Abstract
Background
The PREDICT-HD study seeks to identify clinical and biological markers of Huntington’s disease in premanifest individuals who have undergone predictive genetic testing.
Methods
We compared baseline motor data between gene-expansion carriers (cases) and non gene-expansion carriers (controls) using T-tests and Chi-Square. Cases were categorized as near, mid or far from diagnosis using a CAG-based formula. Striatal volumes were calculated using volumetric MRI measurements. Multiple linear regression associated total motor score, motor domains and individual motor items with estimated diagnosis and striatal volumes.
Results
Elevated total motor scores at baseline were associated with higher genetic probability of disease diagnosis in the near future (partial R2 0.14, p<0.0001) and smaller striatal volumes (partial R2 0.15, p<0.0001). Nearly all motor domain scores showed greater abnormality with increasing proximity to diagnosis, although bradykinesia and chorea were most highly associated with diagnostic immediacy. Among individual motor items, worse scores on finger tapping, tandem gait, Luria, saccade initiation, and chorea show unique association with diagnosis probability.
Conclusions
Even in this premanifest population subtle motor abnormalities were associated with a higher probability of disease diagnosis and smaller striatal volumes. Longitudinal assessment will help inform whether motor items will be useful measures in preventive clinical trials.
Keywords: Huntington’s disease, at-risk, UHDRS
Introduction
Huntington’s disease (HD) is typically an adult-onset, progressive and fatal neurodegenerative disease characterized by the clinical triad of a movement disorder, cognitive decline, and behavioral disturbances. It is autosomal dominant, caused by an expansion of a trinucleotide cytosine-adenine-guanine (CAG) in the 5′-translated region of the IT-15 gene on the short arm of chromosome 4.1 The length of CAG expansion is inversely correlated with age at diagnosis.1, 2 However, the precise point of disease diagnosis is poorly characterized, with clinical abnormalities emerging gradually over many years during a “premanifest” prodromal phase. 3, 7–10 Increasing evidence suggests that neuropathological changes may occur many years prior to the development of clinical changes.4–6 Additionally, the degree of striatal atrophy correlates not only with disease severity in manifest patients11, but also with estimated years to diagnosis in premanifest populations.12–14
The Neurobiological Predictors of Huntington’s Disease (PREDICT-HD) study is designed to prospectively characterize refined clinical, neurobiological and neurobehavioral markers of Huntington’s disease prior to the point of traditional clinical diagnosis in a population known to carry the HD CAG expansion15. Findings from the PREDICT study will identify critically important candidate outcome measures used in clinical trials aimed at delaying the diagnosis of illness. We have recently shown that most clinical indicators in the PREDICT cohort, including motor and neuroimaging markers, show subtle changes one to two decades prior to expected disease diagnosis.16 The present analysis of the PREDICT cohort details the relationship of motor function, probability of disease diagnosis in the near future (based on CAG length and age) and striatal volumes.
Methods
Participant Eligibility
Participants were recruited from 30 sites in the United States, Canada, Australia and Europe. All participants were required to have voluntarily undergone genetic testing for the HD CAG expansion independent from the study. Institutional review boards at each participating site approved the study and each participant signed an informed consent. The Unified Huntington Disease Rating Scale (UHDRS) was used to determine whether each participant met criteria for a diagnosis of HD and only subjects considered premanifest by virtue of scoring a 3 or less on question 17 of the UHDRS (diagnostic confidence) were included in this paper. The diagnostic confidence question asks investigators to rate how confident they are that an individual at-risk for HD meets the definition of the unequivocal presence of an otherwise unexplained movement disorder on a scale from 0 (no abnormalities) to 4 (unequivocal signs of HD, ≥99% confident). Control subjects were individuals who had tested negative for the HD CAG expansion, but were offspring of a parent with HD. Further details of the study have been previously reported.15, 16
Clinical Assessments
All participants underwent detailed motor, cognitive and psychiatric evaluations at baseline.15 Neither participants nor raters were systematically blinded to gene status. The Motor section of the UHDRS was used to assess motor features at baseline and annually thereafter.17 The motor UHDRS is a standardized assessment consisting of 31 items rated on a scale from 0 to 4 with a score of 0 indicating no abnormalities and 4 indicating severe impairment. The maximum possible total score is 124. Based on a factor analysis of the total UHDRS in patients with manifest HD, the motor items have previously been grouped into five factors; oculomotor, bradykinesia, rigidity, dystonia, and chorea.18
Probability of Diagnosis
Estimated years to diagnosis were calculated using a CAG and age based predictive model derived by Langbehn et al. and based on an analysis of 2913 individuals from 40 centers worldwide.19 Consistent with previous reports involving the PREDICT cohort, cases were considered far from diagnosis if their estimated diagnosis was greater than 15 years, mid to diagnosis if their estimated diagnosis was 9–15 years, and near to diagnosis if their estimated diagnosis was less than 9 years. These definitions correspond roughly to tertiles of risk among our participants. The survival formula of Langbehn et al. can also be transformed to a probability of diagnosis within a given future time, based on a participant’s CAG expansion length and current age.16
Magnetic Resonance Imaging
All scans for this project were obtained using a standard multi-mode protocol that included an axial 3D volumetric spoiled gradient echo series (~1×1×1.5 mm voxels) and a dual echo PDT2 (~1×1×3 mm voxels) series. All sites used a General Electric 1.5 Tesla scanner (with the exception of two sites: one using a 1.5 Tesla Siemens and one using a 1.5 Tesla Phillips scanner). Striatal volumes were expressed as percentage of total intracranial volume to control for variation in size.
