Abstract
Oxidative stress and associated reactive oxygen species can modify lipids, proteins, carbohydrates, and nucleic acids, and induce the mitochondrial permeability transition, providing a signal leading to the induction of autophagy, apoptosis, and necrosis. High-mobility group box 1 (HMGB1) protein, a chromatin-binding nuclear protein and damage-associated molecular pattern molecule, is integral to oxidative stress and downstream apoptosis or survival. Accumulation of HMGB1 at sites of oxidative DNA damage can lead to repair of the DNA. As a redox-sensitive protein, HMGB1 contains three cysteines (Cys23, 45, and 106). In the setting of oxidative stress, it can form a Cys23-Cys45 disulfide bond; a role for oxidative homo- or heterodimerization through the Cys106 has been suggested for some of its biologic activities. HMGB1 causes activation of nicotinamide adenine dinucleotide phosphate oxidase and increased reactive oxygen species production in neutrophils. Reduced and oxidized HMGB1 have different roles in extracellular signaling and regulation of immune responses, mediated by signaling through the receptor for advanced glycation end products and/or Toll-like receptors. Antioxidants such as ethyl pyruvate, quercetin, green tea, N-acetylcysteine, and curcumin are protective in the setting of experimental infection/sepsis and injury including ischemia-reperfusion, partly through attenuating HMGB1 release and systemic accumulation. Antioxid. Redox Signal. 14, 1315–1335.
Introduction
Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and a biological system's ability to readily detoxify the reactive intermediates or easily repair the resulting damage (6, 43). ROS have also been identified as important signaling molecules in various pathways regulating both cell survival and cell death (25, 201). High-mobility group box 1 protein (HMGB1) is a highly conserved nuclear protein, acting as a chromatin-binding factor that bends DNA and promotes access to transcriptional protein assemblies on specific DNA targets (133, 150). In addition to its nuclear role, HMGB1 also functions as an extracellular signaling molecule and damage-associated molecular pattern molecule (DAMP) (17, 131, 183), during inflammation (133, 243, 254), cell differentiation (145, 204), cell migration (48, 54, 157, 159, 181), cell proliferation (68, 122, 159, 176, 211), tissue regeneration (61, 122, 172, 233), and tumor development (49, 95, 129, 130, 132, 219, 225, 269). In this review, we focus on the role of HMGB1 in oxidative stress and antioxidant strategies based on targeting HMGB1.
Oxidative Stress, ROS, and Redox Signaling
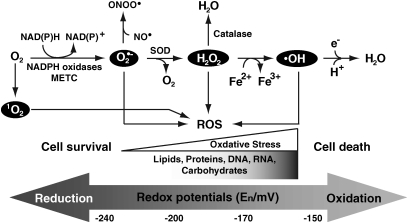
ROS are free radicals that contain the oxygen atom. These very small molecules include superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Fig. 1). They are highly reactive due to the presence of unpaired valence shell electrons. ROS form as a natural byproduct of the normal metabolism of oxygen and have important physiological roles in signal transduction, but in excess can contribute to the mechanisms of disease by dysregulation of signal transduction and/or by oxidative damage to cellular macromolecules (lipids, proteins, DNA, RNA, and carbohydrates) that exceeds the cellular capacity for regeneration or repair (29, 249). The major cellular source of ROS is generated by mitochondrial and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. O2•− is produced by the electron transport chain on the inner mitochondrial membrane, and the rate of production is dependent on mitochondrial potential. In the presence of mitochondrial superoxide dismutase (SOD), O2•− can be converted to H2O2, which can then diffuse out of the mitochondria into the cytoplasm. In the presence of high iron concentrations, H2O2 can form the highly reactive O2•− via the Fenton reaction. Catalase is responsible for converting H2O2 to water and oxygen. O2•− can also react with nitric oxide (NO) to form the highly reactive peroxynitrite (ONOO•). In animals, ROS can influence cell signaling cascades, including protein tyrosine phosphatases (PTPs), tyrosine kinases (PTKs), protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), and nuclear factor (NF)-κB (78).
FIG. 1.
