Abstract
Recent identification of the modular CLS motifs responsible for cyclins A and E localization on centrosomes has revealed a tight linkage between the nuclear and centrosomal cycles. These G1/S cyclins must localize on the centrosome in order for DNA replication to occur in the nucleus, whereas essential DNA replication factors also function on the centrosome to prevent centrosome overduplication. Both events are dependent on the presence of an intact CLS within each cyclin. Here we compare the cyclins A and E CLSs at the structural and functional levels and identify a new cyclin A CLS mutant that disrupts all CLS functions and reduces the affinity of cyclin A for Cdk2. Analysis of interactions of the CLS motif within the cyclin molecules highlights the importance of the cyclin CBOX1 region for Cdk2 binding.
Key words: cyclin A, cyclin E, Cdk2, centrosome, CLS, PSTAIRE, DNA synthesis
Introduction
Localization of proteins in specific cellular compartments at a given time not only ensures their precise regulation and distinct functions, but also may coordinate processes occurring in different regions of the cell. One example of the same proteins being present in two different cellular compartments during the cell cycle is illustrated by the G1/S cyclins A and E: both localize on the centrosome, presumably to regulate centrosome duplication,1,2 and both are present in the nucleus where they regulate DNA replication.3 Recently, we showed that localization of cyclins A and E on centrosomes is also required for the initiation of DNA replication in the nucleus.4,5 Indeed, the chromatin loading of Cdc45, which is necessary for the initiation of DNA replication in the nucleus, is deficient upon displacement of these cyclins from the centrosome.6 Conversely, DNA synthesis can be restored by specifically retargeting cyclins to the centrosome.5 In addition, cyclins A and E directly recruit known DNA replication factors, including MCM5 and Orc1, to centrosomes to regulate centrosome duplication.7–9 Altogether, these findings suggest that the centrosome is an important docking site for proteins controlling the cell cycle. Recent studies showed that complete or partial ablation of the centrosome, as well as the depletion of proteins known to localize on the centrosome, often leads to cell cycle arrest in the subsequent G1 phase.10–12 Furthermore, overduplication of the centrosome is a cause of aneuploidy, a hallmark of cancer.13 Thus understanding the mechanisms targeting proteins to the centrosome to regulate both centrosome duplication and DNA replication is of major importance.
Recently, we described the modular motifs responsible for localization of cyclins A and E on the centrosome.4,5 These CLS (centrosomal localization sequence) motifs are sufficient to target proteins to the centrosome, and expression of either CLS as a GFP-tagged protein displaces both endogenous cyclins from the centrosome and inhibits DNA synthesis.4,5 The CLS of either cyclin is responsible for the recruitment of the DNA replication factor MCM5 to the centrosome via a domain within MCM5 distinct from that involved in DNA replication, and Orc1 interaction with cyclin A also depends on the CLS.7,8 Moreover, expression of Orc1 or MCM5 or of just the MCM5 CLS-interacting domain inhibits centrosome overduplication in mammalian cells.7,9 These results highlight the importance of communication between the nucleus and the centrosome that appears to be dependent on a functional CLS. The importance of the CLS has encouraged further biochemical and structural analysis of the CLS of cyclin A, which was previously studied only in a cyclin A fragment (aa 201–301).5 Although this motif does not display a true consensus sequence with the cyclin E CLS, similarities between the two cyclins in sequence/structure as well as in function are evident. Here we discuss the structural similarities and differences between the two cyclin CLSs and describe a new CLS mutant in full-length cyclin A-deficient in all CLS functions that may be useful for future investigations.