To obtain measures of brain structure, first an approximate rough brain tissue region was obtained using the 3dskull from the AFNI tool suite20. Spatial intensity inhomogeneity correction fields were estimated over the brain tissue region and applied using tools described in Styner et al.21 for each modality. An automated procedure rigidly aligned and resampled the 3 modes of each dataset into a 1mm3 isotropic voxel lattice where a line passing through the anterior commissure (AC) and posterior commissure (PC) is parallel to the horizontal voxel lattice, the inter-hemispheric fissure is aligned with vertical voxel lattice, and the AC point is located at the center of the voxel lattice.
Tissue classification22 is performed using the BRAINS software suite23. Exemplars (2×2×2mm plugs) for grey matter, white matter and cerebrospinal fluid (CSF) are selected by randomly sampling the images and keeping those plugs with low variance under the assumption that they represent a single tissue type. The selected plugs are then assigned to a compartment using k-means clustering. The labeled plugs are then used to define discriminant functions. The discriminant functions are used to classify the multi-modal data, producing an image where each voxel location is labeled with a code representing the grey, white, and CSF composition. The intracranial volume (ICV) measure is composed of all tissue (grey and white matter) and CSF within the cranium, from just under the dura mater and below. Subcortical measures of the caudate, putamen, and thalamus are calculated using the automated neural network segmentation24 tool from the BRAINS package.
The results of this procedure were visually inspected to verify that each stage was completed successfully. Greater than 90% of the scans analyzed passed all stages successfully. Scan failure was not significantly predicted by any of the variables (i.e., HD gene-expansion status or motor severity) that are the subject of this report.
Statistical Analyses
Comparisons between cases and controls were performed using T-tests and Chi-Square. All analyses were adjusted for age and gender. Linear regression models assessed the relationship between either total motor scores, motor domain scores or individual motor items and estimated diagnosis probability or striatal volume. We used diagnosis probability within 5 years rather than estimated years to diagnosis because diagnosis probability has consistently demonstrated approximately linear relationships whereas relationships involving estimated years to diagnosis are generally non-linear and require more complicated statistical models16. To control for starting morphological variability, we used the ratio of striatal volume (caudate plus putamen) to total intracranial volume in analyses involving those measures. Age and gender-adjusted associations with individual motor score components were calculated as partial R2 statistics, derived from the corresponding regression models. We constructed multivariate regression models of the most important motor exam predictors of both diagnosis probability and striatal volume by using backwards selection techniques. Finally, Mantel-Haenszel Chi Square tests were used to assess monotonic trends between motor scores and proximity-to-diagnosis classification groups (far, mid, near). When relevant data were missing, participants were excluded from the analysis (7% of observations). An alpha level of 5% was used for significance testing.
Results
Baseline Characteristics
From October 2002 until October 2007, 929 participants were enrolled and had relevant baseline data available. Of these participants, 733 (79%) were expansion positive (cases), and 196 (21%) were expansion negative (controls). The majority (82%) of the cases were deemed to be either normal or have non-specific motor signs (diagnostic confidence level 0 or 1), 12% had diagnostic confidence level 2, and 6% had diagnostic confidence level 3 on examination at baseline. An additional 30 cases were excluded from analysis because of uncertain specific CAG length information at the time of analysis. For the 733 cases, 277 (38%) were predicted far, 252 (37%) mid, and 184 (25%) being near to estimated age of diagnosis. At the time of data analysis, MRI data were available on 500 cases and 150 controls.
Table 1 summarizes the baseline demographics, motor scores, probability of disease diagnosis and striatal volumes of cases and controls. In addition, demographic data separated by estimated diagnosis categories are given. Cases were slightly younger, had an older age of parental disease diagnosis, had worse total motor scores and worse motor domain scores and smaller striatal volumes than controls (p<0.0001 for all). Worse total motor scores (p<0.0001), worse motor domain scores (p≤0.001 for all domains, except rigidity) and greater striatal atrophy (p<0.001) were associated with closer proximity to age of diagnosis in cases. Younger parental age of diagnosis (p=0.015) and male gender (p = .04) were also associated with closer proximity to age of diagnosis. As a consequence of the group definitions, older age (p<0.0001) and longer CAG repeat length (p<0.0001) were associated with closer proximity to diagnosis. Mean estimated probability of disease diagnosis ranged from 5% in the far from diagnosis group to 20% in the mid to diagnosis group and 46% in the near to diagnosis group.
Table 1.