Generation of free radicals. Superoxide can be generated by specialized enzymes, such as the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases or as a byproduct of cellular metabolism, particularly the mitochondrial electron transport chain (METC). During mitochondrial respiration some electrons go directly to oxygen-forming superoxide anion (O2•−) that leads to the production of other free radicals such as peroxynitrite (ONOO•) through reaction with nitric oxide (NO•), hydrogen peroxide (H2O2) through enzymatic dismutation by superoxide dismutase (SOD), and hydroxyl radical (•OH) by Fenton reaction. Singlet oxygen (1O2) is the diamagnetic form of molecular oxygen (O2). Redox potentials related to these cellular processes are adapted (189).
Redox signaling is the process wherein free radicals, ROS, and other electronically activated species act as messengers in biological systems (251). All forms of life maintain a reducing environment within their cells. This reducing environment is preserved by enzymes that maintain the reduced state through a constant input of metabolism-derived energy. The redox environment within the mitochondrial intermembrane space is maintained separately from the cytosol and matrix (78). Increased and/or sustained levels of oxidative stress and related mediators play a major role in most chronic human diseases, including atherosclerosis (22), diabetes (94), cardiovascular diseases (66), cancer (16), neurodegenerative disorders (14), and chronic liver (246) and lung (161) diseases. Inflammation is a host defense activated by exogenous (i.e., pathogen-associated molecular patterns) or endogenous danger signals (i.e., DAMPs) (131, 134). Inflammation is deeply entangled with redox modulation (28). Triggering of pattern recognition receptors on inflammatory cells induces ROS generation. As a consequence, activated cells mount antioxidant responses to counteract the possible harmful effects of oxidation. When repair is completed, homeostasis is restored. Growing evidence indicates that intra- and extracellular redox affects protein structure, secretion, and function of cytokines such as transforming growth factor beta 1 (13), interleukin-1 (IL-1) (24), IL-4 (199), IL-10 (250), S100 proteins (70), tumor necrosis factor (TNF) (202), and HMGB1 (100, 134, 183). Moreover, Redox also regulates pattern recognition receptors such as the Toll-like receptors (TLRs), and the receptor for advanced glycation endproducts (RAGE) expression and function (35, 91, 151). The thiol-disulfide oxidoreductase thioredoxin-1 (Trx1) (195) and NO (101) are secreted by leukocytes and regulate extracellular redox. Redox of SOD1 in turn regulates caspase-1 and IL-1 release in the setting of endotoxic shock (143). In addition, apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1) is a multifunctional protein involved in reduction and oxidation regulation of HMGB1-induced inflammatory responses (266). Thus, reduction/oxidation of pathogen-associated molecular patterns, DAMPs, and PPRs regulates immunity and inflammation.
Oxidative Stress, Autophagy, Apoptosis, and Necrosis
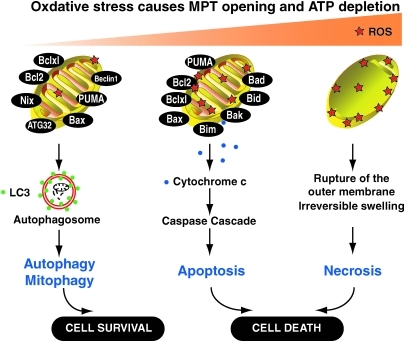
As a major source of ROS production, mitochondria are especially prone to ROS-mediated damage. Such damage can induce the mitochondrial permeability transition caused by opening of nonspecific high conductance permeability transition pores in the mitochondrial inner membrane. ROS themselves also provide a signal leading to the induction of autophagy, apoptosis, and necrosis (Fig. 2).
FIG. 2.
