillic cell lines (53, 72, 158–159). Crosslinking of surface IgD on basophils using an anti-IgD antibody or incubation of the basophilic cell line KU812 with polymerized IgD prepared by glutaldehyde crosslinking could induce the release of cytokines and chemokines without inducing degranulation and the release of histamine (53, 72). Studies on the ability of IgD to function as a chemotactic agent (the so-called cytotaxigen or cytotaxinogen) found that IgD could induce the infiltration of myeloid cells, mainly composed of neutrophils and low numbers of eosinophils but not lymphoid cells, into skin at an early stage of inflammation in patients with contact dermatitis but not skin ulcers (77). Increased infiltration of basophils was also observed in this study, but due to low basophil number and large variations, statistical significance could not be determined. This observation is in line with our finding showing massive infiltration of basophils and/or mast cells in the tonsil of a patient with periodic fever, aphthous-stomatitis, pharyngitis, adenitis (PFAPA) syndrome (53). In addition, crosslinking of surface IgD on basophils from healthy donors in vitro induced the release of antimicrobial and B-cell-stimulating factors, such as LL-37 and BAFF, that augmented microbial killing and B-cell antibody responses. The ability to induce BAFF production, a B-cell homeostatic cytokine, by basophils may explain the observation of peripheral B-cell deficiency observed in IgD−/− mice (136–137). Basophils found in the tonsil of the patient with PFAPA syndrome also contained LL-37 and BAFF. Findings suggesting the connection of IgD and basophils were also obtained in mouse studies long time ago. Injection of a goat polyclonal antibody against mouse IgD results in increased numbers of basophils that produced IL-4 in the bone marrow and spleen (160–163). These studies collectively support the notion that IgD is an important immunomodulatory molecule and that IgD promotes immune defense by inducing the activation and infiltration of IgD-interacting cells into tissues and their production of immune-activating factors, and overactivation of this pathway can cause inflammation and tissue damage (Fig. 4).
Fig. 4. Model of human IgD regulation and function.
B cells from the upper aerodigestive mucosa undergo CSR from Cμ to Cδ through local T-dependent (TD) and T-indepdent (TI) pathways involving CD40L, BAFF, or APRIL together with a unique cocktail of cytokines, including IL-2 and IL-21 or IL-15 and IL-21. CSR requires AID and generates mucosal and circulating IgD plasmablasts that secrete IgD reactive against respiratory bacteria. Mucosal IgD might enhance local immunity after translocating across epithelial cells, whereas circulating IgD binds to basophils. In addition to delivering rapid innate immune signals and alerting the immune system as to the presence of invading bacteria, basophils exposed to IgD-reactive antigens migrate to systemic and mucosal lymphoid organs, perhaps in response to chemotactic factors released by IgD-stimulated mast cells. Tissue-based basophils enhance immune protection by releasing antimicrobial, opsonizing, B-cell-stimulating, chemotactic and pro-inflammatory mediators such as cathelicidin, IL-1β, IL-4, IL-8, IL-13, CD40L, BAFF, APRIL, TNF, and CXCL10, but not histamine. Finally, IgD-armed basophils may regulate B-cell homeostasis through tonic release of the obligatory B cell survival factor BAFF in response to IgD-reactive foreign or autologous antigens.
IgD in diseases
The wide range and high degree of variability of serum IgD concentration among different individuals have rendered the exact role of IgD in various diseases poorly defined. Serum IgD has been considered to be a valuable indicator only for IgD multiple myeloma (164). A good review of the perturbation of serum IgD concentration was provided by Vladutiu (153), and this article will therefore focus on the discussion of IgD’s role in diseases in the context of B-cell and myeloid cell biology. Nonetheless, a careful examination of this body of literature not only sheds light on the immunomodulatory functions of IgD but also reinforces the idea that IgD’s functions may contribute to disease pathogenesis or may be exploited to achieve disease amelioration.
Immunodeficiencies
Individuals with primary immunodeficiencies were found to have either increased or decreased IgD production (165). These patients frequently have accompanying infections; hence, it is unclear whether the perturbation of IgD production in these patients is a result of immunodeficiency per se or a result of infections. Within the entire group of immunodeficiencies, a positive correlation of IgD with IgA and IgE was noted. In human immunodeficiency virus (HIV)-infected people, significantly increased IgD levels were found in asymptomatic or mildly symptomatic individuals (166–169). This hyper-production of IgD became progressively more pronounced in patients with increasingly severe infection and reached its most marked level in patients with AIDS-related complex (ARC) (166), suggesting a possible protective role of IgD in the body’s attempt to cope with severe infection. Remarkably, the production of IgD in HIV-infected people is independent of T-cell defects (167), highlighting the importance of T-cell independent pathways of IgD production. Further indirect evidences of the possible protective role of IgD in immunodeficiency and infection come from selective IgA deficiency, where there were significantly increased numbers of IgD-producing B cells in the upper aerodigestive MALTs (43, 59). Secreted IgD may provide a layer of mucosal protection during IgA deficiency. However, it is important to note that serum IgD concentration was found to be in the normal range of children with selective IgA deficiency (50).
Infections
Many chronic infections can lead to the increase of serum IgD concentration (153, 170), which might suggest some protect roles of IgD in infection. Serum IgD was increased in patients with leprosy (171), tuberculosis (172–173), salmonellosis, infectious hepatitis (174), and malaria (175). IgD reactive to pathogens has been detected in patients with rubella (64) and sub-acute sclerosing panencephalitis (146). It has also been found that IgD can react to various virulence factors of pathogens, including diphtheria toxin, streptococci streptolysin O, M. catarrhalis and H. influenzae adhesin MID (also called Hag), as well as whole bacteria such as M. catarrhalis, H. influenzae, Escherichia coli, and streptococcus group A (53, 147–152, 176). Many patients with elevated IgD had recurrent staphylococcal infections (177), and increased values of serum IgD were found in some patients with viral infections (64, 178). The clinical significance of these specific IgD antibodies is to be determined.
B-cell malignancies
A well-known type of B-cell malignancy related to IgD is IgD multiple myeloma (MM). MM is a cancer of plasma cells characterized by the neoplastic proliferation of a single clone of plasma cells that usually produce a monoclonal Ig that can be found in blood and/or urine, although there has been a longstanding controversy of whether the precursors of myeloma cells are hematopoietic stem cells (179), pre-B cells (180), germinal center (GC) B cells (181), circulating memory cells (182–183), or plasmablasts (184). IgD MM, as the name implies, is the MM of the IgD-producing plasma cells. It is a rare (< 2%) (83, 185) but aggressive form of MM. IgD in the serum of IgD MM patients is always increased and often found to contain a monoclonal IgD protein (86). Sixty to 90% of IgD MM cases are of the λ type (83, 86), a manifestation of the IgD paradox discussed earlier. Although IgD MM cells are suggested to have a GC origin and are frequently observed to have undergone SHM and CSR to IgD (55, 104–105, 186), the exact precursor of the malignant IgD MM cells is not known. The signals involved in the IgD CSR and sustained survival of IgD MM are also poorly understood. Some cases of hairy cell leukemia, a type of mature B-cell neoplastic disease, were also found to express only IgD on the cell surface, suggesting that these cells had undergone CSR (187–189).
Allergic diseases
Elevation of serum IgD has been reported in patients with many allergic diseases, such as atopic diseases, allergic asthma, rhinitis, allergic bronchopulmonary aspergillosis, and antibiotic allergy (158–159, 173, 190–196). Notably, among asthmatic children, such increases were mostly observed during first diagnosis and during disease attack (190, 194). There was evidence suggesting that allergen-specific IgD and allergen-specific IgE were similarly regulated during allergic responses (191). These findings have led to the postulation that the increase of IgD might represent an attempt of the host to block asthma and that IgD might be a protective regulatory factor (190, 194). Indeed, the ability to block IgE-mediated responses has been documented for IgD (197).