Baseline Characteristics of the PREDICT-HD Cohort by Gene Status and Proximity to Diagnosis.
| Variable | Controls (n=196) | Cases | p-value for cases vs. controls | p-value for trend for cases by far, mid and near diagnosis categories | |||
|---|---|---|---|---|---|---|---|
| Far (n=277) | Mid (n=272) | Near (n=184) | All (n=733) | ||||
| Male Gender (%) | 62 (32%) | 90 (32%) | 103(38.%) | 77 (42%) | 270 (37%) | 0.24 | 0.04 |
| Age (sd) | 43.91 (11.37) | 36.85 (8.02) | 42.30 (9.94) | 44.74 (10.24) | 40.85 (9.88) | 0.0008 | <0.0001 |
| CAG repeat length (sd) | 19.98 (3.52) | 41.14 (1.61) | 42.68 (2.18) | 44.34 (3.09) | 42.52 (2.58) | <0.0001 | <0.0001 |
| Parental age of diagnosis (sd) | 44.03 (11.18) | 50.21 (10.21) | 49.40 (10.8) | 47.21 (11.25) | 49.15 (10.75) | <0.0001 | 0.015 |
| UHDRS total motor (sd) | 2.41 (3.06) | 3.47 (3.79) | 4.72 (4.79) | 7.80 (6.74) | 5.02 (5.31) | <0.0001 | <0.0001 |
| UHDRS chorea* (sd) | 0.27 (0.77) | 0.62 (1.26) | 0.92 (1.57) | 1.59 (2.30) | 0.97 (1.73) | <0.0001 | <0.0001 |
| UHDRS dystonia* (sd) | 0.03 (0.19) | 0.06 (0.30) | 0.07 (0.34) | 0.22 (0.78) | 0.10 (0.48) | 0.0005 | 0.001 |
| UHDRS rigidity* (sd) | 0.25 (0.61) | 0.34 (0.70) | 0.32 (0.69) | 0.36 (0.78) | 0.34 (0.72) | 0.08 | 0.79 |
| UHDRS bradykinesia* (sd) | 1.24 (1.79) | 1.33 (1.76) | 2.03 (2.23) | 3.22 (3.02) | 2.07 (2.41) | <0.0001 | <0.0001 |
| UHDRS oculomotor* (sd) | 0.62 (1.28) | 1.12 (1.88) | 1.38 (2.11) | 2.41 (2.65) | 1.54 (2.24) | <0.0001 | <0.0001 |
| Total striatal volume† (sd) | 1.07 (0.10) | 1.02 (0.12) | 0.92 (0.13) | 0.78 (0.14) | 0.92 (0.16) | <0.0001 | <0.0001 |
| Probability of 5 year diagnosis (sd) | N/A | 0.05 (0.03) | 0.20 (0.07) | 0.45 (0.11) | 0.20 (0.17) | <0.0001 | <0.0001 |
Domains based on factors by Marder et al. (2000).(19)
Volumetric MRI presented as a % of intracranial volume, n=500 Cases (Far=174, Mid=172, Near=132) and n=149 Controls.
sd=standard deviation
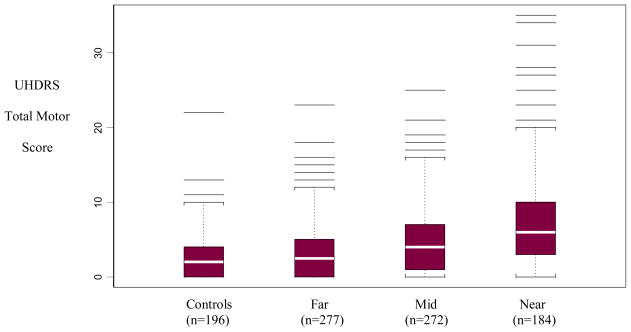
Figure 1 shows the box plots of total motor score for controls and cases by proximity to diagnosis categories (far, mid, near).
Figure 1.
Total Motor Scores for Controls and Cases by Proximity to Diagnosis*
*p<0.0001 for trend by proximity to diagnosis; Multiple horizontal lines are outlying individual values. The box represent the 25–75 percentile (“inter-quartile”) range. The white stripe in the middle of each box is the median.
Motor Assessments and Probability of Diagnosis
Table 2 shows the multivariate regression of total motor scores on probability of disease diagnosis. Worse total motor score at baseline was associated with a greater probability of disease diagnosis (p<0.0001) and accounted for 14% of the variance in the probability of diagnosis.
Table 2.
Relationship of Total Motor UHDRS to Probability of Diagnosis (n=732) and Striatal Volumes (n=500) in Cases*
| Variable | Parameter Estimate (Standard Error) | Partial R2 | p-value |
|---|---|---|---|
| Probability of diagnosis | 0.011 (0.001) | 0.15 | <0.0001 |
| Total striatal volume† | −0.010 (0.001) | 0.15 | <0.0001 |
Controlling for Age and Gender in the linear regression model
Percent of total of intracranial volume
When evaluating each motor domain individually, bradykinesia accounts for 14%, chorea for 6% and oculomotor for 7% of the variance in the probability of diagnosis (see Table 3). If, as an additional step, we simultaneously adjust for all motor domains, only worse scores on the bradykinesia and chorea domains are uniquely associated with a greater probability of diagnosis. Allowing these domains to compete in a backwards-selected reduced model, bradykinesia (p< .0001) and chorea (p = .0005) remain significantly associated with probability of diagnosis. Oculomotor signs were the third most important domain, but were not significant.
