Abstract
Background
Clinical guidelines advocate the routine identification of depressive symptoms for patients with pain in the lumbar or cervical spine, but not for other anatomical regions.
Objective
The purpose of this study was to investigate the prevalence and impact of depressive symptoms for patients with musculoskeletal pain across different anatomical regions.
Design
This was a prospective, associational study.
Methods
Demographic, clinical, depressive symptom (Symptom Checklist 90–Revised), and outcome data were collected by self-report from a convenience sample of 8,304 patients. Frequency of severe depressive symptoms was assessed by chi-square analysis for demographic and clinical variables. An analysis of variance examined the influence of depressive symptoms and anatomical region on intake pain intensity and functional status. Separate hierarchical multiple regression models by anatomical region examined the influence of depressive symptoms on clinical outcomes.
Results
Prevalence of severe depression was higher in women, in industrial and pain clinics, and in patients who reported chronic pain or prior surgery. Lower prevalence rates were found in patients older than 65 years and those who had upper- or lower-extremity pain. Depressive symptoms had a moderate to large effect on pain ratings (Cohen d=0.55–0.87) and a small to large effect on functional status (Cohen d=0.28–0.95). In multivariate analysis, depressive symptoms contributed additional variance to pain intensity and functional status for all anatomical locations, except for discharge values for the cervical region.
Conclusions
Rates of depressive symptoms varied slightly based on anatomical region of musculoskeletal pain. Depressive symptoms had a consistent detrimental influence on outcomes, except on discharge scores for the cervical anatomical region. Expanding screening recommendations for depressive symptoms to include more anatomical regions may be indicated in physical therapy settings.
Depression is recognized as one of the leading causes of disability in the United States and affects approximately 5% to 10% of the American general population.1,2 The point prevalence of pain and depressive symptoms occurring at the same time (commonly referred to as “comorbid pain and depression”) is variable across practice settings. For example, Bair et al3 reported comorbid pain and depressive symptoms ranging from 13% to 85%, with lower rates reported in gynecological clinics and higher rates reported in dental and orofacial pain clinics. The variability in reported prevalence rates could be related to the patient population,3 the nature of the pain disorder,4 or the methods by which depressive symptoms are identified.5,6
In primary care settings, it has been estimated that 27% of patients with musculoskeletal pain conditions also have complaints consistent with major depressive symptoms.3 Researchers who investigated the prevalence of depressive symptoms among patients seeking treatment for musculoskeletal pain in physical therapy settings reported prevalence rates similar to or higher than those in primary care settings.7–10 For example, Hope and Forshaw8 and Haggman et al7 reported that 26% and 40% of patients with low back pain in physical therapy settings, respectively, exhibited depressive signs or symptoms. Higher prevalence rates for depressive symptoms were reported by Werneke and Hart10 (46%) and Lundberg et al9 (47%) for patients seen by physical therapists for low back pain and general musculoskeletal pain, respectively.
Physical therapists are sometimes first-contact clinicians for patients with musculoskeletal pain. Screening is important in that environment because of a strong association of depressive symptoms with poor treatment outcomes, increased medical resource utilization, and decreased work productivity for patients with chronic musculoskeletal pain.11–13 Furthermore, identifying patients who are at high risk for clinical depression is a key component in determining whether referral to another medical provider14,15 or modification of the physical therapy treatment plan is warranted.10,16 Screening for depressive symptoms by physical therapists with the goal of improving patient outcomes is consistent with a secondary prevention approach for limiting the development of chronic musculoskeletal pain syndromes.17 In secondary prevention, biopsychosocial models often are used to guide early identification of psychological distress that can be a predictor of poor patient outcome.18–20 For example, the fear-avoidance model of musculoskeletal pain describes key psychological processes potentially involved in the development of chronic pain.21,22 This model focuses on pain-related fear and catastrophizing; however, it also provides mechanisms that link depressive symptoms with the development of disability.
Specific to the purposes of the current study, the prevalence and impact of depressive symptoms in physical therapy settings have been considered for patients with pain in the low back7,8 but not widely reported for other anatomical regions. We believe investigation of depressive symptoms and pain in other anatomical regions is warranted to further inform physical therapist practice on which patient populations may be most appropriate for screening. Empirical support for our belief comes from a recent population study by Miller and Cano,2 who reported similar depressive symptom rates among all anatomical regions of musculoskeletal pain. These data suggest that there was no predilection for depressive symptoms among those with lumbar or cervical spine pain, yet these are the anatomical regions where identification of depressive symptoms often is emphasized.23–27 Studies that compare depressive symptoms across multiple anatomical regions will provide information on whether screening for patients with musculoskeletal pain is appropriate for subgroups (eg, lumbar or cervical spine pain) or across multiple anatomical regions.
Therefore, the purpose of this study was to investigate the prevalence and impact of depressive symptoms for patients seeking outpatient physical for musculoskeletal pain in 4 different anatomical regions. To investigate whether depressive symptoms had varying influence based on anatomical region of musculoskeletal pain, the aims of this study were: (1) to determine whether rates of severe depressive symptoms differed based on select demographic or clinical factors, (2) to investigate how depressive symptoms influenced intake pain intensity ratings and functional status reports, and (3) to examine how depressive symptoms affected treatment parameters and clinical outcomes. Consistent with our aims, analyses were stratified to determine whether depressive symptoms had differing prevalence and impact based on the anatomical region of musculoskeletal pain.
Method
Overview
This study was an analysis of data collected prospectively from outpatient physical therapy clinics participating with Focus On Therapeutic Outcomes, Inc (FOTO).28–30 Demographic, clinical, and functional status data were collected by self-report from patients with common musculoskeletal diagnoses by use of Patient Inquiry software.* The use of this software for clinical data collection has been described previously.10,28,31–33 These data were collected at initial evaluation (intake) and at the end of rehabilitation (discharge).
