Abstract
Background
Previous studies focused on describing successful sit-to-stand (STS) performance in patients with Parkinson disease (PD). Little is known about why these patients fail to perform this transfer activity.
Objective
This study aimed to determine the role of dynamic stability and limb support in governing successful STS performance in patients with PD and to determine the limits of recovery for discriminating between successful and failed STS trials.
Design
This was a cross-sectional study.
Methods
Twenty-eight patients with PD were instructed to perform the STS task. Kinematic data on 18 successful trials and 14 failed trials (when the patients fell backward) were collected with a motion analysis system. Dynamic stability was determined by the anteroposterior position of the body's center of mass (COM) relative to the base of support (BOS) and by the anteroposterior velocity of the COM relative to the BOS (VelocityCOM/BOS,AP). Limb support was characterized by the hip height (Heighthip).
Results
The findings revealed no between-group (“risers” versus “fallers”) differences in dynamic stability. The fallers shifted their COM in a significantly more anterior position to compensate for their lower VelocityCOM/BOS,AP at seat-off. It was in the vertical direction that the fallers had significantly reduced peak COM velocity (VelocityCOM,vertical) and lower corresponding Heighthip than the risers. Results of a stepwise regression model showed that VelocityCOM/BOS,AP and Heighthip at the instant of peak VelocityCOM,vertical could best predict the STS outcome (success versus failure), with an overall prediction accuracy of 87.5%. The limit differentiating successful from failed STS trials was: Heighthip=−0.814 VelocityCOM/BOS,AP + 0.463.
Limitations
All of the patients were community dwelling and had a moderate level of disease severity. The results cannot be generalized to those who are institutionalized or with advanced PD.
Conclusions
Limb support and ill-timed peak forward COM velocity, rather than dynamic stability, play the dominant roles in determining successful STS performance in patients with PD.
Bradykinesia and postural instability during functional transfers (eg, rising from a chair) are cardinal manifestations that have been linked to a significant increase in fall risk and debilitation in patients with Parkinson disease (PD). The sit-to-stand (STS) task is a complex transfer activity that requires the generation of a propulsive force to accelerate the body in a forward and then upward direction. It also is a self-perturbing and destabilizing task, as the action brings the body from the large base of support (BOS) provided by the buttocks in the sitting position to the small BOS of the feet in the upright stance position. Inability to generate adequate muscle force and poor balance create difficulties in STS performance in patients with PD. Mak and colleagues1,2 previously reported that these patients have reduced peak forward and upward velocities of the body's center of mass (COM) and that they take a prolonged amount of time to perform the STS task. Clinically, 42% to 81% of patients with PD have been found to exhibit difficulty in rising from a chair.3,4 Inability to stand up from a seated position prevents patients with PD from performing upright functional activities such as walking, thus leading to a sedentary lifestyle, physical deconditioning, and functional deterioration and increasing the risk of falls.
Previously published studies have focused on describing successful STS performance in patients with PD. Thus, little is known about why these patients fail to perform such a basic task. It has been proposed that movement stability and hip vertical motion related to the limb support of body weight may play critical roles in resisting falls in young and older people who are healthy during STS performance.5 Dynamic stability describes the neuromuscular system's capacity to restore or maintain a function successfully, despite naturally occurring disturbances and neuromotor control errors.
A theoretical framework that extends the concept of static stability to dynamic conditions6 suggests that dynamic stability can be characterized by the relationship between the body's COM motion state (ie, the combination of COM position and velocity related to the BOS) and the analytically derived stability limits (Fig. 1). A COM state below the limits against backward balance loss is less stable and requires greater correction to recover from that loss. A precursor to backward falling may be insufficient forward momentum to carry the COM forward within the BOS. Conversely, when the COM motion state is above the stability limits against backward balance loss, it is more stable and less likely to result in such loss.7 Although the dynamic stability limits initially were derived theoretically based on a simplified inverted pendulum human model,6 it has since been extensively verified with experimental data derived by our research team8–12 and by others13–17 in a range of task conditions inducing waist pull,16 bimanual pull,17,18 STS slip,19 and gait slip.8,9,11,12 In addition, this new concept of dynamic stability limits has been further confirmed and extended by subsequent model simulation work.11,13,20
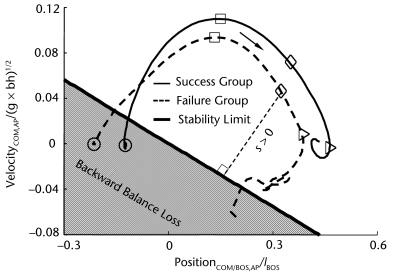
Figure 1.