Autoimmunity
Serum IgD is often increased in patients with autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (174, 198–201), consistent with the observation that many mature B cells class switched to IgD are autoreactive in healthy individuals (145). Anti-nuclear IgD antibodies were found in SLE patients (145, 193, 202–206), and a slight increase of serum IgD was found in patients with Sjögren’s syndrome and in a patient with autoimmune thyroiditis (207). In addition, elevated concentrations of autoantibodies against IgD, mainly of the IgA and IgG classes, were detected in many patients with rheumatoid diseases such as RA, SLE, and mixed connective tissue disease (MCTD) syndrome (208). In mice, naturally occurring IgD that recognizes heat shock proteins (HSPs), such as Hsp70 and gp96, has been found in the serum in an individual-specific and strain-specific manner but does not seem to be related to autoimmunity, as no HSP-reactive IgD was detected in the autoimmune lpr mouse model (209).
Hyper-IgD syndrome and other autoinflammatory syndromes
There is a group of hereditary syndromes called autoinflammatory syndromes or periodic fever syndromes, which include hyper-IgD syndrome (HIDS), familial Mediterranean fever (MFM), TNF receptor-associated periodic syndrome (TRAPS), Muckle-Wells syndrome (MWS) or cryoprin-associated periodic syndrome, familial cold autoinflammatory syndrome (FCAS), neonatal onset multisystem inflammatory disease (NOMID), and PFAPA syndrome (210). These diseases involve recurrent attacks of fever and inflammation in the absence of infection. Many patients also have elevated levels of serum IgD during attacks. Due to the complex etiology of this group of syndromes, this section will limit the review to HIDS, which more specifically and almost always involves the elevation of serum IgD.
Patients with HIDS have recurrent attacks of fever that usually start in infancy (211). During the fever episodes, all patients have elevated serum IgD and frequently also have elevated serum IgA, while between attacks serum IgD level can be normal (211–212). The attacks generally recur every four to six weeks (213–214). HIDS is caused by a deficiency of mevalonate kinase (MvK), a key enzyme in the cholesterol biosynthetic pathway (213, 215). Multiple mutations in the MvK gene have been found in HIDS patients whose MvK activity is reduced to 5% to 15% of the normal level. MvK is located predominantly in peroxisomes (216). It is intriguing why the deficiency of a metabolic enzyme leads to the hyper-production of an Ig and inflammation. There are hypotheses proposing that MvK deficiency leads to the defects in the production of certain downstream metabolites, such as isoprenoids, that are involved in the regulation of immunity and inflammation (217). There have been reports showing exaggerated macrophage activation and dysregulated innate immune responses in HIDS patients (218–219). There are often increased levels of many pro-inflammatory cytokines and acute phage proteins during attacks, such as TNF, IL-6, C-reactive protein (CRP), IL-1β, IL-8, monocyte chemoattractant protein-1 (MCP-1), and IFN-γ during fever attacks. We have observed increased number of IgD class-switched B cells not only in the upper aerodigestive mucosa but also systemic lymphoid organs, such as the spleen, of HIDS patients (Chen and Cerutti, unpublished data). It is important to note the distinction of HIDS and selective IgA deficiency, both of which have increased numbers of IgD-producing B cells in upper aerodigestive mucosal districts. In HIDS, inflammation and fever may be further contributed to by an increase of IgD-producing B cells in the systemic immune system, which is not seen in selective IgA deficiency, a condition with very different clinical presentation (50). These clinical data are consistent with the observation of IgD’s inflammation-inducing capacities, which include its ability to bind to cells of the myeloid lineage in the systemic circulation, such as basophils, and its ability to activate these cells to produce pro-inflammatory cytokines upon cross-linking (53). A mouse model of HIDS was created recently with the deletion of one allele of the MvK gene (220). Multiple matings failed to produce an MvK−/− mouse, probably due to embryonic lethality. MvK+/− mice had significantly elevated levels of serum IgD. Serum TNF levels were also increased, though not statistically significant, and cholesterol levels in tissues and blood and isoprenoids in tissues were normal. This model should prove invaluable in understanding the pathogenesis of HIDS.
Concluding remarks
Having both seen the limelight and been left in oblivion since its discovery almost 50 years ago, IgD still remains an enigmatic player in the immune system. Its perpetuation through hundreds of millions of years of evolution from fish to human clearly evinces its important functions, which remain to be addressed by future research. Clearer pictures of the function of IgD in health and disease will depend on further breakthroughs such as understanding more detailed signals that promote IgD CSR and production in vivo, the B-cell precursors that preferentially switch to IgD under physiological and pathological conditions, as well as the isolation of the IgD receptor and delineation of its signaling pathways in myeloid cells. To that end, we should be able to harness the functions of IgD in order to better combat infection and regulate immunity.
Acknowledgments
The authors are supported by National Institutes of Health research grants AI057653 and AI074378 (to AC), and by a research grant SAF 2008-02725 from the Ministerio de Ciencia e Innovación, Plan Nacional de Investigación Cientifica, Desarollo e Innovación Tecnologica (to AC).
References
- 1.Rowe DS, Fahey JL. A new class of human immunoglobulins. I. A unique myeloma protein. J Exp Med. 1965;121:171–184. doi: 10.1084/jem.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe DS, Fahey JL. A new class of human immunoglobulins. Ii. Normal serum IgD. J Exp Med. 1965;121:185–199. doi: 10.1084/jem.121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black CA. A brief history of the discovery of the immunoglobulins and the origin of the modern immunoglobulin nomenclature. Immunol Cell Biol. 1997;75:65–68. doi: 10.1038/icb.1997.10. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD, van Boxel JA, Asofsky R, Paul WE. Cell membrane IgD: demonstration of IgD on human lymphocytes by enzyme-catalyzed iodination and comparison with cell surface Ig of mouse, guinea pig, and rabbit. J Immunol. 1976;116:1173–1181. [PubMed] [Google Scholar]
- 5.Martin LN, Leslie GA, Hindes R. Lymphocyte surface IgD and IgM in non-human primates. Int Arch Allergy Appl Immunol. 1976;51:320–329. doi: 10.1159/000231606. [DOI] [PubMed] [Google Scholar]
- 6.Ruddick JH, Leslie GA. Structure and biologic functions of human IgD. XI. Identification and ontogeny of a rat lymphocyte immunoglobulin having antigenic cross-reactivity with human IgD. J Immunol. 1977;118:1025–1031. [PubMed] [Google Scholar]
- 7.Finkelman FD, Woods VL, Berning A, Scher I. Demonstration of mouse serum IgD. J Immunol. 1979;123:1253–1259. [PubMed] [Google Scholar]
- 8.Chen CL, Lehmeyer JE, Cooper MD. Evidence for an IgD homologue on chicken lymphocytes. J Immunol. 1982;129:2580–2585. [PubMed] [Google Scholar]
