Abstract
Parkinson's disease (PD) is a major world-wide health problem afflicting millions of the aged population. Factors that act on most or all cell types (pan-cellular factors), particularly genetic mutations and environmental toxins, have dominated public discussions of disease etiology. Although there is compelling evidence supporting an association between disease risk and these factors, the pattern of neuronal pathology and cell loss is difficult to explain without cell-specific factors. This article focuses on recent studies showing that the neurons at greatest risk in PD—substantia nigra pars compacta dopamine neurons—have a distinctive physiological phenotype that could contribute to their vulnerability. The opening of L-type calcium channels during autonomous pacemaking results in sustained calcium entry into the cytoplasm of substantia nigra pars compacta dopamine neurons, resulting in elevated mitochondrial oxidant stress and susceptibility to toxins used to create animal models of PD. This cell-specific stress could increase the negative consequences of pan-cellular factors that broadly challenge either mitochondrial or proteostatic competence. The availability of well-tolerated, orally deliverable antagonists for L-type calcium channels points to a novel neuroprotective strategy that could complement current attempts to boost mitochondrial function in the early stages of the disease. Antioxid. Redox Signal. 14, 1289–1301.
Pan-Cellular Factors in Parkinson's Disease
Studies over the past decade have made great progress in identifying factors that increase disease risk. The vast majority of these is pan-cellular factors, that is, factors that in principle have a broad, negative impact on neuronal and non-neuronal cell types. The four best documented pan-cellular factors are age, genetic mutations, environmental toxins, and inflammation. The biggest risk factor in Parkinson's disease (PD) is age (20, 25). Disease incidence rises exponentially above the age of 65. Because improvements in healthcare are increasing life expectancy, the number of PD patients is expected to grow dramatically in the coming years, reaching over 2 million in the United States by 2030 (27). Why age is such a strong risk factor is unknown, but it is widely speculated that declining mitochondrial function is a key factor (16, 104).
In the last decade, perhaps the greatest single advance in the PD field has been the identification of genes that increase disease risk (34, 68). Although these still account for <10% of all the cases of PD, in some ethnic populations genetic mutations appear to account for a much larger fraction of cases (68). Unfortunately, most of the PD-associated genes are of unknown or poorly understood function. However, this gap is rapidly closing.
One of the emerging themes in the study of genetic risk factors is mitochondrial dysfunction. Three of the genes associated with a recessive, early onset form of the disease (DJ-1, PTEN-induced putative kinase 1 [PINK1], and parkin) are directly linked to mitochondrial function, providing a potential connection with changes associated with aging (104). DJ-1 is a mitochondrially enriched, redox-sensitive protein, giving it the capacity to signal oxidative challenges and potentially coordinate a variety of mitochondrial oxidative defense mechanisms (2, 58). Parkin and PINK1 also have mitochondrial roles. Fruit flies with functional deletions of Parkin have fragmented and apoptotic mitochondria (42); knockout mice have a less dramatic but a clear mitochondrial phenotype (including decreased mitochondrial [respiratory] function, decreased metabolic drive, and increased lipid and protein phosphorylation) (88). PINK1 deletion leads to a similar phenotype in Drosophila as does Parkin deletion–fragmented cristae and apoptotic mitochondria; this phenotype can be rescued by Parkin overexpression, suggesting involvement in some common biochemical pathway (22, 89). Although found both in cytosolic and mitochrondrial preparations, PINK1 has an N-terminus mitochondrial targeting sequence (29). Although the functions of the other genes prominently linked to PD (SNCA, leucine-rich repeat kinase 2 [LRRK2]) remain poorly defined, proteostatic dysfunction resulting in Lewy body (LB) formation is commonly thought to be an essential component of the disease etiology (112).
Another factor linked to PD that should in principle affect all cells is environmental toxin exposure. The proposition that toxins, particularly those that target mitochondria, could be a factor in PD has long been part of the mindset of the field given the ability of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxin (MPTP) and rotenone to reproduce key aspects of the disease phenotype (13, 95). Recent epidemiological studies have found convincing support for a link between pesticide exposure and the risk of developing PD (59, 115).
A fourth pan-cellular factor in PD is inflammation (46, 48, 52). In toxin models of PD, inflammation and resultant oxidant stress are important modulators of cell loss (53, 117–119). In the later stages of the human disease, there are clear signs of microglial activation and inflammation that could contribute to progression (116). Recent work has shown how extrinsic oxidant stress, like that created by inflammation, could result in neuronal death in a cell with high cytosolic calcium levels. Reactive oxygen species (ROS)-mediated activation of protein kinase C beta phosphorylates 66-kDa isoform of the growth factor adapter Shc (p66shc), promoting transport into mitochondria, where it alters calcium responses and promotes apoptosis (93).
In the last year, the proposition that a fifth pan-cellular factor—a viral or prion-like infection—is causative in PD has been advanced (47, 86). In the absence of a direct demonstration of an infectious agent, there are two main pieces of evidence that have been used to argue for this type of process. The first is apparent staging of LB pathology in PD (17); the Braak hypothesis asserts that the pathology progresses from peripheral enteric autonomic ganglia, to the caudal medullary autonomic cell groups and then rostrally into the brain. This apparent progression has been taken as evidence of an infection (47). However, it is far from clear that there is this sort of progression in the majority of PD patients, as the whole hypothesis turns on the supposition that patients with medullary and ganglionic pathology alone would have developed PD had they lived longer. Moreover, there is considerable variability in the regional pattern of LB pathology and LBs have an uncertain connection to the pathophysiology underlying the symptoms of the disease (18, 57). The second piece of evidence is derived from grafting embryonic dopamine (DA) neurons into PD patients (65, 69, 77). In some of these grafts, DA neurons had LBs. Since these neurons were relatively young, the appearance of LBs has been taken as evidence of the spread of a virus or of a prion-like agent from the host. However, these data are open to alternative interpretation. The most obvious of which is that DA neurons are particularly susceptible to the stress of grafting, leading to premature proteostatic dysfunction and LB formation. The apparent restriction of LBs to the DA neurons in the graft is certainly consistent with this explanation and not with an infection model. More importantly, at present, there is no compelling evidence that the pattern of neuronal pathology in PD conforms to the predictions of an infection model. The LB pathology in PD does not follow a nearest neighbor rule. Neurons in the nucleus tractus solitarius, for example, show no signs of pathology in PD in spite of being next to neurons in one of the most vulnerable nuclei (dorsal motor nucleus of the vagus [DMV]). There is no evidence that vulnerability is predicted by synaptic connectivity either. Arguments made that connectivity is an issue consistently ignore the fact that every major neuronal population affected in PD is synaptically coupled to a population of neurons that do not display significant pathology. In view of the dearth of hard scientific support, the infection model of PD is difficult to take seriously.
Thus, studies of pan-cellular factors in PD have identified several potential processes in the etiology of PD, the most compelling of which are mitochondrial and proteostatic dysfunction. What is left unexplained by these studies is the pattern of neuronal dysfunction and loss in PD.
