Abstract
Background
The etiology of ventricular dysfunction in adult congenital heart disease (ACHD) is not well understood. Diffuse fibrosis is a likely common final pathway and is quantifiable using magnetic resonance imaging (MRI).
Methods and Results
ACHD patients (N=50) were studied with cardiac MRI to quantify systemic ventricular volume and function, and diffuse fibrosis. The fibrosis index for a single mid-ventricular plane of the systemic ventricle was quantified by measuring T1 values for blood pool and myocardium before and after administration of gadolinium (0.15 mmol/kg), then adjusted for hematocrit. Results were compared to healthy volunteers (normal controls, N=14) and patients with acquired heart failure (positive controls, N=4). Patients studied (age 37±12 years, 40% female) included 11 with a systemic right ventricle (RV), 17 with tetralogy of Fallot, 10 with cyanosis and 12 with other lesions. The fibrosis index was significantly elevated in ACHD patients compared to normal controls (31.9±4.9% vs. 24.8±2.0%, p=0.001). Values were highest in systemic RV patients (35.0±5.8%, p<0.001) and cyanotic patients (33.7±5.6%, p<0.001). The fibrosis index correlated with end-diastolic volume index (r=0.60, p<0.001) and ventricular ejection fraction (r=−0.53, p<0.001), but not with age, nor oxygen saturation in cyanotic patients. Late gadolinium enhancement did not account for the differences seen.
Conclusions
ACHD patients have evidence of diffuse, extracellular matrix remodeling, similar to patients with acquired heart failure. The fibrosis index may facilitate studies on the mechanisms and treatment of myocardial fibrosis and heart failure in these patients.
Keywords: congenital heart disease, myocardial fibrosis, late gadolinium enhancement, ventricular dysfunction, heart failure
Heart failure in patients with adult congenital heart disease (ACHD) is an increasingly common and life-threatening complication for a substantial portion of patients,1,2, 3,4 Yet etiology is not well understood, and treatment options are largely empiric. Myocardial fibrosis, or abnormal accumulation of extracellular material in the myocardium, is a common final pathway of a number of cardiovascular stresses5, 6 leading to diastolic dysfunction, systolic dysfunction, arrhythmia, and increased mortality; developments which all respond favorably to pharmacotherapy.7–12 Limited studies suggest a similar pathway in ACHD, although evidence for proven response to medical therapy is lacking. Therefore, fibrosis is an ideal pathologic marker on which to focus attention.
In the 1960s, clinicians observed that exercise performance after surgical repair with congenital defects was still below that of normal individuals, and postulated the presence of a “myocardial factor” responsible for the persistent limitation.13 This important observation was likely the first to acknowledge that repair of the anatomic defect could not guarantee full restoration of cardiovascular function. Several years later, pathologists identified changes within the myocardium of ACHD patients including interstitial fibrosis, confirming the notion of the myocardial factor.14,15, 16 Few studies have continued this line of investigation. Surgical samples in young tetralogy of Fallot patients showed fibrosis in 65%.17 Recent interest has been revived by the use of late gadolinium enhancement (LGE) by magnetic resonance imaging (MRI), which has demonstrated macroscopic fibrosis in both the right ventricle (RV) and left ventricle (LV) in ACHD.18–22 However, the method is not conducive to quantify diffuse microscopic fibrosis.
The capacity to detect, quantify, and follow myocardial fibrosis offers tremendous advantage in efforts to study etiology and response to therapy. Quantification of T1 changes before and after gadolinium (Gd) administration during MRI has been successfully employed to differentiate normal and abnormal hearts.23–26 We have further developed this method and used it to quantify myocardial extracellular volume fraction as a marker of extracellular matrix remodeling,27, 28 which we term the “fibrosis index.” The method is objective and independent of cardiac output, gadolinium dose, or imaging timing. It can be widely applied and serially obtained. We sought to apply this method for detection and quantification of fibrosis in ACHD patients.
Methods
Patient Selection
Consecutive adults with ACHD referred for MRI at our institution were studied according to accepted clinical standards including the use of gadolinium. Specifically, though not exclusively, we focused on three diagnostic subgroups; A) patients with a systemic RV (L-transposition of the great arteries or D-transposition with prior atrial redirection surgery, N=11), B) repaired tetralogy of Fallot (N=17), and C) cyanotic heart disease including Eisenmenger syndrome (N=10). These were targeted because systemic ventricular dysfunction is a known complication in each, they are commonly referred for MRI, and because fibrosis has been shown by LGE in all three groups.18–22 Additional patients with other congenital abnormalities with an assumed increased likelihood of diffuse fibrosis were also included (N=12). In addition, we studied patients with dilated or ischemic cardiomyopathy with visible replacement fibrosis (positive controls, N=4) and healthy volunteers (negative controls, N=14). Groups are summarized in Table 1. The protocol was approved by the Institutional Review Board, and healthy volunteers signed informed consent prior to participation.