- 9.Preud'homme JL, Petit I, Barra A, Morel F, Lecron JC, Lelievre E. Structural and functional properties of membrane and secreted IgD. Mol Immunol. 2000;37:871–887. doi: 10.1016/s0161-5890(01)00006-2. [DOI] [PubMed] [Google Scholar]
- 10.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hordvik I, Thevarajan J, Samdal I, Bastani N, Krossoy B. Molecular cloning and phylogenetic analysis of the Atlantic salmon immunoglobulin D gene. Scand J Immunol. 1999;50:202–210. doi: 10.1046/j.1365-3083.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc Natl Acad Sci USA. 2006;103:10723–10728. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambon-Deza F, Espinel CS. IgD in the reptile leopard gecko. Mol Immunol. 2008;45:3470–3476. doi: 10.1016/j.molimm.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Wei Z, et al. Expression of IgM, IgD, and IgY in a reptile, Anolis carolinensis. J Immunol. 2009;183:3858–3864. doi: 10.4049/jimmunol.0803251. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M, Bengten E, Miller NW, Clem LW, Du Pasquier L, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc Natl Acad Sci USA. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha NR, Suetake H, Kikuchi K, Suzuki Y. Fugu immunoglobulin D: a highly unusual gene with unprecedented duplications in its constant region. Immunogenetics. 2004;56:438–447. doi: 10.1007/s00251-004-0693-y. [DOI] [PubMed] [Google Scholar]
- 17.Choi DH, Jang HN, Ha DM, Kim JW, Oh CH, Choi SH. Cloning and expression of partial Japanese flounder (Paralichthys olivaceus) IgD. J Biochem Mol Biol. 2007;40:459–466. doi: 10.5483/bmbrep.2007.40.4.459. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, et al. Identification of IgF, a hinge-region-containing Ig class, and IgD in Xenopus tropicalis. Proc Natl Acad Sci USA. 2006;103:12087–12092. doi: 10.1073/pnas.0600291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirono I, Nam BH, Enomoto J, Uchino K, Aoki T. Cloning and characterisation of a cDNA encoding Japanese flounder Paralichthys olivaceus IgD. Fish Shellfish Immunol. 2003;15:63–70. doi: 10.1016/s1050-4648(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, et al. Artiodactyl IgD: the missing link. J Immunol. 2002;169:4408–4416. doi: 10.4049/jimmunol.169.8.4408. [DOI] [PubMed] [Google Scholar]
- 21.Bengten E, et al. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J Immunol. 2002;169:2488–2497. doi: 10.4049/jimmunol.169.5.2488. [DOI] [PubMed] [Google Scholar]
- 22.Stenvik J, Schroder MB, Olsen K, Zapata A, Jorgensen TO. Expression of immunoglobulin heavy chain transcripts (VH-families, IgM, and IgD) in head kidney and spleen of the Atlantic cod (Gadus morhua L.) Dev Comp Immunol. 2001;25:291–302. doi: 10.1016/s0145-305x(00)00056-2. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg AS, Hughes AL, Guo J, Avila D, McKinney EC, Flajnik MF. A novel "chimeric" antibody class in cartilaginous fish: IgM may not be the primordial immunoglobulin. Eur J Immunol. 1996;26:1123–1129. doi: 10.1002/eji.1830260525. [DOI] [PubMed] [Google Scholar]
- 24.Bengten E, Clem LW, Miller NW, Warr GW, Wilson M. Channel catfish immunoglobulins: repertoire and expression. Dev Comp Immunol. 2006;30:77–92. doi: 10.1016/j.dci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Wang GL, Nie P. IgM, IgD and IgY and their expression pattern in the Chinese soft-shelled turtle Pelodiscus sinensis. Mol Immunol. 2009;46:2124–2132. doi: 10.1016/j.molimm.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Hammarstrom L. Cloning of the complete rat immunoglobulin delta gene: evolutionary implications. Immunology. 2003;108:288–295. doi: 10.1046/j.1365-2567.2003.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Kacskovics I, Rabbani H, Hammarstrom L. Physical mapping of the bovine immunoglobulin heavy chain constant region gene locus. J Biol Chem. 2003;278:35024–35032. doi: 10.1074/jbc.M301337200. [DOI] [PubMed] [Google Scholar]
- 28.Allansmith M, McClellan BH, Butterworth M, Maloney JR. The development of immunoglobulin levels in man. J Pediatr. 1968;72:276–290. doi: 10.1016/s0022-3476(68)80324-5. [DOI] [PubMed] [Google Scholar]
- 29.Buckley CE, 3rd, Dorsey FC. Serum immunoglobulin levels throughout the life-span of healthy man. Ann Intern Med. 1971;75:673–682. doi: 10.7326/0003-4819-75-5-673. [DOI] [PubMed] [Google Scholar]
- 30.Buckley RH, Dees SC, O'Fallon WM. Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics. 1968;41:600–611. [PubMed] [Google Scholar]
- 31.Buckley RH, Younger JB, Brumley GW. Evaluation of serum immunoglobulin concentrations in the perinatal period by use of a standardized method of measurement. J Pediatr. 1969;75:1143–1148. doi: 10.1016/s0022-3476(69)80370-7. [DOI] [PubMed] [Google Scholar]
- 32.Kalff MW. Quantitative determination of serum immunoglobulin levels by single radial immunodiffusion. Clin Biochem. 1970;3:91–104. [PubMed] [Google Scholar]
- 33.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for immunoglobulins A, G, and M: a practical, simple, and clinically relevant approach in a large cohort. J Clin Lab Anal. 1998;12:363–370. doi: 10.1002/(SICI)1098-2825(1998)12:6<363::AID-JCLA6>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Carmen Candia-Plata M, Garcia J, Guzman R, Porath J, Vazquez-Moreno L. Isolation of human serum immunoglobulins with a new salt-promoted adsorbent. J Chromatogr A. 2006;1118:211–217. doi: 10.1016/j.chroma.2006.03.097. [DOI] [PubMed] [Google Scholar]
- 35.Stulik J, Kovarova H, Tichy M, Urban P. Electrophoretic analysis of microheterogeneity of paraproteins in a patient with IgD myeloma. Neoplasma. 1995;42:105–108. [PubMed] [Google Scholar]
- 36.Vitetta ES, Uhr JW. Cell surface immunoglobulin. XIX. Susceptibility of IgD and IgM on murine splenocytes to cleavage by papain. J Immunol. 1976;117:1579–1583. [PubMed] [Google Scholar]
- 37.Mellis SJ, Baenziger JU. Structures of the O-glycosidically linked oligosaccharides of human IgD. J Biol Chem. 1983;258:11557–11563. [PubMed] [Google Scholar]
- 38.Tissot JD, et al. Clonal imbalances of serum immunoglobulins after allogeneic bone marrow transplantation: an analysis by high-resolution two-dimensional gel electrophoresis. Bone Marrow Transplant. 1992;10:347–353. [PubMed] [Google Scholar]
- 39.Brandtzaeg P, Surjan L, Jr, Berdal P. Immunoglobulin-producing cells in clinically normal, hyperplastic and inflamed human palatine tonsils. Acta Otolaryngol Suppl. 1979;360:211–215. doi: 10.3109/00016487809123519. [DOI] [PubMed] [Google Scholar]
- 40.Bahna SL, Heiner DC, Myhre BA. Changes in serum IgD in cigarette smokers. Clin Exp Immunol. 1983;51:624–630. [PMC free article] [PubMed] [Google Scholar]
- 41.Dunnette SL, Gleich GJ, Miller RD, Kyle RA. Measurement of IgD by a double antibody radioimmunoassay: demonstration of an apparent trimodal distribution of IgD levels in normal human sera. J Immunol. 1977;119:1727–1731. [PubMed] [Google Scholar]
- 42.Litwin SD, Zehr BD, Insel RA. Selective concentration of IgD class-specific antibodies in human milk. Clin Exp Immunol. 1990;80:263–267. doi: 10.1111/j.1365-2249.1990.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandtzaeg P, Gjeruldsen ST, Korsrud F, Baklien K, Berdal P, Ek J. The human secretory immune system shows striking heterogeneity with regard to involvement of J chain-positive IgD immunocytes. J Immunol. 1979;122:503–510. [PubMed] [Google Scholar]