Table 3.
| Variable | Probability of diagnosis | Striatal Volume† | ||
|---|---|---|---|---|
| Partial R2ψ (n=732) | p-value | Partial R2ψ (n=490) | p-value | |
| Oculomotor | 0.07 | <0.0001 | 0.09 | <0.0001 |
| Bradykinesia | 0.14 | <0.0001 | 0.11 | <0.0001 |
| Rigidity | 0.01 | 0.05 | 0.01 | 0.02 |
| Dystonia | 0.02 | 0.0001 | 0.02 | 0.002 |
| Chorea | 0.06 | <0.0001 | 0.07 | <0.0001 |
Groupings based on factors by Marder et al. (2000).(19)
Controlling for age and gender in the model.
Percent of total of intracranial volume.
Considering variables one at a time controlling for age and gender.
Table 4 shows the simultaneous multivariate regression of all motor items on probability of diagnosis. Bradykinesia and oculomotor domains are broken into their component motor items. In this analysis where all motor items are included, worse scores on finger tapping, tandem gait, Luria, saccade initiation, and chorea show unique positive association with a greater probability of diagnosis. Controlling for all other motor signs, ocular pursuit had some negative association (p = .01) with diagnosis probability. No changes were noted with backwards variable selection.
Table 4.
Relationship of Individual UHDRS Motor Items and Probability of Diagnosis and Striatal Volumes in Cases.*
| Variable | Probability of diagnosis | Striatal Volumes† | |||||
|---|---|---|---|---|---|---|---|
| Parameter Estimate (× 1000) | SE (× 1000) | p-value | Parameter Estimate (×1000) | SE (×1000) | p-value | ||
| Intercept | −86.79 | 24.93 | 0.0005 | 1148.37 | 30.77 | <.0001 | |
| Age (years) | 6.07 | 0.56 | <.0001 | −50.00 | 0.68 | <.0001 | |
| Gender (f vs m) | −24.84 | 11.14 | 0.03 | 50.76 | 13.31 | 0.0002 | |
| Oculomotor | Pursuits | −18.55 | 7.48 | 0.01 | −3.06 | 8.65 | 0.72 |
| Saccade Initiation | 19.11 | 5.76 | 0.001 | 1.37 | 7.12 | 0.85 | |
| Saccade Velocity | 3.89 | 8.49 | 0.65 | −31.97 | 10.29 | 0.002 | |
| Bradykinesia | Dysarthria | 43.16 | 33.66 | 0.20 | 2.46 | 42.18 | 0.95 |
| Tongue | −15.16 | 18.21 | 0.41 | −32.57 | 19.19 | 0.09 | |
| Finger Taps | 35.06 | 7.23 | <.0001 | −21.66 | 9.07 | 0.02 | |
| RAM | 5.2 | 9.18 | 0.57 | −5.08 | 10.59 | 0.63 | |
| Luria | 21.69 | 7.19 | 0.003 | −5.85 | 9.01 | 0.52 | |
| Gait | 52.76 | 27.77 | 0.06 | −16.16 | 30.85 | 0.60 | |
| Tandem | 61.43 | 13.51 | <.0001 | −35.5 | 15.96 | 0.03 | |
| Pull Test | −21.32 | 11.64 | 0.07 | 16.41 | 14.16 | 0.25 | |
| Body Bradykinesia | −18.81 | 15.53 | 0.23 | 20.42 | 20.02 | 0.31 | |
| Rigidity | 5.08 | 7.68 | 0.51 | −9.01 | 8.68 | 0.30 | |
| Dystonia | 10.32 | 12.06 | 0.39 | −5.74 | 14.05 | 0.68 | |
| Chorea | 9.83 | 3.43 | 0.004 | −9.83 | 3.88 | 0.01 | |
Controlling for age and gender
Percent of total of intracranial volume
Motor Assessments and Striatal Volume
Table 2 shows the regression of total motor scores on striatal volumes. Worse total motor score at baseline was associated with smaller striatal volume (p<0.0001) and accounted for 15% of the variance in striatal volume.
Table 3 shows the regressions of individual motor domains on striatal volume. Similar to the analysis of probability of diagnosis, worse scores on the bradykinesia and chorea domains were associated with smaller striatal volumes, individually accounting for 11% (bradykinesia) and 7% (chorea) of the variance. Oculomotor abnormalities were more closely associated with smaller striatal volumes than probability of diagnosis and accounted for 9% of variance. These domain-striatum associations were unchanged when a multivariate model was chosen by backward selection. Worse scores on oculomotor (p=0.005), bradykinesia (p<0.0001) and chorea (p=0.004) domains were uniquely associated with smaller striatal volumes.
Table 4 shows the simultaneous multivariate regression of the motor items on striatal volume where the bradykinesia and oculomotor domains are broken into their component motor items. In this analysis, worse scores on saccade velocity, finger tapping, tandem gait and chorea are associated with smaller striatal volumes. The reduced model is similar to the full model with only tongue protrusion additionally emerging as potentially significant (p = .04), in addition to saccade velocity (p=0.0004), finger tapping (p=0.001), tandem gait (p=0.02) and chorea (p=0.003).