Table 1.
Subgroup Considerations
| N | M/F | Age (yrs) | EF (%) | EDVi (ml/m2) | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Healthy (neg. control) | 14 | 8/6 | 37.7 ± 11.9 | 66 ± 7 | 79 ± 19 |
| Systemic RV | 11 | 9/2 | 36.1 ± 13.6 | 46 ± 11 | 111 ± 32 |
| Tetralogy | 17 | 8/9 | 34.1 ± 11.7 | 57 ± 12 | 82 ± 16 |
| Cyanosis | 10 | 4/6 | 42.3 ± 10.9 | 54 ± 11 | 142 ± 69 |
| Other Congenital | 12 | 9/3 | 32.9 ± 12.4 | 63 ± 10 | 95 ± 33 |
| Acquired (pos. control) | 4 | 2/2 | 58.3 ± 4.54* | 50 ± 21 | 105 ± 37 |
Study population by subgroup. All values are for the systemic ventricle in each group.
Acquired heart disease patients were older by ANOVA. EF = Ejection Fraction, EDVi = End-diastolic Volume Index. RV = right ventricle.
MRI Protocol
Patients were studied using either a 1.5 Tesla (Philips Achieva) or a 3 Tesla (Philips Intera) scanner, depending solely on scanner availability. Short axis images through the heart were obtained to quantify RV and LV volume and function using established methods.18, 19 Phase velocity flow mapping through the aorta and pulmonary artery were used as confirmation of ventricular stroke volumes. Measurements of T1 were made using a Look-Locker technique29–31 (a gradient echo sequence with a non-slice selective inversion pulse initially after the R wave, followed by segmented gradient echo acquisition for 21 cardiac phases over 2–3 cardiac cycles; temporal resolution 40 ms, slice thickness 8 mm; repetition time > 3 R-to-R intervals) for a single mid-ventricular plane. This type of sequence is often employed for determination of optimal nulling for delayed enhancement (“inversion time scout”). This same Look-Locker acquisition was repeated, using the same mid-ventricular plane, and at various intervals 3–25 minutes after injection of Gd-DTPA (Omniscan, 0.15 mmol/kg), interwoven during the LGE portion of the study. LGE was studied in the usual manner 10–20 minutes after gadolinium administration.18, 19 Total scan times were typically 50–90 minutes depending on the clinical questions addressed. Scans were done according to clinical need, not purely for study purposes.
Fibrosis Index Quantification
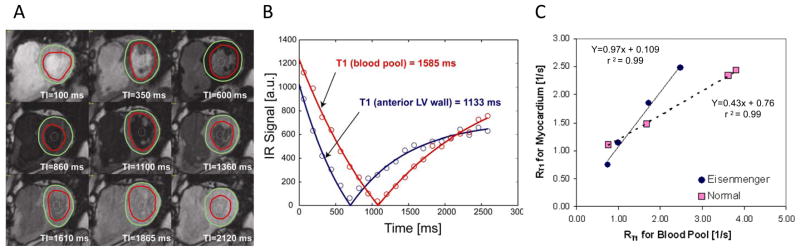
For each T1 sequence the endo- and epicardial borders of the systemic ventricle were traced (Figure 1A) and divided into 6 standard segments (Mass CMR software, Medis, The Netherlands). We did not study the sub-pulmonic ventricle in any patient. The signal intensity vs. time curve for each segment and blood pool was used to determine a segmental T1 through exponential fitting (Figure 1B), and its reciprocal, R1. This was done for each Look-Locker acquisition. The slope of the linear relationship between R1 for myocardium vs. blood from all R1 measurements before and after gadolinium administration defined the partition coefficient for gadolinium, or lambda30 (Figure 1C), and values for all 6 myocardial segments were averaged. To obtain the myocardial volume of distribution of gadolinium, or extracellular volume fraction, the partition coefficient was multiplied by (1-hematocrit/100)27, 31 which we refer to as the “fibrosis index.”
Figure 1.
A) Images from Look-Locker sequence for a patient with tetralogy of Fallot. Regions of interest for the LV myocardium and blood pool are drawn. B) The time vs. signal intensity curves for the blood pool (red) and myocardium (blue), which are fitted to determine the decay constant T1. The inverse, R1, for each is determined before gadolinium administration and at several time points after. C) The relationship of R1 signals between the blood pool and the myocardium before and after gadolinium is linear. The slope of this relationship is the partition coefficient of gadolinium, and determines the fibrosis index. Examples from a patient with cyanosis (Eisenmenger syndrome, blue) and a normal control (pink) are shown. The steeper slope reflects the increase in gadolinium present due to an enlarged extracellular space.