Representative trajectories of the center of mass (COM) motion state (ie, the anteroposterior [AP] position and velocity of the COM relative to the base of support [BOS]) recorded from movement initiation to movement termination during successful (solid line) and failed (dashed line) sit-to-stand trials for patients with Parkinson disease. Movement initiation (circle) was identified as when the angular velocity of any lower-limb segment exceeded 3 standard deviations from its baseline. Movement termination was defined as the instant when the forward COM velocity finally fell below 3 standard deviations of the baseline velocity measured during the period prior to movement initiation. In addition, the instants of the peak AP COM velocity (peak VelocityCOM,AP, square), seat-off (SO, diamond), and the peak vertical COM velocity (peak VelocityCOM,vertical, triangle) are demonstrated. Positions and velocities of COM are related to the heel of the posterior foot and normalized to foot length (lBOS) and , respectively (g represents the gravitational acceleration, and bh represents the body height). The durations from movement initiation to the instant of peak VelocityCOM,AP, from the instant of peak VelocityCOM,AP to SO, and from SO to the instant of peak VelocityCOM,vertical are termed T1, T2, and T3, respectively. Also shown is the model-predicted stability limit against backward balance loss. The thin dashed line indicates the magnitude of the stability against backward balance loss, which was defined as the shortest distance from the given COM motion state to the stability limits (“s” indicates the stability value of the corresponding COM motion). The arrow represents the moving direction of the COM motion state during the task.
Subsequently, the role of dynamic stability and limb support in resisting falls during STS performance has been widely investigated in people who are healthy.7–10,12,15–19 Yet, little effort has been devoted to investigating their role in individuals with PD. It has been reported that patients with PD shift their COM in a more anterior position relative to their BOS prior to seat-off than do control subjects who are healthy,21 a strategy that has been suggested to enhance postural stability (ie, to decrease the risk of a backward fall). The findings of a study by Inkster and Eng21 provide insight into the COM position but no information about COM velocity, a parameter that is equally important in determining dynamic stability during a task. It remains unknown whether COM forward velocity or dynamic stability plays a role in resisting falls during STS performance in patients with PD. Additionally, as previously mentioned, success in completing the STS task also depends upon adequate limb support against gravity. By the standard definition, falls are caused by the vertical descent of the body as a result of limb collapse. Instability coupled with the inability to provide timely and sufficient limb support against gravity makes it impossible to avoid an impending fall even for young adults who are healthy.22 To date, no study has examined the role played by limb support in avoiding falls during STS performance among patients with PD.
Thus, the primary purpose of the present study was to determine the role of dynamic stability and limb support in resisting falls during the STS movement among patients with PD. Because patients with PD are bradykinetic, we hypothesized that their impaired stability results from slow COM movement, a factor that would predispose them to backward balance loss, thus leading to a failed STS trial. We further postulated that inadequate limb support is another factor in STS failure. These hypotheses were tested with the aim of determining the role played by dynamic stability and limb support in governing successful STS performance in patients with PD, as well as the limits of fall recovery that could discriminate between successful and failed STS trials in this patient population. The limits of fall recovery will provide a quantitative basis for evaluating an individual's capacity to resist a fall or assessing his or her risk of a fall during STS performance. They also will offer a better understanding of the mechanisms that underlie incidences of falls among patients with PD.
Method
Participants
All patients with PD attending a movement disorder clinic were diagnosed by a neurologist according to the guidelines of the United Kingdom Parkinson's Disease Society Brain Bank.23 Patients were included if they were stable and taking anti-parkinsonian medications; were able to perform the STS task independently and follow instructions; and had no dyskinesia, orthopedic, arthritic, or heart problems. Disease severity was determined with the Hoehn and Yahr (HY) staging scale.24 Written consent was obtained from all participants prior to data collection, and all tests were performed during the “on medication” phase (ie, within 2 hours of taking anti-parkinsonian medication).
Experimental Protocol
All measurements were carried out at the motion analysis laboratory of The Hong Kong Polytechnic University. Details of the experimental setup and procedure were reported previously.25 Briefly, participants were instructed to sit on a height-adjustable chair with reflective markers attached to 24 anatomical bony landmarks to represent the head, neck, trunk, pelvis, bilateral forearm and hands, and bilateral thighs, shanks, and feet. The height of the chair was based on the individual's knee height, and both knees were kept at 85 degrees of flexion. The participants were instructed to stand up at their natural speed and were provided with no cue to stand up. They were given 2 practice trials, followed by 5 test trials.
Three-dimensional kinematic STS data were recorded using a Vicon motion analysis system (System 370)* with 6 cameras, each containing infrared light-emitting diodes with a recording frequency of 60 Hz. Three strain gauge forceplates (model BEDAS-2)† were used to measure the ground reaction force at a frequency of 60 Hz. The Vicon system was synchronized with the forceplates to capture the kinetic data.
Data Recording and Reduction
The procedures used to process and calculate the following kinematic and kinetic parameters are detailed in a previously published report.25 Briefly, the trajectories of the markers were filtered with a zero-phase lag, bidirectional, fourth-order, low-pass Butterworth filter at a cutoff frequency of 10 Hz. A plug-in gait program within the Vicon motion analysis system was used to determine the coordinates of the body's COM during the chair-rise. The velocity of the body's COM was obtained from the first differentiation of the COM coordinates.
