Abstract
Background
Temporary, antegrade amnesia is one of the core desirable endpoints of general anesthesia. Multiple lines of evidence support a role for the hippocampal θ-rhythm, a synchronized rhythmic oscillation of field potentials at 4–12 Hz, in memory formation. Previous studies have revealed a disruption of the θ-rhythm at surgical levels of anesthesia. We hypothesized that modulation of θ-rhythm would also occur at subhypnotic but amnestic concentrations. Therefore we examined the effect of three inhaled agents on properties of the θ-rhythm that are considered to be critical for the formation of hippocampus-dependent memories.
Methods
We studied the effects of halothane and nitrous oxide, two agents known to modulate different molecular targets (GABAergic vs. non-GABAergic, respectively), and isoflurane (both GABAergic and non-GABAergic targets), on fear-conditioned learning and θ-oscillations in freely behaving rats.
Results
All three anesthetics slowed θ-peak frequency in proportion to their inhibition of fear conditioning (by 1 Hz, 0.7 Hz and 0.5 Hz for 0.32% isoflurane, 60% N2O and 0.24% halothane). The anesthetics inconsistently affected other characteristics of θ-oscillations.
Conclusions
At sub-hypnotic amnestic concentrations, θ-oscillation frequency was the parameter most consistently affected by these three anesthetics. These results are consistent with the hypothesis that modulation of the θ-rhythm contributes to anesthetic-induced amnesia.
Introduction
Amnesia is one of the essential desirable elements of the anesthetic state along with unconsciousness (hypnosis) and immobility. The post-anesthetic recall of contextually rich (episodic) memories in particular is highly undesirable and a potential cause of morbidity.
Current thinking attributes behavioral anesthetic effects to interactions with specific proteins (as opposed to nonspecific effects on lipid membranes). Indeed, anesthetic interactions with numerous plausible molecular targets have been and continue to be extensively documented.1,2 Clinically used inhalational anesthetics are chemically diverse. Despite different receptor-level activity profiles and potencies, all inhaled anesthetics impair learning and memory at concentrations that are subhypnotic and, typically, are only a fraction of the standard ‘surgical’ concentration that is required for immobility.3–6 For example, both the alkane halothane which markedly enhances γ-aminobutyric acid receptor type A-mediated inhibition and the non-γ-aminobutyric acid receptor-ergic gas nitrous oxide suppress inhibitory avoidance training at comparable lipid solubility-corrected concentrations.7 Similarly, the anesthetic isoflurane and the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (F6 or 2N, an experimental drug) suppress fear conditioning (FC) at similar concentrations.5,8 Notably, both are more potent inhibitors of FC to context (hippocampus-dependent) than FC to tone (hippocampus-independent).5,8 The reason for the preferential sensitivity of hippocampus-dependent learning to suppression by anesthetic(like) compounds is not related to specific molecular targets in any obvious pattern: isoflurane enhances γ-aminobutyratergic and inhibits glutamatergic synaptic transmission9,10 and blocks hippocampal long-term potentiation11 while F6 has no known effect on any of these processes.12,13
These observations beg the question whether all anesthetics similarly affect a single amnesia-promoting molecular target or whether their aggregate actions on different targets converge at some higher level of signal integration that is of particular importance for hippocampal learning and memory. The experiments presented in this manuscript investigate the latter possibility.
The hippocampal θ-rhythm is a prominent network activity of the ‘on-line’ hippocampus that can be separated into atropine-sensitive (type-2) and atropine-resistant (type-1) components. Type-1 theta is suppressed by surgical levels of anesthesia.14 Indeed, based on this observation, it was proposed more than 30 years ago that suppression of this non-cholinergic activation of the cerebrum may mediate behavioral effects of anesthesia.14 Since then, substantial evidence has accumulated demonstrating that the θ-rhythm serves an essential network-level role in hippocampal learning and memory (reviewed in references 15,16). For example, θ-oscillations facilitate plasticity17 and support mnemonic processes requiring inter-regional signal integration.18–20 Conversely, suppression of the θ-rhythm impairs learning and memory.21–23
We hypothesized that modulation of type-1 θ-oscillations might serve as a common network-level mechanism of anesthetic-induced impairment of hippocampus-dependent learning and memory. If this were correct, some measure of θ-activity should vary with anesthetic concentrations in the amnestic (but subhypnotic) range. We tested this hypothesis by analyzing the effect of three inhaled anesthetics on type-1 θ-oscillations. We found that θ-frequency (but not other parameters of the θ-rhythm) changed systematically with anesthetic dose. We conclude that these results are consistent with the hypothesis that slowing of θ-frequency correlates with suppression of hippocampus-dependent learning and memory.
Materials and Methods
Behavioral experiments
These experiments were approved by the University of California San Francisco Institutional Animal Care and Use Committee (San Francisco, CA). Naïve adult male Sprague-Dawley rats (Harlan; Indianapolis, IN), aged 70–90 days, were used in these studies. The rats were housed three/cage (42 cm long × 25 cm wide × 20 cm high), and food and water were available ad libitum. The rats were kept on a 12:12-hr light-dark cycle, with lights coming on at 6:00 am, and all experimental procedures took place during the light cycle. All rats were handled daily for approximately 20 s each for a week before each experiment.
An equilibration chamber was used for anesthesia delivery prior to FC. The chamber was similar to the rats’ home cage. It was covered with a plastic slab pierced with two small conduits that allowed inflow and sampling of gases. A 17 cm diameter hole in the slab was capped by a 20 cm high × 17 cm diameter plastic cylinder. This chimney-like structure allowed the escape of excess gases, but more importantly allowed rapid movement of rats into and out of the equilibration chamber with minimal disturbance to the anesthetic concentration. Gas inflow was delivered to the circuit in a 5-l/min oxygen flow through a vaporizer (Isotec 4, Ohmeda; Los Angeles, CA) set to the desired concentration. We used a Gow-Mac gas chromatograph (Gow-Mac Instrument Corp., Bridgewater, NJ) equipped with a flame ionization detector to measure concentrations of the inhaled anesthetics. The 4.6 meter-long, 0.22 cm (ID) column was packed with SF-96. The column temperature was 100°C. The detector was maintained approximately 50°C warmer than the column. The carrier gas flow was nitrogen at a flow of 15–20 mL/min. The detector received 35–38 mL/min hydrogen and 240–320 mL/min air. Primary standards were prepared, and the linearity of the response of the chromatograph was determined. We also used secondary (cylinder) standards referenced to primary standards. An infrared analyzer (Daytex, Instrumentarium Corp.; Helsinki, Finland) was also used to continually monitor the concentration of anesthetics in addition to the sampling described above.