Late Gadolinium Enhancement (LGE)
Areas of late enhancement (unrelated to sites of expected surgical intervention such as a VSD patch) were identified and categorized by location. To determine whether the presence of LGE influenced the fibrosis index, we quantified the area of LGE within the mid-ventricular plane used for the fibrosis index, expressed as a percentage of total myocardial area in the plane (% area LGE). In this quantification, we included any enhancement in the RV-LV junction that overlapped with the region of interest drawn for the fibrosis index.
Statistical Analysis
Results are presented as mean ± SD. One way ANOVA was used to compare fibrosis index values for all ACHD patient subgroups and both positive and negative controls, with Tukey’s post-hoc analysis (SPSS, version 11.0). Age, systemic ventricular ejection fraction (EF), and end-diastolic volume index (EDVi) were also compared in the same manner. Student’s t-test was used to compare patients with vs. without LGE. Pearson’s correlation coefficient was used to correlate the fibrosis index with age, oxygen saturation in cyanotics, ventricular volumes, ejection fraction, and % area of LGE. To test whether the association of EDVi, EF, and age with the fibrosis index varied between diagnostic groups, an interaction between fibrosis index and diagnostic group was included in linear regression models. Non-parametric methods (Kruskal Wallis test with follow-up Mann-Whitney comparisons and Spearmen Correlation coefficients) were also used to assess the sensitivity of the results to standard parametric assumptions. One way ANOVA for repeated measures was used to detect a possible association between mean fibrosis index and the index in specific myocardial segments.
Results
Fifty adult ACHD patients were studied (age 37±12 years, range 18–71, 20 female, 30 male). In addition to the target subgroups (Table 1), we studied 4 patients with pressure overloading (3 with coarctation of the aorta and one with bicuspid aortic stenosis), 5 with volume loading (systemic atrioventricular valvular regurgitation or shunts), 2 with single ventricle Fontan palliation, and 1 with prior Rastelli repair. In addition we studied 4 positive controls and 14 healthy volunteers as negative controls. Positive controls were significantly older. From the entire group (including controls), 25 studies were performed at 3.0 Tesla, and 38 at 1.5 Tesla, with no overall difference in the fibrosis index between them (p=0.40), including no differences between healthy controls specifically.
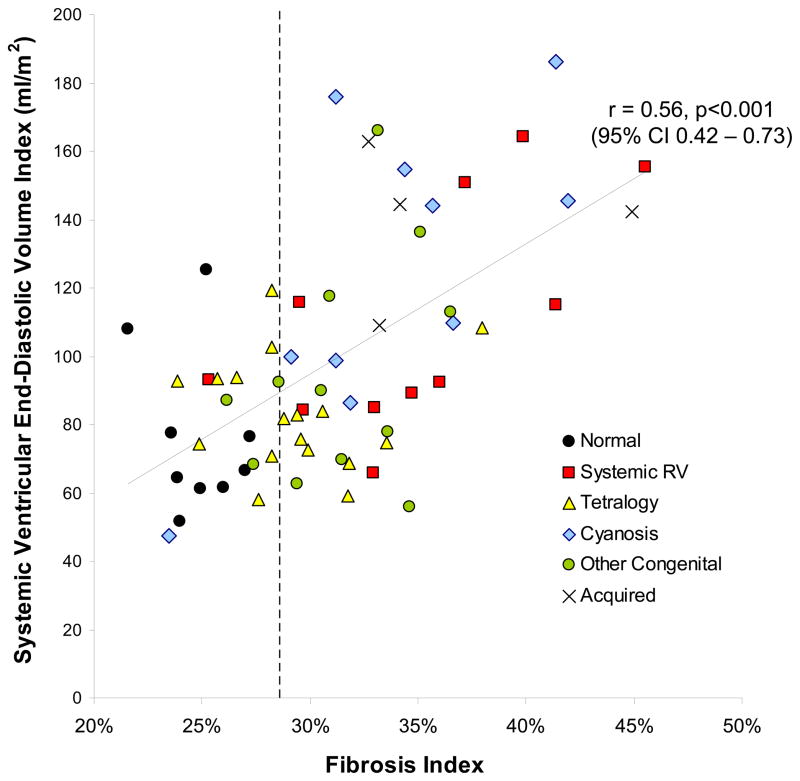
Fibrosis index values for all groups are shown (Table 2). Overall comparisons among groups was highly significant (ANOVA p<0.0001). The fibrosis index was significantly elevated in ACHD patients compared to negative controls (31.9±4.9% vs. 24.8±2.0%, p=0.001) and was comparable to the mean fibrosis index in positive controls (36.2±5.7%, p=0.1). Values were highest in systemic RV patients (35.0±5.8%, p<0.001 vs. normal controls) and cyanotic patients (33.7±5.6%, p<0.001 vs. normal controls). The fibrosis index correlated with EDVi (r=0.60, 95% CI 0.42–0.73, p<0.001, Figure 2) and EF (r=−0.53, 95% CI −0.68 - −0.34, p<0.001, Figure 3), but not with age (r=0.11, 95% CI −0.13–0.34, p=0.37). There was no evidence that the association of EDVi, EF, and age with the fibrosis index varied between diagnostic groups, and therefore the data for ACHD patients were pooled. Results were similar when using non-parametric procedures. As mean + 2 SD for the control group was 28.8%, we defined an abnormal fibrosis index as any value ≥29%.