Dynamic stability was computed from the COM motion state (ie, the instantaneous COM position and velocity), and the COM anteroposterior position (PositionCOM/BOS,AP) and velocity (VelocityCOM/BOS,AP) were determined relative to the heel of the posterior foot. They were normalized by foot length (lBOS) and , respectively, where “g” represents gravitational acceleration and “bh” is body height. Stability was quantified as the shortest distance from the instantaneous COM motion state to the analytically derived stability limits against backward balance loss (Fig. 1). Hip vertical motion related to limb support was quantified by the height of the hip (Heighthip) at midpoint relative to the ground. To validate the use of hip motion to characterize the limb support, the relationships between the resulting change in hip height, ΔHeighthip(t), and the impulse, Impulse(t), of the vertical components of the ground reaction force, Forceground(t), the chair reaction force, Forcechair(t), and the body weight were investigated. The change in hip height and vertical impulse, respectively, were calculated as:
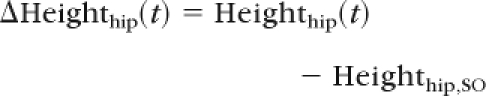 |
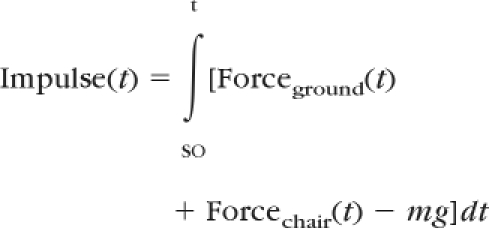 |
where, “m” is the body mass, “SO” represents the instant of seat-off, and “dt” represents a small change in time between 2 analyzed data points (its value was 1/60 seconds in the present study). We found that Impulse(t) was highly correlated with ΔHeighthip(t) at every instant during the ascent phase following seat-off. The relationship across all participants was:
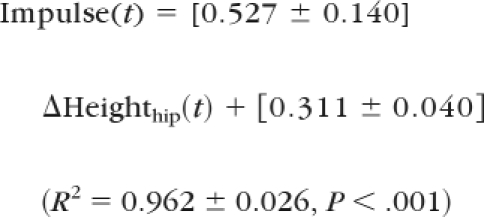 |
Given the high linear correlation between Heighthip and Impulse(t), it is reasonable to anticipate that both characterizations of limb support lead to the same conclusion. Hip height is preferred because it provides a variable that is quantifiable in clinics without the need of force platforms.22 Therefore, we adopted simpler measurement of the two (ie, Heighthip) in examining STS failure in patients with PD.
Outcomes and Events
Each trial was analyzed at 3 points of interest (Figs. 2A, 2B, and 2C): the instant of peak COM forward velocity (VelocityCOM,AP), the instant of seat-off, and the instant of peak COM vertical velocity (VelocityCOM,vertical). Seat-off was determined as the instant at which the force under the chair was less than 10% of the individual's body weight. The durations from movement initiation to peak VelocityCOM,AP (T1), from peak VelocityCOM,AP to seat-off (T2), and from seat-off to the instant of VelocityCOM,vertical (T3) were computed (Fig. 2D). Movement initiation was identified when the angular velocity of any lower-limb segment exceeded 3 standard deviations from its baseline.
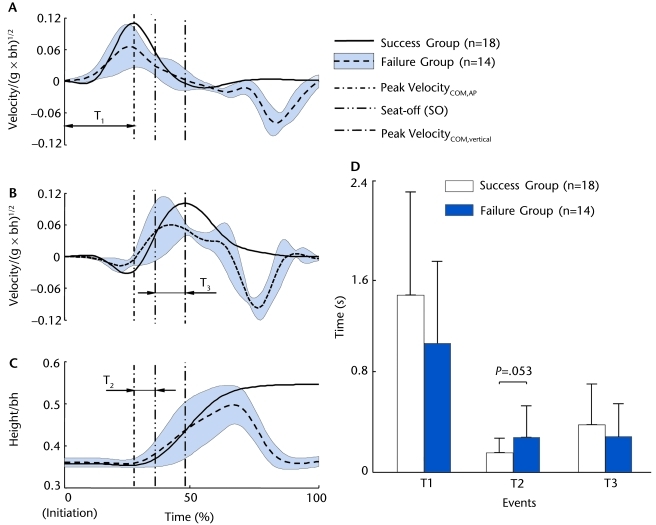
Figure 2.
Group mean of the time history of (A) anteroposterior (AP) velocity of the center of mass (COM) (VelocityCOM,AP), (B) the vertical COM velocity (VelocityCOM,vertical), and (C) the mid-hip point height from movement initiation to movement termination for the patients with Parkinson disease during the sit-to-stand (STS) task for the success group (solid line, n=18) and the failure group (dashed line, n=14). For the failed STS trials, the mean ± standard deviation across all 14 trials of the variables is shown. Also shown are the events of interest (vertical lines) for the STS task. They include the instant of peak VelocityCOM,AP, the instant of seat-off (SO), and the instant of peak VelocityCOM,vertical. The durations between movement initiation and the instant of peak VelocityCOM,AP (T1), between the instant of peak VelocityCOM,AP and SO (T2), and between SO and the instant of peak VelocityCOM,vertical (T3) also are listed. Both velocities are normalized to (g represents the gravitational acceleration, and bh represents the body height). The hip height is normalized to bh. (D) Means and standard deviations of T1, T2, and T3 for both success and failure groups.
The outcome of each trial was classified as either a success or a failure. A trial was considered a failure if the participant unambiguously fell backward and sat back in the chair after the STS task had begun and a success if the participant successfully stood up after taking off from the chair. Those participants who failed to complete the STS task were classified as “fallers” because they came to rest inadvertently on a lower level (ie, the chair) after seat-off to prevent themselves from falling down to the ground.26 Each failed trial was reported as an individual event.
