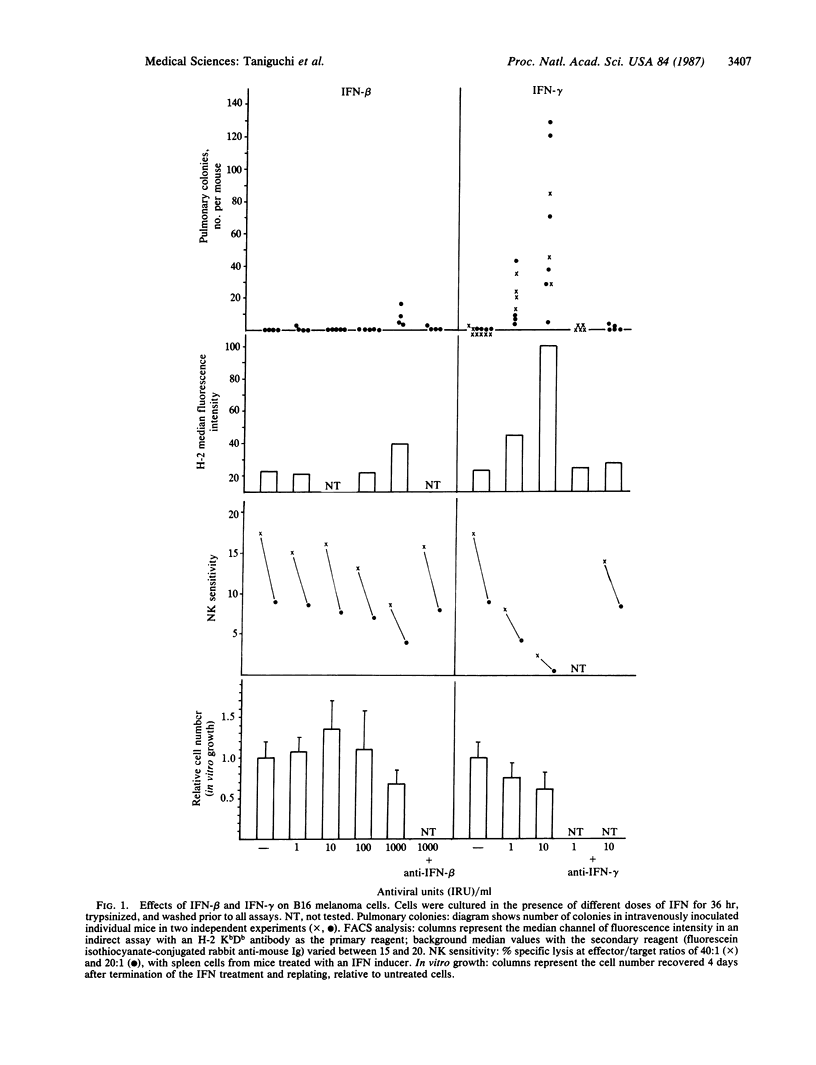
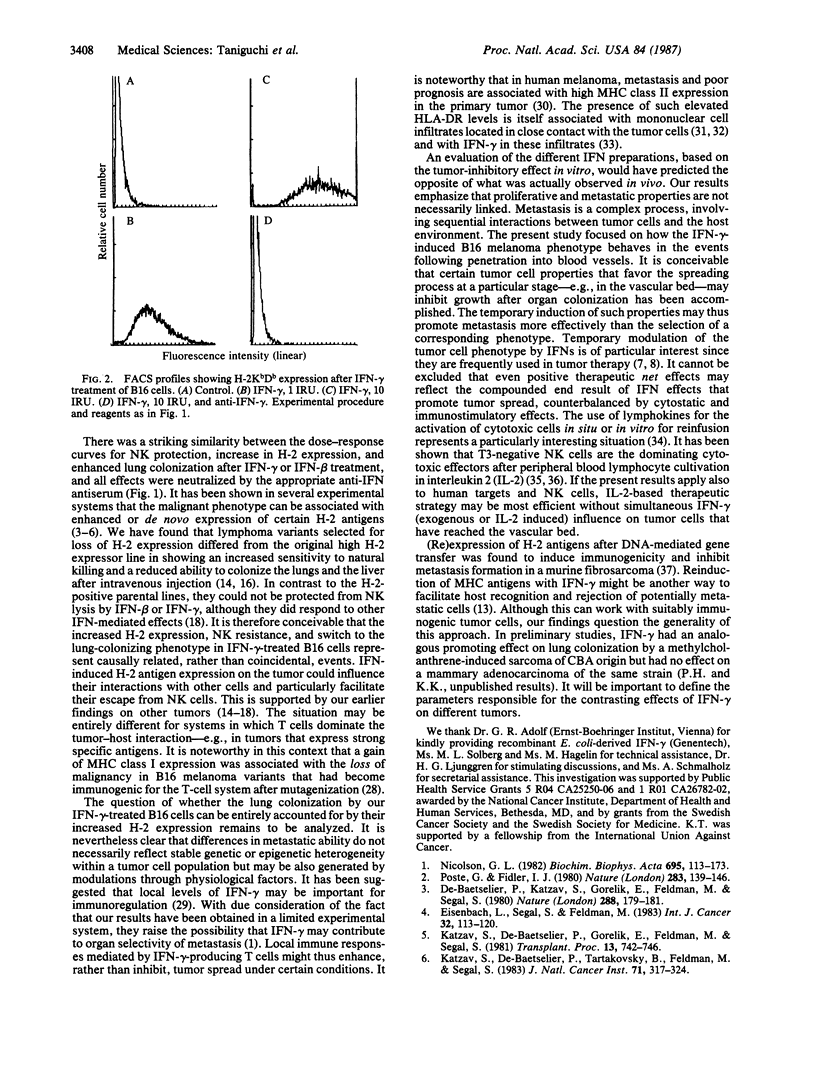
Abstract
Treatment of H-2-deficient nonmetastatic B16 melanoma cells with physiological doses of interferon gamma (IFN-gamma) reduced cellular growth in vitro but induced a shift to the lung-colonizing phenotype as assessed after intravenous injection of the treated cells. As little as 1 antiviral unit of recombinant IFN-gamma per ml induced B16 cells to form 3-40 pulmonary metastases in each injected mouse, whereas a 1000-fold higher concentration of IFN-beta was required to see similar effects. IFN-gamma may induce cell-surface molecules that contribute to the metastatic ability of the tumor cells. The efficient enhancement of metastatic ability after IFN-gamma treatment of the B16 cells was paralleled by an increased H-2 antigen expression and decreased sensitivity to natural killer cells. The experiments support the idea that metastasis may not depend exclusively on stable genetic changes or heterogeneity within a tumor population but may be also influenced through the modulation of the phenotype by physiological or pharmacological agents. The results are also discussed with regard to the role of different effector cells in tumor cell clearance and in relation to lymphokine-based strategies for therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bröcker E. B., Suter L., Sorg C. HLA-DR antigen expression in primary melanomas of the skin. J Invest Dermatol. 1984 Mar;82(3):244–247. doi: 10.1111/1523-1747.ep12260181. [DOI] [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Interferon enhances the susceptibility of virus-infected fibroblasts to cytotoxic T cells. J Exp Med. 1985 Jan 1;161(1):257–262. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baetselier P., Katzav S., Gorelik E., Feldman M., Segal S. Differential expression of H-2 gene products in tumour cells in associated with their metastatogenic properties. Nature. 1980 Nov 13;288(5787):179–181. doi: 10.1038/288179a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Knowles B. B., Wettstein P. J. Immunological surveillance of tumors in the context of major histocompatibility complex restriction of T cell function. Adv Cancer Res. 1984;42:1–65. doi: 10.1016/s0065-230x(08)60455-8. [DOI] [PubMed] [Google Scholar]
- Eisenbach L., Segal S., Feldman M. MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer. 1983 Jul 15;32(1):113–120. doi: 10.1002/ijc.2910320118. [DOI] [PubMed] [Google Scholar]
- Ettinghausen S. E., Rosenberg S. A. The adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. Springer Semin Immunopathol. 1986;9(1):51–71. [PubMed] [Google Scholar]
- Gorelik E., Peppoloni S., Overton R., Herberman R. B. Increase in H-2 antigen expression and immunogenicity of BL6 melanoma cells treated with N-methyl-N'-nitronitrosoguanidine. Cancer Res. 1985 Nov;45(11 Pt 1):5341–5347. [PubMed] [Google Scholar]