Participants
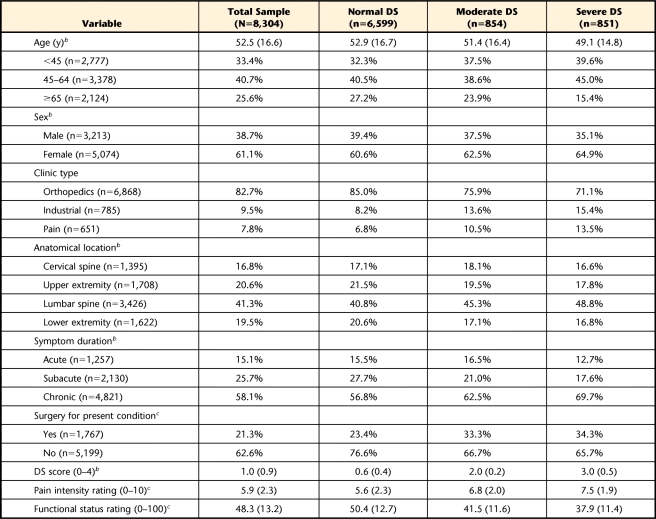
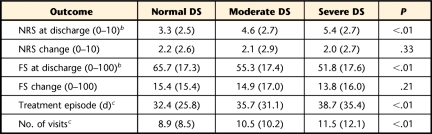
A sample of convenience was collected from 86 outpatient physical therapy clinics in 25 states (in the United States) between July 2003 and July 2009. Patients were selected from the FOTO database based on intake completion of the Symptom Checklist 90–Revised (SCL-90-R) depression scale, resulting in a sample size of 8,304 for this analysis. Descriptive statistics for this sample are reported in Table 1.
Table 1.
Intake Characteristics for Patients Seeking Outpatient Physical Therapy for Musculoskeletal Pain Conditionsa
All data reported as mean (SD) or percentage. DS score=SCL-90-R depression scale score, normal depressive symptoms (DS)=1st–79th percentiles, moderate DS=80th–89th percentiles, severe DS=90th–99th percentiles.
b Missing data less than 5% for category.
c Missing data less than 20% for category.
Measures
Demographic.
Data for age (below 45 years, 45–64 years, and 65 years and older) and sex (male and female) were collected at intake.
Clinical.
Data for type of clinic referred to (orthopedic, industrial, or pain clinic), duration of symptoms (acute [14 days or less], subacute [15–90 days], or chronic [91 days or longer]), and whether musculoskeletal pain was postsurgical (yes or no) were collected at intake.
Anatomical region.
The primary musculoskeletal complaint that led patients to seek physical therapy services was used to code anatomical region. These data were taken from the initial examination when the treating clinician identified the body part affiliated with the primary complaint and that was to be the focus of physical therapy treatment. The individual body regions then were collapsed into 4 anatomical regions to account for the major categories of musculoskeletal pain (cervical, upper extremity, lumbar, and lower extremity).
Depressive symptoms.
The severity of depressive symptoms was assessed with the SCL-90-R depression scale.34,35 The SCL-90-R depression scale has 10 items, with each symptom item rated from 0 (low severity) to 4 (high severity). Individual SCL-90-R depression scale items range from 0 (“not at all depressed”) to 4 (“extremely depressed”). The total score is calculated from the arithmetic mean (total score/10), resulting in a potential range from 0 to 4. Examples of items from the SCL-90-R depression scale include: “In the last day, how much were you distressed by feeling no interest in things?” and “In the last day, how much were you distressed by a feeling of worthlessness?” This scale was used in 2 forms for the purposes of this study. In categorical analyses, the scale was separated into different levels of depressive symptom severity based on previous population normative values36 and referred to as “DS severity” for this report. Normal depressive symptoms were defined as those from 0 to the 79th percentile, moderate symptoms were those from the 80th percentile to the 89th percentile, and severe depressive symptoms were those scoring at the 90th percentile and above. These definitions have been used in previous studies using the SCL-90-R.36–38 In continuous analyses, the SCL-90-R scale was calculated and presented as an averaged item response. This procedure resulted in a total range of 0 to 4 for depressive symptoms and was referred to as “DS score” for this report. The SCL-90-R depression scale scores were collected at intake.
Treatment parameters.
Data for duration of treatment episode (number of calendar days between intake and discharge) and visits to rehabilitation (number of visits) were collected at discharge. Types of interventions were not assessed as part of these analyses, as we had no a priori hypotheses about how depressive symptoms would interact with specific physical therapy interventions.
Outcome measures.
Two clinical outcome measures were included in these analyses. A 0 (“no pain”) to 10 (“worst pain imaginable”) 11-point numerical rating scale (NRS) was used for pain intensity.39,40 In the analyses for this report, we used the current pain intensity rating (ie, pain intensity reported during the physical therapist's examination) from the intake and discharge sessions. Previous psychometric studies have supported the NRS for use as an outcome measure in musculoskeletal pain studies.41–43 Patient self-report of functional status was assessed using computerized adaptive testing methods, as described in previous psychometric studies supporting the use of functional status as an outcome measure.32,43–52 The functional status estimates in the current study ranged from 0 (“very low functioning”) to 100 (“very high functioning”) on a linear metric. In the current analyses, we used the functional status estimate from the intake and discharge sessions.
Data Analysis
All analyses were completed with SPSS statistical software, version 17.0.† Alpha level was set at .01 due to the large sample size and the number of planned analyses. Descriptive analyses were generated and reported in the appropriate metric for continuous and categorical data.
Aim 1.
Severe depressive symptom (ie, scoring at the 90th percentile and above) rates were compared by chi-square analysis for the demographic, clinical, and anatomical region variables. Crude odds ratios (ORs) with 95% confidence intervals (CIs) also were calculated for these variables to generate a clinically relevant magnitude estimate.
Aim 2.
Differences in intake pain intensity and functional levels by DS severity and anatomical region were first investigated by analysis of variance and Bonferroni post hoc testing, as appropriate. This testing was done to determine whether depressive symptoms and anatomical region were main effects or had potential for an interaction effect on these measures. The influence of depressive symptoms on intake pain intensity and functional levels was further investigated by hierarchical multiple regression. Consistent with the aims of this study, individual regression models were created by anatomical region so the contribution of depressive symptoms could be calculated for each anatomical category. The first step of the regression model included demographic and clinical variables (ie, age, sex, clinic type, duration of symptoms, and surgical status), and the second step included the DS score. Separate regression models were created by anatomical region for the dependent variables of NRS and functional status scores.
Aim 3.