Data Analysis
All statistical tests were performed with SPSS version 17.0 software.‡ Statistical significance was set at the .05 level. Mann-Whitney U tests and chi-square tests were used to compare between-group differences in the ordinal variables (eg, HY staging) and the nominal variables (eg, sex), respectively. Descriptive statistics were used to examine the central tendency and variability of all measured variables. Independent t tests were used to compare the between-group differences among the continuous variables, including PositionCOM/BOS,AP, VelocityCOM/BOS,AP, stability, Heighthip at all events, peak VelocityCOM,AP, peak VelocityCOM,vertical, and the 3 time points (T1, T2, and T3).
To identify the most important determinants of the STS outcome, the prediction accuracy of the dependent outcome (success versus failure) was evaluated using univariate logistic regression models in which the variables that demonstrated significant group-related differences were the independent variables. The next step was forward stepwise logistic regression analysis, which was performed by entering all of the independent variables that had exhibited significant group-related differences in the univariate logistic regressions. The likelihood ratio test with a cutoff probability of .05 was used for variable entry, with the limits of fall recovery probability of .5 used for classification.
Role of the Funding Source
The study was supported by a Competitive Research Grant (GYG94), The Hong Kong Polytechnic University, and by National Institutes of Health/National Institute on Aging grant R01-AG029616.
Results
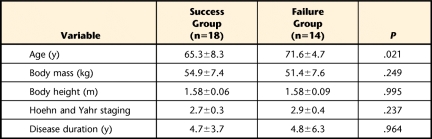
Twenty-eight patients with PD completed the study. Ten participants failed to complete at least 1 of the 5 test trials. Specifically, 7 participants had 1 failed trial, 2 participants had 2 failed trials, and 1 participant had 3 failed trials. Thus, there were 14 failed trials out of 50 STS test trials, and all patients fell back to the chair. For comparison purposes, we randomly selected 1 test trial from each of the 18 participants (STS “risers”) who had successfully completed all of the STS test trials. The fallers were significantly older than the risers (P<.05); however, there were no significant differences between the groups in the other demographic data, in disease severity as reflected by the HY staging score, or in disease duration (Tab. 1).
Table 1.
Demographic Information (Mean±SD) of Patients With Parkinson Disease
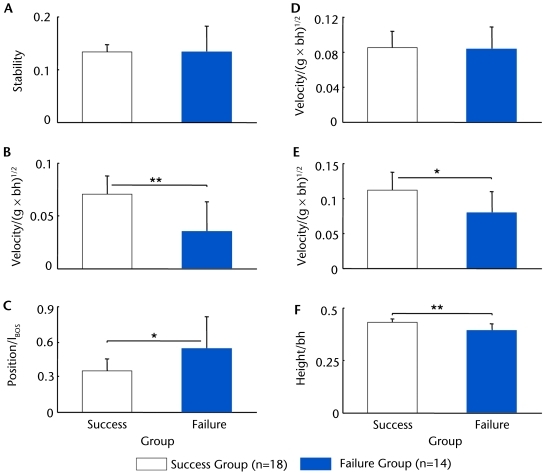
No difference was found in the durations of the T1 and T3 phases between the failure and success groups (P>.05, Fig. 2D), although less time elapsed for the T2 phase in the latter group (P=.053, Fig. 2D). There was no between-group difference in the control of the COM stability (Fig. 3A), although the fallers had a significantly slower VelocityCOM/BOS,AP (by 50.7%, P<.001, 95% confidence interval [CI] of the difference=0.018 to 0.053, Fig. 3B) and more forwardly shifted PositionCOM/BOS,AP compared with the risers (by 54.1%, P<.01, 95% CI=−0.351 to −0.031, Fig. 3C) at seat-off. No between-group difference was found for peak VelocityCOM,AP (Fig. 3D). In contrast, the fallers had significant reductions in peak VelocityCOM,vertical (by 24.1%, P<.01, 95% CI=0.008 to 0.047, Fig. 3E) and Heighthip at the instant of peak VelocityCOM,vertical (by 9.5%, P<.001, 95% CI=0.021 to 0.061, Fig. 3F).
Figure 3.
Comparison of (A) the center of mass (COM) stability at seat-off (SO), (B) the COM anteroposterior (AP) velocity relative to the base of support (BOS) (VelocityCOM,AP) at SO, (C) the COM AP position relative to the BOS (PositionCOM/BOS,AP) at SO, (D) the peak VelocityCOM,AP, (E) the peak COM vertical velocity(VelocityCOM,vertical), and (F) the hip height at the instant of the peak VelocityCOM,vertical during the sit-to-stand task between patients with Parkinson disease who successfully stood up (success group, n=18) and those who fell backward (failure group, n=14). The PositionCOM/BOS,AP is normalized to the foot length (lBOS). All velocities are normalized to , where g represents the gravitational acceleration, and bh represents the body height. The hip height is normalized to bh. *P<.01, **P<.001.