Cell-Specific Factors in PD
The motor symptoms, including bradykinesia, rigidity, and resting tremor, are clearly linked to the degeneration and death of a small group of neurons in the mesencephalon—substantia nigra pars compacta (SNc) DA neurons (49, 99). The palliative efficacy of levodopa or L-3,4-dihydroxyphenylalanine (L-DOPA)—a DA precursor—is testament to the centrality of these neurons in the motor symptoms of PD. These neurons constitute a tiny fraction of all the neurons in the brain (<0.0001%), arguing that there must be cell-specific factors at work in PD. What might these factors be? DA itself has long been viewed as a culprit, as oxidation of cytosolic DA (and its metabolites) is damaging (40, 112). However, there are reasons to doubt this type of cellular stress alone is responsible for the loss of DA neurons in PD. First, there is considerable regional variability in the vulnerability of DA neurons in PD, with some being devoid of pathological markers (24, 55, 62, 75, 103). Second, L-DOPA administration (which relieves symptoms by elevating DA levels in PD patients) does not appear to accelerate disease progression (30), suggesting that DA is not a significant source of reactive oxidant stress, at least in the short term. Sulzer and colleagues have recently reported that calcium entry through L-type channels stimulates DA metabolism in SNc DA neurons, pushing cytosolic DA concentrations into a toxic range with L-DOPA loading (81). For this mechanism to be relevant to selective vulnerability, one would have to posit that modest elevations in cytosolic DA over decades lead to an accumulation of cellular defects that ultimately produces cell death. If true, treating patients in the early stages of the disease with direct acting agonists, rather than L-DOPA, should lead to a slower progression of the disease. That said, the frank death or phenotypic decline of a variety of nondopaminergic neurons in PD argues that DA itself is not likely to be the principal cell-specific risk factor in the disease.
Another distinctive feature of SNc DA neurons, and many of the other neurons that succumb in PD (e.g., locus ceruleus [LC] neurons), is their enormous axonal field. Recent anatomical work has estimated that a typical SNc DA neuron has mean axonal length of 470,000 μm (74). Each axon supports ∼370,000 synapses, orders of magnitude higher than the number supported by cortical pyramidal neurons for example (3). Sustaining such a large field must elevate axonal protein trafficking and proteostatic stress. Given that alpha-synuclein is largely a synaptic protein, its trafficking must be elevated in SNc DA neurons, potentially contributing to the axonal pathology seen in PD patients (33). Moreover, because synaptic terminals are metabolically demanding specializations that typically require mitochondria, sustaining this axonal field could create a mitochondrial sink for these neurons, lowering mitochondrial density in the somodendritic region and lowering spare oxidative capacity, creating an energy crisis (85). In fact, mitochondrial density in the somatodendritic region of SNc DA neurons appears to be abnormally low (71). Diminished oxidative reserve capacity could increase the production of damaging superoxide by mitochondria, contributing to their decline with age.
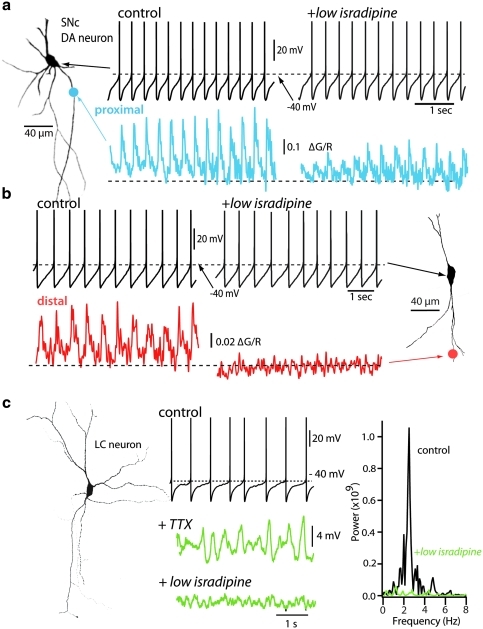
Our work in the last few years has focused on the potential role of physiological phenotype. Unlike the vast majority of neurons in the brain, adult SNc DA neurons are autonomously active, generating regular, broad action potentials (2–4 Hz) in the absence of synaptic input (21, 39, 43, 84). This pacemaking activity is believed to be important to maintaining ambient DA levels in regions that are innervated by these neurons, particularly the striatum (102). Although most neurons rely exclusively on monovalent cation channels to drive pacemaking, SNc DA neurons also engage L-type ion channels that allow calcium to enter the cytoplasm (15, 92, 96), leading to oscillations in intracellular calcium concentrations (21, 43, 125) (Fig. 1a, b). The L-type calcium channels used by SNc DA neurons in pacemaking have a distinctive Cav1.3 pore-forming subunit encoded by Cacna1d (21, 111). Cav1.3 calcium channels are relatively rare, constituting only about 10% of the all the L-type calcium channels found in the brain (107). Channels with this subunit differ from other L-type calcium channels in that they open at relatively hyperpolarized potentials, allowing them to contribute to the mechanisms driving the membrane potential to spike threshold underlying autonomous pacemaking (21, 43, 96).
FIG. 1.
Physiological properties of substantia nigra pars compacta (SNc) and locus ceruleus (LC) neurons. (a, b) SNc dopamine (DA) neurons pacemaker spikes are coupled to calcium transients sensitive to L-type calcium channel blockers. Shown in the left, a reconstruction of an SNc DA neuron filled with the red dye Alexa594 (50 μM) and the calcium-sensitive Fluo4 (200 μM). Shown to the right of the DA neuron, representative pacemaking traces before and after bath application of the L-type calcium channel blocker (5 μM isradipine), and below pacemaking traces, time-matched dendritic calcium transients from (a) proximal and (b) distal dendrite (>80 μm away from soma). Calcium transients in distal dendrites were abolished by application of isradipine, yet the pacemaking firing was insensitive to this calcium channel blocker. (c) Shown on the left is a biocytin-filled LC neuron illustrating extensive dendritic arbor. Middle panel shows pacemaking firing from an LC neuron before and after blockade of sodium spikes with 1 μM tetrodotoxin (TTX). Membrane potential oscillations (green trace) were eliminated by bath application of 5 μM isradipine. (a, b) Adapted with permission from earlier published work [© Guzman et al. (43)]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The sustained engagement of Cav1.3 calcium channels during pacemaking comes at an apparent metabolic cost to SNc DA neurons. Because of its involvement in cellular processes ranging from the regulation of enzyme activity to programmed cell death, calcium is under very tight homeostatic control, with a cytosolic set point near 100 nM—10,000 times lower than the concentration of calcium in the extracellular space (12, 87, 101). Calcium entering neurons is rapidly sequestered or pumped back across the steep plasma membrane concentration gradient; this process requires energy stored in adenosine triphosphate (ATP) or in ion gradients that are maintained with ATP-dependent pumps, like the Na-K ATPase. In most neurons, calcium channel opening is a rare event, occurring primarily during very brief action potentials. This makes the task and the metabolic cost to the cell readily manageable, but in SNc DA neurons, where Cav1.3 calcium channels are open much of the time, the magnitude and the spatial extent of calcium influx are much larger (125). Preliminary studies using transgenic mice that express a mitochondrially targeted redox-sensitive variant of green fluorescent protein under control of the tyrosine hydroxylase promoter have revealed that indeed mitochondria in SNc DA neurons have a high basal oxidant stress that is a direct consequence of opening of L-type calcium channels. Further, calcium entry (and presumably the concomitant oxidant stress) increases the vulnerability of SNc DA neurons to toxins (MPTP, 6-hydroxydopamine, and rotenone) used to create animal models of PD (21).