Differences in treatment parameters and clinical outcomes by DS severity and anatomical region were first investigated by analysis of variance and post hoc testing, as appropriate. In the initial univariate analyses discharge scores, NRS and functional status change scores (intake to discharge), duration of treatment episode, and number of visits were the dependent variables. Depressive symptoms' influence on discharge pain intensity ratings and functional status scores was further investigated by hierarchical multiple regression models so appropriate confounding factors (ie, age, sex, clinic type, duration of symptoms, and surgical status) could be accounted for in the multivariate analysis. Again, the regression analyses were done separately by anatomical region so that the contribution of depressive symptoms could be calculated for each anatomical category. The first step of the regression model included demographic and clinical variables, as well as the baseline NRS or functional status score (depending upon the model). The second step of the regression model included the DS score, and separate regression models were created by anatomical region for the dependent variables of discharge NRS and functional status scores.
Role of the Funding Source
This study was supported by the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (grant HD043730).
Results
Aim 1
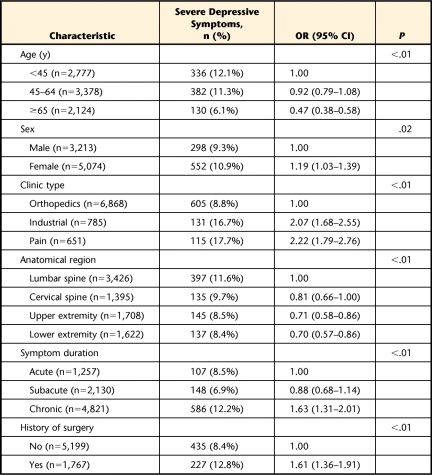
Variability in DS severity rates based on demographic and clinical data is reported in Table 2. Increased frequency of reporting severe depressive symptoms was associated with being female (OR=1.19), seeking treatment at an industrial clinic (OR=2.07) or pain clinic (OR=2.22), having chronic symptoms (OR=1.63), and having surgery for the present condition (OR=1.61). Decreased frequency of reporting severe depressive symptoms was associated with the 65 years and older age group (OR=0.47) and having musculoskeletal pain in the upper extremity (OR=0.71) or the lower extremity (OR=0.70).
Table 2.
Univariate Association of Intake Severe Depressive Symptoms With Demographic and Clinical Factorsa
OR=odds ratio, 95% CI=95% confidence interval, severe depressive symptoms=90th–99th percentiles.
Aim 2
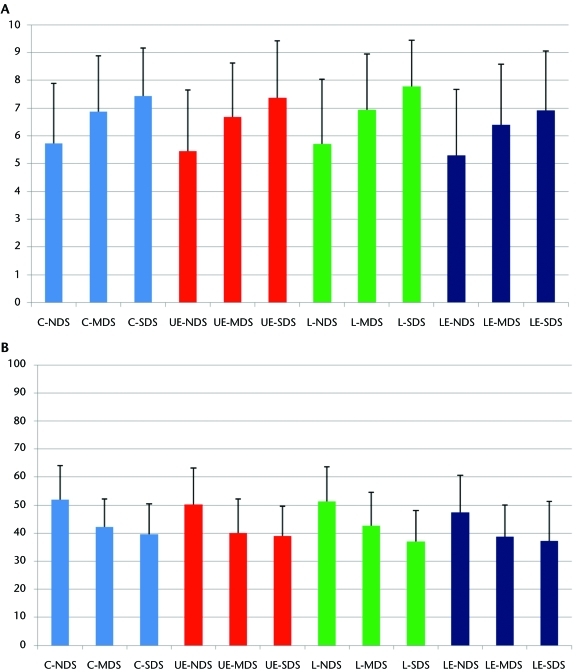
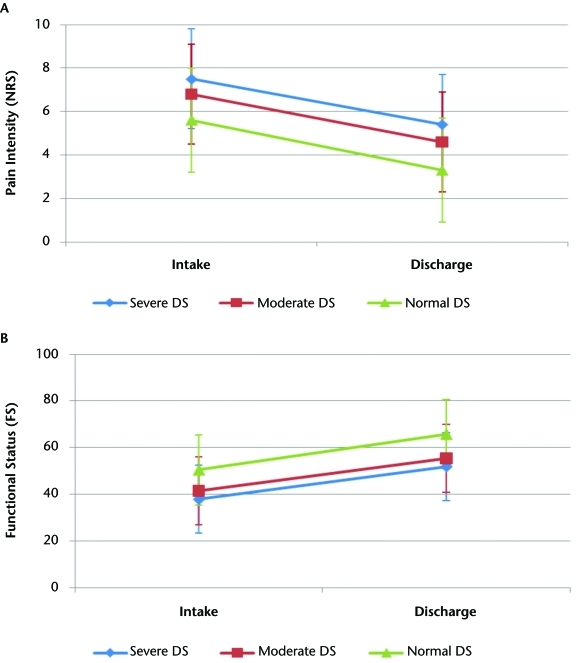
There was no interaction between DS severity and anatomical region for intake pain intensity and functional status. There were main effects for both DS severity and anatomical region (P<.01 for both main effects). These results are depicted graphically in Figures 1A and 1B. Higher mean pain intensity scores were associated with the lumbar (6.07) and cervical (6.02) spine categories, in comparison with the upper-extremity (5.88) and lower-extremity (5.54) categories. The effects from anatomical region were small, ranging from 0.05 to 0.24 (Cohen d).53 Severe depressive symptoms had the highest mean pain intensity rating (7.48), followed by moderate depressive symptoms (6.78) and normal depressive symptoms (5.57). The effects from DS severity were moderate to large, ranging from 0.55 to 0.87 (Cohen d).53 The lowest mean functional status score was reported for the lower-extremity category (45.8), whereas the highest mean functional status score was reported for the cervical spine category (49.9). The upper-extremity (48.4) and lumbar (48.9) categories had similar mean functional status scores. The effects from anatomical region were small, ranging from 0.04 to 0.31 (Cohen d).53 Severe depressive symptoms had the lowest mean functional status score (37.9), followed by moderate depressive symptoms (41.5) and normal depressive symptoms (50.4). The effects from DS severity were small to large, ranging from 0.28 to 0.95 (Cohen d).53
Figure 1.