Pathways activated in response to different degree of oxidative stress. When low oxidative stress is just enough to lead to the mitochondrial permeability transition (MPT), and autophagy and mitophagy are likely induced (261). As the oxidative damage increases, molecules such as cytochrome c may be released from mitochondria activating the caspase cascade and triggering apoptosis. At the extreme, oxidative stress causes severe MPT or even the rupture of the mitochondrial membrane, and neither autophagy nor apoptosis can provide an adequate response. The Bcl-2 proteins are a family of proteins involved in the response to autophagy and apoptosis, and play important roles in regulating MPT. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Macroautophagy (subsequently referred to simply as autophagy) is the sequestration of organelles and long-lived proteins in a double-membrane vesicle, called an autophagosome or autophagic vacuole, inside the cell (106). The primary function of autophagy is to recycle cellular components to sustain metabolism during nutrient deprivation and to prevent the accumulation of damaged, toxic proteins, and organelles during stress. In most situations, autophagy promotes survival in response to stress and starvation. Mitochondrial quality control is important in maintaining proper cellular homeostasis. Selective mitochondrial degradation by autophagy (mitophagy) is suggested to have an important role in quality control (224, 261). Indeed, many stimuli that induce ROS generation also induce autophagy, including nutrient starvation, mitochondrial toxins, and hypoxia (193). ROS formation in the mitochondria is a fundamental regulatory event promoting autophagy and mitophagy (102, 192). The major endogenous source of cellular ROS is the mitochondrial electron transport chain (192). ROS can induce autophagy through several distinct mechanisms involving catalase or caspase activation of autophagy-related gene 4 (Atg4) and disturbances in the mitochondrial electron transport chain (11). Nip3-like protein X (Nix) and Atg32 are mitochondrial proteins that confer selectivity during mitophagy in erythroid cells and yeast, respectively (97, 187).
Chaperone-mediated autophagy (CMA), a selective mechanism for degradation of cytosolic proteins in lysosomes, contributes to the removal of altered proteins as part of the cellular quality-control systems (99). In CMA, only those proteins that have a consensus peptide sequence are recognized by the binding of a heat shock protein 70 (HSP70)-containing chaperone/co-chaperone complex. This CMA substrate/chaperone complex then moves to the lysosomes, where the CMA receptor lysosome-associated membrane protein type-2A (LAMP-2A) recognizes it. The protein is unfolded and translocated across the lysosome membrane assisted by the lysosomal hsc70 on the other side (99). Induction of mild-oxidative stress in cells increases the degradation of proteins via CMA (99, 103). Part of the enhanced CMA directly results from the oxidative modification of the CMA substrates, which are more readily degraded through this pathway, when compared with their unmodified counterparts (99, 103). It is possible that partial unfolding, typically associated with oxidative damage, could expose hidden CMA-targeting motifs, facilitating their recognition by the cytosolic chaperone complex (99).
Excessive ROS production and adenosine triphosphate depletion from uncoupling of oxidative phosphorylation promotes necrotic cell death. Release of cytochrome c (Cyt c) after mitochondrial swelling activates caspases and initiates apoptotic cell death (193, 261) (Fig. 2). Bcl-2 family members (antiapoptotic [i.e., Bcl-2 and Bcl-XL] or pro- apoptotic [i.e., Bax, Bid, Bad, Bim, Bak, NOXA, and the p53-upregulated mediator of apoptosis, PUMA]) (179) play important roles in regulation of the mitochondrial outer membrane permeabilization and MPT (Fig. 2). Both Bcl-2 and Bcl-XL negatively regulate Beclin1-dependent autophagy (152, 163). In addition, PUMA itself can induce autophagy that leads to the selective removal of mitochondria. This function of PUMA depends on Bax/Bak and can be reproduced by overexpression of Bax (260). Cytoskeletal reorganization is also an important regulator of apoptosis and autophagy (20, 64, 149, 174). Recently, WAVE1, an actin cytoskeleton regulator, was found to be associated with mitochondrial Bcl-2, and its depletion led to mitochondrial release of Bcl-2, and phosphorylation of Bcl-2 (96). Depletion of WAVE1 expression increased anticancer drug-induced production of ROS in leukemia cells (96). It is not clear whether WAVE1 is involved in autophagy and/or mitophagy.
Cysteine and Disulfide Bonds
Cysteine is uniquely suited to sensing a range of redox signals as the thiol side-chain (-SH) can be oxidized to several different reversible redox states such as disulphide (R-S-S-R); sulphenic acid (R-SOH); and S-nitrosothiol (R-SNO). Importantly, not all cysteines are equally reactive. The protein environment of the thiol has a key role in determining this reactivity by affecting the ionization state of the thiol (the thiolate ion is much more reactive to peroxides than the protonated form) and its overall accessibility (65).