Structural Similarity of the CLS in Cyclin A and Cyclin E
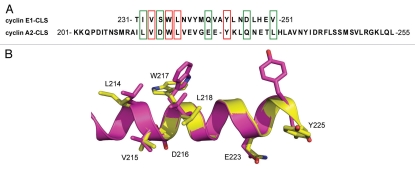
The cyclin E CLS has previously been located in the α1′ helix, at the beginning of the C-terminal cyclin box fold (aa residues 231–251),4 whereas the cyclin A CLS was recently found within the α1 helix, at the beginning of the N-terminal cyclin box fold (aa residues 201–255).5 Remarkably, although these motifs are located in different parts of the cyclin box folds, they share both a similar structure and sequence. Figure 1 shows the sequence alignment and superimposition of the alpha-helical regions of the CLSs from cyclins E1 and A2. Several of the residues are identical in cyclin E1 and cyclin A2, and the remaining residues are highly similar in size and hydrophilicity (Fig. 1A). In particular, a five amino acid sequence directly following the MRAIL motif in cyclin A is highly conserved in cyclin E (red and green boxes) and includes some of the cyclin E1 residues S234 W235 N237 Q241 (SWNQ) essential for CLS function (reviewed in ref. 4 and see below). Also, four other residues that are evenly distributed along the second half of the cyclin E CLS have similar counterparts in cyclin A (boxes). Superimposition of the cyclins E and A CLS structures also shows similarities between the two motifs, particularly around the beginning of the cyclin E CLS (Fig. 1B). Notably, some of these residues are exposed at the surface of the CLS (e.g., D216/S234, V219/N237, E223/Q241, Q228/E247) and may contribute to interaction with CLS binding partners, while other residues may be critical for maintenance of the CLS structure (e.g., V215/V234, W217/W235, L218/L236, Y225/Y244).
Figure 1.
The CLS domains of cyclins A and E are similar. (A) Amino acid sequence alignment for cyclin E1 and cyclin A2 CLSs. Identical residues are boxed in red and similar residues are boxed in green. (B) Ribbon diagram showing superimposition of cyclins E (yellow, PDB ID 1W98) and A (magenta, PDB ID 1VIN) CLSs in the conserved region. Identical and similar residues are represented as stick models.
CLS Loss of Function Mutations in Full Length Cyclin A
We previously showed that mutation of four residues exposed at the surface of the cyclin A CLS to arginine (IEEK-R mutant) abolished localization of the expressed cyclin A CLS domain (aa 201–255 or aa 201–301) on centrosomes.5 These mutations also inhibited all other known phenotypes associated with cyclin A CLS expression in cells: displacement of endogenous cyclins from the centrosome and inhibition of DNA replication.5 However, mutation of these four residues within full length cyclin A did not efficiently affect cyclin A localization (unpublished data). In contrast, our group previously reported that mutation of four residues in the cyclin E CLS domain (SWNQ-A mutant) was sufficient to disrupt the centrosomal localization of both the CLS domain (aa 231–251) and full-length cyclin E.4 Therefore, to rigorously compare the cyclins A and E CLSs, we investigated other mutations within the cyclin A CLS that could potentially disrupt centrosomal localization of the full-length protein. By aligning the cyclins A and E CLSs, it is evident that the mutation in cyclin A equivalent to the cyclin E S234 W235 N237 Q241 (SWNQ-A) CLS mutant would be D216 W217 V219 E223 (cyclin A-DWVE-A) (Fig. 2A and residues boxed in red). CLS constructs carrying these mutations did not localize on centrosomes with high efficiency (<10% compared to 82% for the wild-type CLS, aa 201–301) (Fig. 2B and upper parts). Importantly, even in full-length cyclin A, these mutations almost completely suppressed centrosomal localization (<10% compared to 95% for the wild-type full length cyclin A) (Fig. 2B and lower parts). Thus the cyclin A equivalent of the cyclin E SWNQ-A mutant also exhibits the same loss-of-function for centrosomal localization.
Figure 2.
Mutation of equivalent CLS residues in cyclins A and E inhibits centrosomal localization. (A) Amino acid sequence alignment of the cyclin E1 and cyclin A2 CLSs in the conserved regions. Conserved residues are boxed in blue. Residues that lead to loss of CLS function when mutated into alanine are boxed in red. In the text, the resulting mutants are named SWNQ-A for cyclin E and DWVE-A for cyclin A. (B) Xenopus S3 cells were transfected with DNA constructs encoding either EGFP-fused-cyclin A CLS wild-type (Wt) domain (aa 201–255) or with a mutant CLS domain (DWVE-A) (upper parts). Lower parts, cells transfected with EGFP-fused-full-length (Fl) cyclin A with a wild-type CLS (wt) or mutant CLS (DWVE-A). Cells were methanol-fixed and stained for γ-tubulin as a marker for centrosomes, and EGFP was monitored by direct fluorescence. Arrows indicate the position of centrosomes. Insets: magnification of the centrosomal region in the merged image. Scale bars, 10 µm.