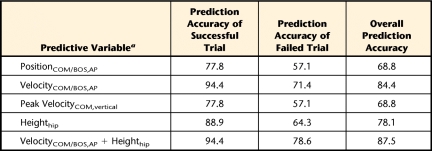
When compared with the other predictive variables, VelocityCOM/BOS,AP at seat-off and Heighthip at the instant of peak VelocityCOM,vertical displayed greater accuracy in predicting the STS outcome (ie, success or failure) (Tab. 2). More specifically, the overall prediction accuracies of STS outcome for VelocityCOM/BOS,AP and Heighthip were 84.4% and 78.1%, respectively (Tab. 2). The stepwise logistic regression, in which all 4 of the significant variables were entered, confirmed that the best prediction was achieved by including VelocityCOM/BOS,AP at seat-off and Heighthip at the instant of peak VelocityCOM,vertical in the model. The prediction probability of failed STS for an individual with PD can be evaluated by these 2 predictive variables using the following equation:
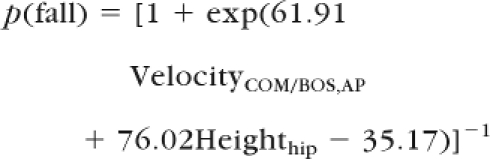 |
Table 2.
Prediction Accuracy (%) of the Sit-to-Stand Outcome (Success Versus Failure) Based on Each Predictive Variable Showing a Significant Difference Between Success and Failure Groups
a PositionCOM/BOS,AP=center of mass (COM) anteroposterior position relative to the base of support (BOS) at seat-off, VelocityCOM/BOS,AP=COM anteroposterior velocity relative to that of the BOS at seat-off, VelocityCOM,vertical=COM vertical velocity, Heighthip=hip height.
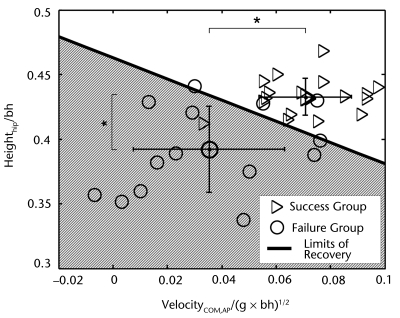
Based on this logistic regression equation, the limits of fall recovery at which failed trials can be distinguished from successful trials with a high degree of accuracy during STS performance among patients with PD in the motion state space (ie, VelocityCOM/BOS,AP − Heighthip, Fig. 4) can be stated as Heighthip=−0.814 VelocityCOM/BOS,AP + 0.463.
Figure 4.
The relationship among the center of mass (COM) anteroposterior (AP) velocity relative the base of support (BOS) (VelocityCOM,AP) at seat-off, the hip height (Heighthip) at the instant of the peak COM vertical velocity, and the outcome (success vs failure) during the sit-to-stand (STS) task for patients with Parkinson disease, including 18 successful trials and 14 failed trials. Individuals within the shaded area would be classified as likely to fall by the derived logistic regression model: p(fall) = [1 + exp(61.91VelocityCOM/BOS,AP + 76.02Heighthip − 35.17)]−1 (P<.05 for all model coefficients). The limits of recovery differentiating successful and failed trials (the thick solid line) was derived based on the logistic regression equation. It is Heighthip=−0.814 VelocityCOM/BOS,AP + 0.463. This limits of recovery correctly classified 87.5% of the STS task outcome. Also shown is the mean and standard deviation of the 2 predictive variables for both groups. *P<.001.
Discussion
This is the first study to examine the role played by dynamic stability and limb support in resisting falls during STS performance among patients with PD. These results have clearly shown that fallers and risers were not significantly different in stability control. Even more striking is the fact that the fallers were able to properly forward shift their COM position to offset their tendency of a lowered COM velocity at the critical moment of seat-off compared with the risers. Furthermore, this lower COM velocity did not result from a lower peak COM velocity, which was similar in magnitude but merely occurred slightly earlier than that of the risers. The synthesis of these findings leads to the somewhat unanticipated conclusion that the fallers and the risers had similar ability in the control of dynamic stability. Thus, our hypothesis that dynamic COM stability is a critical predictor of STS outcome in patients with PD was not supported. Because of the significant between-group differences in Heighthip at that moment, we conclude that these failed chair-rises must result from inadequate limb support generated by the fallers.
In retrospect, this finding may not really be that extraordinary, as earlier findings indicated that even the natural aging process could have significantly altered the vertical ascent characteristics,27 predisposing older adults to a great likelihood of falls in the STS slip.28 Nonetheless, Riley et al29 observed 2 types of STS failure—sit-back and step failure—in older individuals. They thought that the former is associated with the inadequate generation of forward momentum and the latter is associated with insufficient upward momentum. In both types of STS failure, the body's COM is located behind the BOS at seat-off.29 Thus, one could expect PositionCOM/BOS,AP to be closer to the rear of the BOS during seat-off among those patients with PD who failed the STS trials. On the contrary, however, the fallers shifted their COM in a more anterior position within their BOS than did the risers. The fallers were significantly older than the risers in this study. It is possible that a more advanced age, together with PD, may have resulted in their greater trunk stiffness,30 greater trunk rigidity,31 and more weakness in the trunk extensor muscles30,32 among the fallers compared with the risers.