Depressive symptoms category and anatomical location: (A) association with intake pain intensity (0–10 scale); (B) association with intake functional status (0–100 scale). C=cervical (blue); UE=upper-extremity (red); L=lumbar (green); LE=lower extremity (black); NDS=normal depressive symptoms (1st–79th percentiles); MDS=moderate depressive symptoms (80th–89th percentiles); SDS=severe depressive symptoms (90th–99th percentiles).
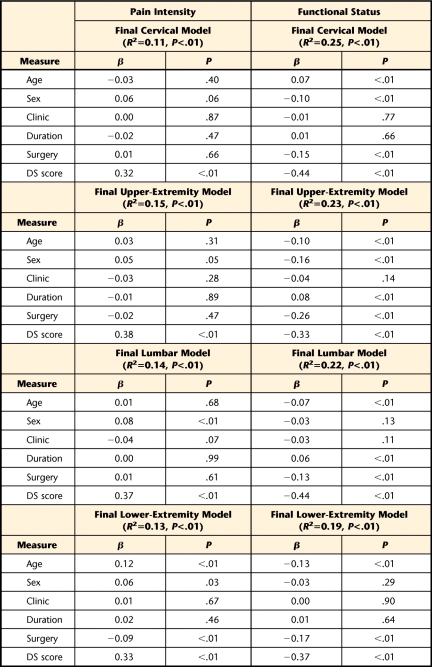
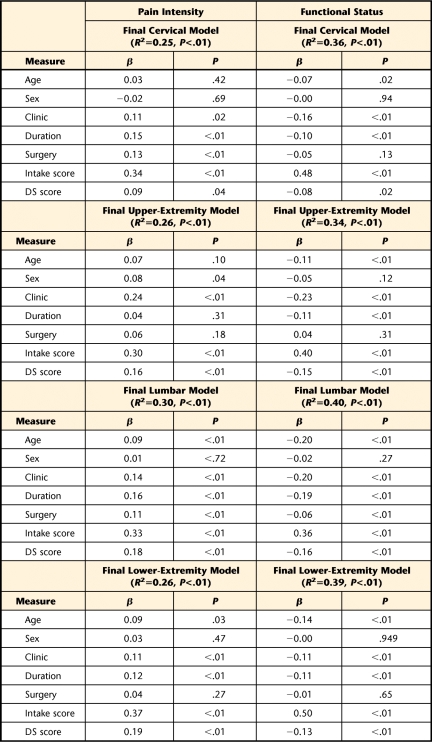
The final hierarchical regression models reporting overall variance for intake pain intensity ratings and functional status are reported in Table 3. Differences in variance explained by step 1 (demographic and clinical variables) and step 2 (depressive symptoms) are reported in the text for the pain intensity and functional status models. In the first step, demographic and clinical variables contributed 1.3%, 1.1%, 1.7%, and 2.6% variance to intake pain intensity scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the second step, the DS score contributed 10.0%, 13.5%, 12.6%, and 10.4% additional variance for intake pain intensity scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the first step, demographic and clinical variables contributed 6.7%, 12.8%, 4.7%, and 5.7% variance in intake functional scores to cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the second step, the DS score contributed 18.6%, 10.4%, 17.8%, and 13.2% additional variance to intake functional scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. Presence of depressive symptoms was a statistically significant addition to all anatomical models, and in the final models, depressive symptom was the strongest individual contributor to intake pain intensity ratings and functional status scores for all anatomical locations (Tab. 3).
Table 3.
Depressive Symptoms' Contribution to Intake Pain Intensity Ratings and Functional Status Scores for all Anatomical Locationsa
Age entered as continuous measure; sex coded 0=male, 1=female, clinic coded 0=orthopedic clinic, 1=pain or industrial clinic; duration coded 0=acute or subacute, 1=chronic; surgery coded 0=no surgery for present condition, 1=surgery for present condition; DS score=SCL-90-R depression scale score.
Aim 3
The mean duration of the treatment episode was 33.3 days (SD=27.4), and the mean number of visits was 9.3 (SD=9.0). Variability in treatment parameters and clinical outcomes based on depressive symptom category is reported in Table 4. These results were similar across anatomical region, so only collapsed data were reported for ease of interpretation. There was a strong association among discharge pain intensity, functional status, and depressive symptoms. Depressive symptom severity was associated with the highest pain intensity and lowest functional status, with significant differences between each depressive symptom category. Depressive symptom severity was not associated with changes in outcome, as all groups reported similar amounts of change for pain intensity and functional status. The results for pain intensity ratings and functional status scores are reported graphically in Figures 2A and 2B. Finally, severe and moderate depressive symptoms were associated with longer treatment duration and more clinic visits, in comparison with normal depressive symptoms. There were no statistical differences in treatment duration or visits for those participants with severe and moderate depressive symptoms.
Table 4.
Univariate Association of Intake Depressive Symptoms With Clinical Outcomesa
All data reported as mean (SD). NRS=numerical rating scale for pain intensity; FS=functional status assessed by computerized adaptive testing; normal depressive symptoms (DS)=1st–79th percentiles; moderate DS=80th–89th percentiles; severe DS=90th–99th percentiles.
b Post hoc differences among all 3 DS groups.
c Post hoc differences between normal and severe DS groups and normal and moderate DS groups only.
Figure 2.
Depressive symptoms' influence on: (A) pain intensity outcomes (collapsed across all anatomical locations) and (B) functional status outcomes (collapsed across all anatomical locations). Normal DS=normal depressive symptoms (1st–79th percentiles); moderate DS=moderate depressive symptoms (80th–89th percentiles); severe DS=severe depressive symptoms (90th–99th percentiles); NRS=numerical rating scale for pain intensity; FS=functional status assessed by computerized adaptive testing.
The final hierarchical regression models reporting overall variance for discharge pain intensity ratings and functional status scores are reported in Table 5. Differences in variance for step 1 (demographic and clinical variables) and step 2 (depressive symptoms) are reported in the text for the pain intensity and functional status models. In the first step of the models, the demographic, clinical, and baseline pain intensity variables contributed 24.2%, 23.5%, 27.3%, and 23.0% variance to discharge pain intensity scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the second step, the DS score contributed 0.7%, 2.2%, 2.4%, and 3.0% additional variance to discharge pain intensity scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the first step, the demographic and clinical variables contributed 35.0%, 32.3%, 38.0%, and 37.2% variance to discharge functional status scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the second step, the DS score contributed 0.5%, 1.9%, 2.1%, and 1.5% additional variance to discharge functional status scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. In the final models, the intake pain intensity ratings and functional status scores were the strongest predictors of discharge scores in their respective models. Depressive symptoms were a statistically significant addition to discharge pain intensity ratings and functional status scores, except for the cervical anatomical region (Tab. 5).