Discussion
In this cross sectional analysis of premanifest HD CAG-expansion-positive participants and expansion-negative controls enrolled in the PREDICT-HD study, total motor ratings distinguished cases from controls. This is despite only slight abnormalities detected on examination in cases (mean total motor of 4.98 +/−5.23 out of a total possible score of 124). These differences appear to be driven largely by the group that was near to their estimated diagnosis, with the vast majority of other cases having normal to near normal motor examinations. These findings confirm previous smaller studies that have shown that subtle motor abnormalities distinguish expansion positive premanifest HD individuals from controls.7, 25–27
Cases with closer estimated proximity to diagnosis had worse total motor scores and worse scores on the motor domains than individuals further from estimated diagnosis. This was most apparent for total motor scores, the chorea domain, the bradykinesia domain and the oculomotor domain. Consistent with our findings is evidence suggesting that chorea26 and quantitative measures of oculomotility may be sensitive in premanifest HD.9, 28, 29 Although dystonia was also associated with proximity to diagnosis, it was uncommon in all premanifest groups, consistent with the literature30. Although rigidity was not a sensitive measure of premanifest disease in our cohort, it may warrant further investigation in a young-diagnosis sample.31
Striatal volumes were smaller in cases than controls, with increasing atrophy associated with closer proximity to diagnosis. This confirms previous findings that striatal atrophy occurs early and may predate diagnosis by years.14, 32, 33
Similarly, among premanifest gene expansion carriers, higher (worse) total motor scores at baseline were predictive of a greater probability of diagnosis and smaller striatal volumes. Despite significant univariate relationships between all domains and proximity to diagnosis, however, only worse bradykinesia and chorea domain scores were uniquely associated with a greater probability of diagnosis and smaller striatal volumes. More specifically, chorea and greater impairment on the bradykinesia items of tongue protrusion, finger tapping, and tandem gait were separately associated with a greater probability of diagnosis in cases after accounting for all other aspects of the motor exam. Although the domain score for oculomotor items was not significant, other investigators have purported difficulty with clinically assessing ocular motility9, 28.
In regards to the association of individual motor items and striatal volumes, only finger tapping and tandem gait (amongst the Bradykinesia items), saccade velocity (amongst the Oculomotor items) and chorea scores were inversely associated with striatal volume. These findings suggest that striatal volumes and probability of diagnosis may reflect slightly different aspects of the motor exam.
Although the sample volunteered from among the population of gene-tested, premanifest persons at risk for HD, there may be a slight selection bias because persons at risk for a young age of diagnosis may be less able to participate. While it may be that younger individuals with earlier diagnosis may be systematically different than the population enrolled in PREDICT-HD, this cohort is similar to the population at-risk and will likely be representative of individuals enrolled in preventive trials. (It is unknown whether data from clinical studies and trials in adults will generalize to the juvenile form of HD.)
A limitation is a lack of prospective validation of diagnosis probabilities derived from the Langbehn et al. formula (or any other HD age-of onset formula). Continued longitudinal assessment of this cohort will ultimately address the validity of the estimated diagnosis formula and the relationship of motor abnormalities to actual disease diagnosis.
In this cross-sectional analysis of the PREDICT cohort, subtle motor abnormalities are present in premanifest HD gene expansion carriers. These motor abnormalities distinguish cases from controls and, among cases, are associated with closer proximity to estimated disease diagnosis and greater striatal atrophy. These findings suggest that the UHDRS motor examination may be a useful outcome measure in clinical trials aimed at delaying diagnosis of illness (i.e. disease onset) in premanifest HD. Continued longitudinal follow-up of the PREDICT cohort through disease diagnosis will be necessary to better determine which motor domains and items are sensitive to change over time and are predictive of actual diagnosis. Ultimately, multidimensional outcomes including motor, cognitive, behavioral and imaging domains may be necessary in preventive trials.
Acknowledgments
National Institutes of Health [NS40068]; CHDI, Inc.