Disulfide bonds play an important role in the folding and stability of proteins, particularly those secreted into the extracellular medium (197). Disulfide bonds in proteins are formed after oxidation of the thiol groups of cysteine residues (Fig. 3). The other sulfur-containing amino acid (AA), methionine, cannot form disulfide bonds. More aggressive oxidants convert cysteine to the corresponding sulfinic acid and irreversibly oxidized sulfonic acid. Cysteine residues play a valuable role by crosslinking proteins, which increases the rigidity of proteins and also functions to confer proteolytic resistance. Since protein export is a bioenergetically costly process, minimizing its necessity is advantageous. Inside the cell, disulfide bridges between cysteine residues within a polypeptide support the protein's secondary structure.
FIG. 3.
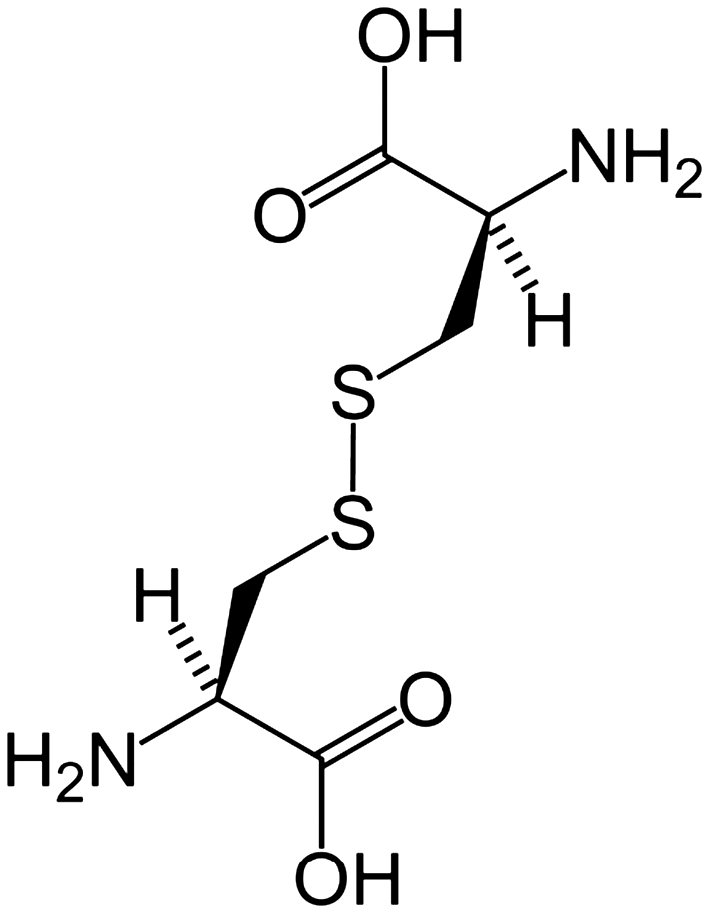
Cystine is composed of two cysteines linked by a disulfide bond. Rarely is it found as a free amino acid, but rather as a consequence of proteolysis.
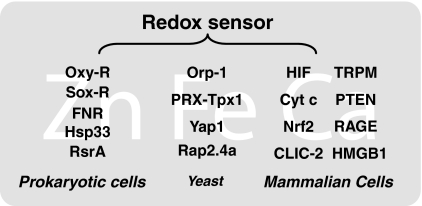
Redox Sensors
Redox sensors were first described in bacteria, including the OxyR and SoxR redox-sensitive transcription factors, the chaperone molecule Hsp33, the oxygen sensor FNR, and others (Fig. 4). All of these redox receptors have a structure designed to sense specific ROS, oxidants, or other reactive intermediates. These ancestral redox sensors can essentially contribute to rapid mechanisms designed to deal with ROS and to make critical adjustments allowing survival of the bacteria. During evolutionary development, these simple bacterial sensors have been replaced with more specifically designed proteins, such as yeast thiol peroxidases (enzymes belonging to the family of peroxyredoxins or GPXs), transcription factor Yap1 (9), and Rap2.4a (198), which contribute to ROS signaling. In mammals, the hypoxia-inducible transcription factors (8), Cyt c (247), nuclear factor-erythroid-2-related factor 2 (212), the type 2 chloride intracellular channel (86), the transient receptor potential melastatin (140), phosphatase and tensin homolog (34), RAGE (35), and HMGB1 (77, 185) regulate ROS-homeostasis by sensing cellular redox status (Fig. 4).
FIG. 4.