Another reason to suspect that calcium is an important factor is the inverse correlation between expression of the mobile calcium buffering protein calbindin and vulnerability in PD, as well as in animal models of the disease (35). Calbindin expression is high in DA neurons of the ventral tegmental area as well as the dorsal tier of the SNc, both areas that are relatively resistant in PD. What is less clear is precisely why this should be the case. One possibility is that calbindin reduces calcium entry into the endoplasmic reticulum, holding it for plasma membrane extrusion mechanisms and avoiding double pumping.
The glutamatergic synaptic input to SNc DA neurons could also contribute to their vulnerability. In vivo, SNc DA neurons spike in at least two other modes that are created by superimposing synaptic input on the basal pacemaking activity (120). From a functional standpoint as well as from the standpoint of neurodegeneration, the most interesting of these is the burst mode. Because of its association reward prediction errors (105), elevated release of DA in the striatum and the induction of long-term synaptic plasticity, this burst has generated a great deal of experimental attention. A bevy of studies have recently focused on the mechanisms underlying the burst. These studies have established the necessity of N-methyl-D-aspartate (NMDA) receptor activation in burst generation (14, 26, 127). Because pacemaking keeps the membrane potential of SNc DA neurons in a voltage range where magnesium block of NMDA receptors is ineffective, even modest glutamatergic input is capable of producing substantial NMDA receptor currents. Could calcium entry through NMDA receptors synergize with that through Cav1.3 channels engaged by pacemaking to create a metabolic tipping point for mitochondria? Excitotoxicity has long been hypothesized to be a factor in the etiology of PD (6, 41, 108), but the engagement of NMDA receptors and the elevation in cytosolic calcium concentration this brings about has been envisioned to be a relatively late stage event, coming only when cells were unable to maintain a stable, hyperpolarized membrane potential. Nevertheless, SNc DA neurons are pacemakers that do not have a stable hyperpolarized membrane potential when they are healthy, meaning that NMDA receptors should be more easily recruited. Another factor in this equation is the intracellular calcium stores. Metabotropic glutamate receptor (mGluR) activation mobilizes these stores (80), forcing neurons to re-sequester calcium (at the expense of ATP). Because of the sustained calcium entry into SNc DA neurons during pacemaking, these stores are fully charged, which adds to the burden created by mGluR activation. In this way, pacemaking and regular activation of NMDA and mGluRs should create a sustained calcium storm in SNc DA neurons. Although mitochondria appear capable of weathering this storm in the short-term, the elevation in oxidant stress created by the need to supply a steady stream of ATP to pumps should slowly increase damage to their DNA and accelerate their aging (11, 98). Figure 2 summarizes the cell-specific risk factors that could elevate oxidant stress in SNc DA neurons.
FIG. 2.
Calcium signaling in SNc DA neurons could lead to mitochondrial oxidant stress, accelerated aging, and eventual cell death. The steep concentration gradient for calcium allows it to cross the plasma membrane readily into cells through open pores, like L-type Cav1.3 calcium channels, or by synaptic activation of N-methyl-D-aspartate (NMDA) receptors. In addition, the model considers the importance of metabotropic glutamate receptors (mGluRs) as regulators of intracellular calcium via endoplasmic reticulum (ER) calcium stores. Once inside neurons, calcium is either transported back across the plasma membrane or sequestered in intracellular organelles. Calcium is transported across the plasma membrane through either the calcium-ATPase (PMCA) or through a sodium/calcium exchanger (NCX) that relies upon the Na+ gradient. Calcium is rapidly sequestered either by ionic interactions with buffering proteins or by transport into cytosolic organelles (i.e., the mitochondria and the ER). The ER uses high-affinity sarcoendoplasmic reticulum calcium ATPase (SERCA) pumps that depend upon ATP to take calcium from the cytoplasm into the ER lumen. Calcium flows back into the cytoplasm after opening of inositol triphosphate receptors (IP3R) and ryanodine receptors (RyRs) studding the ER membrane. Mitochondria are often found in close apposition to the ER and plasma membrane, creating a region of high (but localized) calcium concentration that drives calcium into the matrix of mitochondria through a calcium uniporter. ER membrane fuse with mitochondria to create mitochondrial-associated membrane (MAM) pores that allows direct communication between the ER and mitochondrion. Calcium can leave the mitochondrion through a number of mechanisms. The dominant mitochondrial calcium efflux path in neurons is through mitochrondrial NCXs. Calcium release through higher conductance ion channels, like the mitochondrial permeability transition pore (mPTP), has also been proposed. The mPTP is posited to have two conductance states: a low conductance state that is reversible and participates in physiological calcium handling, and a high conductance state that is irreversible and leads to mitochondrial swelling and loss of molecules like cytochrome C that trigger apoptosis. Also shown schematically are elements of the tricarboxylic acid (TCA) cycle that produces reducing equivalents for the electron transport chain complexes I–IV. The electrochemical gradient created drives adenosine triphosphate (ATP) synthase and the conversion of ATP from adenosine diphosphate (ADP) delivered to the matrix by the adenine nucleotide transporter (ANT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
PD, ROS, and Aging
One of the oldest and most popular theories of aging is that it is a direct consequence of accumulated mtDNA and organelle damage produced by ROS and related reactive molecules generated by the electron transport chain in the course of oxidative phosphorylation (44, 123). A corollary of this hypothesis is that the rate of aging is directly related to metabolic rate. There is no obvious reason not to extend this organismal postulate to individual cells. The reliance of SNc DA neurons on a metabolically expensive strategy to generate autonomous activity that taxes mitochondria should mean that they age more rapidly than other types of neuron. Is then PD simply a reflection of accelerated aging in neurons that rely too heavily upon Ca2+ channels to do their business? Age is undoubtedly the single strongest risk factor for PD (20, 37). Stereological estimates of normal aging related cell death in humans argue that SNc DA neurons at a higher risk than other neurons in the absence of environmental toxins or pathogens, as they are lost at a significantly higher rate than many other types of neurons (some of which show no appreciable loss over a 6–7-decade span) (109). In mammals with significantly shorter lifespans, loss of SNc DA neurons with age has not been seen reliably, but there is a clear decline in phenotypic markers with age that matches that seen in PD, as well as an increased susceptibility to toxins (4, 23, 54, 60, 61, 76). Taken together, these studies make a case that SNc DA neurons age more rapidly than the vast majority of the neurons in the brain.
This wear-and-tear theory suggests that PD is first and foremost a consequence of aging. Why then do some people become symptomatic in their 50s, others in their 60s or 70s, or not at all in an 80- or 90-year life? Genetic factors certainly could account for a large part of this variation (20, 79, 112). These factors could increase the rate at which vulnerable neurons age by compromising mitochondrial function or Ca2+ homeostasis. Several of the genetic mutations associated with early onset forms of PD are directly linked to mitochondrial dysfunction (104). Unfortunately, animal models of these forms of PD have failed to recapitulate the disease. For example, mutations of DJ-1 (PARK7) are associated with an early onset form of PD. DJ-1 is redox sensitive, giving it the capacity to signal oxidative challenges and potentially coordinate a variety of mitochondrial oxidative defense mechanisms (58). Although deleting DJ-1 increases the sensitivity of cells generally to oxidative challenge, it remains to be determined how it compromises SNc DA neurons under more physiological conditions. Studies of DJ-1 knockouts have not revealed any loss of SNc DA neurons or a fundamental shift in their physiology, only a modest deficit in DA release during burst stimulation that is difficult to tie to an increased vulnerability (38), but there have been no studies in these mice of mitochondrial physiology in mature SNc DA neurons where Ca2+ influx during pacemaking has fully developed. This could prove to be critical to an understanding of the mechanisms that could accelerate the loss of these cells over decades.