Table 5.
Intake Depressive Symptoms' Association With Discharge Pain Intensity Ratings and Functional Status Scoresa
Age entered as continuous measure; sex coded 0=male, 1=female; clinic coded 0=orthopedic clinic, 1=pain or industrial clinic; duration coded 0=acute or subacute, 1=chronic; surgery coded 0=no surgery for present condition, 1=surgery for present condition; intake score=pain intensity or functional status depending on model; DS score= SCL-90-R depression scale score.
From the original cohort, only 4,037 patients (48.6%) provided data at the discharge rehabilitation session. Patients providing discharge data compared with patients without discharge data were more likely to be in the older age categories, have received treatment at an orthopedic clinic, have acute or subacute symptom duration, have cervical or lumbar anatomical region, and have normal DS severity (P<.01 for all of these comparisons). The consequences of the low overall follow-up rate and differences between those patients who provided and those who did not provide discharge data were further investigated with a sensitivity analysis. First, missing discharge NRS and functional status scores were imputed with linear interpolation and linear trend at a point. These are 2 different methods offered in SPSS to replace missing values from a time series analysis. The actual and imputed discharge scores then were included in parallel regression analyses to assess the potential of confounding due to the low and differential follow-up rates.
There were no substantial differences among the imputed models, so only the linear trend at a point results are presented. For pain intensity, the models with imputed data contributed a total of 12.5%, 12.1%, 13.9%, and 10.1% total variance to discharge scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. For functional status, the models with imputed data contributed a total of 22.4%, 18.6%, 21.9%, and 19.1% total variance to discharge scores for cervical, upper-extremity, lumbar, and lower-extremity categories, respectively. Similar to the results for the original models, DS score was a unique contributor in the final imputed models for pain and functional status for all anatomical regions, except the cervical region.
Discussion
This study described the prevalence and impact of depressive symptoms for a large sample of patients seeking treatment in outpatient physical therapy settings. The primary aim of these analyses was to determine whether depressive symptoms were a psychological factor specific to subgroups of patients with musculoskeletal pain based on anatomical region. Collectively, our results suggested that depressive symptoms have a strong association with pain intensity ratings and functional status scores across all anatomical regions of musculoskeletal pain assessed in this study. These data provide “proof of concept” suggesting that screening for depressive symptoms should not be focused on specific anatomical regions, especially if prevention of chronic musculoskeletal pain is a goal.
Comparisons with prevalence rates reported in other studies are difficult because of the differences in depressive symptom measures used across studies.3–6 Furthermore, the depression measure in our study has used percentile values to determine moderate and severe scores in previous investigations.37,38 Therefore, our interpretation does not focus on absolute prevalence rates of depressive symptoms. Instead, we focused on the relative differences in prevalence rates across anatomical regions for this particular cohort. Results of the prevalence analyses indicated that similar rates of severe depressive symptoms are to be expected for patients with lumbar and cervical conditions, whereas lower rates are to be expected for those with upper-extremity and lower-extremity conditions. For example, the highest rate of severe depressive symptoms was associated with the lumbar spine category, whereas the lowest rate was associated with the lower-extremity category (Tab. 2). Although these differences in prevalence rates for anatomical region were statistically significant, their magnitude also must be considered when interpreting these results. In this case, the absolute difference in severe depressive rates was small for even the largest difference, as was the decreased odds of having depressive symptoms for patients with lower-extremity conditions (OR=0.70). These prevalence data suggest that anatomical region has an association with severe depressive symptom rates. However, the potentially small difference in prevalence rates does not support a strong association between anatomical region and severe depressive symptom rates, at least when defined by the SCL-90-R.
A consistent pattern emerged when severity of depressive symptoms and anatomical region were considered for their influence on intake pain intensity and functional status. First, no interactions were detected, meaning that severity of depressive symptoms had a similar association with intake pain intensity and functional status across all anatomical locations. Second, both depressive symptom severity and anatomical region had a statistically significant association with intake pain intensity and functional status. Third, depressive symptom severity had a strong association with intake pain intensity and functional status. Specific to the purposes of the current study, depressive symptom severity had larger effect sizes compared with anatomical region of musculoskeletal pain for pain intensity and functional status (Figs. 1A and 1B).
The focus of this study was on depressive symptoms and anatomical location, but other factors were associated with severe depressive symptom rates. Indeed, when demographic and clinical factors were considered, there was an increased association between depressive symptoms and sex, clinic type, duration of symptoms, and surgery for the current condition. In contrast, decreased rates of depressive symptoms were associated with the oldest age category. These findings are interesting and are consistent with the findings of other studies on depressive symptoms.2,3,54 Although these variables were not directly related to the aims of this article, they were retained in the multivariate analyses, so our conclusions about depressive symptom scores were made based on models that contained potentially confounding demographic and clinical variables.
Depressive symptom scores had a consistently strong association in the multivariate regression models for intake pain intensity and functional status. The amount of variance contributed by DS scores varied across anatomical regions for pain intensity (10.0%–13.5%) and functional status (10.4%–18.6%). The additional variance contributed was statistically significant for each anatomical location, and the depressive symptom variable was the strongest individual contributor to pain intensity and functional status in the final models. These data suggest that depressive symptoms continued to be an important contributor to the initial clinical presentation, even after accounting for other relevant demographic and clinical factors. Patients with more-severe depressive symptoms would be expected to have higher pain intensity ratings and lower functional status scores across all anatomical locations. Although these are strong associations, it is important to realize that our analyses did not account for the temporal aspect. Therefore, we do not know whether depressive symptoms preceded these pain intensity ratings and functional status scores or whether the scores preceded the depressive symptoms.