- 44.Surjan L, Jr, Brandtzaeg P, Berdal P. Immunoglobulin systems of human tonsils. II. Patients with chronic tonsillitis or tonsillar hyperplasia: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol. 1978;31:382–390. [PMC free article] [PubMed] [Google Scholar]
- 45.Korsrud FR, Brandtzaeg P. Quantitative immunohistochemistry of immunoglobulin- and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology. 1980;39:129–140. [PMC free article] [PubMed] [Google Scholar]
- 46.Cederqvist LL, Ewool LC, Bonsnes RW, Litwin SD. Detectability and pattern of immunoglobulins in normal amniotic fluid throughout gestation. Am J Obstet Gynecol. 1978;130:220–224. doi: 10.1016/0002-9378(78)90370-8. [DOI] [PubMed] [Google Scholar]
- 47.Brandtzaeg P, Korsrud FR. Significance of different J chain profiles in human tissues: generation of IgA and IgM with binding site for secretory component is related to the J chain expressing capacity of the total local immunocyte population, including IgG and IgD producing cells, and depends on the clinical state of the tissue. Clin Exp Immunol. 1984;58:709–718. [PMC free article] [PubMed] [Google Scholar]
- 48.Brandtzaeg P, et al. Production and secretion of immunoglobulins in the gastrointestinal tract. Ann Allergy. 1987;59:21–39. [PubMed] [Google Scholar]
- 49.Bjerke K, Brandtzaeg P, Rognum TO. Distribution of immunoglobulin producing cells is different in normal human appendix and colon mucosa. Gut. 1986;27:667–674. doi: 10.1136/gut.27.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plebani A, et al. IgM and IgD concentrations in the serum and secretions of children with selective IgA deficiency. Clin Exp Immunol. 1983;53:689–696. [PMC free article] [PubMed] [Google Scholar]
- 51.Korsrud FR, Brandtzaeg P. Immunohistochemical evaluation of J-chain expression by intra- and extra-follicular immunoglobulin-producing human tonsillar cells. Scand J Immunol. 1981;13:271–280. doi: 10.1111/j.1365-3083.1981.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- 53.Chen K, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu YJ, et al. Normal human IgD+IgM- germinal center B cells can express up to 80 mutations in the variable region of their IgD transcripts. Immunity. 1996;4:603–613. doi: 10.1016/s1074-7613(00)80486-0. [DOI] [PubMed] [Google Scholar]
- 55.Arpin C, et al. The normal counterpart of IgD myeloma cells in germinal center displays extensively mutated IgVH gene, Cmu-Cdelta switch, and lambda light chain expression. J Exp Med. 1998;187:1169–1178. doi: 10.1084/jem.187.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 57.Brandtzaeg P, Nilssen DE. Mucosal aspects of primary B cell deficiency and gastrointestinal infections. Curr Opin Gastroenterol. 1995;11:532–540. [Google Scholar]
- 58.Brandtzaeg P. The role of humoral mucosal immunity in the induction and maintenance of chronic airway infections. Am J Respir Crit Care Med. 1995;151:2081–2086. doi: 10.1164/ajrccm.151.6.7767561. discussion 2086–2087. [DOI] [PubMed] [Google Scholar]
- 59.Brandtzaeg P, Carlsen HS, Farstad IN. The human mucosal B-cell system. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- 60.Cederqvist LL, Ewool LC, Litwin SD. IgD and the fetal immune response. Scand J Immunol. 1977;6:821–825. doi: 10.1111/j.1365-3083.1977.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 61.Salimonu LS, Ladipo OA, Adeniran SO, Osukoya BO. Serum immunoglobulin levels in normal, premature and postmature newborns and their mothers. Int J Gynaecol Obstet. 1978;16:119–123. doi: 10.1002/j.1879-3479.1978.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 62.Zegers BJ, van der Giessen M, Reerink-Brongers EE, Stoop JW. The serum IgG subclass levels in healthy infants of 13--62 weeks of age. Clin Chim Acta. 1980;101:265–269. doi: 10.1016/0009-8981(80)90252-1. [DOI] [PubMed] [Google Scholar]
- 63.Avrech OM, Samra Z, Lazarovich Z, Caspi E, Jacobovich A, Sompolinsky D. Efficacy of the placental barrier for immunoglobulins: correlations between maternal, paternal and fetal immunoglobulin levels. Int Arch Allergy Immunol. 1994;103:160–165. doi: 10.1159/000236622. [DOI] [PubMed] [Google Scholar]
- 64.Salonen EM, Hovi T, Meurman O, Vesikari T, Vaheri A. Kinetics of specific IgA, IgD, IgE, IgG, and IgM antibody responses in rubella. J Med Virol. 1985;16:1–9. doi: 10.1002/jmv.1890160102. [DOI] [PubMed] [Google Scholar]
- 65.Rogentine GN, Jr, Rowe DS, Bradley J, Waldmann TA, Fahey JL. Metabolism of human immunoglobulin D (IgD) J Clin Invest. 1966;45:1467–1478. doi: 10.1172/JCI105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudders RA, Andersen J. IgD-Fc receptors on normal and neoplastic human B lymphocytes. Clin Exp Immunol. 1982;50:579–586. [PMC free article] [PubMed] [Google Scholar]
- 67.Swenson CD, Thorbecke GJ. The effect of aging on IgD receptor expression by T cells and its functional implications. Immunol Rev. 1997;160:145–157. doi: 10.1111/j.1600-065x.1997.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 68.Swenson CD, et al. Human T cell IgD receptors react with O-glycans on both human IgD and IgA1. Eur J Immunol. 1998;28:2366–2372. doi: 10.1002/(SICI)1521-4141(199808)28:08<2366::AID-IMMU2366>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 69.Tamma SM, Coico RF. IgD-receptor (IgD-R) cross-linking partially protects murine T cells from dexamethasone-induced apoptosis. J Leukoc Biol. 2003;73:764–770. doi: 10.1189/jlb.1002492. [DOI] [PubMed] [Google Scholar]
- 70.Lakshmi Tamma SM, Wu Y, Toporovsky I, Lima V, Coico RF. IgD receptor-mediated signal transduction in T cells. Cell Immunol. 2001;207:110–117. doi: 10.1006/cimm.2000.1747. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y, Lakshmi Tamma SM, Lima V, Coico R. Facilitated antigen presentation by B cells expressing IgD when responding T cells express IgD-receptors. Cell Immunol. 1999;192:194–202. doi: 10.1006/cimm.1998.1453. [DOI] [PubMed] [Google Scholar]
- 72.Sechet B, Meseri-Delwail A, Arock M, Wijdenes J, Lecron JC, Sarrouilhe D. Immunoglobulin D enhances interleukin-6 release from the KU812 human prebasophil cell line. Gen Physiol Biophys. 2003;22:255–263. [PubMed] [Google Scholar]
- 73.Berretty PJ, Cormane RH. Immunofluorescence studies on eosinophilic granulocytes. Br J Dermatol. 1979;101:309–314. doi: 10.1111/j.1365-2133.1979.tb05624.x. [DOI] [PubMed] [Google Scholar]
- 74.Walsh GM, Kay AB. Binding of immunoglobulin classes and subclasses to human neutrophils and eosinophils. Clin Exp Immunol. 1986;63:466–472. [PMC free article] [PubMed] [Google Scholar]
- 75.Lawrence DA, WO Weigle, Spiegelberg HL. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975;55:368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunyadi J, Hamerlinck F, Cormane RH. Immunoglobulin and complement bearing polymorphonuclear leukocytes in allergic contact dermatitis and psoriasis vulgaris. Br J Dermatol. 1976;94:417–422. doi: 10.1111/j.1365-2133.1976.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 77.Dikeacou TC, van Joost T, Cormane RH. The recruitment of inflammatory cells using the skin-window technique. Arch Dermatol Res. 1979;265:1–7. doi: 10.1007/BF00412695. [DOI] [PubMed] [Google Scholar]