It also is possible that the fallers may have adopted this hip flexion strategy to compensate for their low VelocityCOM/BOS,AP in order to decrease the risk of a backward fall. Previous studies have suggested that the use of the exaggerated hip flexion strategy can increase postural stability during seat-off in patients with PD 21 and older individuals.33,34 However, the patients who had too little COM forward velocity because of more-severe bradykinesia still fell backward, even after they had anteriorly shifted their COM to achieve the same level of stability as the successful patients. This finding suggests that sole reliance on shifting the COM to a more anterior position may provide insufficient compensation for speed deficits, thus eventually leading to failure to complete the STS task. To rise from a chair successfully and avoid a backward fall, patients have to generate adequate COM forward velocity relative to the BOS at seat-off. In patients with PD, it is VelocityCOM/BOS,AP, not PositionCOM/BOS,AP or COM stability, that plays an important role in determining the success of a chair-rise.
Our results do support our second hypothesis, that is, that hip height (Heighthip), an indicator of limb support, at the instant of peak VelocityCOM,vertical is one determinant of successful STS performance. After the instant of seat-off, sufficient limb support plays a dominant role in successful vertical ascent.5 Bradykinesia, a cardinal sign of basal ganglia dysfunction, can contribute to insufficient limb support (ie, a lower Heighthip). Mak and Hui-Chan2 previously reported that hip and knee extension torques were reduced by 10% and the built-up rate of both were markedly reduced (by 50%) during STS performance in patients with PD. Inadequate limb support also can be related to muscle weakness,35 and patients with PD have been found to have reduced concentric muscle strength (force-generating capacity) in their hip extensors and knee extensors, as well as reduced isometric bilateral leg extensor strength.36–38 Hip extension muscle strength has been further reported to be correlated with STS time.36 Problems in producing appropriate muscle force and a reduced rate of joint torque production may be related to an inability to recruit, or inconsistency in recruiting, motor unit activity, which is attributed to the central mechanisms.39,40 Other reported factors include an increase in agonist-antagonist coactivation41,42 and rigidity in the agonist or antagonist muscles.43,44
It is noteworthy that all participants had a comparable peak VelocityCOM,AP (Fig. 3D), whereby the fallers appeared to reach their peak VelocityCOM,AP earlier than the risers (Fig. 2D). Although this difference in timing did not reach a level of statistical significance (Fig. 2D), the offset was sufficient to cause a lower VelocityCOM/BOS,AP at seat-off among the fallers (Fig. 2B). This finding is consistent with previous findings on gait initiation, which demonstrated that although patients with PD are slow, their spatiotemporal pattern is preserved.45–47 These patients appear to have no problem in selecting an appropriate motor program, although they may encounter difficulties in sequencing that program.46,48
An intriguing finding of the current study is the determination of the limits for discriminating successful from failed STS performance in patients with PD by using their VelocityCOM/BOS,AP at seat-off and their Heighthip at the instant of peak VelocityCOM,vertical (Fig. 4). The combination of these 2 predictive variables was able to classify 78.6% of the failed trials and 94.4% of the successful trials (Tab. 2). Ideally, a risk prediction model should not only yield a yes/no answer, but also offer an estimate of risk severity. The limits of fall recovery derived in this study provide a theoretical basis for quantifying the severity of risk of falling in patients with PD during the STS task. Such severity can be calculated as the shortest distance from the given combination of VelocityCOM/BOS,AP and Heighthip to the limits of fall recovery. In the current study, the newly computed limits of fall recovery and index were able to verify with accuracy that the further the VelocityCOM/BOS,AP and the Heighthip combination fell below the limits of fall recovery, the greater the likelihood of an actual backward fall. In contrast, the further above the limits of fall recovery this combination was, the less likely the occurrence of such a fall.
The negative slope of the limits of fall recovery (−0.814) implies a trade-off between VelocityCOM/BOS,AP at seat-off and Heighthip at the instant of peak VelocityCOM,vertical (Fig. 4). For instance, when a person had a high VelocityCOM/BOS,AP, he or she was able to stand up successfully even with a low Heighthip. Conversely, a person with a high Heighthip was able to stand up successfully even when the VelocityCOM/BOS,AP was low. However, our fallers had a slower VelocityCOM/BOS,AP at seat-off and a low Heighthip at the instant of peak VelocityCOM,vertical relative to the risers (Figs. 3 and 4) and, therefore, failed to complete the STS task. In line with the limits of fall recovery identified in this study, to increase STS success, patients must maintain dynamic stability by increasing their COM forward velocity at seat-off, not by solely by shifting their COM to a more anterior position, as previous studies have suggested.21,34 The ability to provide sufficient limb support after seat-off also is crucial in determining a successful chair-rise in patients with PD.
The findings of this study suggest that control of the timing of the peak forward COM velocity and improvement in lower-extremity muscle strength can facilitate successful STS performance in patients with PD. The use of audiovisual cues has been found to increase the forward and upward velocities of body COM during STS performance in these patients.25,49 Other studies have shown that balance and strength training can enhance their postural control and muscle strength.50,51 Hass et al52 reported that 12 weeks of combined moderate resistive exercise training and creatine monohydrate supplements could speed up STS performance in patients with PD. Further randomized controlled trials are needed to examine effective strategies for enhancing STS performance in this patient population.
To conclude, the fallers adapted well by shifting their COM forward to compensate for their slower seat-off velocity to enhance their COM stability against backward falling, such that their stability was comparable to that of the risers. Their failure in the STS task was primarily attributable to their poor timing in the generation of the peak COM forward velocity coupled with weak limb support, as indicated by a lower Heighthip. The limits of fall recovery derived in this study provide a quantitative basis for assessing risk of falling in patients with PD during the STS task and provide guidelines for developing effective and efficient interventions to reduce the incidence of falls in this patient population.