Examples of redox sensors in prokaryotic cells, yeast, and mammal cells. There are significant interactions between zinc (Zn), iron (Fe), calcium (Ca), and redox species.
Zinc/cysteine coordination environments in proteins are redox-active. Oxidation of the sulfur ligands mobilizes zinc, whereas reduction of the oxidized ligands enhances zinc binding, providing redox control over the availability of zinc ions (137). Iron also is utilized as a sensor of cellular redox status. Iron-based sensors incorporate Fe-S clusters, heme, and mononuclear iron sites, acting as switches to control protein activity in response to changes in cellular redox balance (156). Moreover, there are significant interactions between calcium and redox species, and these interactions modify a variety of proteins that participate in signaling transduction pathways and in other fundamental cellular functions that determine cell life or death (73).
HMGB1 Basics
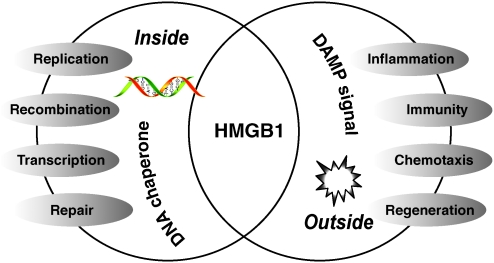
The HMG-1 protein was first purified from nuclei ∼40 years ago and termed HMG protein because of its rapid mobility on electrophoresis gels (62). HMG-1 was subsequently renamed HMGB1 by a nomenclature committee (26). It is constitutively expressed in many types of cells, and a large pool of preformed HMGB1 is stored in the nucleus owing to the presence of two lysine-rich nuclear localization sequences (21). As a DNA chaperone, HMGB1 participates in DNA replication, recombination, transcription, and repair. HMGB1 also interacts with and enhances the activities of a number of transcription factors including p53 (12, 89, 130, 142, 208), p73 (209, 235), the retinoblastoma protein (45, 90), members of the Rel/NF-κB family (3, 23), and nuclear hormone receptors including the estrogen receptor (38, 51, 239, 271). HMGB1-gene-deficient mice are born with several defects and die shortly after birth of hypoglycemia initially believed to be caused by deficient glucocorticoid receptor function (27). In addition to its nuclear role, HMGB1 also functions as an extracellular signaling molecule during inflammation, immunity, cell differentiation, cell migration, and tissue regeneration (46, 49, 133, 150) (Fig. 5).
FIG. 5.
Functions of high-mobility group box 1 (HMGB1). HMGB1 is present in almost all metazoans and plants. As a DNA chaperone, HMGB1 participates in DNA replication, recombination, transcription, and repair. HMGB1 is passively released from necrotic cells and is actively secreted by inflammatory cells, mediating the response to inflammation, immunity, chemotaxis, and tissue regeneration. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Structure of the Redox-Sensitive HMGB1 Protein
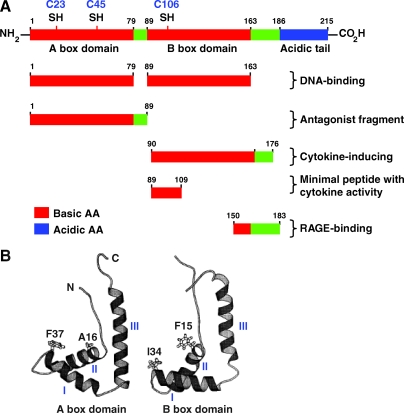
HMGB1 is a 215-AA protein of ∼30 kDa. Structurally, HMGB1 is composed of three domains: two positively charged domains (A box and B box) and a negatively charged carboxyl terminus (the acidic tail). HMGB1 adopts a closed, dynamic but compact conformation, shown by nuclear magnetic resonance spectroscopy and small angle X-ray scattering (207). The long acidic tail of HMGB1 protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes (107). Moreover, the C-terminal acidic tail has a higher affinity for the B box and that A box–tail interactions are preferentially disrupted (207). Functionally, the A and B boxes are DNA-binding domains. HMG-box proteins are targeted to particular DNA sites in chromatin by either protein–protein interactions or recognition of specific DNA structures. Recombinant analysis shows that the B box contains cytokine activity by inducing macrophage secretion of proinflammatory cytokines (116). This cytokine activity is antagonized by the recombinant A box. The first 21-AA residues (AA89–109) of the recombinant B box represent the minimal peptide sequence that retains cytokine-like activity (116). The protein structure involved in binding of HMGB1 with RAGE is located between AA residues 150 and 183. A recent study suggests that the C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis (11).