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters (October 2007 data cut)
David Ames, MD, Edmond Chiu, MD, Phyllis Chua, MD, Olga Yastrubetskaya, PhD, Phillip Dingjan, M. Psych., Kristy Draper D. Psych, Nellie Georgiou-Karistianis PhD, Anita Goh, D. Psych, Angela Komiti and Christel Lemmon (The University of Melbourne, Kew, Victoria, Australia);
Henry Paulson, MD, Kimberly Bastic, BA, Rachel Conybeare, BS, Clare Humphreys, Peg Nopoulos, MD, Robert Rodnitzky, MD, Ergun Uc, MD, BA, Leigh Beglinger, PhD, Kevin Duff, PhD, Vincent A. Magnotta, PhD, Nicholas Doucette, BA, Sarah French, MA, Andrew Juhl, BS, Harisa Kuburas, BA, Ania Mikos, BA, Becky Reese, BS, Beth Turner and Sara Van Der Heiden, BA and (University of Iowa Hospitals and Clinics, Iowa City, Iowa, USA);
Lynn Raymond, MD, PhD, Joji Decolongon, MSC (University of British Columbia, Vancouver, British Columbia, Canada);
Adam Rosenblatt, MD, Christopher Ross, MD, PhD, Abhijit Agarwal, MBBS, MPH, Lisa Gourley, Barnett Shpritz, BS, MA, OD, Kristine Wajda, Arnold Bakker, MA and Robin Miller, MS (Johns Hopkins University, Baltimore, Maryland, USA);
William M. Mallonee, MD, Greg Suter, BA, David Palmer, MD and Judy Addison, MA (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Randi Jones, PhD, Joan Harrison, RN, J. Timothy Greenamyre, MD PhD and Claudia Testa MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA);
Elizabeth McCusker, MD, Jane Griffith, RN, Bernadette Bibb, PhD, Catherine Hayes, PhD and Kylie Richardson, B LIB (Westmead Hospital, Wentworthville, Australia);
Ali Samii, MD, Hillary Lipe, ARNP, Thomas Bird, MD, Rebecca Logsdon, PhD and Kurt Weaver, PhD, Katherine Field, BA (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Bernhard G. Landwehrmeyer, MD, Katrin Barth, Anke Niess, RN, Sonja Trautmann, Daniel Ecker, MD and Christine Held, RN (University of Ulm, Ulm, Germany);
Mark Guttman, MD, Sheryl Elliott, RN, Zelda Fonariov, MSW, Christine Giambattista, BSW, Sandra Russell, BSW, Jose Sebastian, MSW, Rustom Sethna, MD, Rosa Ip, Deanna Shaddick, Alanna Sheinberg, BA and Janice Stober, BA, BSW (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman, MD, Russell Carroll, Arik Johnson, MD and George Jackson, MD, PhD (University of California, Los Angeles Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, MD, PhD, Mira Guzijan, MAand Katherine Rose, BS, (University of California San Francisco, California, USA);
Tom Warner, MD, PhD, Stefan Kloppel, MD, Maggie Burrows, RN, BA, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, Sarah Tabrizi, MD PhD and Charlotte Golding, PhD (National Hospital for Neurology and Neurosurgery, London, UK);
Roger A Barker, BA, MBBS, MRCP, Sarah Mason, BSC and Emma Smith, BSC (Cambridge Centre for Brain Repair, Cambridge, UK);
Anne Rosser, MD, PhD, MRCP, Jenny Naji, PhD, BSC, Kathy Price, RN and Olivia Jane Handley, PhD, BS (Cardiff University, Cardiff, Wales, UK);
Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, Mary Lou Klimek, RN, BN, MA, and Dolen Kirstein, BSC (University of Calgary, Calgary, Alberta, Canada);
Diana Rosas, MD, MS, Melissa Bennett, Jay Frishman, CCRP, Yoshio Kaneko, BA, Talia Landau, BA, Martha Lausier, CNRN, Lindsay Muir, Lauren Murphy, BA, Anne Young, MD, PhD, Colleen Skeuse, BA, Natlie Balkema, BS, Wouter Hoogenboom, MSC, Catherine Leveroni, PhD, Janet Sherman, PhD and Alexandra Zaleta (Massachusetts General Hospital, Boston, Massachusetts, USA);
Peter Panegyres, MB, BS, PhD, Carmela Connor, BP, MP, DP, Mark Woodman BSC and Rachel Zombor (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia);
Joel Perlmutter, MD, Stacey Barton, MSW, LCSW and Melinda Kavanaugh, MSW, LCSW (Washington University, St. Louis, Missouri, USA);
Sheila A Simpson, MD, Gwen Keenan, MA, Alexandra Ure, BSC and Fiona Summers, DClinPsychol (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, MD, Rhona Macleod, RN, PhD, Andrea Sollom, MA and Elizabeth Howard, MD (University of Manchester, Manchester, UK)
Kimberly Quaid, PhD, Melissa Wesson, MS, Joanne Wojcieszek, MD and Xabier Beristain, MD (Indiana University School of Medicine, Indianapolis, IN);
Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, Jennifer Williamson, MS, Carol Moskowitz, MS, RNC and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA);
Peter Como, PhD, Amy Chesire, Charlyne Hickey, RN, MS, Carol Zimmerman, RN, Timothy Couniham, MD, Frederick Marshall, MD, Christina Burton, LPN and Mary Wodarski, BA (University of Rochester, Rochester, New York, USA);
Vicki Wheelock, MD, Terry Tempkin, RNC, MSN and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA);
Joseph Jankovic, MD, Christine Hunter, RN, CCRC, William Ondo, MD and Carrie Martin, LMSW-ACP (Baylor College of Medicine, Houston, Texas, USA);
Justo Garcia de Yebenes, MD, Monica Bascunana Garde, Marta Fatas, Christine Schwartz, Dr. Juan Fernandez Urdanibia and Dr. Cristina Gonzalez Gordaliza. (Hospital Ramon y Cajal, Madrid, Spain);
Lauren Seeberger, MD, Alan Diamond, DO, Deborah Judd, RN, Terri Lee Kasunic, RN, Lisa Mellick, Dawn Miracle, BS, MS, Sherrie Montellano, MA, Rajeev Kumar, MD and Jay Schneiders, PhD (Colorado Neurological Institute, Englewood, Colorado, USA);
Martha Nance, MD, Dawn Radtke, RN, Deanna Norberg, BA and David Tupper, PhD (Hennepin County Medical Center, Minneapolis, Minnesota, USA);
Wayne Martin, MD, Pamela King, BScN, RN, Marguerite Wieler, MSc, PT, Sheri Foster and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada);
Richard Dubinsky, MD, Carolyn Gray, RN, CCRC and Phillis Switzer (University of Kansas Medical Center, Kansas City, Kansas, USA).