Table 2.
Fibrosis index
| Mean ± SD | Min. | Max. | p vs Healthy | p vs Acquired | |
|---|---|---|---|---|---|
| Healthy (neg. control) | 24.8% ± 2.0% | 21.6% | 28.1% | -- | <0.001 |
| Systemic RV | 35.0% ± 5.8% | 25.3% | 45.5% | <0.001 | 0.997 |
| Tetralogy | 29.2% ± 3.4% | 23.9% | 38.0% | 0.051 | 0.039 |
| Cyanosis | 33.7% ± 5.6% | 23.5% | 41.9% | <0.001 | 0.926 |
| Other Congenital | 31.4% ± 3.2% | 26.2% | 36.5% | 0.002 | 0.436 |
| Acquired (pos. control) | 36.2% ± 5.8% | 32.7% | 44.9% | 0.003 | -- |
Results of the fibrosis index by subgroup. Omnibus p-value <0 .0001. Tukey’s method used for follow-up comparisons. RV=right ventricle.
Figure 2.
Relationship between systemic ventricular volume index and the fibrosis index (r=0.60, 95% CI 0.42–0.73, p<0.001). Dotted vertical line is the upper limit of normal.
Figure 3.
Relationship between systemic ventricular ejection fraction and the fibrosis index is linear. (r=-0.53, 95% CI -0.68 - -0.34, p<0.001). Dotted vertical line is the upper limit of normal.
Subgroup Differences
Of the 11 systemic RV patients, 10 had abnormal fibrosis index values. There was a positive relationship between EDVi (morphologic RV) and the fibrosis index, (r=0.65, p=0.03) and a non-significant trend with EF (r=−0.40, p=0.2). There was no association with age. Two patients had severe tricuspid regurgitation, including the patient with the highest value of 46%, who had an ejection fraction of 45%. There was no association with tricuspid valve regurgitant fraction (estimated by stroke volume comparisons).
All tetralogy patients had been repaired and none were cyanotic. The mean fibrosis index for the LV was higher than for healthy controls (29.2±3.4%), though this did not reach statistical significance (p=0.051). The value was abnormal in 9 of the 17 patients. The patient with the highest fibrosis index (value of 38%), had severe LV dysfunction (EF 30%). Overall there was no correlation with LV EDVi or EF as seen in the other subgroups. There was also no correlation between the fibrosis index (for the LV) and RV size or function. We did not measure the fibrosis index for the RV. There was a non-significant correlation between the pulmonary valve regurgitant fraction and the fibrosis index (r=0.44, p=0.086). Patients with an abnormal fibrosis index (N=9) compared to those with normal values (N=8) more often had moderate to severe pulmonary regurgitation (p=0.02, Fisher’s exact test), and had fewer prior cardiac surgeries (range 1- 3 surgeries, p=0.034 by chi-square test). We found no significant differences in prior shunt placement, age at time of shunt placement, or age at time of repair.
In order to determine the influence of the RV on septal fibrosis in tetralogy of Fallot, we compared fibrosis index values of the 6 segments of the myocardial wall in all 17 patients using a random effects model. Range of fibrosis index measures for the tetralogy of Fallot patients by segment were as follows: Anterior 21.7–37.1, Anterior-septal 23.7–39.5, Inferior-septal 21.1–41.5, Inferior 24.9–40.8, Lateral 23.1–34.9, Posterior 23.5–33.2 %. Similar ranges for normal controls were: Anterior 20.6–29.3, Anterior-septal 18.7–29.9, Inferior-septal 21.4–29.5, Inferior 21.6 − 31.5, Lateral 19.6–27.1, Posterior 19.6–26.3 %. ANOVA for repeated measures indicated that in tetralogy patients there was a significant variation of the means of the fibrosis index among segments (p=0.009). In healthy volunteers the same test did not indicate any significant variation between segments (p=0.127). The largest values for the fibrosis segment in tetralogy patients were observed in the inferior segments. Post-hoc paired comparisons confirmed that among the 6 myocardial segments the inferior segment had higher fibrosis index values compared with all other segments (p<0.05 with Bonferroni correction) except the inferior-septal segment. Relative to the anterior segment this difference of fibrosis index only amounted to a trend (p<0.1). Therefore, the septum was not disproportionately affected compared to other myocardial segments.