Depressive symptoms also influenced treatment parameters and clinical outcomes, but with some contingencies worth noting. In the univariate analyses, severe depressive symptoms were associated with higher discharge pain intensity ratings, lower discharge functional status scores, longer treatment duration, and more clinic visits. However, there was no association between depressive symptoms and change in pain intensity ratings or functional status scores. Thus, although depressive symptoms were associated with both intake and discharge status, they were not associated with improvement experienced during physical therapy (Figs. 2A and 2B). In the multivariate analyses controlling for demographic factors, clinical factors, and baseline scores, depressive symptoms contributed to discharge scores, but they explained smaller amounts of variance for pain intensity (2.2%–3.0%) and functional status (1.5%–2.1%). As expected, intake pain intensity ratings and functional status scores were the strongest contributors to their respective discharge scores. Conversely, in a secondary analysis of a randomized controlled trial in patients with comorbid pain and depression, Ang et al55 did not find baseline severity of depression a predictor of 3-month pain intensity. They presumed other highly predictive factors (eg, treatment arm) reduced the additional predictive value of the depression scores in the multivariate model. Although our results generally were consistent across anatomical location, the addition of depressive symptoms did not add to the models for the cervical spine in our multivariate analyses of discharge scores. This finding is consistent with a recent published evidence summary for neck pain in which psychological influence was present in the general population, but not for other neck pain subgroups.56–58
Limitations
These analyses are based on a large data set, which provides some confidence that these results are robust; however, there are still some important limitations to consider when interpreting these findings. First, this was a sample of convenience, and patients were included in the analysis only if they completed the SCL-90-R during their outpatient physical therapy visit. We did not have access to patients who did not complete the SCL-90-R for statistical comparison to determine whether there were systematic differences for patients included in the analysis. Therefore, the consideration of selection bias was not directly addressed in this analysis and tempers the ability to generalize these findings. Second, even though more than 4,000 patients provided discharge data, the 46.7% completion rate was low. This rate is comparable to those reported in large data set studies using similar methods.28,33 Differences were noted between those patients who completed follow-up and those who did not. The sensitivity analyses with imputed data produced models with lower amounts of variance explained when compared with the original models. Therefore, the low and differential follow-up rates may have resulted in models that overestimated the total variance accounted for in pain intensity and functional status. However, the sensitivity analyses also indicated that depressive symptoms remained a unique contributor in the imputed models, suggesting that the follow-up concerns did not alter that aspect of the models.
Another limitation is that our analyses did not consider potential differences within each of our 4 anatomical region categories. For example, we did not report on any potential differences between patients with hip- and knee-related diagnoses. Additionally, we did not have other psychological variables, such as pain-related fear and pain catastrophizing, that have been studied for their influence on musculoskeletal pain.22 Therefore, our results must be interpreted specifically to depressive symptoms. Finally, our analyses did not consider the type of intervention received during physical therapy, so we cannot directly address interactions between depressive symptoms and specific physical therapy interventions. Therefore, these data are best interpreted to reflect the influence of depressive symptoms on general physical therapy responses. Future studies might investigate whether differences exist within these anatomical locations, include more psychological factors, and investigate how depressive symptoms influence responses to specific intervention protocols. Identification of specific interventions that are associated with better clinical outcomes for those with severe depressive symptoms would be a high priority for guiding future clinical decision making.
Clinical Relevance
Clinical guidelines support screening for psychological distress (including depression) in patients with lumbar spine conditions23,24,27 and, in certain cases, for cervical spine conditions.25,26 Screening for psychological distress is not routinely included in clinical guidelines for patients with upper-extremity and lower-extremity conditions.59–61 Our results suggest that screening recommendations for depressive symptoms in physical therapy settings should be expanded to those anatomical regions if the goal is to prevent the development of chronic musculoskeletal pain syndromes.
A limitation of the current study is that we cannot make specific clinical recommendations. Even though our measure of depressive symptoms (SCL-90-R) has been validated previously, there are no clinical cutoffs established for which referral is appropriate for patients with musculoskeletal pain conditions. Our cutoff for severe depressive symptoms was based on population distribution used in previous studies,36,37 and these cutoff scores may not have direct application. Future studies in this area should focus on developing and refining clinically relevant depressive symptom screening procedures for different anatomical regions of musculoskeletal pain.
Furthermore, future studies are needed to determine which behavioral interventions are most appropriate for improving outcomes for patients who are identified as at risk due to elevated depressive symptoms.16,32 Effective intervention strategies aimed at improving depressive symptoms have yet to be elucidated in physical therapy settings, and it is not clear how co-management with other health care professionals is part of this strategy. It has been suggested that addressing both depressive symptoms and pain complaints concurrently may improve pain, depressive symptoms, and disability outcomes in other settings.62 However, more work needs to be done in physical therapy settings before clinical recommendations can be made about treatment after depressive symptom screening.
Conclusion
Prevalence of depressive symptoms varied by anatomical region for patients seeking physical therapy for musculoskeletal pain, but the magnitude of this anatomical variation was small. Depressive symptoms had a consistent detrimental association with both intake and discharge scores for pain intensity and functional status.
The Bottom Line
What do we already know about this topic?
Previous studies from primary care and physical therapy settings indicated that the presence of major depressive symptoms with musculoskeletal pain can be a predictor of poor outcomes, especially for patients with lumbar or cervical spine pain.
What new information does this study offer?
This study used a large sample to build on previous studies by investigating the role of depressive symptoms in different anatomical regions of musculoskeletal pain, including upper- and lower-extremity locations. Although there was slightly higher prevalence of severe depressive symptoms for cervical and lumbar spine pain regions, severe depressive symptoms were associated with higher pain intensity ratings and lower functional status scores across all anatomical regions.
If you're a patient, what might these findings mean for you?
Patients with elevated depressive symptoms may have poorer outcomes from musculoskeletal pain, regardless of anatomical location of pain. Patients may receive a screening for depressive symptoms when seeking outpatient physical therapy.
Footnotes
Dr George, Dr Beneciuk, Ms Valencia, and Dr Hart provided concept/idea/research design. Dr George, Mr Coronado, Mr Werneke, and Dr Hart provided writing. Dr Hart provided data collection. Dr George, Mr Coronado, and Dr Beneciuk provided data analysis. Mr Coronado provided clerical support. Dr George, Mr Coronado, Ms Valencia, and Dr Hart provided consultation (including review of manuscript before submission).
The Focus on Therapeutic Outcomes, Inc (FOTO) Institutional Review Board for the Protection of Human Subjects approved the original collection of the data from clinical encounters. The Institutional Review Board for Protection of Human Subjects at the University of Florida approved the use of the data in de-identified form for these analyses.