The FC chambers (32 cm L × 25 cm W × 25 cm H) were constructed of clear acrylic. These airtight chambers allowed the continuous delivery of inhaled anesthetics at a constant concentration by vaporizer. Two 2 cm diameter conduits on opposing sides of the chamber allowed inflow and the outflow of gases to all four chambers. The grid floor used to deliver shock was composed of 19 stainless steel bars, each 4 mm in diameter, spaced 16 mm center to center. These floors were connected to a shock delivery system (Med Associates, St. Albans, VT). The chambers were wiped down with a pine-scented cleaner (5% Pine Scented Disinfectant, Midland, Inc.; Sweetwater, TN) before and after each session. In the room in which training took place, the overhead fluorescent bulbs were left on and a ventilation fan provided background noise (65 db). The appearance, odor, and texture of the chambers and room comprised the training context.
Four rats, counterbalanced for group assignment, were trained at a time. After a 3 min baseline exploratory period in the chambers, rats received three 30 s tones (2,000 Hz, 90 db) co-terminating with a shock (1 mA, 2 s) pairings, separated by 60 s and were removed from the chamber after an additional 30 s.
The following day, rats were tested for fear to the training context and fear to tone without anesthetics. For the context test, each rat was placed back in the chamber in which it was trained for a period of 8 min (in the absence of tone and shock). For the tone test, groups of four rats were transported in separate plastic pots (14 cm high × 15.5 cm diameter) to a different context in a separate room. The test chambers were triangular in shape with an acrylic floor (28 cm L × 25 cm W) and two acrylic sidewalls (28 cm L × 22 cm W) at a 45°angle. The chambers were equipped with a speaker and were wiped down with acetic acid (1%, Fisher Scientific; St. Louis, MO) before and after each session. The room appeared dark to the rats, being lit by a single red 30 W red bulb. White noise (65 dB) was used for background noise. The order of the context and tone tests was counterbalanced, so that half of each treatment group was tested to context first and tone second and vice versa. “Freezing”, defined as the absence of all movement except that necessary for respiration, is an innate defensive fear response in rodents and a reliable measure of learned fear.24,25 Each rat’s freezing behavior was scored by an observer blinded to the treatment history every 8 s during the observation period (totaling 60 observations per rat for each experiment) and a percentage was calculated by dividing the number of freezing observations by the total number possible during the observation period.
Experiments were conducted with separate groups of 8 rats for each of the three agents as summarized in Table 1. One rat in the 1% halothane group died during the equilibration portion of the experiment prior to training, leaving this group with seven rats. We considered the minimal alveolar concentration for halothane, isoflurane and nitrous oxide to be 1.1%, 1.38%,26 and 221%.27
Table 1.
Experimental Design and Protocol for Fear Conditioning Experiments
| Experiment | Number of Rats | DAY 1 | DAY 2 | ||
|---|---|---|---|---|---|
| Anesthetic Concentration | TRAINING | TEST | |||
| Halothane | 8 | 0% | 3 Tone-Shock Pairings | Context/Tone Test | Tone/Context Test |
| 8 | 0.25% | ||||
| 8 | 0.5% | ||||
| 7 | 1% | ||||
| Isoflurane | 8 | 0% | |||
| 8 | 0.3% | ||||
| 8 | 0.6% | ||||
| 8 | 0.9% | ||||
| Nitrous Oxide | 8 | 0% | |||
| 8 | 30% | ||||
| 8 | 45% | ||||
| 8 | 60% | ||||
| 8 | 75% | ||||
Statistical Analysis
For FC, we used nonlinear regression (SPSS; Chicago, IL) to calculate EC50 values and the maximum value of the dose response curve for context and tone conditional freezing.
The following equation was used in the regression, with Freezing=the percentage of time rats displayed freezing behavior during the observation period, A=the maximal freezing value, and n=the Hill coefficient.
Results of FC experiments are presented as mean ± standard error.
Electrophysiological experiments
All experiments were conducted according to the guidelines laid out in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) and were approved by the University of Wisconsin Animal Care and Use Committee, Madison, Wisconsin and have been described previously.28
Animal husbandry
Young adult male Sprague-Dawley rats were housed in the animal care facility of the University of Wisconsin with free access to rat chow and water. Thirteen rats underwent electrode implantation. After the implantation of the electrode, the animals recovered for at least 7 days before being exposed to volatile agents. After each exposure, the animals were observed until full recovery and then transferred back into their home cage. Each animal was exposed to an agent only once per day.
Electrode preparation
Linear microwire array recording electrodes with four sites spaced 200 μm apart were fabricated from 30 μm formvar-insulated nichrome wire.29 Briefly, a piece of polytetrafluoroethylene tubing of 300 μm inner diameter was cut along its long axis and one half of the tubing was used as a form for the electrode array. Four holes were punched in the tubing at 200 μm intervals, and a single piece of 35 μm formvar-insulated NiChrome wire (CFW-188-0012-HFV, California Fine Wire, Grover Beach, CA) was passed through each hole. Each wire was bent at a 90 degree angle to lie parallel to the inside wall of the form. The hemicylinder of tubing with the microwire bundle was filled with epoxy resin (Epoxylite 6001-M, Epoxylite Corp., St. Louis, MO) and heat cured. After discarding the polytetrafluoroethylene tubing, the hardened resin hemicylinder formed the shank of the electrode, and the wires were then broken off at right angles to the long axis of the array to form the recording sites. Finally, the resin matrix was sharpened at the tip to facilitate penetration of the brain, with the closest electrode approximately 25 μm away from the tip. The bare wires of the electrode array were connected to a Neuralynx EIB-16 interface board with an Neuralynx HS-16 Omnetics nanoconnector (Neuralynx, Tucson, AZ) suitable for connecting to a small headstage.