Three cysteines are encoded at positions 23, 45, and 106 (Fig. 6). These two Cys23-Cys45 residues can rapidly form an intramolecular disulfide bond with the standard redox potential as low as −237 mV. This suggests that the cellular glutathione system alone is not enough to keep HMGB1 completely reduced within the cell (185). The reduction of oxidized HMGB1 by thioredoxin is sufficient to maintain most of the HMGB1 in a reduced form, whereas the reaction is far slower than other reducing reactions mediated by thioredoxin. The low efficiency of the thioredoxin–HMGB1 reaction, together with the protein stabilization by the Cys23-Cys45 disulfide bond, might lead to accumulation of the oxidized form of the HMGB1 protein in cells under oxidative stress (185). Replacement of Cys23 and/or 45 with serines did not affect the nuclear distribution of the mutant proteins, whereas C106S and triple cysteine mutations impaired nuclear localization of HMGB1 (77), allowing some of the protein entry to the cytosol. C106 is required for HMGB1 binding to TLR-4 and activation of cytokine release in macrophages (256). These various functional domains of HMGB1 are retained conservatively in many species, suggesting multiple critical roles in its biological function intracellularly and extracellularly, precluding changes in the molecule.
FIG. 6.
Structure of the HMGB1 protein. (A) HMGB1 is a conserved chromosomal protein composed of two similar DNA binding domains (A and B box) linked by a short basic stretch to an acidic C-terminal tail of 30 residues. There are oxidation-sensitive unpaired cysteines at positions 23, 45, and 106. (B) Helical secondary structure of A box domain and B box domain (226). Green shades are neutral amino acids; red-shaded amino acids are basic and blue-shaded amino acids are acidic residues. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Our recent studies demonstrated that HMGB1 promotes autophagy and cell survival in cancer chemotherapy and nutrition depletion (214, 215, 220). Reduced exogenous HMGB1 increases autophagy and oxidized HMGB1 increases apoptosis (214, 220). Mutation of cysteine 106, but not the vicinal cysteines 23 and 45, of HMGB1 promotes cytosolic localization and sustained autophagy (215). Moreover, the intramolecular disulfide bridge (C23/45) of HMGB1 is required for binding to Beclin 1 and sustaining autophagy (215). These findings suggest that redox of HMGB1 regulates autophagy.
HMGB1 and Oxidative DNA Damage Repair
HMGB1 proteins are constitutively expressed in the nucleus of both cancer and normal cells. HMGB1's affinity for a number of different DNA structures has been measured. In addition to supercoiled, single-stranded, B- and Z-DNA, it binds preferentially to DNA mini-circles, four-way junctions, looped structures, hemicatenated DNA, and triplex DNA (87, 113, 153, 254). Native HMGB1 extracted from tumor cells inhibits DNA replication and this effect is reduced after acetylation and completely abolished after removal of the acidic C-terminal tail. Recombinant HMGB1, however, fails to inhibit replication, but it acquires this property after in vitro phosphorylation by PKC (229). A nuclear protein complex containing HMGB1 and HMGB2, Hsp70, ERp60, and glyceraldehyde-3-phosphate dehydrogenase is involved in the cytotoxic response to DNA modified by incorporation of anticancer nucleoside analogs (110), suggesting that HMGB1 plays a critical role in DNA repair. Accumulation of HMGB1 is found at sites of oxidative DNA damage in live cells, thus defining HMGB1 as a component of an early DNA damage response (167). Reduced histone acetylation after DNA damage in HMGB1-deficient cells indicates a role for HMGB1 in DNA damage-induced chromatin remodeling (112). Mutations in mitochondrial DNA and oxidative stress both contribute to aging, which is the greatest risk factor for neurodegenerative diseases (123). It is not clear what the role of HMGB1 is in mitochondrial DNA mutation although HMGB1 can localize in mitochondria during human endothelial cell Toxoplasma gondii infection (210). Recent studies have identified the cross-link of HMGB1 with a DNA base excision repair intermediate, indicating that this protein is involved in base excision repair pathway (63, 128). Moreover, HMGB1 facilitates trinucleotide repeat CAG expansion by stimulating APE1 and flap endonuclease 1 in forming single strand breaks and ligatable nicks (127, 128). However, the molecular mechanisms underlying its effect on flap endonuclease 1 remain to be elucidated.