- 78.Drenth JP, Goertz J, Daha MR, van der Meer JW. Immunoglobulin D enhances the release of tumor necrosis factor-alpha, and interleukin-1 beta as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology. 1996;88:355–362. doi: 10.1046/j.1365-2567.1996.d01-672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sjoberg O. Presence of receptors for IgD on human T and non-T lymphocytes. Scand J Immunol. 1980;11:377–382. doi: 10.1111/j.1365-3083.1980.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 80.Fine JM, Rivat C, Lambin P, Ropartz C. Monoclonal IgD. A comparative study of 60 sera with IgD "M" component. Biomedicine. 1974;211:119–125. [PubMed] [Google Scholar]
- 81.Pernis B, Governa M, Rowe DS. Light chain types in plasma cells that produce IgD. Immunology. 1969;16:685–689. [PMC free article] [PubMed] [Google Scholar]
- 82.Hobbs JR, Corbett AA. Younger age of presentation and extraosseous tumour in IgD myelomatosis. Br Med J. 1969;1:412–414. doi: 10.1136/bmj.1.5641.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jancelewicz Z, Takatsuki K, Sugai S, Pruzanski W. IgD multiple myeloma. Review of 133 cases. Arch Intern Med. 1975;135:87–93. [PubMed] [Google Scholar]
- 84.Fibbe WE, Jansen J. Prognostic factors in IgD myeloma: a study of 21 cases. Scand J Haematol. 1984;33:471–475. doi: 10.1111/j.1600-0609.1984.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 85.Shimamoto Y, Anami Y, Yamaguchi M. A new risk grouping for IgD myeloma based on analysis of 165 Japanese patients. Eur J Haematol. 1991;47:262–267. doi: 10.1111/j.1600-0609.1991.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 86.Blade J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12:2398–2404. doi: 10.1200/JCO.1994.12.11.2398. [DOI] [PubMed] [Google Scholar]
- 87.Arpin C, et al. Human peripheral B cell development. sIgM-IgD+CD38+ hypermutated germinal center centroblasts preferentially express Ig lambda light chain and have undergone mu-to-delta switch. Ann NY Acad Sci. 1997;815:193–198. doi: 10.1111/j.1749-6632.1997.tb52060.x. [DOI] [PubMed] [Google Scholar]
- 88.Litwin SD, Zehr BD. In vitro studies on human IgD. II. IgD-secreting cells preferentially elaborate IgD, lambda molecules. Eur J Immunol. 1987;17:491–495. doi: 10.1002/eji.1830170409. [DOI] [PubMed] [Google Scholar]
- 89.Litwin SD, Zehr BD. Membrane IgD-positive B cells of "low-IgD serum phenotype" individuals fail to secrete IgD and fail to shift to preferential lambda light-chain expression in vitro. J Clin Immunol. 1987;7:114–120. doi: 10.1007/BF00916005. [DOI] [PubMed] [Google Scholar]
- 90.Litwin SD, Zehr BD. In vitro studies on human IgD. III. Immunologic features of individuals with high sera IgD and spontaneous IgD biosynthesis. Clin Immunol Immunopathol. 1988;47:75–83. doi: 10.1016/0090-1229(88)90146-8. [DOI] [PubMed] [Google Scholar]
- 91.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441:E4. doi: 10.1038/nature04875. discussion E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zouali M. Receptor editing and receptor revision in rheumatic autoimmune diseases. Trends Immunol. 2008;29:103–109. doi: 10.1016/j.it.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 93.van der Burg M, Bende RJ, Aarts WM, Langerak AW, van Dongen JJ, van Noesel CJ. Biased Iglambda expression in hypermutated IgD multiple myelomas does not result from receptor revision. Leukemia. 2002;16:1358–1361. doi: 10.1038/sj.leu.2402513. [DOI] [PubMed] [Google Scholar]
- 94.Derudder E, et al. Development of immunoglobulin lambda-chain-positive B cells, but not editing of immunoglobulin kappa-chain, depends on NF-kappaB signals. Nat Immunol. 2009;10:647–654. doi: 10.1038/ni.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bengten E, Wilson M, Miller N, Clem LW, Pilstrom L, Warr GW. Immunoglobulin isotypes: structure, function, and genetics. Curr Top Microbiol Immunol. 2000;248:189–219. doi: 10.1007/978-3-642-59674-2_9. [DOI] [PubMed] [Google Scholar]
- 96.Miller NW, Rycyzyn MA, Wilson MR, Warr GW, Naftel JP, Clem LW. Development and characterization of channel catfish long term B cell lines. J Immunol. 1994;152:2180–2189. [PubMed] [Google Scholar]
- 97.Kerr WG, Hendershot LM, Burrows PD. Regulation of IgM and IgD expression in human B-lineage cells. J Immunol. 1991;146:3314–3321. [PubMed] [Google Scholar]
- 98.Weiss EA, Michael A, Yuan D. Role of transcriptional termination in the regulation of mu mRNA expression in B lymphocytes. J Immunol. 1989;143:1046–1052. [PubMed] [Google Scholar]
- 99.Yuan D. Regulation of immunoglobulin D synthesis in murine neonatal B lymphocytes. Mol Cell Biol. 1986;6:1015–1022. doi: 10.1128/mcb.6.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim M, Qiu P, Abuodeh R, Chen J, Yuan D. Differential regulation of transcription termination occurring at two different sites on the micro-delta gene complex. Int Immunol. 1999;11:813–824. doi: 10.1093/intimm/11.5.813. [DOI] [PubMed] [Google Scholar]
- 101.Yuan D, Witte PL, Tan J, Hawley J, Dang T. Regulation of IgM and IgD heavy chain gene expression: effect of abrogation of intergenic transcriptional termination. J Immunol. 1996;157:2073–2081. [PubMed] [Google Scholar]
- 102.Peterson ML, Perry RP. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989;9:726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takagaki Y, Seipelt RL, Peterson ML, Manley JL. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B cell differentiation. Cell. 1996;87:941–952. doi: 10.1016/s0092-8674(00)82000-0. [DOI] [PubMed] [Google Scholar]
- 104.Kluin PM, et al. IgD class switching: identification of a novel recombination site in neoplastic and normal B cells. Eur J Immunol. 1995;25:3504–3508. doi: 10.1002/eji.1830251244. [DOI] [PubMed] [Google Scholar]
- 105.White MB, Word CJ, Humphries CG, Blattner FR, Tucker PW. Immunoglobulin D switching can occur through homologous recombination in human B cells. Mol Cell Biol. 1990;10:3690–3699. doi: 10.1128/mcb.10.7.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seifert M, Steimle-Grauer SA, Goossens T, Hansmann ML, Brauninger A, Kuppers R. A model for the development of human IgD-only B cells: Genotypic analyses suggest their generation in superantigen driven immune responses. Mol Immunol. 2009;46:630–639. doi: 10.1016/j.molimm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 107.Lin LC, Putnam FW. Primary structure of the Fc region of human immunoglobulin D: implications for evolutionary origin and biological function. Proc Natl Acad Sci USA. 1981;78:504–508. doi: 10.1073/pnas.78.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mellis SJ, Baenziger JU. Structures of the oligosaccharides present at the three asparagine-linked glycosylation sites of human IgD. J Biol Chem. 1983;258:11546–11556. [PubMed] [Google Scholar]
- 109.Amin AR, et al. The immunoaugmenting properties of murine IgD reside in its C delta 1 and C delta 3 regions: potential role for IgD-associated glycans. Int Immunol. 1993;5:607–614. doi: 10.1093/intimm/5.6.607. [DOI] [PubMed] [Google Scholar]
- 110.Gala FA, Morrison SL. The role of constant region carbohydrate in the assembly and secretion of human IgD and IgA1. J Biol Chem. 2002;277:29005–29011. doi: 10.1074/jbc.M203258200. [DOI] [PubMed] [Google Scholar]
- 111.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974;249:7260–7269. [PubMed] [Google Scholar]