Electrode implantation
For the implantation, animals were anesthetized with isoflurane and placed in a stereotactic apparatus. Miniature stainless steel machine screws were inserted through the right and left frontal, right parietal and left occipital bones and advanced until contact was made with the dura. They served as anchors for the electrode assembly and as surface electrodes, with the screw in the occipital bone used as animal ground for reference. An additional hole was drilled 3.0 mm caudal to Bregma and 2.0 mm lateral to the midline and the electrode array was advanced 2.6 mm below the surface of the cortex. After an animal had undergone all planned experiments, it was sacrificed to verify the location of the electrode. Histological examination showed that electrodes were successfully implanted into the CA1 region of the dorsal hippocampus in eight rats and the signal from the electrode closest to the hippocampal fissure was used for field-potential analysis.
Experimental procedure
On the day of an experiment, the animal was placed into a custom-made 10-liter chamber made of acrylic glass and equipped with ports for drug injection, gas sampling and connection to the recording equipment. The footprint of the area that could be explored by the rat was 19 × 15 cm, which did not allow running. Adhesive tape sealed the lid of the chamber and all ports. Five minutes of acclimation were followed by a 15 minute control period, after which a loading dose of the drug to be tested was injected. Distilled water was injected for control experiments. A fan accelerated the evaporation and distribution of volatile agents that were deposited into a glass Petri dish via a polytetrafluoroethylene tube. Soda lime scavenged carbon dioxide. We took gas samples from the chamber with airtight glass syringes for gas chromatography at 5, 15, 22.5 and 30 min after the initial drug injection. The last three measurements were averaged and considered to represent the tested concentration. The concentration of the drug was kept approximately constant by injecting additional small boluses 10 and 20 min after the initial injection. In preliminary experiments we determined that, with this protocol, it was possible to maintain stable concentrations (within 20% of the reported concentrations) of the agent throughout the 30 min of drug exposure. Drug washout was achieved by suctioning the chamber and allowing fresh air to enter the chamber. The oxygen concentration in the chamber was constantly monitored with a gas analyzer (POET II, Criticare Systems Inc, Waukesha WI) and maintained above 20%. In most experiments, a dedicated but electroencephalogram-naive observer scored the animal’s behavior. In addition, we videotaped the animal for post-hoc analysis. The 15 minutes before drug injection (0–15 min), before drug removal (30 to 45 min), and before the end of the experiment (60–75 min after beginning of the recording) were defined as the “control”, “test”, and “recovery” time periods for analysis.
Behavioral scoring
For the electrophysiological experiments, the observer classified the behavior as immobile, exploring, grooming or undefined. Exploring was considered any behavior that involved movement of the animal’s head or body that was not grooming-related, and included walking, sniffing or manipulating any of the objects present in the tray. Grooming included scratching, face- and paw-washing. Immobile did not include assumption of the sleep posture (curling up), which was very infrequent, and which we classified as undefined. In practice, only ‘exploring’ and ‘immobile’ behaviors, corresponding to type I and type II behaviors, respectively,14 were observed with sufficient duration and frequency for subsequent data analysis.
Data Acquisition and Analysis
Electroencephalographic signals were amplified by a unity-gain HS-16 headstage preamplifier to reduce movement-related artifacts, a Lynx-8 second-stage amplifier (both from Neuralynx), bandpassed between 1 and 325 Hz, and digitized at 1000 Hz using a DigiData 1200 A/D converter (Axon Instruments, Foster City, CA). Data acquisition and processing were controlled with the pClamp software suite (Axon Instruments) and stored for analysis on a pentium-based personal computer.
Data analysis was performed primarily with custom-written routines in Matlab (The MathWorks, Natick, MA). Origin (MicroCal, Northampton, MA) and Instat (GraphPad Software, San Diego, CA) were used for graphical presentation and statistical analysis. In a preprocessing step, the raw data were passed through a digital bandpass filter with an attenuation of 40 dB/octave (IIR Butterworth in conjunction with the Matlab filtfilt forward and reverse filtering routine resulting in zero phase shift) designed for the extraction of signals in the θ-frequency band. The −3 dB (corner) frequencies were 4 and 12 Hz. Artifacts occurred primarily from mechanical causes and were excluded from analysis together with adjacent data points (± 0.5 – 1 sec, as necessary). Subsequently, data were sorted by behavior and the raw and filtered data were subdivided into segments of 4,096 points each (~4 s) with 30% overlap (1,365 points). Shorter data segments were not analyzed. All parameters described below were computed for each segment and then averaged with other segments obtained for the same drug condition and behavior for a given animal. The number of 4,096-point-segments underlying each spectrogram ranged from one (isoflurane at high concentrations) to over 100 (drug-free control, 30% N2O) and is indicated for each spectrogram shown.
For spectral analysis, we multiplied the data segments with a Hamming window (4096 points) and then computed their fast Fourier transforms, from which the power spectral density was derived, i.e. generally corresponding to the Welch method. From the power spectral density, we extracted the power in distinct frequency bands (the integral of the power spectral density within the frequency ranges detailed above) as well as amplitude and frequency of the θ peak. Autocorrelograms were produced with a modified Matlab routine (xcorr) and were normalized such that autocorrelations at zero lag were identical to 1.
Statistical analysis
Unless indicated otherwise, all numerical results are expressed as changes relative to time- and behavior-matched control experiments (exposure to distilled water). Only those animals that had been exposed to a specific drug were included in that drug’s control group. Linear regression lines were fitted according to the model: Yi=A+BXi, with the parameters A (intercept) and B (slope) estimated by the method of least squares minimization. The correlation between drug concentration and change in the measured parameter was considered significant if the null hypothesis of zero slope could be rejected at p <0.05. We assumed a normal distribution for all θ-related variables. Excel (Microsoft Office Systems, Redmond, WA) and Origin (OriginLab Corporation, Northampton, MA) were used for statistical analysis. The results are reported as mean ± standard deviation. We compared drug effects on theta parameters using two-tailed paired t-tests.