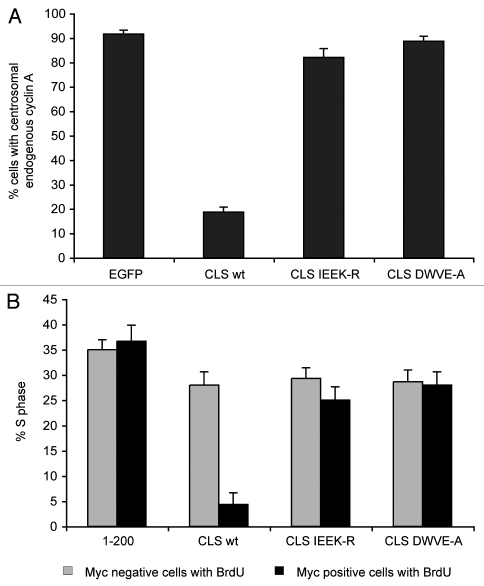
Expression of the cyclin E CLS wild type, but not the SWNQ-A mutant, displaces both endogenous cyclins E and A from centrosomes, indicating that a similar CLS-dependent mechanism is responsible for localization of both cyclins.4 Similar results were obtained by expressing the wild-type and mutant (IEEK-R) cyclin A CLSs in cells (reviewed in ref. 5 and Fig. 3A). Here we show that the cyclin A CLS DWVE-A mutant, equivalent to the cyclin E CLS SWNQ-A mutant, does not displace either endogenous cyclin from the centrosome (Fig. 3A). We also reported previously that expression of the cyclin E CLS wild type, but not the SWNQ-A mutant, inhibited DNA replication by arresting the cells at the G1/S boundary. This arrest was a consequence of the failure to load Cdc45 onto origins of replication.6 Similar results were later obtained when expressing the cyclin A CLS wild-type or mutant (IEEK-R) domains in cells (reviewed in ref. 5 and Fig. 3B). We show here that the Myc-tagged-cyclin A CLS DWVE-A mutant also has no significant effect on DNA replication and S-phase progression (Fig. 3B). Thus the cyclin A CLS DWVE-A mutant, like the cyclin E SWNQ-A mutant, is a loss-of-function mutant for all known phenotypes associated with expression of the CLS.
Figure 3.
The mutated cyclin A CLS DWVE-A does not displace endogenous cyclin A from centrosomes or inhibit DNA replication. (A) CHO-K1 cells were transfected with DNA constructs encoding the EGFP- cyclin A CLS domain wt or carrying CLS loss-of-function mutations (IEEK-R and DWVE-A). The vector encoding EGFP alone was used as a negative control. Centrosomal localization of endogenous cyclin A in cells expressing these constructs was monitored and compared with the localization of endogenous cyclin A in cells expressing EGFP alone. Bars indicate mean ± SEM (n = 3). (B) CHO-K1 cells were transfected with DNA constructs encoding the Myc-cyclin A CLS domain wt or carrying CLS loss-of-function mutants (IEEK-R and DWVE-A). BrdU incorporation into DNA was monitored as a marker of S phase and compared with BrdU incorporation in cells expressing a non-centrosomally-localized cyclin A fragment (aa 1–200). Black bars represent BrdU incorporation in cells expressing the Myc-construct (Myc positive cells), whereas grey bars represent BrdU incorporation in cells not expressing Myc-constructs (Myc negative cells) from the same field. Bars indicate mean ± SEM (n = 3).