Two other genes linked to familial PD have unequivocal linkages to mitochondria. One is Parkin (PARK2). Fruit flies with functional deletions of Parkin have fragmented and apoptotic mitochondria (42); knockout mice have a less dramatic but a clear mitochondrial phenotype (including decreased mitochondrial [respiratory] function, decreased metabolic drive, and increased lipid and protein phosphorylation) (88). Another is PINK1 (PARK6). Its deletion leads to an identical phenotype in Drosophila as does Parkin deletion–fragmented cristae and apoptotic mitochondria; this phenotype can be rescued by Parkin overexpression, suggesting involvement in some common biochemical pathway (22, 89). Although found both in cytosolic and mitochrondrial preparations, PINK1 has an N-terminus mitochondrial targeting sequence (29). PINK1 deletion also compromises DA release during burst stimulation, like DJ-1 (63). It is not difficult to infer that loss of function mutations in either of these genes should have their biggest impact in a cell type that had a high basal level of metabolic activity, creating a mitochondrial oxidant stress, but to date, this has not been convincingly demonstrated.
PD Is Not Just a Disease of Dopaminergic Neurons in the SNc
In PD loss of neurons in multiple brain regions paralleling the loss of DA neurons in the SNc occurs (17, 32, 36, 56, 121). Two of the cell-specific risk factors standout as common to the other cell types that succumb in PD: autonomous or spontaneous activity with broad action potentials and a depolarized membrane potential that promotes the opening of NMDA receptors. Even though current data set is fragmented, neurons in the DMV, LC, raphe nuclei, pedunculopontine nucleus, lateral hypothalamus, tuberomammillary nucleus, basal forebrain, and olfactory bulb all have these two physiological characteristics, while having widely different transmitters and axonal fields. DMV cholinergic neurons, which are thought to be among the earliest neurons with LBs in PD, are spontaneously active (122); this activity is autonomously generated and depends upon L-type calcium channels (unpublished observations). LC noradrenergic neurons, like SNc DA neurons, have large axonal arbors and are autonomous pacemakers (with broad spikes) that engage L-type calcium channels (124) (Fig. 1c). Serotonergic neurons in the raphe nuclei have broad spikes and are calcium-dependent autonomous pacemakers (19). This is also true of pedunculopontine nucleus cholinergic neurons (114). Tuberomammillary and lateral hypothalamic (orexin expressing) neurons are spontaneously active (70, 110, 126). Tuberomammillary neurons engage L-type calcium channels in this process (110, 113, 124) (this question has not been addressed in orexin neurons). Basal forebrain cholinergic are lost in PD (17). These neurons have large axonal terminal fields, are spontaneously active in brain slices, and have prominent calcium channel currents (83). Moreover, with aging there are significant and deleterious changes in calcium homeostasis in these neurons (82). DA neurons in the olfactory bulb are a slightly different case from the standpoint of pathology. These neurons are calcium-dependent autonomous pacemakers (91). However, there are no signs of cell loss in the olfactory bulb in spite of deficits in olfaction being a harbinger of the motor symptoms in PD (50, 94). This does not mean that a reliance upon calcium is a bad thing though, as this region is capable of adult neurogenesis (90). Although much needs to be done, the shared physiological characteristics of these seven vulnerable cell types points to a common mechanism underlying their slow functional decline with age: calcium mediated stress.
The Interplay Between Pan-Cellular and Cell-Specific Risk Factors in PD
Age is the strongest of the pan-cellular risk factors. One of the oldest and most popular theories of aging is that it is a direct consequence of accumulated mtDNA and organelle damage produced by ROS and related reactive molecules generated by the electron transport chain in the course of oxidative phosphorylation (45, 123). A corollary of this hypothesis is that the rate of aging is directly related to metabolic rate. There is no obvious reason not to extend this organismal postulate to individual cells. The reliance of SNc DA neurons on a metabolically expensive strategy to generate autonomous activity that taxes mitochondria should mean that they age more rapidly than other types of neuron—creating a positive interaction between cell-specific and pan-cellular risk factors. This perspective predicts that there should be functional impairment or loss of SNc DA neurons with normal aging. Stereological estimates of normal aging-related cell death in humans argue that SNc DA neurons at a higher risk than many other types of neuron (109). In mammals with significantly shorter lifespans, loss of SNc DA neurons with age has not been seen reliably, but there is a clear decline in phenotypic markers with age that matches that seen in PD, as well as an increased susceptibility to toxins (4, 23, 54, 61, 76). There is also an aging-related decline in SNc mitochondrial function (5), some of which could easily be attributed to the accumulation of mitochondrial DNA mutations with normal aging (10).
There could be a positive interaction between cell-specific mitochondrial stress and the other pan-cellular risk factors. As with aging, an interaction of this sort could help create a tipping point in the pathogenesis of PD that would results in a selective pattern of degeneration. Consider the loss of DJ-1 function that compromise mitochondrial oxidant defenses. In most neurons, oxidant defense engagement is likely to be mild, episodic, and readily endured even without a fully functional defense system, but in cell types like SNc DA neurons, where oxidant stress appears to be sustained, compromising oxidant defenses could come at higher long term cost. Mutations that diminish proteostatic competence (e.g., alpha-synuclein overexpression) could also exert their primary effects on cellular viability through increasing ATP utilization. It would be of considerable interest to see if overexpression of alpha-synuclein increased mitochondrial oxidant stress. Further, broadly acting environmental toxins that partially compromise mitochondrial function should have a bigger impact on cell types that have high mitochondrial demands. Lastly, inflammation in the late stages of the disease should significantly increase the production of ROS in and around surviving neurons. If these neurons are already overproducing ROS because of cell-specific factors, their oxidant defenses could be overwhelmed, leading to apoptosis.
Targeting Dihydropyridine-Sensitive L-Type Calcium Channels as a Potential Therapeutic Strategy for PD
Current therapeutic strategies are ostensibly targeting the pan-cellular factors (e.g., Coenzyme Q10). Attacking the cell-specific factors is another strategy. Certain risk factors, like DA and axonal terminal field, are not malleable, at least not without compromising brain function. One factor that does appear to be a viable target is calcium entry through L-type calcium channels during autonomous or spontaneous spiking. These channels are antagonized by dihydropyridines (DHPs) that are approved for human use. DHPs have a very modest side-effect profile and have been used for decades to treat hypertension (28).
There are two basic questions that have to be answered before moving ahead with this kind of a neuroprotective strategy in the early stages of PD. The first is whether antagonizing L-type calcium channels will significantly impair the ability of SNc DA neurons to perform their duties. The second is whether neuroprotective concentrations of DHP can be achieved in the brain of PD patients.