Results
Three diverse anesthetics differentially suppress FC
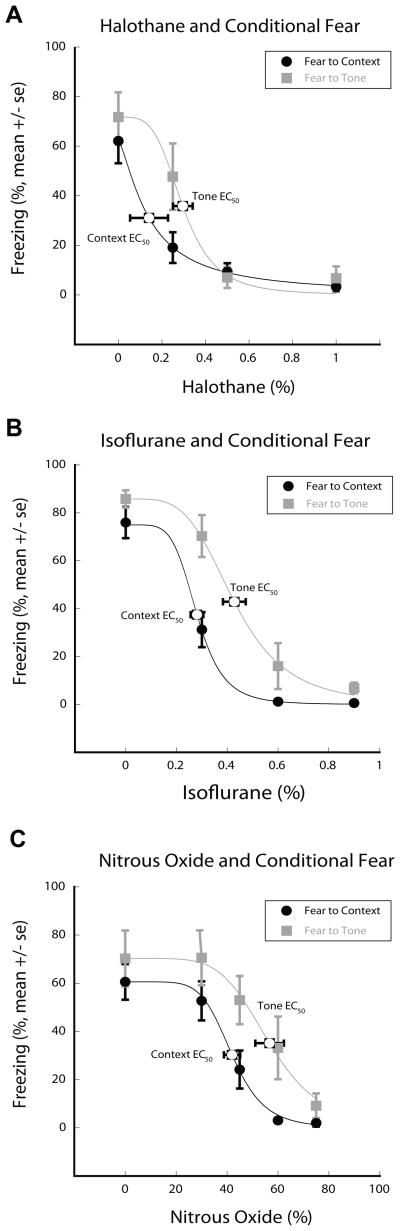
We examined the amnestic potency of isoflurane, halothane and nitrous oxide by testing the animals at drug concentrations which, based on published data5 and our own preliminary results, we expected to bracket the EC50 concentrations for FC to context and to tone.
As shown in figure 1, all three anesthetics incrementally suppressed freezing to context and tone, reaching essentially complete suppression of learning and memory at the highest concentrations tested. For all three drugs FC to context was more sensitive to inhibition than FC to tone. The EC50 concentrations derived from the concentration response relationships for halothane, isoflurane and nitrous oxide were (in % of inspired gas) 0.14± 0.09, 0.28± 0.03, 42±3 and 0.30± 0.05, 0.43± 0.05 and 57± 6 for FC to context and tone, respectively.
Figure 1.
Separation of θ- vs. non-θ-states
The electrophysiological data were obtained from 8 animals in which we confirmed the correct location of the recording electrode by histology that was congruent with the local field potential patterns.
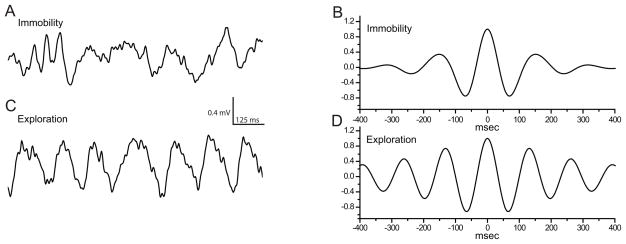
The behavioral state determines the pattern of hippocampal local field potentials in the awake, unrestrained animal. Type-1 θ-rhythm is typically present during behaviors generically described as ‘exploratory’. Our behavioral classification of ‘exploring’ vs. ‘immobile’ allowed a separation of high- and low-theta states as illustrated in figure 2. During immobility, local field potentials in the hippocampus were characterized by irregular, lower-frequency activity (fig. 2A). The irregular nature of the fluctuations is reflected in the rapid damping of the side-peaks in the autocorrelogram (fig. 2B). By contrast, during behavior classified as ‘exploring’ the hippocampal electroencephalogram was characterized by prominent, rhythmic activity in the θ-band (fig. 2C). The regularity of this oscillation is illustrated by the presence of multiple peaks in the autocorrelation (fig. 2D). θ-peak frequency under drug-free control conditions was 7.3± 0.24 Hz for ‘exploring’. The peak frequency during ‘immobile’ was slower at 6.2± 0.3 Hz (7.1–7.6 and 5.6–6.8 Hz, n=8; p=0.00018), indicating effective separation of behavioral states with respect to the θ-rhythm as type-2 (dominant during ‘immobile) is slower than type-1 θ-rhythm. Only θ-exploring (type-1) was used for further analysis.
Figure 2.
θ-rhythm persists under amnesia-inducing conditions
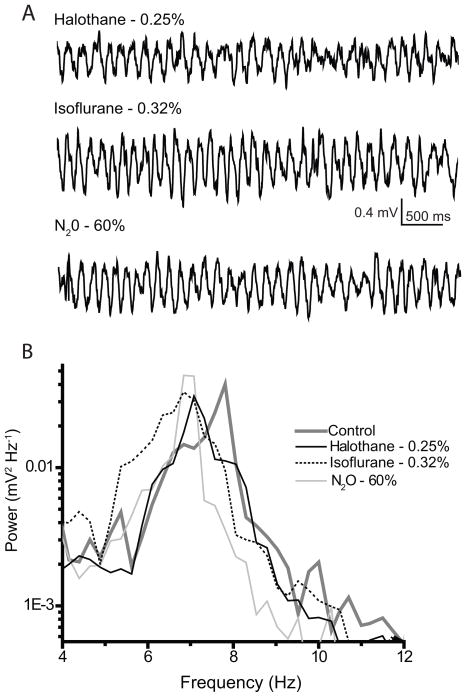
Exploratory activity occurred in the presence of memory-impairing concentrations of anesthetic agents accompanied by prominent rhythmic activity in the θ-band (fig. 3A). Power spectra obtained from these experiments reveal that peak amplitude and power (area under the curve) were not substantially altered, but that frequency of the θ-peak was slowed by isoflurane, nitrous oxide and halothane (fig. 3B). The illustrated example shows the effect of the drugs at concentrations that suppress hippocampus-dependent memory to a comparable degree (fig. 1).
Figure 3.