Differential Contributions of the Cyclin A and E CLSs to Cdk Binding
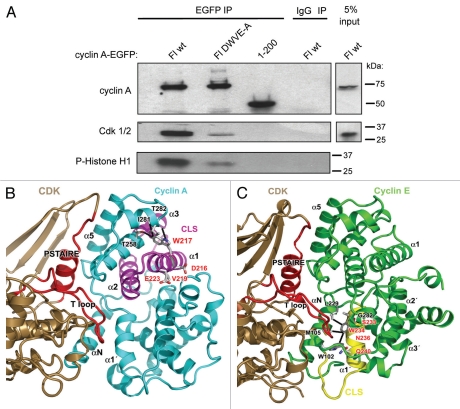
Another important characteristic of the cyclin E SWNQ-A mutant is the retention of nearly wild-type affinity for Cdk2.4 Similarly, the IEEK-R mutation in full-length cyclin A2 did not efficiently disrupt binding to Cdk or inhibit Cdk activity (data not shown). In order to determine whether the effects of the DWVE-A mutation in cyclin A were independent of Cdk binding, the cyclin A-EGFP constructs described above were expressed in Chinese Hamster Ovary (CHO-K1) cells, then immunoprecipitated through their EGFP tag and examined for Cdk binding by co-immunoprecipitation (Fig. 4A and upper and middle parts respectively). In addition, the precipitates were analyzed for histone H1 kinase activity (lower part). Unlike the IEEK-R mutant, the DWVE-A mutant immunoprecipitated far less Cdk1/2 than the WT protein (Fig. 4A). The lower level of immunoprecipitated Cdk was also consistent with the lower level of histone H1 phosphorylation observed in the kinase assay.
Figure 4.
Mutations within the cyclin A CLS reduce Cdk binding. (A) CHO-K1 cells were transfected with DNA constructs encoding EGFP-fused cyclin A Fl wt, cyclin A Fl DWVE-A or cyclin A aa 1–200 (control without a CLS). The resulting EGFP-proteins were immunoprecipitated with anti EGFP antibodies, and the efficiency of immunoprecipitation monitored by western blotting using anti-cyclin A antibodies (upper part). The binding to Cdk in the immunoprecipitate was monitored by western blot with PSTAIRE antibodies (middle part), whereas Cdk kinase activity was assayed in the immune-complex using histone H1 as a substrate (lower part, autoradiograph). (B) Ribbon diagram showing the interaction between Cdk2 (brown) and cyclin A (cyan) in the cyclin A-Cdk2 complex (PDB 1JSU). The site of the mutated residues within the cyclin A CLS region (magenta) and the major contact regions of the Cdk PSTAIRE helix and T loop are shown in red. Mutation in the cyclin A CLS, and particularly the substitution of W217 to alanine may affect the conformation of the surrounding α-helices (α3 and α4) that have multiple direct contacts (T258, I281, T282) with this residue and indirectly participate in the interaction with Cdk2 through helix α5. (C) Ribbon diagram showing the interaction between Cdk2 (brown) and cyclin E (green) in the cyclin E-Cdk2 complex (PDB 1W98). The site of the cyclin E CLS region (yellow) containing the SWNQ mutated residues, and the Cdk PSTAIRE helix and T loop are shown in red. This view highlights the proximity of the SWNQ residues to the N-helix, where cyclin E W234 contacts L229, M105 and W102, and the tip of the T loop, but does not contact the PSTAIRE helix of Cdk2.
In order to understand why the DWVE-A mutant has a greater impact on Cdk binding than the IEEK-R mutant, we examined the reported structures of the Cdk2-cyclin E1 complex (PDB ID 1w98),14 and the Cdk2-Cyclin A-p27Kip1 complex (PDB ID 1jsu).15 Three of the substituted residues (Asp216, Val219 and Glu223) lie on the surface of cyclin A2 and mutating them to alanine would not be expected to have an impact on the structure of cyclin A2 (Fig. 4B). Furthermore, they are unlikely to have any direct interaction with Cdk1/2 because they are located on the opposite face of the cyclin-box fold from the Cdk1/2 binding site. However, although Trp217 in α helix 1 does not interact directly with Cdk1/2, a substitution to alanine would be predicted to have an indirect effect on the structure of the N-terminal cyclin-box fold. Trp217 forms hydrogen bonds with Thr282 and has van der Waals interactions with Thr258 and Ile281, which are all located in α helix 3 and would be lost with the alanine substitution. The loss of these interactions could destabilize the cyclin-box fold and thus likely decrease the binding affinity of Cdk to this domain of the cyclin. In contrast, the residues SWNQ-A in cyclin E that do not significantly alter CDK binding or activity are located in the CBOX2 region of the cyclin and are not expected to affect this interaction (Fig. 4C).