Active Secretion and Passive Release of HMGB1
Immune cells actively release HMGB1 in response to exogenous bacterial products (such as endotoxin or CpG-DNA) (84, 241) or endogenous host stimuli (i.e., TNF, IFN-γ, or H2O2) (175, 221, 222). Cytolytic cells, both natural killer (NK) cells and specific T-cells, induce HMGB1 release from melanoma cell lines (83). The redox potential in the endoplasmic reticulum (ER), where most extracellular proteins become oxidized before secretion, is −180 mV (185, 191). If HMGB1 is secreted through the ER, 99% of the released HMGB1 should be in the oxidized form in equilibrium. HMGB1 lacks a leader peptide and is thus not secreted via the classical ER-Golgi secretory pathway (241). HMGB1 secretion from monocytes/macrophages depends on relocalization from the nucleus to special cytoplasmic organelles, the secretory lysosomes (21, 59, 175). Unless the vesicle provides an environment as oxidative as the ER, both reduced and oxidized forms may exist when HMGB1 molecules are released into the extracellular space. The released HMGB1 in the reduced form should be a short-lived species because of the extracellular oxidative environment (185). However, the reduced extracellular microenvironment generated by cysteine and redox enzymes, possibly secreted by stressed cells (184) or passively released by necrotic cells, prevents the oxidation of HMGB1, prolonging its extracellular lifespan and activity (28). Notably, these two forms of HMGB1 likely have different roles in extracellular signaling (133, 164).
The initial phase of HMGB1 secretion requires an inflammatory signal such as lipopolysaccharide (LPS), IL-1, or TNF to the monocyte (241). This signal will lead to acetylation of lysine residues, which causes an accumulation of HMGB1 in the cytoplasm and blocks reentry to the nuclear compartment (21). In addition, serine phosphorylation might be another requisite step for HMGB1 nucleocytoplasmic translocation (263). The phosphorylation of HMGB1 is potentially also mediated by the calcium/calmodulin-dependent protein kinase IV (272) because calcium/calmodulin-dependent protein kinase IV can be translocated to the nucleus after endotoxin stimulation, where it can potentially bind and phosphorylate HMGB1 (272). In addition, HMGB1 can be passively released from necrotic cells (190) or cells infected by viruses (i.e., West Nile, salmon anemia, dengue, and influenza viruses) or mycobacteria (67, 76) and similarly triggers inflammatory response. In vitro, apoptotic cells activate macrophages to release HMGB1 (169). Monoclonal antibodies directed against HMGB1 conferred protection against organ damage but did not prevent the accumulation of apoptotic cells in the spleen. Thus, HMGB1 production is downstream of apoptosis on the final common pathway to organ damage in severe sepsis (169).
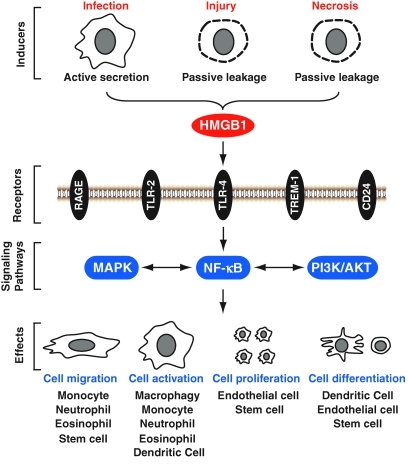
HMGB1 has been shown to bind to at least five different surface receptors expressed on immune cells, namely, the receptor for advanced glycosylation endproducts (RAGE) (95, 173, 206), TLR-2 (264), TLR-4 (139, 264), triggering receptor expressed on myeloid cells-1 (52), and CD24 (30). After interaction they activate MAPKs, NF-κB, and phosphoinositide 3-kinases/AKT signaling pathways (Fig. 7). The absence of HMGB1 severely impairs the activation of TLR-3, TLR-7, and TLR-9 by their cognate nucleic acids (254). HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses (254).
FIG. 7.