θ-frequency is reduced by diverse agents
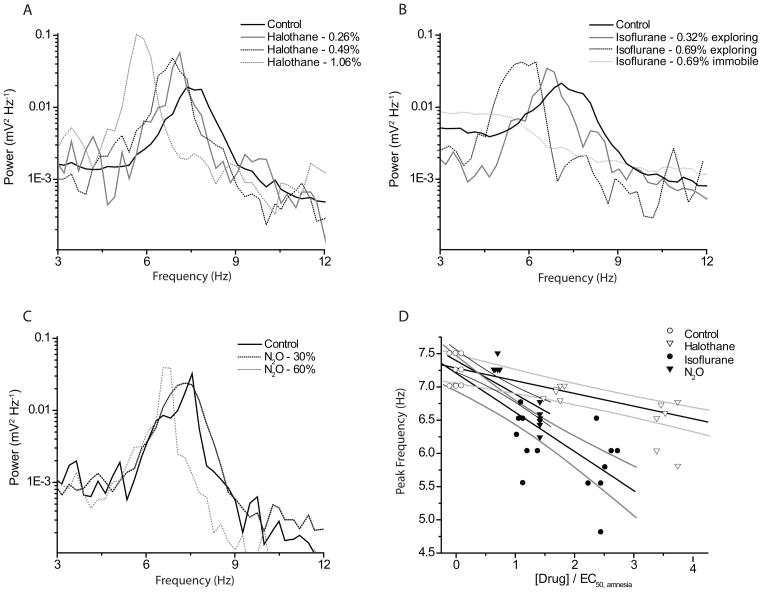
All tested anesthetic agents caused a concentration-dependent slowing of θ-peak frequency as shown in the spectra of figure 4A–C. For all anesthetics, we observed significant slowing at the lowest concentration producing amnesia. Isoflurane had the most pronounced effect on peak frequency: 0.32±0.03% slowed the θ-peak frequency by one Hz (7.3± 0.2 to 6.3±0.4 Hz, n=7; p=0.00029). 60% N2O slowed the θ-peak by 0.7 Hz (7.4± 0.2 Hz to 6.7± 0.2 Hz, n=8, p=0.0013) and halothane (0.24± 0.01%) reduced the frequency by 0.5 Hz (7.4± 0.2 to 6.9± 0.1 Hz, n= 6, p=0.0108).
Figure 4.
In order to facilitate the comparison between agents with different potencies, we ‘normalized’ the effect on θ-frequency to the individual agents’ amnestic EC50 for inhibition of FC to context as determined in the behavioral experiments. The results for all experiments are summarized as a concentration-response relationship in figure 4D. The correlation coefficients for all three least-squares linear fits were similar and significantly different from zero (R= 0.84, 0.80 and 0.80 for isoflurane, nitrous oxide and halothane, p<0.001 for each). The slopes of the regression lines were steeper for isoflurane and nitrous oxide (−0.59 and −0.5) than for halothane (−0.19).
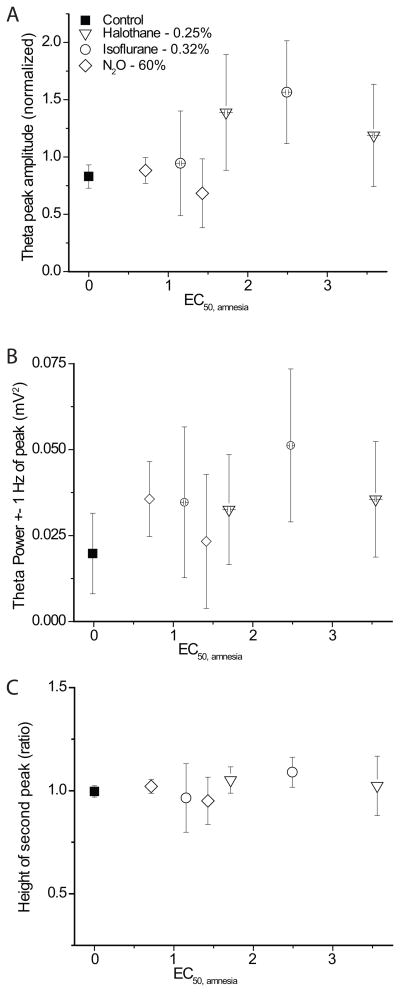
Anesthetic effects on θ-power and rhythmicity
In order to test for a relationship with respect to anesthetic-induced amnesia, we analyzed three measures of θ-power (amplitude of θ-peak, the spectral power within ± 1 Hz of the peak and the power in the 4–12Hz band) analogously to θ-frequency and grouped them by concentration. The summaries are shown in figures 5A and B (power 4–12 not shown). In contrast to the effect on θ-frequency, no common systematic change is apparent for the three drugs.
Figure 5.
We also investigated whether anesthetics affected the rhythmicity of θ-oscillations. We expressed the rhythmicity as the height of the 2nd peak in the autocorrelogram (fig. 2B and D). As can be seen from figure 5C, none of the agents had a systematic effect on the regularity of θ-rhythm during exploratory activity.
Discussion
Our two principal findings from this study are that 1) isoflurane, halothane, and nitrous oxide all inhibited hippocampus-dependent more effectively than hippocampus- independent FC, and 2) each agent slowed θ-frequency in proportion to its impairment of FC. These results are consistent with the hypothesis that a variety of agent-specific interactions with molecular targets converge to a uniform effect on an important network characteristic that is essential to proper hippocampal function during learning: in this case, θ–rhythm frequency. The degree of slowing observed at equivalently amnestic concentrations, however, varied - possibly reflecting the diversity of affected molecular targets.