We previously showed that the cyclin A CLS is largely contained within the α1 helix of the N-terminal cyclin box fold (CBOX1), whereas the cyclin E CLS is contained in the opposite part of the cyclin molecule, within the α1′ helix in the C-terminal cyclin box fold (CBOX2).4,5 Despite this difference, the cyclin A CLS, like the cyclin E CLS, can function as a modular domain to localize the protein to the centrosome in a Cdk2-independent manner. However, the studies here suggest that the binding of Cdk1/2 to the cyclins is much more sensitive to mutations in the CLS located in the CBOX1 domain (cyclin A) than the CBOX2 domain (cyclin E).
By visual inspection of the crystal structures of cyclins E and A complexed with Cdk2 (Fig. 4B and C), it is evident that the cyclin E CLS is located closer to the activation loop (T loop) of Cdk2 than the cyclin A CLS. It might therefore have been expected that mutation of the cyclin E CLS would affect Cdk binding or activity, whereas only the cyclin A CLS mutations were found to significantly affect the cyclin-Cdk interaction. The binding of cyclins to Cdks involves an extensive interface that includes both the cyclin box fold as well as the N-terminal helix region of the cyclins.14 Each cyclin box is stabilized by a hydrophobic core formed by residues in alpha helices 1, 2 and 3 in CBOX1 and 1′, 2′ and 3′ in CBOX2.16 The CBOX1 region of cyclin A is more highly conserved among cyclins than CBOX2, particularly in those regions that contact the Cdk.14,17 The primary regions of the Cdk that contact CBOX1 are the PSTAIRE helix (residues 42–57) (shown in red in Fig. 4B and C), the T loop (residues 146–166) (also shown in red in Fig. 4B and C) and alpha helix 5. Cyclin binding induces several specific conformational changes in the T-loop and PSTAIRE region of the kinase that enhance enzymatic activity.14,16,18
Visual inspection of protein-protein interfaces does not necessarily indicate the magnitude of the interface contribution to binding affinity for any given region, but substitution of residues in the PSTAIRE and T loop of Cdk2 abolishes cyclin binding, which indicates that the interactions with CBOX1 in the formation of the cyclin A-Cdk2 complex are essential.19,20 Furthermore, substitution of the arginine in the MRAIL sequence in cyclin A (a subdomain of the CLS) abolishes binding to Cdk2, which further indicates that the CBOX1 interaction is critical.21 These considerations may explain why the Trp217 substitution drastically decreases Cdk2 binding, as this residue occupies an important position in the core of CBOX1 and likely contributes to its overall stability. The observation that the Cyclin E-Cdk2 interaction is not disrupted by mutations to the CLS is consistent with these CLS substitutions only altering interactions with the Cyclin N-helix that do not prevent the N-helix from binding to the CDK (Fig. 4C). Furthermore, there are fewer additional contacts made by Cyclin E to the CDK, and these are much less important for the overall affinity of the interaction than the contacts with the PSTAIRE helix and the T loop.