Steering Committee
Jane Paulsen, PhD, Principal Investigator, Douglas Langbehn, MD, PhD and Hans Johnson, PhD (University of Iowa Hospitals and Clinics, Iowa City, IA); Elizabeth Aylward, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, WA); Kevin Biglan, MD, Karl Kieburtz, MD, David Oakes, PhD, Ira Shoulson, MD (University of Rochester, Rochester, NY); Mark Guttman, MD (The Centre for Addiction and Mental Health, University of Toronto, Markham, ON, Canada); Michael Hayden, MD, PhD (University of British Columbia, Vancouver, BC, Canada); Bernhard G. Landwehrmeyer, MD (University of Ulm, Ulm, Germany); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); Christopher Ross, MD, PhD (Johns Hopkins University, Baltimore MD); Julie Stout, PhD (Indiana University, Bloomington, IN, USA and Monash University, Victoria, Australia).
Study Coordination Center
Steve Blanchard, MSHA, Christine Anderson, BA, Ann Dudler, Elizabeth Penziner, MA, Anne Leserman, MSW, LISW, Bryan Ludwig, BA, Brenda McAreavy, Gerald Murray, PhD, Carissa Nehl, BS, Stacie Vik, BA, Chiachi Wang, MS, and Christine Werling (University of Iowa)
Clinical Trials Coordination Center
Keith Bourgeois, BS, Catherine Covert, MA, Susan Daigneault, Elaine Julian-Baros, CCRC, Kay Meyers, BS, Karen Rothenburgh, Beverly Olsen, BA, Constance Orme, BA, Tori Ross, MA, Joseph Weber, BS, and Hongwei Zhao, PhD (University of Rochester, Rochester, NY.)
Cognitive Coordination Center
Julie C. Stout, PhD, Sarah Queller, PhD, Shannon A. Johnson, PhD, J. Colin Campbell, BS, Eric Peters, BS, Noelle E. Carlozzi, PhD, and Terren Green, BA, Shelley N. Swain, MA, David Caughlin, BS, Bethany Ward-Bluhm, BS., Kathryn Whitlock, MS (Indiana University, Bloomington, Indiana, USA; Monash University, Victoria, Australia; and Dalhousie University, Halifax, Canada)
Recruitment and Retention Committee
Jane Paulson, PhD, Elizabeth Penziner, MA, Stacie Vik, BA, (University of Iowa, USA); Abhijit Agarwal, MBBS, MPH, Amanda Barnes, BS (Johns Hopkins University, USA); Greg Suter, BA (Hereditary Neurological Disease Center, USA); Randi Jones, PhD (Emory University, USA); Jane Griffith, RN (Westmead Hospital, AU); Hillary Lipe, ARNP (University of Washington, USA); Katrin Barth (University of Ulm, GE); Michelle Fox, MS (University of California, Los Angeles, USA); Mira Guzijan, MA, Andrea Zanko, MS (University of California San Francisco, USA); Jenny Naji, PhD (Cardiff University, UK); Rachel Zombor, MSW (Graylands, Selby-Lemnos & Special Care Health Services, AU); Melinda Kavanaugh (Washington University, USA); Amy Chesire, Elaine Julian-Baros, CCRC, Elise Kayson, MS, RNC (University of Rochester, USA); Terry Tempkin, RNC, MSN (University of California Davis, USA); Martha Nance, MD (Hennepin County Medical Center, USA); Kimberly Quaid, PhD (Indiana University, USA); and Julie Stout, PhD (Indiana University, Bloomington, IN, USA and Monash University, Victoria, Australia).
Event Monitoring Committee
Jane Paulsen, PhD William Coryell, MD (University of Iowa, USA); Christopher Ross, MD, PhD (Johns Hopkins University, Baltimore MD); Elise Kayson, MS, RNC, Aileen Shinaman, JD (University of Rochester, USA); Terry Tempkin, RNC, ANP (University of California Davis, USA); Martha Nance, MD (Hennepin County Medical Center, USA); Kimberly Quaid, PhD (Indiana University, USA); Julie Stout, PhD (Indiana University, Bloomington, IN, USA and Monash University, Victoria, Australia); and Cheryl Erwin, JD, PhD (McGovern Center for Health, Humanities and the Human Spirit, USA).
Footnotes
Disclosure: The authors report no conflicts of interest.