Are L-type calcium channels necessary for pacemaking?
Several groups over the last 15 years have argued that voltage-dependent calcium channels were necessary (1, 78, 84, 125). Considering the sensitivity of pacemaking to DHPs, it has been inferred that these channels were L-type channels. In support of this view, SNc DA neurons robustly express L-type channels having a Cav1.3 pore-forming subunit with the kind of gating properties necessary to drive a sub-threshold membrane potential oscillation thought to underlie pacemaking (21, 43, 66). However, the concentrations of DHPs necessary to slow or stop pacemaking are more than three orders of magnitude higher than the equilibrium binding constant of most DHPs for Cav1.3 channels—raising basic questions about the necessity of these channels for pacemaking.
As a first step toward understanding what concentration of DHP is sufficient to antagonize L-type calcium channels in SNc DA neurons, a modulated receptor model was constructed using the framework proposed by Bean (8). In this model, the channel was assumed to have high and low affinity states governed by voltage-dependent inactivation (Fig. 3a). The macroscopic balance between these states was governed by voltage-dependent inactivation of the Cav1.3 calcium channel. Estimates of this parameter were taken from the work of Koschak et al. (66), in which channels were heterologously expressed in a cell type that was readily voltage-clamped (Fig. 3b). Because isradipine has a relatively high affinity for the Cav1.3 calcium channels thought to underlie pacemaking, our initial calculations modeled its actions (107). The apparent dissociation constant (Kapp) of isradipine as a function of membrane voltage was computed using the formula proposed by Bean (1984) by assuming that the dissociation constant (KD) for high affinity state was equal to that estimated from equilibrium binding studies (0.48 nM) and the dissociation constant for the low affinity state was a thousand fold higher (480 nM) (107) (Fig. 3c). Next, an all-points histogram of membrane potential during pacemaking was generated (Fig. 3d); this distribution had two modes: one near −60 mV and another near −40 mV. Considering this result, the relationship between isradipine concentration and the fraction of Cav1.3 channels available (not antagonized) was computed at − 50, −60, and (for comparison) −90 mV (Fig. 3e). This calculation showed that at either −50 or −60 mV, more than 90% of the Cav1.3 channels should be antagonized by 100 nM isradipine, whereas at −90 mV only about 30% of the channels should be antagonized. For the purposes of comparison, the dose–response curve for another commonly used, but less potent, DHP (nifedipine) was calculated at a potential of −60 mV. Considering this calculation, at equilibrium one micromolar nifedipine should antagonize >90% of the Cav1.3 channel population during pacemaking (Fig. 3f). These calculations strongly argue that at equilibrium, submicromolar concentrations of isradipine and other DHPs should effectively suppress Cav1.3 calcium channel currents and disrupt pacemaking that depends upon them. These calculations also show that serum concentrations of isradipine found in patients taking the medication for hypertension (∼5 nM) should antagonize roughly half of the Cav1.3 channels in SNc DA neurons if we assume equilibrium across the blood–brain barrier (BBB).
FIG. 3.
Nanomolar concentrations of isradipine should produce a significant antagonism of Cav1.3 calcium channels. (a) Model of the modulated receptor model of dihydropyridine drug action. C stands for a closed state of the channel; C* stands for a closed, drug-bound state. The dissociation constant (Kc) for this reaction is assumed to be 500 nM. I stands for the inactivated state of the channel. Transitions between C and I states are controlled by membrane voltage. I* stands for the inactivated, drug-bound state; the dissociation constant (Ki) for this reaction is assumed to be 0.5 nM. (b) Steady-state inactivation of Cav1.3 channels as a function of membrane voltage; this describes the transition of channels from the C to I states in (a). (c) Plot of the apparent dissociation constant as a function of transmembrane voltage computed from the equation Kapp = 1/[C/Kc + (1 − C)/Ki], where C is the fraction of the channels in the closed state from (b) and Kc and Ki are from (a). (d) Recording of an SNc DA neuron and an all-points histogram of membrane potential showing that these neurons reside at potentials more depolarized than −60 mV. (e) Plot of the fraction of available channels as a function of drug concentration for three different membrane potentials. The plots reveal that with a holding potential of −60 mV, roughly half the channels are antagonized by 5 nM isradipine; only a small percentage of the channels will be antagonized by this drug concentration if the membrane potential is at −90 mV (as would be the case in a striatal medium spiny neuron). (f) Plot of the fraction of Cav1.3 channels available as a function of isradipine concentration (green line) at −60 mV or of a lower affinity antagonist nifedipine (red line). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Based upon these results, we re-examined the role of Cav1.3 channels in pacemaking. The problem is that we needed a measure of Cav1.3 channel function that was independent of pacemaking. We turned to calcium imaging using two photon laser scanning microscopy, allowing us to monitor pacemaking neurons deep in a brain slice from the mesencephalon. In these dual recordings, the dendritic calcium concentration oscillate in phase with somatic spiking. Bath application of 200 nM isradipine for 20 min (to allow an equilibrium to be achieved 50–100 μm below the surface of the slice) eliminated the dendritic calcium oscillation, but had no impact on pacemaking rate—providing a clear dissociation between Cav1.3 channel opening and pacemaking.
Does this mean that Cav1.3 channels are completely superfluous? Modeling the pacemaking process revealed that there is a rich interplay of ionic conductances that lead to autonomous spiking. Many of the conductances play similar roles, so that deficiencies in one can be compensated for to maintain the correct spiking rate. For example, when Cav1.3 channels are blocked in our model, outward currents through small conductance calcium-dependent potassium channels decline and the trajectory of the after hyperpolarization changes leading to stronger engagement of cationic hyperpolarization activated cyclic nucleotide (HCN) channels. The net effect on pacemaking is minimal. Similarly, if HCN channels are blocked by themselves, Cav1.3 current increases to compensate. However, if both HCN and Cav1.3 channels are blocked, pacemaking stops because the cell cannot generate enough inward current near spike threshold.
This behavior of the model was verified experimentally in SNc DA neurons. The takeaway message is that SNc DA neurons are well designed, with a robust network of ion channels to support pacemaking. It is a fail-safe system because pacemaking is so important to the functioning of the basal ganglia.
Our contention that Cav1.3 channels are not necessary for pacemaking has been challenged in a recent article (97). The crux of their argument is that they can experimentally restart pacemaking that has been halted with high concentrations of DHP by using dynamic current clamp to reintroduce Cav1.3 channels into the soma. The basic problem with this argument is that there is nothing unique about the inward conductance created by the dynamic clamp; introducing a Nav1 channel or an HCN channel would produce the same outcome. So, in the end all the authors demonstrate that pacemaking is robust in the sense that it can be generated in several ways.
On the basis of findings from our group and others, the question that still remains is, could antagonizing L-type calcium channels prevent or slow PD in a normal lifespan? Calcium channel antagonists (CCAs), including the DHPs used in animal studies, are commonly used in clinical practice to treat hypertension, creating a potential database to be mined. A case–control study of hypertensive patients found a significant reduction in the observed risk of PD with CCA use, but not with medications that reduce blood pressure in other ways (9). More recently, a large Danish data set has been examined (100). The authors agreed with the main conclusions of the Becker et al. study but extended their findings by showing that only DHPs that cross the BBB are associated with reduced PD risk (∼30%). Given the short period of treatment in many cases (∼2 years), variable dosing, and low relative affinity of DHPs for Cav1.3 calcium channels (compared to Cav1.2 channels) (28, 67, 73), this is a surprisingly strong association and lends further credence to the proposition that a BBB permeable and potent Cav1.3 antagonist could be a very effective neuroprotective agent.