A portion of the data were presented at the 13th World Congress of the International Association for the Study of Pain; August 29–September 2, 2010; Montreal, Quebec, Canada.
Dr Beneciuk received support from the National Institutes of Health T-32 Neural Plasticity Research Training Fellowship (grant HD043730) while preparing the manuscript.
Dr Hart is an employee of and an investor in FOTO, the database management company that manages the data analyzed in the study.
Focus on Therapeutic Outcomes, Inc, PO Box 11444, Knoxville, TN 37939.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133 [DOI] [PubMed] [Google Scholar]
- 2. Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–627 [DOI] [PubMed] [Google Scholar]
- 3. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445 [DOI] [PubMed] [Google Scholar]
- 4. Taylor R, Lovibond PF, Nicholas MK, et al. The utility of somatic items in the assessment of depression in patients with chronic pain: a comparison of the Zung Self-Rating Depression Scale and the Depression Anxiety Stress Scales in chronic pain and clinical and community samples. Clin J Pain. 2005;21:91–100 [DOI] [PubMed] [Google Scholar]
- 5. Roger PR, Johnson-Greene D. Comparison of assessment measures for post-stroke depression. Clin Neuropsychol. 2009;23:780–793 [DOI] [PubMed] [Google Scholar]
- 6. Vahle VJ, Andresen EM, Hagglund KJ. Depression measures in outcomes research. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S53–S62 [DOI] [PubMed] [Google Scholar]
- 7. Haggman S, Maher CG, Refshauge KM. Screening for symptoms of depression by physical therapists managing low back pain. Phys Ther. 2004;84:1157–1166 [PubMed] [Google Scholar]
- 8. Hope P, Forshaw M. Assessment of psychological distress is important in patients presenting with low back pain. Physiotherapy. 1999;85:563–570 [Google Scholar]
- 9. Lundberg M, Larsson M, Ostlund H, Styf J. Kinesiophobia among patients with musculoskeletal pain in primary healthcare. J Rehabil Med. 2006;38:37–43 [DOI] [PubMed] [Google Scholar]
- 10. Werneke MW, Hart DL. Centralization: association between repeated end-range pain responses and behavioral signs in patients with acute non-specific low back pain. J Rehabil Med. 2005;37:286–290 [DOI] [PubMed] [Google Scholar]
- 11. Keeley P, Creed F, Tomenson B, et al. Psychosocial predictors of health-related quality of life and health service utilisation in people with chronic low back pain. Pain. 2008;135:142–150 [DOI] [PubMed] [Google Scholar]
- 12. Sullivan MJ, Adams H, Thibault P, et al. Initial depression severity and the trajectory of recovery following cognitive-behavioral intervention for work disability. J Occup Rehabil. 2006;16:63–74 [DOI] [PubMed] [Google Scholar]
- 13. Lotters F, Franche RL, Hogg-Johnson S, et al. The prognostic value of depressive symptoms, fear-avoidance, and self-efficacy for duration of lost-time benefits in workers with musculoskeletal disorders. Occup Environ Med. 2006;63:794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boissonnault WG. Primary Care for the Physical Therapist: Examination and Triage. 2nd ed. St Louis, MO: Elsevier; 2010 [Google Scholar]
- 15. Goodman CC, Snyder TE. Differential Diagnosis in Physical Therapy. 4th ed. Philadelphia, PA: Saunders; 2007 [Google Scholar]
- 16. Christiansen D, Larsen K, Kudsk Jensen O, Vinther Nielsen C. Pain responses in repeated end-range spinal movements and psychological factors in sick-listed patients with low back pain: is there an association? J Rehabil Med. 2009;41:545–549 [DOI] [PubMed] [Google Scholar]
- 17. Sullivan MJ, Adams H, Rhodenizer T, Stanish WD. A psychosocial risk factor: targeted intervention for the prevention of chronic pain and disability following whiplash injury. Phys Ther. 2006;86:8–18 [DOI] [PubMed] [Google Scholar]
- 18. Burton AK, Tillotson KM, Main CJ, Hollis S. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine (Phila Pa 1976). 1995;20:722–728 [DOI] [PubMed] [Google Scholar]
- 19. Feleus A, Bierma-Zeinstra SM, Miedema HS, et al. Prognostic indicators for non-recovery of non-traumatic complaints at arm, neck and shoulder in general practice: 6 months follow-up. Rheumatology (Oxford). 2007;46:169–176 [DOI] [PubMed] [Google Scholar]
- 20. Feleus A, van Dalen T, Bierma-Zeinstra SM, et al. Kinesiophobia in patients with non-traumatic arm, neck and shoulder complaints: a prospective cohort study in general practice. BMC Musculoskelet Disord. 2007;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372 [DOI] [PubMed] [Google Scholar]
- 22. Leeuw M, Goossens ME, Linton SJ, et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94 [DOI] [PubMed] [Google Scholar]
- 23. Airaksinen O, Brox JI, Cedraschi C, et al. Chapter 4: European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(suppl 2):S192–S300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society [erratum in Ann Intern Med. 2008;148:247–248]. Ann Intern Med. 2007;147:478–491 [DOI] [PubMed] [Google Scholar]
- 25. Cote P, van der Velde G, Cassidy JD, et al. The burden and determinants of neck pain in workers: results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S60–S74 [DOI] [PubMed] [Google Scholar]
- 26. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S39–S51 [DOI] [PubMed] [Google Scholar]
- 27. van Tulder M, Becker A, Bekkering T, et al. Chapter 3: European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(suppl 2):S169–S191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deutscher D, Hart DL, Dickstein R, et al. Implementing an integrated electronic outcomes and electronic health record process to create a foundation for clinical practice improvement. Phys Ther. 2008;88:270–285 [DOI] [PubMed] [Google Scholar]
- 29. Swinkels IC, Hart DL, Deutscher D, et al. Comparing patient characteristics and treatment processes in patients receiving physical therapy in the United States, Israel and the Netherlands: cross sectional analyses of data from three clinical databases. BMC Health Serv Res. 2008;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swinkels IC, van den Ende CH, de Bakker D, et al. Clinical databases in physical therapy. Physiother Theory Pract. 2007;23:153–167 [DOI] [PubMed] [Google Scholar]