In cyanotic patients (mean oxygen saturation 80 ± 4%), 9 out of 10 had abnormal values. There was a strong correlation between the fibrosis index and systemic EDVi (r=0.72, p=0.019) and EF (r=−0.86, p<0.001). There was no correlation of the fibrosis index with resting oxygen saturation (mean 80 ± 4%), or age. Even after exclusion of cyanotic patients from the rest of the cohort, relationships of the fibrosis index with EDVi and EF remained significant for the remaining patients (r=0.493, p<0.001 and r=−0.451, p=0.001 respectively).
Ten of the 12 other ACHD patients studied had abnormal fibrosis coefficient values; including all 3 coarctation patients, 5 patients with volume loading, and both Fontan patients. One patient with a prior surgical repair of a VSD was normal, as was a patient with bicuspid aortic valve stenosis.
Late Gadolinium Enhancement (LGE)
Of the total ACHD cohort, two patients (1 cyanotic and 1 systemic RV) had poor LGE data from difficulty nulling and were not included. Of the remaining, LGE was present in the systemic ventricle in 27 (56%), specifically 5/10 transposition patients, 12/17 tetralogy patients, 5/9 cyanotic patients, and 5/12 other ACHD patients. The most common LGE finding was enhancement at the RV-LV junction, and in 12 patients (7 with tetralogy) this was the only area of enhancement present. The majority of other positive LGE findings involved small portions of septum or papillary muscle. Only one patient had transmural enhancement consistent with a small myocardial infarction, known from a perioperative coronary embolism. There was no difference in the fibrosis index between patients with vs. without LGE (31.2±4.8% vs. 33.2±5.1%, p=0.17). All patients with acquired heart disease had visible LGE within the plane selected for the fibrosis index.
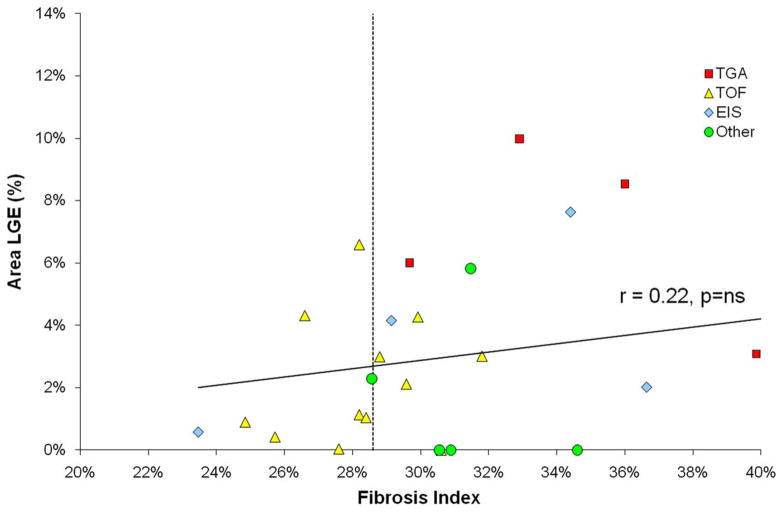
The % area LGE in ACHD patients was significantly lower than the amount seen in acquired heart disease (3.0±2.8% vs. 14.1±6.3%, p<0.001). For all ACHD patients, there was no correlation between the fibrosis index and % area LGE (r=0.22, Figure 4). In 5 patients, the LGE findings were not present in the plane used for LGE. Even excluding these patients, there was no relationship between % area LGE and the fibrosis index (r=0.27).
Figure 4.
Relationship between fibrosis index and the area LGE (area of positive late enhancement / total area of myocardium in the selected plane) for ACHD patients. There was no correlation, and many patients with significantly elevated fibrosis index had little/no detectable LGE. By comparison, patients with acquired heart disease had area LGE values of 16–20% (data not shown). Dotted vertical line is the upper limit of normal.
Discussion
ACHD patients show evidence of myocardial extracellular matrix expansion causing an enlargement of the volume of distribution of gadolinium, namely the fibrosis index. We found a relationship between this index and ventricular volume and systolic function across a subset of ACHD patients. This is congruous with histopathologic studies demonstrating diffuse fibrosis in ACHD.14, 16, 17 Together with other data showing neurohormonal activation,32 exercise intolerance,33 and mortality4 similar to patients with acquired heart failure, our findings provide additional evidence that ACHD is a form of progressive heart failure with a pathologic process similar to other etiologies.