Studies of cyclin-Cdk structures and Cdk inhibitors also provide a coherent explanation for the differential effect of cyclin box perturbations within the CLS region on Cdk binding seen for cyclins A2 and E1. Protein inhibitors of Cdk kinase activity alter the structure of the residues at the cyclin-Cdk interface. In a previous study we showed that the the MRAIL region which the Cdk inhibitor p27KIP1 binds to is contained within the cyclin A CLS,5 and overexpression of p27KIP1 removes cyclin A, but not cyclin E, from the centrosome. As another example, the structure of the p18-Cdk6-cyclin K ternary complex shows that the interaction between p18 and cyclin K occurs on the side of the cyclin opposed to the one contacting the Cdk6 catalytic cleft. Therefore, p18 does not directly block the cyclin K-Cdk6-interacting region, but the binding of p18 perturbs the structure of the cyclin-Cdk interface in precisely the same region of the cyclin fold that would be impacted by the DWVE-A cyclin A CLS mutant.22 In a related system, the cyclin-Cdk Pho85-Pho80 complex,23 the cyclin contains only one cyclin box, which corresponds to CBOX1 that contains the cyclin A2 CLS. These distinct cyclin-Cdk complexes also point to the dominance of the CBOX1 region in cyclin-Cdk interaction. The large defect in Cdk binding observed for the DWVE-A compared to the SWNQ-A mutant is consistent with this accumulated evidence that in cyclin-Cdk complexes the CBOX1 region is considerably more important for Cdk binding affinity than the CBOX2 region.
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and by National Institutes of Health grant GM79154 (to M.E.A.C.).
References
- 1.Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 2.Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 3.Woo RA, Poon RY. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle. 2003;2:316–324. [PubMed] [Google Scholar]
- 4.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 5.Pascreau G, Eckerdt F, Churchill ME, Maller JL. Discovery of a distinct domain in cyclin A sufficient for centrosomal localization independently of Cdk binding. Proc Natl Acad Sci USA. 2010;107:2932–2937. doi: 10.1073/pnas.0914874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson RL, Maller JL. Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis. Curr Biol. 2010;20:856–860. doi: 10.1016/j.cub.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson RL, Maller JL. Cyclin E-dependent localization of MCM5 regulates centrosome duplication. J Cell Sci. 2008;121:3224–3232. doi: 10.1242/jcs.034702. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson RL, Pascreau G, Maller JL. The cyclin A centrosomal localization sequence recruits MCM5 and Orc1 to regulate centrosome reduplication. J Cell Sci. 2010;123:2743–2749. doi: 10.1242/jcs.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemerly AS, Prasanth SG, Siddiqui K, Stillman B. Orc1 controls centriole and centrosome copy number in human cells. Science. 2009;323:789–793. doi: 10.1126/science.1166745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- 11.Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- 13.D'Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 14.Honda R, Lowe ED, Dubinina E, Skamnaki V, Cook A, Brown NR, et al. The structure of cyclin E1/CDK2: implications for CDK2 activation and CDK2-independent roles. EMBO J. 2005;24:452–463. doi: 10.1038/sj.emboj.7600554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 16.Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown NR, Noble ME, Endicott JA, Garman EF, Wakatsuki S, Mitchell E, et al. The crystal structure of cyclin A. Structure. 1995;3:1235–1247. doi: 10.1016/s0969-2126(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 18.Heitz F, Morris MC, Fesquet D, Cavadore JC, Doree M, Divita G. Interactions of cyclins with cyclin-dependent kinases: A common interactive mechanism. Biochemistry. 1997;36:4995–5003. doi: 10.1021/bi962349y. [DOI] [PubMed] [Google Scholar]
- 19.Ducommun B, Brambilla P, Felix MA, Franza BR, Jr, Karsenti E, Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991;10:3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcote MJ, Knighton DR, Basi G, Sowadski JM, Brambilla P, Draetta G, et al. A three-dimensional model of the Cdc2 protein kinase: localization of cyclin- and Suc1-binding regions and phosphorylation sites. Mol Cell Biol. 1993;13:5122–5131. doi: 10.1128/mcb.13.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi H, Stewart E, Poon R, Adamczewski JP, Gannon J, Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell. 1992;3:1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeffrey PD, Tong L, Pavletich NP. Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 2000;14:3115–3125. doi: 10.1101/gad.851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang K, Ferrin-O'Connell I, Zhang W, Leonard GA, O'Shea EK, Quiocho FA. Structure of the Pho85-Pho80 CDK-cyclin complex of the phosphate-responsive signal transduction pathway. Mol Cell. 2007;28:614–623. doi: 10.1016/j.molcel.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]