That said, these studies are not a substitute for a controlled clinical trial. In the absence of a selective Cav1.3 CCA, the DHP isradipine is the most attractive drug for such a trial. Isradipine has a relatively higher affinity for Cav1.3 calcium channels than the other known DHP and has good brain bioavailability (107). At the doses used to treat hypertension, isradipine has relatively minor side effects (31). The question is whether it will prove neuroprotective at doses tolerated by the general population. Pharmacokinetic studies by our group have found that serum concentrations of isradipine achieved in mice that are protected (∼1–2 ng/ml) against MPTP and 6-hydroxydopamine toxicity are very close to those achieved in humans with a very well-tolerated daily dose (10 mg/day, Dynacirc CR). As shown above, these isradipine concentrations should antagonize around half of the Cav1.3 channels in a pacemaking neuron, suggesting that neuroprotection is achievable.
It is also worth considering how DHPs might be used in combination with other drugs that are being tested in clinical neuroprotection trials for PD. Although early trials with creatine, coenzyme Q10, and other antioxidant supplements have been disappointing (51), they share the hypothesis that oxidant stress exacerbates the symptoms and progression of PD. Coenzyme Q10 is an electron acceptor for complexes I and II that appears compromised in PD patients (64, 106) and is neuroprotective in animal models of PD (6, 7). Creatine is a substrate for ATP production that can both improve mitochondrial efficiency and reduce oxidant stress by buffering fluctuations in cellular energy production (64). Both approaches are aimed at improving mitochondrial function rather than attacking the source of stress on mitochondria. Rasagline or deprenyl also could prove to have neuroprotective effects by virtue not of their ability to inhibit monoamine oxidase B, but by their ability to induce the expression of antioxidant defenses (72). Because their sites of action differ within the chain of events leading to oxidant stress and mitochondrial dysfunction, a combination therapy could prove more effective than any one therapy alone.
Conclusions
Even though pan-cellular risk factors dominate current thinking about the etiology of PD, there are compelling reasons to believe that cell-specific factors are important as well. From a theoretical standpoint, it is very difficult to explain the pattern of neuronal pathology in PD without these factors. While transmitter or anatomical phenotype might contribute to the vulnerability of SNc DA neurons, the trait with the clearest mechanistic path to cellular aging and degeneration is the engagement of L-type calcium channels in the generation of autonomous spiking. The sustained entry of calcium undoubtedly taxes the ATP-dependent pumps responsible for keeping its concentration low, and in so doing creates a burden on mitochondrial oxidative phosphorylation. An inevitable consequence of oxidative phosphorylation is the production of superoxide capable of damaging DNA and proteins. Although this metabolic stress is not sufficient to disable SNc DA neurons in the short run, it is possible that in the long run it exacerbates the normal aging-related decline in mitochondrial function, resulting in persistent energy shortages that compromise proteostatic competence. Because L-type channels are not necessary for SNc DA neurons to do their job, L-type channel antagonists seem to be a viable neuroprotective strategy. These drugs are well tolerated and safe, and their use is associated with a diminished risk of PD. Because this physiological phenotype is not unique to SNc DA neurons but appears to be shared by many of the neurons that succumb in PD, these antagonists could confer protection well beyond the SNc.
Abbreviations Used
- ADP
adenosine diphosphate
- ANT
adenine nucleotide transporter
- ATP
adenosine triphosphate
- BBB
blood–brain barrier
- CCAs
calcium channel antagonists
- DA
dopamine
- DHP
dihydropyridines
- DMV
dorsal motor nucleus of the vagus
- ER
endoplasmic reticulum
- ETC
electron transport chain
- Glu
glutamate
- HCN
hyperpolarization activated cyclic nucleotide
- IP3R
inositol triphosphate receptors
- LB
Lewy body
- LC
locus ceruleus
- L-DOPA
levodopa or L-3,4-dihydroxy-phenylalanine
- LRRK2
leucine-rich repeat kinase 2
- MAM
mitochondrial-associated membrane
- mGluR
metabotropic glutamate receptors
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxin
- mPTP
mitochondrial permeability transition pore
- Na-K ATPase
sodium/potassium ATPase
- NCX
sodium/calcium exchanger
- NMDA
N-methyl-D-aspartate
- PD
Parkinson's disease
- Pi
inorganic phosphate
- PINK-1
PTEN-induced putative kinase 1
- PLC
phospholipase C
- PMCA
plasma membrane calcium ATPase
- ROS
reactive oxygen species
- RyRs
ryanodine receptors
- SNc
substantia nigra pars compacta
- SNCA
gene name for alpha-synuclein
- TCA
tricarboxylic acid
- TTX
tetrodotoxin
- UCP
uncoupling proteins
- UP
mitochondrial calcium uniporter
- VDAC
voltage-dependent anion channel
References
- 1.Amini B. Clark JW., Jr. Canavier CC. Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol. 1999;82:2249–2261. doi: 10.1152/jn.1999.82.5.2249. [DOI] [PubMed] [Google Scholar]
- 2.Andres-Mateos E. Perier C. Zhang L. Blanchard-Fillion B. Greco TM. Thomas B. Ko HS. Sasaki M. Ischiropoulos H. Przedborski S. Dawson TM. Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbuthnott GW. Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Backman L. Ginovart N. Dixon RA. Wahlin TB. Wahlin A. Halldin C. Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 6.Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 7.Beal MF. Matthews RT. Tieleman A. Shults CW. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3,tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998;783:109–114. doi: 10.1016/s0006-8993(97)01192-x. [DOI] [PubMed] [Google Scholar]
- 8.Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984;81:6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker C. Jick SS. Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- 10.Bender A. Krishnan KJ. Morris CM. Taylor GA. Reeve AK. Perry RH. Jaros E. Hersheson JS. Betts J. Klopstock T. Taylor RW. Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 11.Bender A. Schwarzkopf RM. McMillan A. Krishnan KJ. Rieder G. Neumann M. Elstner M. Turnbull DM. Klopstock T. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol. 2008;255:1231–1235. doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ. Lipp P. Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 13.Betarbet R. Sherer TB. MacKenzie G. Garcia-Osuna M. Panov AV. Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 14.Blythe SN. Wokosin D. Atherton JF. Bevan MD. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 2009;29:15531–15541. doi: 10.1523/JNEUROSCI.2961-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonci A. Grillner P. Mercuri NB. Bernardi G. L-Type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J Neurosci. 1998;18:6693–6703. doi: 10.1523/JNEUROSCI.18-17-06693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boumezbeur F. Mason GF. de Graaf RA. Behar KL. Cline GW. Shulman GI. Rothman DL. Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braak H. Ghebremedhin E. Rub U. Bratzke H. Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 18.Burke RE. Dauer WT. Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burlhis TM. Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- 20.Calne DB. Langston JW. Aetiology of Parkinson's disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- 21.Chan CS. Guzman JN. Ilijic E. Mercer JN. Rick C. Tkatch T. Meredith GE. Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 22.Clark IE. Dodson MW. Jiang C. Cao JH. Huh JR. Seol JH. Yoo SJ. Hay BA. Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 23.Collier TJ. Lipton J. Daley BF. Palfi S. Chu Y. Sortwell C. Bakay RA. Sladek JR., Jr. Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damier P. Hirsch EC. Agid Y. Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122((Pt 8)):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 25.de Lau LM. Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 26.Deister CA. Teagarden MA. Wilson CJ. Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci. 2009;29:15888–15897. doi: 10.1523/JNEUROSCI.4053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorsey ER. Constantinescu R. Thompson JP. Biglan KM. Holloway RG. Kieburtz K. Marshall FJ. Ravina BM. Schifitto G. Siderowf A. Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberg MJ. Brox A. Bestawros AN. Calcium channel blockers: an update. Am J Med. 2004;116:35–43. doi: 10.1016/j.amjmed.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Exner N. Treske B. Paquet D. Holmstrom K. Schiesling C. Gispert S. Carballo-Carbajal I. Berg D. Hoepken HH. Gasser T. Kruger R. Winklhofer KF. Vogel F. Reichert AS. Auburger G. Kahle PJ. Schmid B. Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 31.Fitton A. Benfield P. Isradipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs. 1990;40:31–74. doi: 10.2165/00003495-199040010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Fronczek R. Overeem S. Lee SY. Hegeman IM. van Pelt J. van Duinen SG. Lammers GJ. Swaab DF. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 33.Galvin JE. Uryu K. Lee VM. Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasser T. Mendelian forms of Parkinson's disease. Biochim Biophys Acta. 2009;1792:587–596. doi: 10.1016/j.bbadis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 35.German DC. Manaye KF. Sonsalla PK. Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 36.German DC. Manaye KF. White CL., 3rd Woodward DJ. McIntire DD. Smith WK. Kalaria RN. Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- 37.Gibb WR. Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991;54:388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg MS. Pisani A. Haburcak M. Vortherms TA. Kitada T. Costa C. Tong Y. Martella G. Tscherter A. Martins A. Bernardi G. Roth BL. Pothos EN. Calabresi P. Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 39.Grace AA. Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenamyre JT. Hastings TG. Biomedicine. Parkinson's—divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- 41.Greenamyre JT. O'Brien CF. N-methyl-D-aspartate antagonists in the treatment of Parkinson's disease. Arch Neurol. 1991;48:977–981. doi: 10.1001/archneur.1991.00530210109030. [DOI] [PubMed] [Google Scholar]
- 42.Greene JC. Whitworth AJ. Kuo I. Andrews LA. Feany MB. Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzman JN. Sanchez-Padilla J. Chan CS. Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 45.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann A. Hunot S. Hirsch EC. Inflammation and dopaminergic neuronal loss in Parkinson's disease: a complex matter. Exp Neurol. 2003;184:561–564. doi: 10.1016/j.expneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Hawkes CH. Del Tredici K. Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirsch EC. Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 49.Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–964. [PubMed] [Google Scholar]
- 50.Huisman E. Uylings HB. Hoogland PV. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson's disease patients. Mov Disord. 2008;23:1407–1413. doi: 10.1002/mds.22009. [DOI] [PubMed] [Google Scholar]
- 51.Hung AY. Schwarzschild MA. Clinical trials for neuroprotection in Parkinson's disease: overcoming angst and futility? Curr Opin Neurol. 2007;20:477–483. doi: 10.1097/WCO.0b013e32826388d6. [DOI] [PubMed] [Google Scholar]
- 52.Hunot S. Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–S58. doi: 10.1002/ana.10481. discussion S58–S60. [DOI] [PubMed] [Google Scholar]
- 53.Hunot S. Vila M. Teismann P. Davis RJ. Hirsch EC. Przedborski S. Rakic P. Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa T. Dhawan V. Kazumata K. Chaly T. Mandel F. Neumeyer J. Margouleff C. Babchyck B. Zanzi I. Eidelberg D. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–1765. [PubMed] [Google Scholar]
- 55.Ito H. Goto S. Sakamoto S. Hirano A. Calbindin-D28k in the basal ganglia of patients with parkinsonism. Ann Neurol. 1992;32:543–550. doi: 10.1002/ana.410320410. [DOI] [PubMed] [Google Scholar]
- 56.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 58.Kahle PJ. Waak J. Gasser T. DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Kamel F. Tanner C. Umbach D. Hoppin J. Alavanja M. Blair A. Comyns K. Goldman S. Korell M. Langston J. Ross G. Sandler D. Pesticide exposure and self-reported Parkinson's disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 60.Kanaan NM. Kordower JH. Collier TJ. Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J Comp Neurol. 2007;502:683–700. doi: 10.1002/cne.21333. [DOI] [PubMed] [Google Scholar]
- 61.Kanaan NM. Kordower JH. Collier TJ. Age-related changes in dopamine transporters and accumulation of 3-nitrotyrosine in rhesus monkey midbrain dopamine neurons: relevance in selective neuronal vulnerability to degeneration. Eur J Neurosci. 2008;27:3205–3215. doi: 10.1111/j.1460-9568.2008.06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kish SJ. Shannak K. Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 63.Kitada T. Pisani A. Porter DR. Yamaguchi H. Tscherter A. Martella G. Bonsi P. Zhang C. Pothos EN. Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klivenyi P. Ferrante RJ. Matthews RT. Bogdanov MB. Klein AM. Andreassen OA. Mueller G. Wermer M. Kaddurah-Daouk R. Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 65.Kordower JH. Chu Y. Hauser RA. Freeman TB. Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 66.Koschak A. Reimer D. Huber I. Grabner M. Glossmann H. Engel J. Striessnig J. Alpha 1D (Cav1.3) subunits can form l-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 67.Kupsch A. Sautter J. Schwarz J. Riederer P. Gerlach M. Oertel WH. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 1996;741:185–196. doi: 10.1016/s0006-8993(96)00917-1. [DOI] [PubMed] [Google Scholar]
- 68.Lees AJ. Hardy J. Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 69.Li JY. Englund E. Holton JL. Soulet D. Hagell P. Lees AJ. Lashley T. Quinn NP. Rehncrona S. Bjorklund A. Widner H. Revesz T. Lindvall O. Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 70.Li Y. Gao XB. Sakurai T. van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 71.Liang CL. Wang TT. Luby-Phelps K. German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson's disease. Exp Neurol. 2007;203:370–380. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 72.Magyar K. Szende B. (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 2004;25:233–242. doi: 10.1016/S0161-813X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 73.Mannhold R. Rekker RF. Sonntag C. ter Laak AM. Dross K. Polymeropoulos EE. Comparative evaluation of the predictive power of calculation procedures for molecular lipophilicity. J Pharm Sci. 1995;84:1410–1419. doi: 10.1002/jps.2600841206. [DOI] [PubMed] [Google Scholar]
- 74.Matsuda W. Furuta T. Nakamura KC. Hioki H. Fujiyama F. Arai R. Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matzuk MM. Saper CB. Preservation of hypothalamic dopaminergic neurons in Parkinson's disease. Ann Neurol. 1985;18:552–555. doi: 10.1002/ana.410180507. [DOI] [PubMed] [Google Scholar]
- 76.McCormack AL. Di Monte DA. Delfani K. Irwin I. DeLanney LE. Langston WJ. Janson AM. Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol. 2004;471:387–395. doi: 10.1002/cne.20036. [DOI] [PubMed] [Google Scholar]