The Bottom Line
What do we already know about this topic?
Patients with Parkinson disease (PD) are known to have difficulties in rising from a chair. However, it is unknown whether the failure to complete the sit-to-stand task is due to impaired postural stability or to inadequate limb support.
What new information does this study offer?
Failure to rise from a chair is more likely due to weak limb support against gravity than to poor control of postural stability. However, patients who are unsuccessful at rising to a standing position tend to demonstrate poor timing of the peak (maximum) velocity of the body's center of mass in the forward direction.
If you're a patient, what might these findings mean for you?
The following rehabilitation strategies could help facilitate successful sit-to-stand transfers in patients with PD: (1) strengthening the knee and hip extensor muscles and (2) improving the timing of maximum forward velocity of the body closer to the point in the standing movement when the body lifts off the chair.
Footnotes
All authors provided concept/idea/research design and writing. Dr Mak provided data collection, project management, fund procurement, participants, facilities/equipment, institutional liaisons, and consultation (including review of manuscript before submission). Dr Mak and Dr Yang provided data analysis.
The study was approved by the Ethics Committee of The Hong Kong Polytechnic University.
The study was supported by a Competitive Research Grant (GYG94), The Hong Kong Polytechnic University, and by National Institutes of Health/National Institute on Aging grant R01-AG029616.
Oxford Metrics Group, 14 Minns Business Park, West Way, Oxford OX2 0JB, United Kingdom.
Advanced Medical Technology Inc, 141 California St, Newton, MA 02158.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1. Mak MK, Levin O, Mizrahi J, Hui-Chan CW. Joint torques during sit-to-stand in healthy subjects and people with Parkinson's disease. Clin Biomech (Bristol, Avon). 2003;18:197–206 [DOI] [PubMed] [Google Scholar]
- 2. Mak MK, Hui-Chan CW. The speed of sit-to-stand can be modulated in Parkinson's disease. Clin Neurophysiol. 2005;116:780–789 [DOI] [PubMed] [Google Scholar]
- 3. Brod M, Mendelsohn GA, Roberts B. Patient's experiences of Parkinson's disease. J Gerontol B Psychol Sci Soc Sci. 1998;53:213–222 [DOI] [PubMed] [Google Scholar]
- 4. Munton JS, Ellis MI, Chamberlain MA, Wright V. An investigation into the problems of easy chairs used by the arthritic and the elderly. Rheumatol Rehabil. 1981;20:164–173 [DOI] [PubMed] [Google Scholar]
- 5. Pavol MJ, Pai YC. Deficient limb support is a major contributor to age differences in falling. J Biomech. 2007;40:1318–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pai YC, Patton J. Center of mass velocity-position predictions for balance control [erratum in: J Biomech. 1998;31:199]. J Biomech. 1997;30:347–354 [DOI] [PubMed] [Google Scholar]
- 7. Pai YC, Wening JD, Runtz EF, et al. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol. 2003;90:755–762 [DOI] [PubMed] [Google Scholar]
- 8. Bhatt T, Pai YC. Immediate and latent interlimb transfer of gait stability adaptation following repeated exposure to slips. J Mot Behav. 2008;40:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatt T, Wening JD, Pai YC. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res. 2006;170:61–73 [DOI] [PubMed] [Google Scholar]
- 10. Pai YC, Wening JD, Runtz EF, et al. Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol. 2003;90:755–762 [DOI] [PubMed] [Google Scholar]
- 11. Yang F, Anderson FC, Pai YC. Predicted threshold against backward balance loss in gait. J Biomech. 2007;40:804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang F, Anderson FC, Pai YC. Predicted threshold against backward balance loss following a slip in gait. J Biomech. 2008;41:1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8 [DOI] [PubMed] [Google Scholar]
- 14. Mille ML, Rogers MW, Martinez K, et al. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90:666–674 [DOI] [PubMed] [Google Scholar]
- 15. Pai YC, Maki BE, Iqbal K, et al. Thresholds for step initiation induced by support-surface translation: a dynamic center-of-mass model provides much better prediction than a static model. J Biomech. 2000;33:387–392 [DOI] [PubMed] [Google Scholar]
- 16. Pai YC, Rogers MW, Patton J, et al. Static versus dynamic predictions of protective stepping following waist-pull perturbations in young and older adults. J Biomech. 1998;31:1111–1118 [DOI] [PubMed] [Google Scholar]
- 17. Patton JL, Pai YC, Lee WA. Evaluation of a model that determines the stability limits of dynamic balance. Gait Posture. 1999;9:38–49 [DOI] [PubMed] [Google Scholar]
- 18. Patton JL, Lee WA, Pai YC. Relative stability improves with experience in a dynamic standing task. Exp Brain Res. 2000;135:117–126 [DOI] [PubMed] [Google Scholar]
- 19. Pai YC. Movement termination and stability in standing. Exerc Sport Sci Rev. 2003;31:19–25 [DOI] [PubMed] [Google Scholar]