- 112.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 113.Takahashi N, Tetaert D, Debuire B, Lin LC, Putnam FW. Complete amino acid sequence of the delta heavy chain of human immunoglobulin D. Proc Natl Acad Sci USA. 1982;79:2850–2854. doi: 10.1073/pnas.79.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith AC, de Wolff JF, Molyneux K, Feehally J, Barratt J. O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol. 2006;17:1192–1199. doi: 10.1681/ASN.2005101115. [DOI] [PubMed] [Google Scholar]
- 115.Spiegelberg HL. The structure and biology of human IgD. Immunol Rev. 1977;37:3–24. doi: 10.1111/j.1600-065x.1977.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 116.Griffiths RW, Gleich GJ. Proteolytic degradation of IgD and its relation to molecular conformation. J Biol Chem. 1972;247:4543–4548. [PubMed] [Google Scholar]
- 117.Goyert SM, Hugli TE, Spiegelberg HL. Sites of "spontaneous" degradation of IgD. J Immunol. 1977;118:2138–2144. [PubMed] [Google Scholar]
- 118.Lin LC, Putnam FW. Structural studies of human IgD: isolation by a two-step purification procedure and characterization by chemical and enzymatic fragmentation. Proc Natl Acad Sci USA. 1979;76:6572–6576. doi: 10.1073/pnas.76.12.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Z, Almogren A, Furtado PB, Chowdhury B, Kerr MA, Perkins SJ. Semi-extended solution structure of human myeloma immunoglobulin D determined by constrained X-ray scattering. J Mol Biol. 2005;353:155–173. doi: 10.1016/j.jmb.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 120.Loset GA, Roux KH, Zhu P, Michaelsen TE, Sandlie I. Differential segmental flexibility and reach dictate the antigen binding mode of chimeric IgD and IgM: implications for the function of the B cell receptor. J Immunol. 2004;172:2925–2934. doi: 10.4049/jimmunol.172.5.2925. [DOI] [PubMed] [Google Scholar]
- 121.Van Boxel JA, Paul WE, Terry WD, Green I. Communications. IgD-bearing human lymphocytes. J Immunol. 1972;109:648–651. [PubMed] [Google Scholar]
- 122.Rowe DS, Hug K, Faulk WP, McCormick JN, Gerber H. IgD on the surface of peripheral blood lymphocytes of the human newborn. Nat New Biol. 1973;242:155–157. doi: 10.1038/newbio242155a0. [DOI] [PubMed] [Google Scholar]
- 123.Rowe DS, Hug K, Forni L, Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973;138:965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abney ER, Parkhouse RM. Candidate for immunoglobulin D present on murine B lymphocytes. Nature. 1974;252:600–602. doi: 10.1038/252600a0. [DOI] [PubMed] [Google Scholar]
- 125.Melcher U, Vitetta ES, McWilliams M, Lamm ME, Phillips-Quagliata JM, Uhr JW. Cell surface immunoglobulin. X. Identification of an IgD-like molecule on the surface of murine splenocytes. J Exp Med. 1974;140:1427–1431. doi: 10.1084/jem.140.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim KM, Reth M. The B cell antigen receptor of class IgD induces a stronger and more prolonged protein tyrosine phosphorylation than that of class IgM. J Exp Med. 1995;181:1005–1014. doi: 10.1084/jem.181.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim KM, Reth M. Function of B-cell antigen receptor of different classes. Immunol Lett. 1995;44:81–85. doi: 10.1016/0165-2478(94)00219-h. [DOI] [PubMed] [Google Scholar]
- 128.Kim KM, Reth M. Signaling difference between class IgM and IgD antigen receptors. Ann NY Acad Sci. 1995;766:81–88. doi: 10.1111/j.1749-6632.1995.tb26651.x. [DOI] [PubMed] [Google Scholar]
- 129.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ales-Martinez JE, Warner GL, Scott DW. Immunoglobulins D and M mediate signals that are qualitatively different in B cells with an immature phenotype. Proc Natl Acad Sci USA. 1988;85:6919–6923. doi: 10.1073/pnas.85.18.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peckham D, Andersen-Nissen E, Finkelman FD, Stunz LL, Ashman RF. Difference in apoptosis induction between surface IgD and IgM. Int Immunol. 2001;13:285–295. doi: 10.1093/intimm/13.3.285. [DOI] [PubMed] [Google Scholar]
- 132.Geisberger R, Crameri R, Achatz G. Models of signal transduction through the B-cell antigen receptor. Immunology. 2003;110:401–410. doi: 10.1111/j.1365-2567.2003.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heltemes LM, Manser T. Level of B cell antigen receptor surface expression influences both positive and negative selection of B cells during primary development. J Immunol. 2002;169:1283–1292. doi: 10.4049/jimmunol.169.3.1283. [DOI] [PubMed] [Google Scholar]
- 134.Iglesias A, Lamers M, Kohler G. Expression of immunoglobulin delta chain causes allelic exclusion in transgenic mice. Nature. 1987;330:482–484. doi: 10.1038/330482a0. [DOI] [PubMed] [Google Scholar]
- 135.Lutz C, et al. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797–801. doi: 10.1038/31716. [DOI] [PubMed] [Google Scholar]
- 136.Nitschke L, Kosco MH, Kohler G, Lamers MC. Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc Natl Acad Sci USA. 1993;90:1887–1891. doi: 10.1073/pnas.90.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roes J, Rajewsky K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J Exp Med. 1993;177:45–55. doi: 10.1084/jem.177.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118:429–437. doi: 10.1111/j.1365-2567.2006.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan D, Dang T, Bibi R. Inappropriate expression of IgD from a transgene inhibits the function of antigen-specific memory B cells. Cell Immunol. 2001;211:61–70. doi: 10.1006/cimm.2001.1812. [DOI] [PubMed] [Google Scholar]
- 140.Glynne R, Akkaraju S, Healy JI, Rayner J, Goodnow CC, Mack DH. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature. 2000;403:672–676. doi: 10.1038/35001102. [DOI] [PubMed] [Google Scholar]
- 141.Cohen P, Ziff M, Vitetta ES. Characterization of a B cell defect in the NZB mouse manifested by an increased ratio of surface IgM to IgD. J Immunol. 1978;121:973–977. [PubMed] [Google Scholar]
- 142.Duty JA, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wienands J, Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992;356:246–248. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- 144.Chaturvedi A, Siddiqui Z, Bayiroglu F, Rao KV. A GPI-linked isoform of the IgD receptor regulates resting B cell activation. Nat Immunol. 2002;3:951–957. doi: 10.1038/ni839. [DOI] [PubMed] [Google Scholar]
- 145.Koelsch K, et al. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luster MI, Armen RC, Hallum JV, Leslie GA. Measles virus-specific IgD antibodies in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci USA. 1976;73:1297–1299. doi: 10.1073/pnas.73.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heiner DC, Rose B. A study of antibody responses by radioimmunodiffusion with demonstration of gamma D antigen-binding activity in four sera. J Immunol. 1970;104:691–697. [PubMed] [Google Scholar]
- 148.Jefferis R, Mathews JB. Studies of human IgD myeloma proteins. Proteolytic digestion patterns. Immunochemistry. 1977;14:171–178. doi: 10.1016/0019-2791(77)90191-4. [DOI] [PubMed] [Google Scholar]
- 149.Sewell HF, Chambers L, Maxwell V, Matthews JB, Jefferis R. The natural antibody response to E. coli includes antibodies of the IgD class. Clin Exp Immunol. 1978;31:104–110. [PMC free article] [PubMed] [Google Scholar]
- 150.Swierczynska Z, Wozniczko-Orlowska G, Maldyk H. An IgD myeloma protein with anti-streptolysin O activity. Immunochemistry. 1976;13:379–382. doi: 10.1016/0019-2791(76)90371-2. [DOI] [PubMed] [Google Scholar]