θ-rhythm and memory
Evidence accumulated over the last decades provides extensive support for the importance of the θ-rhythm in learning and memory. Some of the early evidence for a role of θ-oscillations was obtained from rabbits undergoing classic (delay) conditioning. Even though this learning task is not strictly hippocampus-dependent, rabbits acquired the learning task faster if θ-rhythm dominated their hippocampal electroencephalogram just prior to the presentation of the paired stimuli.30,31 Conversely, administration of the conditioned-unconditioned stimulus pair during non-theta states slowed learning, while synchronizing the stimuli with spontaneous θ-oscillations dramatically accelerated acquisition of the conditioned reflex.32,33 Because the importance of the hippocampus increases when a learning task requires temporal processing, even non-spatial tasks that are made discontiguous (e.g. by imposing an empty ‘trace’ interval between the conditioned and unconditioned stimulus) become hippocampus-dependent. Under the ‘trace’ paradigm, neuronal responsiveness in the hippocampus to a conditioned stimulus developed prior to the behavioral expression of the conditioned reflex in those individuals that acquired the reflex.34 Hence it is not surprising that θ-power positively correlated with learning in trace eyeblink conditioning in the early stages of learning.35
While most of the published work did not discriminate between the influence of θ-power and θ-frequency, recent data provides support for a role of θ-frequency per se for the rate of learning. Baseline θ-frequency recorded during quiet waking and paradoxical sleep (i.e. not during learning) positively correlated with the rate of learning in rats subjected to an operant conditioning paradigm.36 Hence, both higher θ-frequency and increased θ-power at baseline appear to improve learning performance.
The results of these observational studies are complemented by investigations that actively modulated theθ-rhythm by pharmacological, physical or genetic means and observed the effects on learning and memory. Injection of tetracaine into the medial septum ablated hippocampal theta while preserving ‘upstream’ rhythmicity leading to severe learning impairment that, however, was largely reversible by rhythmic exogenous stimulation of the fornix that generated θ-like synchronized activity in the hippocampus.23 Pan and McNaughton21 slowed θ-oscillations by either injection of chlordiazepoxide or systemic cooling and tested learning and memory using a Morris water maze. They found that hippocampus-dependent learning was very sensitive to changes in θ-frequency: slowing of θ-oscillations by only 0.35–0.5 Hz 37 already produced a measurable memory impairment, and slowing of θ-oscillations by 1 Hz produced a substantial impairment. 21 Similarly, mice with a deletion of the γ-aminobutyric acid transporter type 1 displayed slowed type-1 θ-rhythm, reduced long-term potentiation and impaired hippocampal learning.38 By contrast, drug-induced acceleration of θ-frequency by 5–10% above baseline improved learning.39 These results thus indicate that learning is sensitive to changes in θ-frequency by amounts that are comparable to those produced by the amnestic concentration of the agents that we studied.
Changes in θ-frequency may not, however, be the only means by which drugs can influence memory via θ rhythm modulation. The cannabinoid CP55940 was shown to impair learning in the hippocampus-dependent delayed spatial alternation task in proportion to the degree of selective suppression of θ-power, a correlation that was independent of the locomotion speed.22 Similarly, we showed previously that suppression of θ-power without a change in θ-frequency produced by the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (F6, 2N)28 also correlated with impairment of contextual FC.8 In fact, the muscarinic receptor blocker scopolamine even accelerates θ-oscillations while impairing learning and memory.40,41 Scopolamine does, however, also suppress θ-power. Therefore, scopolamine’s suppression of θ-power may override its effect on θ-frequency, resulting in a net pro-amnestic effect.
Anesthetic-induced suppression of learning and memory
Considering the importance of amnesia as an anesthetic endpoint during surgical procedures under general anesthesia, its mechanisms have not been studied extensively. From the variety of learning types that exist in mammals, those subserving what is commonly referred to as episodic and episodic-like memory in humans and animals, respectively, are tightly linked to the intact functioning of the medial temporal lobe in general and the hippocampal formation in particular. Contextual FC requires an intact hippocampus and is the primary target of our investigation (note that the terms episodic, explicit, conscious and declarative memory are closely related and require intact hippocampal processing). However, in order to place our experiments into the framework of existing literature, we also tested FC to tone as a hippocampus-independent control paradigm.
From the existing, rather piecemeal, data on the amnestic profiles of the three drugs tested in our experiments the following picture emerges: early clinical observations42,43 and studies in human volunteers44,45 found that nitrous oxide suppressed episodic memory at subhypnotic concentrations and that conscious memory appeared to be more sensitive to interference than other forms of memory. The higher sensitivity of hippocampus-dependent learning was confirmed in animal experiments using eyeblink conditioning, an experimental assay capable to separately assess hippocampus-dependent (trace) vs. independent (delay) learning.46 Thus, even though episodic memory in humans may differ qualitatively from that in animals, experimental evidence supports the notion that differences in the sensitivity of various memory systems to nitrous oxide exist in multiple species.
Most data on isoflurane-induced memory suppression are derived from FC-based experiments. Similarly to nitrous oxide, isoflurane impaired learning and memory at subhypnotic concentrations and contextual (i.e. hippocampus-dependent) FC was more sensitive than cued (i.e. hippocampus-independent) FC. Interestingly, the EC50 of isoflurane for contextual FC that we determined in rats (0.28%) is close to its EC50 for suppression of episodic memory in human volunteers (0.24%).44
Less is known about halothane. The existing data only suggests that very low halothane concentrations (behaviorally sub-hypnotic but not further defined) do not affect acquisition (learning) but substantially impair retention (memory),47 while even ‘surgical’ concentrations of halothane (up to 2%, but not further specified) fail to completely suppress trace conditioning to tone (a hippocampus-independent task).48 We found that halothane showed the greatest discrimination between the two forms of FC with a more than two-fold difference in its EC50ies (0.14±0.09 and 0.3 ± 0.04%, respectively). An alternate way of expressing this finding is that halothane is the weakest inhibitor of hippocampus-independent learning (relative to its lipid solubility), as found in a recent study using inhibitory avoidance training as a learning paradigm.7
When tested in our hands under identical conditions, the three anesthetics suppressed FC to context at lower concentrations than FC to tone (EC50Tone / EC50Context were 1.35, 1.5 and 2.1 for nitrous oxide, isoflurane and halothane, respectively fig. 1). A similar differential amnestic profile was previously also reported for the nonimmobilizer 1,2-dichlohexafluorocyclobutane (EC50Tone / EC50Context, 1.7).8 We interpret these results as indicating that hippocampal processing increases the susceptibility of learning and memory to interference. Based on our electrophysiological findings, we suggest that this might be attributable to the dependence of the hippocampus on large-scale synchronization by the θ-rhythm, a dependence not shared by other important memory-processing structures, including the amygdala. An alternative (but not mutually exclusive) explanation is that the local expression of critical types of receptors or other circuit elements that produce or regulate synaptic plasticity are more sensitive to anesthetics in the hippocampus than in the amygdala. For this to be the cause, however, one would have to postulate region-specific differences in the sensitivity of receptors to the tested drugs. Such quantitative studies have not been performed.