- 31. Hart DL, Werneke MW, George SZ, et al. Screening for elevated levels of fear-avoidance beliefs regarding work or physical activities in people receiving outpatient therapy. Phys Ther. 2009;89:770–785 [DOI] [PubMed] [Google Scholar]
- 32. Werneke MW, Hart DL, George SZ, et al. Clinical outcomes for patients classified by fear-avoidance beliefs and centralization phenomenon. Arch Phys Med Rehabil. 2009;90:768–777 [DOI] [PubMed] [Google Scholar]
- 33. Deutscher D, Horn SD, Dickstein R, et al. Associations between treatment processes, patient characteristics, and outcomes in outpatient physical therapy practice. Arch Phys Med Rehabil. 2009;90:1349–1363 [DOI] [PubMed] [Google Scholar]
- 34. Derogatis LR. SCL-90: Administration, Scoring and Procedures Manual–II. Townsen, MD: Clinical Psychometrics Research; 1983 [Google Scholar]
- 35. Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289 [DOI] [PubMed] [Google Scholar]
- 36. Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–183 [DOI] [PubMed] [Google Scholar]
- 37. Von Korff M, Le Resche L, Dworkin SF. First onset of common pain symptoms: a prospective study of depression as a risk factor. Pain. 1993;55:251–258 [DOI] [PubMed] [Google Scholar]
- 38. Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149 [DOI] [PubMed] [Google Scholar]
- 39. Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58:387–392 [DOI] [PubMed] [Google Scholar]
- 40. Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162 [DOI] [PubMed] [Google Scholar]
- 41. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158 [DOI] [PubMed] [Google Scholar]
- 42. Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30:1331–1334 [DOI] [PubMed] [Google Scholar]
- 43. Mintken PE, Glynn P, Cleland JA. Psychometric properties of the Shortened Disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and numeric pain rating scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18:920–926 [DOI] [PubMed] [Google Scholar]
- 44. Hart DL, Werneke M, Wang YC, et al. Computerized adaptive test for patients with lumbar spine impairments produced valid and sensitive measures of function. Spine (Phila Pa 1976). 2010. June 30 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45. Hart DL, Wang YC, Stratford PW, Mioduski JE. A computerized adaptive test for patients with hip impairments produced valid and responsive measures of function. Arch Phys Med Rehabil. 2008;89:2129–2139 [DOI] [PubMed] [Google Scholar]
- 46. Hart DL, Wang YC, Stratford PW, Mioduski JE. Computerized adaptive test for patients with foot or ankle impairments produced valid and responsive measures of function. Qual Life Res. 2008;17:1081–1091 [DOI] [PubMed] [Google Scholar]
- 47. Hart DL, Wang YC, Stratford PW, Mioduski JE. Computerized adaptive test for patients with knee impairments produced valid and responsive measures of function. J Clin Epidemiol. 2008;61:1113–1124 [DOI] [PubMed] [Google Scholar]
- 48. Hart DL, Mioduski JE, Werneke MW, Stratford PW. Simulated computerized adaptive test for patients with lumbar spine impairments was efficient and produced valid measures of function. J Clin Epidemiol. 2006;59:947–956 [DOI] [PubMed] [Google Scholar]
- 49. Hart DL, Cook KF, Mioduski JE, et al. Simulated computerized adaptive test for patients with shoulder impairments was efficient and produced valid measures of function. J Clin Epidemiol. 2006;59:290–298 [DOI] [PubMed] [Google Scholar]
- 50. Hart DL, Wang YC, Cook KF, Mioduski JE. A computerized adaptive test for patients with shoulder impairments produced responsive measures of function. Phys Ther. 2010;90:928–938 [DOI] [PubMed] [Google Scholar]
- 51. Hart DL, Connolly JB. Pay-for-performance for physical therapy and occupational therapy: Medicare part B services. Final report. Grant #18-P-93066/9–01: Health & Human Services/Centers for Medicare & Medicaid Services. 2006. Available at: http://www.cms.hhs.gov/TherapyServices/downloads/P4PFinalReport06–01–06.pdf Accessed May 15, 2009
- 52. Hart DL, Mioduski JE, Stratford PW. Simulated computerized adaptive tests for measuring functional status were efficient with good discriminant validity in patients with hip, knee, or foot/ankle impairments. J Clin Epidemiol. 2005;58:629–638 [DOI] [PubMed] [Google Scholar]
- 53. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 54. Keogh E, McCracken LM, Eccleston C. Gender moderates the association between depression and disability in chronic pain patients. Eur J Pain. 2006;10:413–422 [DOI] [PubMed] [Google Scholar]
- 55. Ang DC, Bair MJ, Damush TM, et al. Predictors of pain outcomes in patients with chronic musculoskeletal pain co-morbid with depression: results from a randomized controlled trial. Pain Med. 2010;11:482–491 [DOI] [PubMed] [Google Scholar]
- 56. Carroll LJ, Hogg-Johnson S, Cote P, et al. Course and prognostic factors for neck pain in workers: results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S93–S100 [DOI] [PubMed] [Google Scholar]
- 57. Carroll LJ, Hogg-Johnson S, van der Velde G, et al. Course and prognostic factors for neck pain in the general population: results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S75–S82 [DOI] [PubMed] [Google Scholar]
- 58. Carroll LJ, Holm LW, Hogg-Johnson S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33(4 suppl):S83–S92 [DOI] [PubMed] [Google Scholar]
- 59. Cibulka MT, White DM, Woehrle J, et al. Hip pain and mobility deficits—hip osteoarthritis: clinical practice guidelines linked to the International Classification of Functioning, Disability and Health from the Orthopaedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2009;39:A1–A25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McPoil TG, Martin RL, Cornwall MW, et al. Heel pain—plantar fasciitis: clinical practice guildelines linked to the International Classification of Function, Disability and Health from the Orthopaedic Section of the American Physical Therapy Association [erratum in J Orthop Sports Phys Ther. 2008;38:648]. J Orthop Sports Phys Ther. 2008;38:A1–A18 [DOI] [PubMed] [Google Scholar]
- 61. Logerstedt DS, Snyder-Mackler L, Ritter RC, et al. Knee stability and movement coordination impairments: knee ligament sprain. J Orthop Sports Phys Ther. 2010;40:A1–A37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301:2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]