- 151.Forsgren A, et al. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J Immunol. 2001;167:2112–2120. doi: 10.4049/jimmunol.167.4.2112. [DOI] [PubMed] [Google Scholar]
- 152.Ronander E, Brant M, Janson H, Sheldon J, Forsgren A, Riesbeck K. Identification of a novel Haemophilus influenzae protein important for adhesion to epithelial cells. Microbes Infect. 2008;10:87–96. doi: 10.1016/j.micinf.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 153.Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7:131–140. doi: 10.1128/cdli.7.2.131-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Listi F, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann NY Acad Sci. 2006;1089:487–495. doi: 10.1196/annals.1386.013. [DOI] [PubMed] [Google Scholar]
- 155.Kiyotaki C, Shimizu A, Watanabe S, Yamamura Y. Superoxide production from human polymorphonuclear leucocytes stimulated with immunoglobulins of different classes and fragments of IgG bound to polystyrene dishes. Immunology. 1978;35:613–618. [PMC free article] [PubMed] [Google Scholar]
- 156.Wilson BJ, Kocvara H. A simple rapid method for layering blood on Ficoll-Isopaque gradients. J Immunol Methods. 1975;9:67–68. doi: 10.1016/0022-1759(75)90036-8. [DOI] [PubMed] [Google Scholar]
- 157.Boyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- 158.Xu YP, Hu BX, Yu YJ, Pan HF. The relationship of allergic asthma and basophil surface IgD. Shanghai J Immunol. 1986;6:164–165. al. e. [Google Scholar]
- 159.Peng Z, Fisher R, Adkinson NF., Jr Total serum IgD is increased in atopic subjects. Allergy. 1991;46:436–444. doi: 10.1111/j.1398-9995.1991.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 160.Conrad DH, Ben-Sasson SZ, Le Gros G, Finkelman FD, Paul WE. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J Exp Med. 1990;171:1497–1508. doi: 10.1084/jem.171.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dvorak AM, Seder RA, Paul WE, Kissell-Rainville S, Plaut M, Galli SJ. Ultrastructural characteristics of Fc epsilon R-positive basophils in the spleen and bone marrow of mice immunized with goat anti-mouse IgD antibody. Lab Invest. 1993;68:708–715. [PubMed] [Google Scholar]
- 162.Seder RA, et al. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci USA. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Finkelman FD, Snapper CM, Mountz JD, Katona IM. Polyclonal activation of the murine immune system by a goat antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J Immunol. 1987;138:2826–2830. [PubMed] [Google Scholar]
- 164.Vladutiu AO, Netto D. Is quantitation of serum IgD clinically useful? Clin Chem. 1982;28:1409–1410. [PubMed] [Google Scholar]
- 165.Litzman J, Ward AM, Wild G, Znojil V, Morgan G. Serum IgD levels in children under investigation for and with defined immunodeficiency. Int Arch Allergy Immunol. 1997;114:54–58. doi: 10.1159/000237643. [DOI] [PubMed] [Google Scholar]
- 166.Mizuma H, et al. Serum IgD elevation is an early marker of B cell activation during infection with the human immunodeficiency viruses. Clin Exp Immunol. 1987;68:5–14. [PMC free article] [PubMed] [Google Scholar]
- 167.Mizuma H, Litwin S, Zolla-Pazner S. B-cell activation in HIV infection: relationship of spontaneous immunoglobulin secretion to various immunological parameters. Clin Exp Immunol. 1988;71:410–416. [PMC free article] [PubMed] [Google Scholar]
- 168.Chess Q, Daniels J, North E, Macris NT. Serum immunoglobulin elevations in the acquired immunodeficiency syndrome (AIDS): IgG, IgA, IgM, and IgD. Diagn Immunol. 1984;2:148–153. [PubMed] [Google Scholar]
- 169.Papadopoulos NM, Frieri M. The presence of immunoglobulin D in endocrine disorders and diseases of immunoregulation, including the acquired immunodeficiency syndrome. Clin Immunol Immunopathol. 1984;32:248–252. doi: 10.1016/0090-1229(84)90125-9. [DOI] [PubMed] [Google Scholar]
- 170.Rowe DS, Crabbe PA, Turner MW. Immunoglobulin D in serum, body fluids and lymphoid tissues. Clin Exp Immunol. 1968;3:477–490. [PMC free article] [PubMed] [Google Scholar]
- 171.Sirisinha S, Charupatana C, Ramasoota T. Serum immunoglobulins in leprosy patients with different spectra of clinical manifestations. Proc Soc Exp Biol Med. 1972;140:1062–1068. doi: 10.3181/00379727-140-36612. [DOI] [PubMed] [Google Scholar]
- 172.Buckley CE, Trayer HR. Serum IgD concentrations in sarcoidosis and tuberculosis. Clin Exp Immunol. 1972;10:257–265. [PMC free article] [PubMed] [Google Scholar]
- 173.Kikindjanin V. IgD values in children suffering from acute, recurrent and chronic respiratory diseases. Allerg Immunol (Leipz) 1981;27:203–206. [PubMed] [Google Scholar]
- 174.Rostenberg I, Penaloza R. Serum IgG and IgD and levels in some infectious and noninfectious diseases. Clin Chim Acta. 1978;85:319–321. doi: 10.1016/0009-8981(78)90310-8. [DOI] [PubMed] [Google Scholar]
- 175.Colwell EJ, Bernier GM, Fife EH., Jr Serum immunoglobulin D and malaria antibodies in South Vietnamese residents. Trans R Soc Trop Med Hyg. 1971;65:310–314. doi: 10.1016/0035-9203(71)90005-8. [DOI] [PubMed] [Google Scholar]
- 176.Forsgren A, Grubb AO. Many bacterial species bind human IgD. J Immunol. 1979;122:1468–1472. [PubMed] [Google Scholar]
- 177.Buckley RH, Fiscus SA. Serum IgD and IgE concentrations in immunodeficiency diseases. J Clin Invest. 1975;55:157–165. doi: 10.1172/JCI107906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roman S. Two different techniques for serum IgD determination, comparative study: some pathological circumstances capable of increasing IgD levels. Rev Roumaine Biochim. 1982;19:141–149. [Google Scholar]
- 179.Epstein J, Xiao HQ, He XY. Markers of multiple hematopoietic-cell lineages in multiple myeloma. N Engl J Med. 1990;322:664–668. doi: 10.1056/NEJM199003083221005. [DOI] [PubMed] [Google Scholar]
- 180.Kubagawa H, Vogler LB, Capra JD, Conrad ME, Lawton AR, Cooper MD. Studies on the clonal origin of multiple myeloma. Use of individually specific (idiotype) antibodies to trace the oncogenic event to its earliest point of expression in B-cell differentiation. J Exp Med. 1979;150:792–807. doi: 10.1084/jem.150.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stevenson FK, Sahota SS. B cell maturation in relation to multiple myeloma. Pathol Biol (Paris) 1999;47:89–97. [PubMed] [Google Scholar]
- 182.Jensen GS, Mant MJ, Belch AJ, Berenson JR, Ruether BA, Pilarski LM. Selective expression of CD45 isoforms defines CALLA+ monoclonal B-lineage cells in peripheral blood from myeloma patients as late stage B cells. Blood. 1991;78:711–719. [PubMed] [Google Scholar]
- 183.Sahota SS, Leo R, Hamblin TJ, Stevenson FK. Ig VH gene mutational patterns indicate different tumor cell status in human myeloma and monoclonal gammopathy of undetermined significance. Blood. 1996;87:746–755. [PubMed] [Google Scholar]
- 184.MacLennan ICM, Chan EYT. The origin of bone marrow plasma cells. In: Obrams GI, Potter M, editors. Epidemiology and Biology of Multiple Myeloma. Berlin: Springer-Verlag; 1991. pp. 129–135. [Google Scholar]
- 185.Valenzuela R, Govindarajan S, Tubbs R, Deodhar S, Bukowski R. IgD myeloma. Report of a case with unusual clinical and immunologic features. Am J Clin Pathol. 1979;72:246–250. doi: 10.1093/ajcp/72.2.246. [DOI] [PubMed] [Google Scholar]