- 20. Wu M, Ji L, Jin D, Pai YC. Minimal step length necessary for recovery of forward balance loss with a single step. J Biomech. 2007;40:1559–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inkster LM, Eng JJ. Postural control during a sit-to-stand task in individuals with mild Parkinson's disease. Exp Brain Res. 2004;154:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang F, Bhatt T, Pai YC. Role of stability and limb support in recovery against a fall following a novel slip induced in different daily activities. J Biomech. 2009;42:1903–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 25. Mak MK, Hui-Chan CW. Cued task-specific training is better than exercise in improving sit-to-stand in patients with Parkinson's disease: a randomized controlled trial. Mov Disord. 2008;23:501–509 [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization Falls. Available at: http://www.who.int/mediacentre/factsheets/fs344/en/
- 27. Pai YC, Naughton BJ, Chang RW, Rogers MW. Control of body centre of mass momentum during sit-to-stand among young and elderly adults. Gait Posture. 1994;2:109–116 [Google Scholar]
- 28. Pai YC, Yang F, Wening JD, Pavol MJ. Mechanisms of limb collapse following a slip among young and older adults. J Biomech. 2006;39:2194–2204 [DOI] [PubMed] [Google Scholar]
- 29. Riley PO, Krebs DE, Popat RA. Biomechanical analysis of failed sit-to-stand. IEEE Trans Rehabil Eng. 1997;5:353–359 [DOI] [PubMed] [Google Scholar]
- 30. Bridgewater KJ, Sharpe MH. Trunk muscle performance in early Parkinson's disease. Phys Ther. 1998;78:566–576 [DOI] [PubMed] [Google Scholar]
- 31. Mak MK, Wong EC, Hui-Chan CW. Quantitative measurement of trunk rigidity in Parkinsonian patients. J Neurol. 2007;254:202–209 [DOI] [PubMed] [Google Scholar]
- 32. Pang MY, Mak MK. Trunk muscle strength, but not trunk rigidity, is independently associated with bone mineral density of the lumbar spine in patients with Parkinson's disease. Mov Disord. 2009;24:1176–1182 [DOI] [PubMed] [Google Scholar]
- 33. Alexander NB, Schulz AB, Ashton-Miller JA, et al. Muscle strength and rising from a chair in older adults. Muscle Nerve Suppl. 1997;5:S56–S59 [PubMed] [Google Scholar]
- 34. Schultz AB, Alexander NB, Ashton-Miller JA. Biomechanical analyses of rising from a chair. J Biomech. 1992;12:1383–1391 [DOI] [PubMed] [Google Scholar]
- 35. Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23:1–11 [DOI] [PubMed] [Google Scholar]
- 36. Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson's disease and relates to the ability to rise from a chair. Mov Disord. 2003;18:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nallegowda M, Singh U, Handa G, et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: a pilot study. Am J Phys Med Rehabil. 2004;83:898–908 [DOI] [PubMed] [Google Scholar]
- 38. Paasuke M, Mottus K, Ereline J, et al. Lower limb performance in older female patients with Parkinson's disease. Aging Clin Exp Res. 2002;14:185–191 [DOI] [PubMed] [Google Scholar]
- 39. Dengler R, Konstanzer A, Gillespie J, et al. Behavior of motor units in Parkinsonism. Adv Neurol. 1990;53:167–173 [PubMed] [Google Scholar]
- 40. Glendinning DS, Enoka RM. Motor units behavior in Parkinson's disease. Phys Ther. 1994;74:61–70 [DOI] [PubMed] [Google Scholar]
- 41. Beckley DJ, Bloem BR, van Dijk JG, et al. Electrophysiological correlates of postural instability in Parkinson's disease. Electroencephalogr Clin Neurophysiol. 1991;81:263–268 [DOI] [PubMed] [Google Scholar]
- 42. Horak FB, Nutt JG, Nashner LM. Postural inflexibility in Parkinsonian subjects. J Neurol Sci. 1992;111:46–58 [DOI] [PubMed] [Google Scholar]
- 43. Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity: evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–449 [DOI] [PubMed] [Google Scholar]
- 44. Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in Parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396 [DOI] [PubMed] [Google Scholar]
- 45. Halliday SE, Winter DA, Frank JS, et al. The initiation of gait in young, elderly, and Parkinson's disease subjects. Gait Posture. 1998;8:8–14 [DOI] [PubMed] [Google Scholar]
- 46. Marsden CD. Parkinson's disease. Lancet. 1990;335:948–952 [DOI] [PubMed] [Google Scholar]
- 47. Rosin R, Topka H, Dichgans J. Gait initiation in Parkinson's disease. Mov Disord. 1997;12:682–690 [DOI] [PubMed] [Google Scholar]
- 48. Marsden CD. The enigma of the basal ganglia and movement. Trends Neurosci. 1980;3:284–287 [Google Scholar]
- 49. Mak MK, Hui-Chan CW. Audio-visual cues can enhance sit-to-stand and in patients with Parkinson's disease. Mov Disord. 2004;19:1012–1019 [DOI] [PubMed] [Google Scholar]
- 50. Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson's disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33:14–26 [DOI] [PubMed] [Google Scholar]
- 51. Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003;84:1109–1117 [DOI] [PubMed] [Google Scholar]
- 52. Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson's disease: a randomized trial. Neurorehabil Neural Repair. 2007;21:107–115 [DOI] [PubMed] [Google Scholar]