We employed a technique for assessing diffuse fibrosis based on changes of T1 after gadolinium administration. Gd-contrast based changes in myocardial R1 accurately reflect Gd concentration and relative distribution volume with good reproducibility,23,24 and correlate with histological evidence of fibrosis even in the absence of LGE.25 The methodology avoids the necessity of defining “normal” myocardium for nulling as is done with LGE imaging.34 However, reliance on T1 alone as an indicator of extracellular remodelling requires consistency in gadolinium dosing and timing of post-contrast image acquisition.34, 35 The method used here overcomes this by measuring the myocardial partition coefficient for Gd-based contrast whereby the R1 changes in myocardium are normalized by the R1 changes in the blood. Correcting for hematocrit to account for changes in plasma Gd concentration gives the fibrosis index, a measure of extracellular volume fraction. Increased extracellular volume implies the presence of diffuse interstitial fibrosis. The normal range in our study agrees with other estimates of the extracellular myocardial volume.26 Our data cannot exclude the possibility that replacement fibrosis (or frank myocyte necrosis) contributed to the observed findings. However, elevation of the fibrosis index was not explained by visible LGE, and was found diffusely across wall segments. Although the presence of LGE will raise the fibrosis index, as we found in positive controls, the ACHD patients had fibrosis index values far greater than what could be explained by visible LGE alone (Figure 4), and 21 patients had no LGE in the selected plane despite an elevated fibrosis index. These findings are consistent with the interpretation that diffuse microscopic fibrosis is present.
The amount of LGE seen in target subgroups was generally consistent with previous reports.18–20 Although degree of LGE in tetralogy and D-transposition patients correlates with adverse clinical variables,18, 19 the clinical relevance of LGE in Eisenmenger patients remains undetermined.20 One possible reason for this may be the difficulty of quantifying LGE when the fibrotic change may be much more diffuse, as has been described in cardiac amyloidosis.36 Indeed, difficulty nulling may reflect the diffuse nature of extracellular myocardial change, and nulling with shorter inversion times would effectively suppress the evidence of diffuse fibrosis.
Our use of positive controls specifically included patients with visible fibrosis on LGE, including patients with both ischemic and non-ischemic cardiomyopathy. We recognize that not all non-ischemic cardiomyopathy patients will necessarily have visible fibrosis by LGE.37, 38 Therefore, we also made comparisons on patients with idiopathic cardiomyopathy from our previously study.39 We identified 7 patients (5 men, age 59±16 years) with reduced LV function (LVEF 32±14%) and quantified the fibrosis index for each. The fibrosis index (mean 31.4±4.6%) was significantly higher than our control group (p<0.001), but not different from our ACHD cohort nor positive controls (p=0.78 and p=0.16 respectively). There were significant correlations within this group between the fibrosis index and EDVi and EF (r=0.51 and −0.59 respectively), and the index did not increase with age. These confirmatory findings also support the similarities between ACHD and acquired heart failure.
Specific Subgroup Considerations
Extracellular remodeling was present in all three diagnostic subgroups (though less so in tetralogy) despite likely differences in the pathogenesis of myocyte dysfunction in each such as volume and/or pressure loading, abnormal ventricular geometry, previous cardiopulmonary bypass, or chronic myocardial ischemia. The finding that diffuse interstitial fibrosis is present to some degree in all three groups underscores this as a common final pathway and invites further study into specific inciting mechanisms in each subgroup.
We found comparatively less fibrosis in the tetralogy group, which generally had less evidence of heart failure. Although systolic dysfunction is more common in the RV in this group, we measured fibrosis in the LV. LV dysfunction is increasingly common and has prognostic significance,40, 41 though very little is known about its etiology. In practical terms, it is harder to reliably demarcate the thin-walled RV. In this limited subgroup, we found no evidence of interventricular interaction that might explain the development of fibrosis in the LV through electromechanical and/or neurohormonal coupling with the RV. However, the finding of more severe PR in patients with higher LV fibrosis does suggest a role of RV loading.
Other patients included those with pressure loading. All coarctation patients were previously repaired and did not have restenosis at the time of the study. The bicuspid valve patient had moderate stenosis, though the fibrosis value was not elevated as we would have expected. The patient was asymptomatic and it is possible that fibrosis had not yet occurred in response to the load.
Clinical Implications and Future Directions
The importance of our data lies not only in demonstration of diffuse fibrosis but also in the potential application of the fibrosis index as a tool for future studies. The quantification of diffuse fibrosis serially over time has tremendous implications for both therapeutic and mechanistic studies.