How could slowing of the θ-rhythm affect learning and memory? Hippocampal synaptic plasticity (and, by extension, hippocampus-dependent learning and memory) is known to depend on precise synchronization of cell spiking with the phase of ongoing network activity.49 This dependence may not be shared by other forms of learning, as there is neither direct evidence for hippocampus-independent θ-rhythm generation nor for θ-phase synchrony playing as important a role in the amygdala as it does in the hippocampus and in the intimately related prefrontal cortex18 during memory encoding.50 If slowing of θ-oscillations is particularly disruptive for learning-related processes in the hippocampus, a prediction testable in future experiments would be that a systemically administered drug that inhibits long-term potentiation exclusively by direct N-methyl-D-aspartic acid receptor blockade should be equally effective in both structures and for both types of FC. Also, the present experiments were conducted in different groups of animals that were exposed to anesthetics while either measuring hippocampal oscillations or conducting fear conditioning experiments. By simultaneously measuring hippocampal oscillations and behavioral responses, it may possible to extend this correlation to the performance of individual animals rather than populations.
Is there a single molecular target that mediates effects on θ-rhythm?
We postulated that the actions of several inhaled agents on a diverse set of molecular targets converge on the θ-oscillation circuitry to produce a common network-level effect: a disruption of the endogenous temporal metric that interferes with the distinctive ‘what-where-when’ associations of hippocampus-dependent episodic declarative memory.51,52 However, is it possible that modulation of a single molecular target is responsible for the observed effect? One such candidate could be a member of the K2P channel family. TREK-1 potassium channels are enhanced by halothane and nitrous oxide 53 and TREK-1 knock-outs are less sensitive to immobility and loss of righting reflex54 (but see55 for a critique of a causal relationship between mutation and anesthetic resistance). However, despite extensive studies, there is no reported phenotype of TREK-1 knock-outs with respect to either learning, memory or electroencephalographic patterns. Nevertheless, considering the heavy expression of TREK-1 in the septum,56 an important contribution of these channels to anesthetic-induced changes in θ-rhythm is a possibility. Alternatively, and perhaps more likely, a range of actions on different targets by each anesthetic may lead to slowing of the θ-rhythm.
Summary
In summary, we have found a correlation between the concentration-dependent suppression of hippocampus-dependent learning and a reduced frequency of the hippocampal θ-rhythm. This correlation, which was present for three different inhaled anesthetics that modulate different sets of molecular targets, supports the hypothesis that modulation of the hippocampal θ-rhythm contributes to anesthetic-induced amnesia.
Acknowledgments
Supported by grant NS056411 National Institutes of Health, Bethesda, MD (to RAP), GM47818 (to VR, EIE, MP and RAP), and by the Department of Anesthesiology, University of Wisconsin-Madison.
We thank Ms. Marnie Filstein BA, administrative assistant, Department of Anesthesiology, University of Wisconsin for excellent secretarial help and Harald Hentschke, Ph.D., Assistant Professor, Department of Anesthesiology, University of Tübingen, Germany for continued help with data analysis.
Footnotes
Presented, in part, at the American Society of Anesthesiologists annual meeting in San Francisco, CA Oct. 13–17, 2007 and at the Society for Neuroscience annual meeting in San Diego, CA Nov. 3–8 2007.
Summary statement: three inhalational anesthetics with different molecular activity profiles slowed θ-peak frequency in proportion to their impairment of hippocampal-dependent memory.
References
- 1.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 3.Chortkoff BS, Bennett HL, Eger EI., 2nd Subanesthetic concentrations of isoflurane suppress learning as defined by the category-example task. Anesthesiology. 1993;79:16–22. doi: 10.1097/00000542-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chortkoff BS, Gonsowski CT, Bennett HL, Levinson B, Crankshaw DP, Dutton RC, Ionescu P, Block RI, Eger EI., 2nd Subanesthetic concentrations of desflurane and propofol suppress recall of emotionally charged information. Anesth Analg. 1995;81:728–36. doi: 10.1097/00000539-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI., 2nd The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology. 2001;94:514–9. doi: 10.1097/00000542-200103000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Gonsowski CT, Chortkoff BS, Eger EI, 2nd, Bennett HL, Weiskopf RB. Subanesthetic concentrations of desflurane and isoflurane suppress explicit and implicit learning. Anesth Analg. 1995;80:568–72. doi: 10.1097/00000539-199503000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Alkire MT, Gorski LA. Relative amnesic potency of five inhalational anesthetics follows the Meyer-Overton rule. Anesthesiology. 2004;101:417–29. doi: 10.1097/00000542-200408000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI. Short-term memory resists the depressant effect of the nonimmobilizer 1-2-dichlorohexafluorocyclobutane (2N) more than long-term memory. Anesth Analg. 2002;94:631–9. doi: 10.1097/00000539-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa K, MacIver MB. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92:228–36. doi: 10.1097/00000542-200001000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa K, MacIver MB. Agent-selective effects of volatile anesthetics on GABA(A) receptor-mediated synaptic inhibition in hippocampal interneurons. Anesthesiology. 2001;94:340–7. doi: 10.1097/00000542-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Simon W, Hapfelmeier G, Kochs E, Zieglgansberger W, Rammes G. Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology. 2001;94:1058–65. doi: 10.1097/00000542-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Chesney MA, Perouansky M, Pearce RA. Differential uptake of volatile agents into brain tissue in vitro. Measurement and application of a diffusion model to determine concentration profiles in brain slices. Anesthesiology. 2003;99:122–30. doi: 10.1097/00000542-200307000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Perouansky M, Pearce RA. Effects on synaptic inhibition in the hippocampus do not underlie the amnestic and convulsive properties of the nonimmobilizer 1,2-dichlorohexafluorocyclobutane. Anesthesiology. 2004;101:66–74. doi: 10.1097/00000542-200407000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: Relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- 15.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 16.Klimesch W, Freunberger R, Sauseng P. Oscillatory mechanisms of process binding in memory. Neurosci Biobehav Rev. 2010;34:1015–22. doi: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Masquelier T, Hugues E, Deco G, Thorpe SJ. Oscillations, phase-of-firing coding, and spike timing-dependent plasticity: An efficient learning scheme. J Neurosci. 2009;29:13484–93. doi: 10.1523/JNEUROSCI.2207-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–51. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Paz R, Bauer EP, Pare D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learn Mem. 2008;15:524–31. doi: 10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–80. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan WX, McNaughton N. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res. 1997;764:101–8. doi: 10.1016/s0006-8993(97)00431-9. [DOI] [PubMed] [Google Scholar]