- Grönberg A., Kiessling R., Masucci G., Guevara L. A., Eriksson E., Klein G. Gamma-interferon (IFN-gamma) produced during effector and target interactions renders target cells less susceptible to NK-cell-mediated lysis. Int J Cancer. 1983 Nov 15;32(5):609–616. doi: 10.1002/ijc.2910320515. [DOI] [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Hayashi H., Tanaka K., Jay F., Khoury G., Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985 Nov;43(1):263–267. doi: 10.1016/0092-8674(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Katzav S., De Baetselier P., Gorelik E., Feldman M., Segal S. Immunogenetic control of metastasis formation by a methylcholanthrene-induced tumor (T10) in mice: differential expression of H-2 gene products. Transplant Proc. 1981 Mar;13(1 Pt 2):742–746. [PubMed] [Google Scholar]
- Katzav S., De Baetselier P., Tartakovsky B., Feldman M., Segal S. Alterations in major histocompatibility complex phenotypes of mouse cloned T10 sarcoma cells: association with shifts from nonmetastatic to metastatic cells. J Natl Cancer Inst. 1983 Aug;71(2):317–324. [PubMed] [Google Scholar]
- Klein G., Klein E. Immune surveillance against virus-induced tumors and nonrejectability of spontaneous tumors: contrasting consequences of host versus tumor evolution. Proc Natl Acad Sci U S A. 1977 May;74(5):2121–2125. doi: 10.1073/pnas.74.5.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986 Apr-Jun;13(2-3):141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985 Dec 1;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek G. E., Taniguchi K., Ljunggren H. G., Grönberg A., Kiessling R., Klein G., Kärre K. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol. 1985 Dec;135(6):4281–4288. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Strander H., Cantell K., Carlström G., Jakobsson P. A. Clinical and laboratory investigations on man: systemic administration of potent interferon to man. J Natl Cancer Inst. 1973 Sep;51(3):733–742. doi: 10.1093/jnci/51.3.733. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Kärre K., Klein G. Lung colonization and metastasis by disseminated B16 melanoma cells: H-2 associated control at the level of the host and the tumor cell. Int J Cancer. 1985 Oct 15;36(4):503–510. doi: 10.1002/ijc.2910360415. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Wallach D. Interferon-induced resistance to the killing by NK cells: a preferential effect of IFN-gamma. Cell Immunol. 1983 Feb 1;75(2):390–395. doi: 10.1016/0008-8749(83)90337-4. [DOI] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Karre K., Hansson M., Kunkel L. A., Kiessling R. W. Interferon-mediated protection of normal and tumor target cells against lysis by mouse natural killer cells. J Immunol. 1981 Jan;126(1):219–225. [PubMed] [Google Scholar]