Therapeutically, studies of specific medications known to attenuate or reverse fibrosis can employ this method for detecting change non-invasively in specific patient groups. To date, studies have generally not succeeded in showing a favorable impact of these drugs using clinical endpoints.42, 43 An exception to this was the report that tetralogy patients with restrictive RV physiology randomized to lisinopril vs. placebo for 6 months had a favorable increase in LV ejection fraction.44 This is interesting in light of our data, which suggest a possible rationale for the findings; namely that restriction is known to be associated with fibrosis, and fibrosis should improve with ACE inhibitor therapy. Small studies with surrogate endpoints such as the fibrosis index will be easier to fund and coordinate. These in turn can provide important pilot data to support larger multicenter studies with hard clinical endpoints including exercise capacity, arrhythmia, or even mortality, which are tremendously lacking in the field.
Mechanistically, the identification of key inciting events that trigger fibrogenesis as well as their biochemical pathways will manifest differences between subgroups, and be the key to prevention. Using the fibrosis index in either humans or animal models coupled with biomarkers of inflammation, neurohormonal activation, or collagen deposition, we may demonstrate to what extent fibrogenesis is induced by pressure loading, myocardial ischemia, cardiopulmonary bypass, or microvascular thrombosis. In addition, the method can be applied to other patient groups susceptible to ventricular dysfunction.
Limitations
Our study employs an approach that is relatively novel. Only a single mid-ventricular slice was quantified, and analysis is time-consuming. The fibrosis index measures extracellular volume, which may be increased from processes other than fibrosis (such as acute inflammation or infiltrative processes). The relation between the partition coefficient of gadolinium (on which the fibrosis index is based) and collagen volume fraction (the gold standard for quantifying fibrosis) has only been tested ex-vivo,28 which by necessity employs a different method of gadolinium diffusion. Obtaining histologic confirmation was not feasible here, although correlation between histology and T1 measurements has been demonstrated.25 Only systolic function was measured, although diastolic function would be expected to correlate with fibrosis. Further study will be required for additional validation. The method requires exclusion of pacemaker/ICD patients. Since the need for pacing and/or arrhythmia may be associated with myocardial fibrosis, this selection bias may exclude patients with more extreme fibrosis. Our study has limited power, and thus the lack of association/correlation does not necessarily mean that an association would not be found with a larger sample size. This is especially relevant for tests involving the 4 positive controls.
Conclusions
In congenital heart disease, it is becoming increasingly evident that protection of the myocardium should be given as much attention as the hemodynamics early on in a patient’s life, both for preservation of ventricular function and improved survival. This study demonstrates that remodeling of the extracellular matrix, or myocardial fibrosis, which can be quantified serially and non-invasively, is associated with adverse ventricular enlargement and declining ventricular function across different forms of congenital heart disease. The data confirm that myocardial fibrosis is a common final pathway leading to myocardial dysfunction, and opens further possibilities for much needed research into both the etiology and treatment of these patients.
Acknowledgments
We wish to thank Dawn Peters, PhD, Division of Biostatistics at Oregon Health and Science University for statistical consultation, and acknowledge the Oregon Clinical and Translational Research Institute (grant number UL1 RR024140 01, from the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research), in providing funding for her contribution.
Sources of Funding
Research funding and partial salary support for Dr. Broberg were provided in part by a grant from the American Heart Association. Dr. Chugh was funded in part by National Heart Lung and Blood Institute R01HL088416 and 3R01HL088416-03S.
Footnotes
Disclosures
None.