- 77.Mendez I. Vinuela A. Astradsson A. Mukhida K. Hallett P. Robertson H. Tierney T. Holness R. Dagher A. Trojanowski JQ. Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mercuri NB. Bonci A. Calabresi P. Stratta F. Stefani A. Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br J Pharmacol. 1994;113:831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore DJ. West AB. Dawson VL. Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 80.Morikawa H. Khodakhah K. Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosharov EV. Larsen KE. Kanter E. Phillips KA. Wilson K. Schmitz Y. Krantz DE. Kobayashi K. Edwards RH. Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murchison D. Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell. 2007;6:297–305. doi: 10.1111/j.1474-9726.2007.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murchison D. Griffith WH. Low-voltage activated calcium currents increase in basal forebrain neurons from aged rats. J Neurophysiol. 1995;74:876–887. doi: 10.1152/jn.1995.74.2.876. [DOI] [PubMed] [Google Scholar]
- 84.Nedergaard S. Flatman JA. Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- 85.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 86.Olanow CW. Gracies JM. Goetz CG. Stoessl AJ. Freeman T. Kordower JH. Godbold J. Obeso JA. Clinical pattern and risk factors for dyskinesias following fetal nigral transplantation in Parkinson's disease: a double blind video-based analysis. Mov Disord. 2009;24:336–343. doi: 10.1002/mds.22208. [DOI] [PubMed] [Google Scholar]
- 87.Orrenius S. Zhivotovsky B. Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 88.Palacino JJ. Sagi D. Goldberg MS. Krauss S. Motz C. Wacker M. Klose J. Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 89.Park J. Lee SB. Lee S. Kim Y. Song S. Kim S. Bae E. Kim J. Shong M. Kim JM. Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 90.Pignatelli A. Ackman JB. Vigetti D. Beltrami AP. Zucchini S. Belluzzi O. A potential reservoir of immature dopaminergic replacement neurons in the adult mammalian olfactory bulb. Pflugers Arch. 2009;457:899–915. doi: 10.1007/s00424-008-0535-0. [DOI] [PubMed] [Google Scholar]
- 91.Pignatelli A. Kobayashi K. Okano H. Belluzzi O. Functional properties of dopaminergic neurones in the mouse olfactory bulb. J Physiol. 2005;564:501–514. doi: 10.1113/jphysiol.2005.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ping HX. Shepard PD. Apamin-sensitive Ca(2+)-activated K+ channels regulate pacemaker activity in nigral dopamine neurons. Neuroreport. 1996;7:809–814. doi: 10.1097/00001756-199602290-00031. [DOI] [PubMed] [Google Scholar]
- 93.Pinton P. Rimessi A. Marchi S. Orsini F. Migliaccio E. Giorgio M. Contursi C. Minucci S. Mantovani F. Wieckowski MR. Del Sal G. Pelicci PG. Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 94.Postuma RB. Lang AE. Massicotte-Marquez J. Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–851. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 95.Przedborski S. Tieu K. Perier C. Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 96.Puopolo M. Raviola E. Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Putzier I. Kullmann PH. Horn JP. Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reeve AK. Krishnan KJ. Turnbull DM. Age related mitochondrial degenerative disorders in humans. Biotechnol J. 2008;3:750–756. doi: 10.1002/biot.200800066. [DOI] [PubMed] [Google Scholar]
- 99.Riederer P. Wuketich S. Time course of nigrostriatal degeneration in Parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 100.Ritz B. Qian L. Rhodes SL. Schernhammer E. Olsen J. Friis S. L-Type calcium channel blockers and Parkinson's disease in Denmark. Ann Neurol. 2010;67:600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rizzuto R. Intracellular Ca(2+) pools in neuronal signalling. Curr Opin Neurobiol. 2001;11:306–311. doi: 10.1016/s0959-4388(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 102.Romo R. Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- 103.Saper CB. Sorrentino DM. German DC. de Lacalle S. Medullary catecholaminergic neurons in the normal human brain and in Parkinson's disease. Ann Neurol. 1991;29:577–584. doi: 10.1002/ana.410290602. [DOI] [PubMed] [Google Scholar]
- 104.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 105.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 106.Shults CW. Haas RH. Passov D. Beal MF. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol. 1997;42:261–264. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- 107.Sinnegger-Brauns MJ. Huber IG. Koschak A. Wild C. Obermair GJ. Einzinger U. Hoda JC. Sartori SB. Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 2009;75:407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- 108.Sonsalla PK. Albers DS. Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids. 1998;14:69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- 109.Stark AK. Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 2004;318:81–92. doi: 10.1007/s00441-004-0972-9. [DOI] [PubMed] [Google Scholar]
- 110.Stevens DR. Haas HL. Calcium-dependent prepotentials contribute to spontaneous activity in rat tuberomammillary neurons. J Physiol. 1996;493((Pt 3)):747–754. doi: 10.1113/jphysiol.1996.sp021419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Striessnig J. Koschak A. Sinnegger-Brauns MJ. Hetzenauer A. Nguyen NK. Busquet P. Pelster G. Singewald N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–909. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- 112.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Taddese A. Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 114.Takakusaki K. Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience. 1997;78:771–794. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 115.Tanner CM. Ross GW. Jewell SA. Hauser RA. Jankovic J. Factor SA. Bressman S. Deligtisch A. Marras C. Lyons KE. Bhudhikanok GS. Roucoux DF. Meng C. Abbott RD. Langston JW. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol. 2009;66:1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- 116.Tansey MG. Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teismann P. Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 118.Teismann P. Tieu K. Choi DK. Wu DC. Naini A. Hunot S. Vila M. Jackson-Lewis V. Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teismann P. Tieu K. Cohen O. Choi DK. Wu DC. Marks D. Vila M. Jackson-Lewis V. Przedborski S. Pathogenic role of glial cells in Parkinson's disease. Mov Disord. 2003;18:121–129. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- 120.Tepper JM. Sawyer SF. Groves PM. Electrophysiologically identified nigral dopaminergic neurons intracellularly labeled with HRP: light-microscopic analysis. J Neurosci. 1987;7:2794–2806. doi: 10.1523/JNEUROSCI.07-09-02794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thannickal TC. Lai YY. Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Travagli RA. Gillis RA. Hyperpolarization-activated currents, IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol. 1994;71:1308–1317. doi: 10.1152/jn.1994.71.4.1308. [DOI] [PubMed] [Google Scholar]
- 123.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams JT. North RA. Shefner SA. Nishi S. Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- 125.Wilson CJ. Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol. 2000;83:3084–3100. doi: 10.1152/jn.2000.83.5.3084. [DOI] [PubMed] [Google Scholar]
- 126.Yamanaka A. Beuckmann CT. Willie JT. Hara J. Tsujino N. Mieda M. Tominaga M. Yagami K. Sugiyama F. Goto K. Yanagisawa M. Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 127.Zweifel LS. Parker JG. Lobb CJ. Rainwater A. Wall VZ. Fadok JP. Darvas M. Kim MJ. Mizumori SJ. Paladini CA. Phillips PE. Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]