- 186.Moore KW, et al. Expression of IgD may use both DNA rearrangement and RNA splicing mechanisms. Proc Natl Acad Sci USA. 1981;78:1800–1804. doi: 10.1073/pnas.78.3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Golomb HM, Davis S, Wilson C, Vardiman J. Surface immunoglobulins on hairy cells of 55 patients with hairy cell leukemia. Am J Hematol. 1982;12:397–401. doi: 10.1002/ajh.2830120411. [DOI] [PubMed] [Google Scholar]
- 188.Fu SM, Winchester RJ, Rai KR, Kunkel HG. Hairy cell leukemia: proliferation of a cell with phagocytic and B-lymphocyte properties. Scand J Immunol. 1974;3:847–851. doi: 10.1111/j.1365-3083.1974.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 189.Rubin AD, Douglas SD, Chessin LN, Glade PR, Dameshek W. Chronic reticulolymphocytic leukemia. Reclassification of "leukemic reticuloendotheliosis" through functional characterization of the circulating mononuclear cells. Am J Med. 1969;47:149–162. doi: 10.1016/0002-9343(69)90249-6. [DOI] [PubMed] [Google Scholar]
- 190.Najam FI, Giasuddin AS, Shembesh AH. Immunoglobulin isotypes in childhood asthma. Indian J Pediatr. 1999;66:337–344. doi: 10.1007/BF02845519. [DOI] [PubMed] [Google Scholar]
- 191.Zhang M, Niehus J, Brunnee T, Kleine-Tebbe J, O'Connor A, Kunkel G. Measurement of allergen-specific IgD and correlation with allergen-specific IgE. Scand J Immunol. 1994;40:502–508. doi: 10.1111/j.1365-3083.1994.tb03496.x. [DOI] [PubMed] [Google Scholar]
- 192.Kohler PF, Farr RS. Quantitative comparison of immunoglobulins in atopic (reaginic) and nonatopic (nonreaginic) individuals: higher gamma-D levels in atopic sera. J Allergy. 1967;39:311–322. doi: 10.1016/0021-8707(67)90095-0. [DOI] [PubMed] [Google Scholar]
- 193.Luster MI, Leslie GA, Bardana EJ., Jr Structure and biological functions of human IgD. VI. Serum IgD in patients with allergic bronchopulmonary aspergillosis. Int Arch Allergy Appl Immunol. 1976;50:212–219. doi: 10.1159/000231498. [DOI] [PubMed] [Google Scholar]
- 194.Salpietro DC, Masaracchio A, Turiaco A, Di Bella MR, Toscano V, Merlino MV. Serum IgD levels in children with atopic asthma. A longitudinal study. Minerva Pediatr. 2001;53:1–5. [PubMed] [Google Scholar]
- 195.Spector SL. The role of allergy in sinusitis in adults. J Allergy Clin Immunol. 1992;90:518–520. doi: 10.1016/0091-6749(92)90178-5. [DOI] [PubMed] [Google Scholar]
- 196.Gleich GJ, Stankievic RR. Measurement of penicilloyl antibodies by radioimmunoprecipitation. Immunochemistry. 1969;6:85–99. doi: 10.1016/0019-2791(69)90181-5. [DOI] [PubMed] [Google Scholar]
- 197.Bringel H, Vela C, Urena V, Gurbindo D, Garcia R, Lahoz C. IgD antibodies: in vitro blocking activity of IgE mediated reactions. Clin Allergy. 1982;12:37–46. doi: 10.1111/j.1365-2222.1982.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 198.Schmidt K, Mueller-Eckhard C. Antinuclear autoantibodies of IgD class: an analysis of 82 patients. Z Immunitaetsforsch. 1973;145:385–391. [PubMed] [Google Scholar]
- 199.Lertora JJ, Gomez-Perez FJ, Leslie GA. Structure and biological functions of human IgD. V. Insulin antibodies of the IgD class in sera from some diabetic patients. Int Arch Allergy Appl Immunol. 1975;49:597–606. doi: 10.1159/000231441. [DOI] [PubMed] [Google Scholar]
- 200.Leslie GA, Martin LN. Structure and function of serum and membrane immunoglobulin D (IgD) Contemp Top Mol Immunol. 1978;7:1–49. doi: 10.1007/978-1-4757-0779-3_1. [DOI] [PubMed] [Google Scholar]
- 201.Onodera S, Shibata A, Miura AB, Suzuki A, Sakamoto S. Quantitative determination of serum immunoglobulin D in gammopathy. Tohoku J Exp Med. 1968;95:145–151. doi: 10.1620/tjem.95.145. [DOI] [PubMed] [Google Scholar]
- 202.Gleich GJ, Bieger RC, Stankievic R. Antigen combining activity associated with immunoglobulin D. Science. 1969;165:606. doi: 10.1126/science.165.3893.606. [DOI] [PubMed] [Google Scholar]
- 203.Ritchie RF. Two new antinuclear antibodies: their relationship to the homogeneous immunofluorescent pattern. Arthritis Rheum. 1968;11:37–43. doi: 10.1002/art.1780110105. [DOI] [PubMed] [Google Scholar]
- 204.Hassfeld W, et al. Demonstration of a new antinuclear antibody (anti-RA33) that is highly specific for rheumatoid arthritis. Arthritis Rheum. 1989;32:1515–1520. doi: 10.1002/anr.1780321204. [DOI] [PubMed] [Google Scholar]
- 205.Sehgal S, Sharma BK. Antinuclear antibody activity of IgD immunoglobulins. Indian J Med Res. 1986;83:610–613. [PubMed] [Google Scholar]
- 206.Wiik A, Permin H. The prevalence and possible significance of IgD granulocyte-specific antinuclear antibodies in neutropenic and non-neutropenic cases of rheumatoid arthritis. Acta Pathol Microbiol Scand C. 1978;86:19–22. doi: 10.1111/j.1699-0463.1978.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 207.Kantor GL, Van Herle AJ, Barnett EV. Auto-antibodies of the IgD class. Clin Exp Immunol. 1970;6:951–962. [PMC free article] [PubMed] [Google Scholar]
- 208.Pope RM, Keightley R, McDuffy S. Circulating autoantibodies to IgD in rheumatic diseases. J Immunol. 1982;128:1860–1863. [PubMed] [Google Scholar]
- 209.Menoret A, Chandawarkar RY, Srivastava PK. Natural autoantibodies against heat-shock proteins hsp70 and gp96: implications for immunotherapy using heat-shock proteins. Immunology. 2000;101:364–370. doi: 10.1046/j.1365-2567.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R86–R98. doi: 10.1152/ajpregu.00504.2006. [DOI] [PubMed] [Google Scholar]
- 211.Drenth JP, Haagsma CJ, van der Meer JW. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine (Baltimore) 1994;73:133–144. [PubMed] [Google Scholar]
- 212.Drenth JP, Boom BW, Toonstra J, Van der Meer JW. Cutaneous manifestations and histologic findings in the hyperimmunoglobulinemia D syndrome. International Hyper IgD Study Group, Arch Dermatol. 1994;130:59–65. [PubMed] [Google Scholar]
- 213.Drenth JP, van der Meer JW. Hereditary periodic fever. N Engl J Med. 2001;345:1748–1757. doi: 10.1056/NEJMra010200. [DOI] [PubMed] [Google Scholar]
- 214.Simon A, et al. Mevalonate kinase deficiency: Evidence for a phenotypic continuum. Neurology. 2004;62:994–997. doi: 10.1212/01.wnl.0000115390.33405.f7. [DOI] [PubMed] [Google Scholar]
- 215.Drenth JP, et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 216.Biardi L, et al. Mevalonate kinase is predominantly localized in peroxisomes and is defective in patients with peroxisome deficiency disorders. J Biol Chem. 1994;269:1197–1205. [PubMed] [Google Scholar]
- 217.Houten SM, Frenkel J, Waterham HR. Isoprenoid biosynthesis in hereditary periodic fever syndromes and inflammation. Cell Mol Life Sci. 2003;60:1118–1134. doi: 10.1007/s00018-003-2296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bodar EJ, Drenth JP, van der Meer JW, Simon A. Dysregulation of innate immunity: hereditary periodic fever syndromes. Br J Haematol. 2009;144:279–302. doi: 10.1111/j.1365-2141.2008.07036.x. [DOI] [PubMed] [Google Scholar]
- 219.Rigante D, et al. First report of macrophage activation syndrome in hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheum. 2007;56:658–661. doi: 10.1002/art.22409. [DOI] [PubMed] [Google Scholar]
- 220.Hager EJ, et al. Deletion of a single mevalonate kinase (Mvk) allele yields a murine model of hyper-IgD syndrome. J Inherit Metab Dis. 2007;30:888–895. doi: 10.1007/s10545-007-0776-7. [DOI] [PubMed] [Google Scholar]