References
- 1.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J. 2003;24:970–976. doi: 10.1016/s0195-668x(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 3.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 4.Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol. 2007;50:1263–1271. doi: 10.1016/j.jacc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 6.Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34:1577–1584. doi: 10.1006/jmcc.2002.2088. [DOI] [PubMed] [Google Scholar]
- 7.Chan AK, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WW, Wong JT, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–596. doi: 10.1016/j.jacc.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 8.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 9.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 10.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–310. doi: 10.1016/s0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 12.Anand K, Mooss AN, Mohiuddin SM. Aldosterone inhibition reduces the risk of sudden cardiac death in patients with heart failure. J Renin Angiotensin Aldosterone Syst. 2006;7:15–19. doi: 10.3317/jraas.2006.001. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh HD, Cohen AI. Pulmonary stenosis; the importance of the myocardial factor in determining the clinical course and surgical results. Am Heart J. 1963;65:715–716. [Google Scholar]
- 14.Jones M, Ferrans VJ, Morrow AG, Roberts WC. Ultrastructure of crista supraventricularis muscle in patients with congenital heart diseases associated with right ventricular outflow tract obstruction. Circulation. 1975;51:39–67. doi: 10.1161/01.cir.51.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Jones M, Ferrans VJ. Myocardial degeneration in congenital heart disease. Comparison of morphologic findings in young and old patients with congenital heart disease associated with muscular obstruction to right ventricular outflow. Am J Cardiol. 1977;39:1051–1063. doi: 10.1016/s0002-9149(77)80221-x. [DOI] [PubMed] [Google Scholar]
- 16.Jones M, Ferrans VJ. Myocardial ultrastructure in children and adults with congenital heart disease. Cardiovasc Clin. 1979;10:501–530. [PubMed] [Google Scholar]
- 17.Chowdhury UK, Sathia S, Ray R, Singh R, Pradeep KK, Venugopal P. Histopathology of the right ventricular outflow tract and its relationship to clinical outcomes and arrhythmias in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2006;132:270–277. doi: 10.1016/j.jtcvs.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, Gatzoulis MA, Kilner PJ. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 19.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 20.Broberg CS, Prasad SK, Gatzoulis MA. The interventricular relationship with Eisenmenger syndrome: Findings with cardiac magnetic resonance and late gadolinium enhancement. J Am Coll Cardiol. 2007;49:263A. (Abstract) [Google Scholar]
- 21.Oosterhof T, Mulder BJ, Vliegen HW, de Roos A. Corrected tetralogy of Fallot: delayed enhancement in right ventricular outflow tract. Radiology. 2005;237:868–871. doi: 10.1148/radiol.2373041324. [DOI] [PubMed] [Google Scholar]
- 22.Hartke LP, Gilkeson RC, O'Riordan MA, Siwik ES. Evaluation of right ventricular fibrosis in adult congenital heart disease using gadolinium-enhanced magnetic resonance imaging: initial experience in patients with right ventricular loading conditions. Congenit Heart Dis. 2006;1:192–201. doi: 10.1111/j.1747-0803.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 23.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238:1004–1012. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 24.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 25.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta S, Kaye D, Taylor A. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 26.Pack NA, Dibella EV, Wilson BD, McGann CJ. Quantitative myocardial distribution volume from dynamic contrast-enhanced MRI. Magn Reson Imaging. 2008;26:532–542. doi: 10.1016/j.mri.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24:61–68. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 29.Look D, Locker D. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1976;41:250–251. [Google Scholar]
- 30.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Lorenz CH, Holburn GE, Overholser KA. Regional measurement of the Gd-DTPA tissue partition coefficient in canine myocardium. Magn Reson Med. 1997;38:541–545. doi: 10.1002/mrm.1910380406. [DOI] [PubMed] [Google Scholar]
- 32.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJ, Anker SD, Gatzoulis MA. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106:92–99. doi: 10.1161/01.cir.0000020009.30736.3f. [DOI] [PubMed] [Google Scholar]
- 33.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole-Wilson PA, Francis DP, Gatzoulis MA. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich M. There is more than shape and function. J Am Coll Cardiol. 2008;52:1581–1583. doi: 10.1016/j.jacc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Thornhill RE, Prato FS, Wisenberg G, Moran GR, Sykes J. Determining the extent to which delayed-enhancement images reflect the partition-coefficient of Gd-DTPA in canine studies of reperfused and unreperfused myocardial infarction. Magn Reson Med. 2004;52:1069–1079. doi: 10.1002/mrm.20236. [DOI] [PubMed] [Google Scholar]
- 36.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 37.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 38.Assomull RG, Pennell DJ, Prasad SK. Cardiovascular magnetic resonance in the evaluation of heart failure. Heart. 2007;93:985–992. doi: 10.1136/hrt.2003.025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerosch-Herold M, Sheridan D, Kushner J, Nauman D, Burgess D, Dutton D, Hershberger R. Cardiac magnetic resonance contrast enhancement differentiates patients affected with familial dilated cardiomyopathy from asymptomatic relatives. J Cardiovasc Magn Reson. 2006;8:154–155. [Google Scholar]
- 40.Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, del Nido PJ, Geva T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 41.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 43.Vonder Muhll I, Liu P, Webb G. Applying standard therapies to new targets: the use of ACE inhibitors and B-blockers for heart failure in adults with congenital heart disease. Int J Cardiol. 2004;97 (Suppl 1):25–33. doi: 10.1016/j.ijcard.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Babu-Narayan S, Uebing A, Davlouros PA, Path MK, Davidson S, Bayne S, Dimopoulos K, Pennell DJ, Gibson DG, Kilner PJ, Li W, Gatzoulis MA. Potential therapeutic role of ACE inhibitors in repaired tetralogy of Fallot and pulmonary regurgitation - the appropriate study (ACE inhibitors for potential prevention of the deleterious effects of pulmonary regurgitation in adults with repaired tetralogy of Fallot). International Symposium on Adult Congenital Heart Disease; Toronto, CA. 2008. (Oral Presentation) [Google Scholar]