Documentation of author roles:
1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique
3. Manuscript: A. Writing the first draft, B. Review and Critique
Kevin M. Biglan, MD, MPH: 1C 2C 3A
Christopher A. Ross, MD, PhD: 1BC 2C 3B
Douglas R. Langbehn, MD, PhD: 1BC 2AB 3B
Elizabeth H. Aylward, PhD: 1BC 2C 3B
Julie C. Stout, PhD: 1BC 2C 3B
Sarah Queller, PhD: 1C 2C 3B
Noelle E. Carlozzi, PhD: 1C 2C 3B
Kevin Duff, PhD: 1C 2C 3B
Leigh J. Beglinger, PhD: 1C 2C 3B
Jane S. Paulsen, PhD: 1ABC 2AC 3B
PREDICT-HD Investigators of the Huntington Study Group: 1C 2C 3B
Statistical Analysis: Performed by Douglas Langbehn, MD, PhD at The University of Iowa
References
- 1.Duyao M, Ambrose C, Myers R, et al. Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nature genetics. 1993;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 2.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen JS, Zhao H, Stout JC, et al. Clinical markers of early disease in persons near onset of Huntington’s disease. Neurology. 2001;57(4):658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- 4.Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44(5):823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Tortosa E, MacDonald ME, Friend JC, et al. Quantitative neuropathological changes in presymptomatic Huntington’s disease. Annals of neurology. 2001;49(1):29–34. [PubMed] [Google Scholar]
- 6.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 7.Thieben MJ, Duggins AJ, Good CD, et al. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125(Pt 8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 8.Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. Journal of neurology, neurosurgery, and psychiatry. 2005;76(5):650–655. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blekher T, Johnson SA, Marshall J, et al. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67(3):394–399. doi: 10.1212/01.wnl.0000227890.87398.c1. [DOI] [PubMed] [Google Scholar]
- 10.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biological psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Harris GJ, Pearlson GD, Peyser CE, et al. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington’s disease. Annals of neurology. 1992;31(1):69–75. doi: 10.1002/ana.410310113. [DOI] [PubMed] [Google Scholar]
- 12.Aylward EH, Codori AM, Rosenblatt A, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov Disord. 2000;15(3):552–560. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Archives of neurology. 1996;53(12):1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 14.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the Predict-HD study. Archives of neurology. 2006;63(6):883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of neurology, neurosurgery, and psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 18.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group Neurology. 2000;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 19.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clinical Genetics. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Styner M, Brechbuhler C, Szekely G, Gerig G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE transactions on medical imaging. 2000;19(3):153–165. doi: 10.1109/42.845174. [DOI] [PubMed] [Google Scholar]
- 22.Harris G, Andreasen NC, Cizadlo T, et al. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. Journal of computer assisted tomography. 1999;23(1):144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 24.Magnotta VA, Bockholt HJ, Johnson HJ, Christensen GE, Andreasen NC. Subcortical, cerebellar, and magnetic resonance based consistent brain image registration. NeuroImage. 2003;19(2 Pt 1):233–245. doi: 10.1016/s1053-8119(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood SC, Siemers E, Bond C, Conneally PM, Christian JC, Foroud T. Confirmation of subtle motor changes among presymptomatic carriers of the Huntington disease gene. Archives of neurology. 2000;57(7):1040–1044. doi: 10.1001/archneur.57.7.1040. [DOI] [PubMed] [Google Scholar]
- 26.McCusker E, Richards F, Sillence D, Wilson M, Trent RJ. Huntington’s disease: neurological assessment of potential gene carriers presenting for predictive DNA testing. J Clin Neurosci. 2000;7(1):38–41. doi: 10.1054/jocn.1998.0151. [DOI] [PubMed] [Google Scholar]
- 27.Penney JB, Jr, Young AB, Shoulson I, et al. Huntington’s disease in Venezuela: 7 years of follow-up on symptomatic and asymptomatic individuals. Mov Disord. 1990;5(2):93–99. doi: 10.1002/mds.870050202. [DOI] [PubMed] [Google Scholar]
- 28.Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology. 2006;67(3):485–487. doi: 10.1212/01.wnl.0000218215.43328.88. [DOI] [PubMed] [Google Scholar]
- 29.Biglan KM, Halmagyi M. The eyes as a window into disease prevention. Neurology. 2006;67(3):376–377. doi: 10.1212/01.wnl.0000232764.18332.b6. [DOI] [PubMed] [Google Scholar]
- 30.Feigin A, Kieburtz K, Bordwell K, et al. Functional decline in Huntington’s disease. Mov Disord. 1995;10(2):211–214. doi: 10.1002/mds.870100213. [DOI] [PubMed] [Google Scholar]
- 31.Siesling S, Vegter-van der Vlis M, Roos RA. Juvenile Huntington disease in the Netherlands. Pediatric neurology. 1997;17(1):37–43. doi: 10.1016/s0887-8994(97)00069-6. [DOI] [PubMed] [Google Scholar]
- 32.Reading SA, Yassa MA, Bakker A, et al. Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry research. 2005;140(1):55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Paulsen JS, Zimbelman JL, Hinton SC, et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington’s Disease. Ajnr. 2004;25(10):1715–1721. [PMC free article] [PubMed] [Google Scholar]