- 22.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–33. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 23.McNaughton N, Ruan M, Woodnorth MA. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–10. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- 24.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 25.Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–2. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- 26.White PF, Johnston RR, Eger EI. Determination of anesthetic requirement in rats. Anesthesiology. 1974;40:52–7. doi: 10.1097/00000542-197401000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Gong D, Fang Z, Ionescu P, Laster MJ, Terrell RC, Eger EI. Rat strain minimally influences anesthetic and convulsant requirements of inhaled compounds in rats. Anesth Analg. 1998;87:963–6. doi: 10.1097/00000539-199810000-00040. [DOI] [PubMed] [Google Scholar]
- 28.Perouansky M, Hentschke H, Perkins M, Pearce RA. Amnesic concentrations of the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (F6, 2N) and isoflurane alter hippocampal theta oscillations in vivo. Anesthesiology. 2007;106:1168–76. doi: 10.1097/01.anes.0000267600.09764.af. [DOI] [PubMed] [Google Scholar]
- 29.Jellema T, Weijnen JA. A slim needle-shaped multiwire microelectrode for intracerebral recording. J Neurosci Methods. 1991;40:203–9. doi: 10.1016/0165-0270(91)90069-c. [DOI] [PubMed] [Google Scholar]
- 30.Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- 31.Nokia MS, Penttonen M, Korhonen T, Wikgren J. Hippocampal theta (3–8Hz) activity during classical eyeblink conditioning in rabbits. Neurobiol Learn Mem. 2008;90:62–70. doi: 10.1016/j.nlm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci USA. 2002;99:1616–20. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci. 2004;118:403–11. doi: 10.1037/0735-7044.118.2.403. [DOI] [PubMed] [Google Scholar]
- 34.McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–96. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Nokia MS, Penttonen M, Korhonen T, Wikgren J. Hippocampal theta-band activity and trace eyeblink conditioning in rabbits. Behav Neurosci. 2009;123:631–40. doi: 10.1037/a0015334. [DOI] [PubMed] [Google Scholar]
- 36.Santos LM, Dzirasa K, Kubo R, Silva MT, Ribeiro S, Sameshima K, Valle AC, Timo-Iaria C. Baseline hippocampal theta oscillation speeds correlate with rate of operant task acquisition. Behav Brain Res. 2008;190:152–5. doi: 10.1016/j.bbr.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Whishaw IQ, Vanderwolf CH. Hippocampal EEG and behavior: Effects of variation in body temperature and relation of EEG to vibrissae movement, swimming and shivering. Physiol Behav. 1971;6:391–7. doi: 10.1016/0031-9384(71)90172-7. [DOI] [PubMed] [Google Scholar]
- 38.Gong N, Li Y, Cai GQ, Niu RF, Fang Q, Wu K, Chen Z, Lin LN, Xu L, Fei J, Xu TL. GABA transporter-1 activity modulates hippocampal theta oscillation and theta burst stimulation-induced long-term potentiation. J Neurosci. 2009;29:15836–45. doi: 10.1523/JNEUROSCI.4643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci. 1995;15:2445–52. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Givens B, Olton DS. Local modulation of basal forebrain: Effects on working and reference memory. J Neurosci. 1994;14:3578–87. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markowska AL, Olton DS, Givens B. Cholinergic manipulations in the medial septal area: Age-related effects on working memory and hippocampal electrophysiology. J Neurosci. 1995;15:2063–73. doi: 10.1523/JNEUROSCI.15-03-02063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970;42:535–42. doi: 10.1093/bja/42.6.535. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal G, Sikh SS. Awareness during anaesthesia. A prospective study. Br J Anaesth. 1977;49:835–8. doi: 10.1093/bja/49.8.835. [DOI] [PubMed] [Google Scholar]
- 44.Dwyer R, Bennett HL, Eger EI, 2nd, Heilbron D. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology. 1992;77:888–98. doi: 10.1097/00000542-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Block RI, Ghoneim MM, Pathak D, Kumar V, Hinrichs JV. Effects of a subanesthetic concentration of nitrous oxide on overt and covert assessments of memory and associative processes. Psychopharmacology (Berl) 1988;96:324–31. doi: 10.1007/BF00216058. [DOI] [PubMed] [Google Scholar]
- 46.Ghoneim MM, El Zahaby HM, Block RI. Classical conditioning during nitrous oxide treatment: Influence of varying the interstimulus interval. Pharmacol Biochem Behav. 1999;62:449–55. doi: 10.1016/s0091-3057(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 47.Rosman E, Quartermain D, Pang R, Turndorf H. Halothane anesthesia causes state-dependent retrieval failure in mice. Physiol Behav. 1992;52:449–53. doi: 10.1016/0031-9384(92)90330-5. [DOI] [PubMed] [Google Scholar]
- 48.Pang R, Turndorf H, Quartermain D. Pavlovian fear conditioning in mice anesthetized with halothane. Physiol Behav. 1996;59:873–5. doi: 10.1016/0031-9384(95)02137-x. [DOI] [PubMed] [Google Scholar]
- 49.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–7. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 50.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working Memory Performance Correlates with Prefrontal-Hippocampal Theta Interactions but not with Prefrontal Neuron Firing Rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devito LM, Eichenbaum H. Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.09.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 53.Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–52. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 54.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K(+) channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eger EI, Raines DE, Shafer SL, Hemmings HC, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–48. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, Meadows HJ. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]