Extracellular HMGB1 functions as a damage-associated molecular pattern molecule signal. HMGB1 is passively released from injury and necrotic cells and is actively secreted by inflammatory cells, binding with high affinity to several receptors, including the receptor for advanced glycation end products (RAGE), Toll-like receptors (TLR)-2, TLR-4, triggering receptor expressed on myeloid cells-1 (TREM-1), and CD24, mediating the response to cell migration, cell activation, cell proliferation, and cell differentiation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Once released, extracellular HMGB1 mediates a wide range of biological responses in diverse cell types and tissues. In vitro, extracellular HMGB1 can activate macrophages and monocytes (4), and promote dendritic cell (DC) maturation (47, 147, 182). In vivo, HMGB1 causes acute lung inflammation and epithelial-cell barrier leaking (2, 188). Moreover, increased levels of HMGB1 are found in patients with sepsis and other major inflammatory diseases, including rheumatoid arthritis and meningitis (213, 223, 241). Accumulating evidence indicates that HMGB1 is capable of stimulating migration of neurite (54), smooth muscle cells (41), tumor cells (79), mesoangioblast stem cells (158), monocytes (181), DCs (255), and neutrophils (155, 242). HMGB1 could interact with phosphatidylserine on cell surface of apoptotic neutrophils and consequently inhibit phagocytotic elimination of apoptotic neutrophils by macrophages (125). Notably, HMGB1 is capable of attracting stem cells (159) and may be important for skeletal muscle, heart tissue repair, and regeneration (42, 61, 119, 180). In vitro, HMGB1 induces migration and proliferation of both adult and embryonic mesoangioblasts, and disrupts the barrier function of endothelial monolayers (159). Moreover, HMGB1 induces human primary cardiac fibroblasts migration and proliferation (180). HMGB1 is capable of stimulating differentiation of DC (47), erythroleukemia cell (205), neuroblastoma cell (162), myoblasts (177), and endogenous cardiac stem cell (122).
Hsp72 Inhibits HMGB1 Release and Function in Oxidative Stress
ROS are produced as a normal product of cellular metabolism. In particular, one major contributor to oxidative damage is H2O2, which is converted from superoxide that leaks from the mitochondria. H2O2 induces both active and passive HMGB1 release from macrophage and monocyte cultures in a time- and dose-dependent fashion (222). At nontoxic doses, H2O2 induced HMGB1 cytoplasmic translocation and active release within 3–24 h. At higher concentrations, however, H2O2 exhibits cytotoxicity for macrophages and monocyte cell cultures, and subsequently triggers both active and passive HMGB1 release. In addition, H2O2 stimulates interaction of HMGB1 with a nuclear export factor, chromosome region maintenance 1 (CRM1) protein homolog, in macrophage/monocyte cultures. Inhibitors specific for the c-jun N-terminal kinase, MEK-extracellular signal-regulated kinase (ERK), but not p38 MAPKs, abrogate H2O2-induced active HMGB1 release (222). These findings establish an important role for oxidative stress in inducing active HMGB1 release potentially through an MAPK- and CRM1-dependent mechanism (Fig. 8).
FIG. 8.
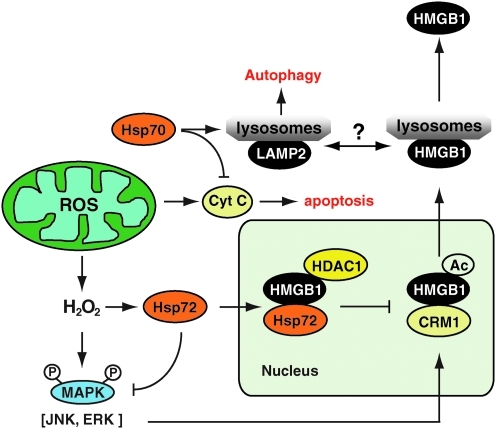
Hsp72 regulates oxidative stress-induced HMGB1 cytoplasmic translocation and release. Upon stimulation with oxidative stress (i.e., H2O2), mitogen-activated protein kinase (MAPK) signal pathways are activated to trigger potential acetylation of HMGB1. On the other hand, oxidative stress also induces nuclear translocation of Hsp72, which directly or indirectly interacts with various nuclear proteins (such as HMGB1 and histone deacetylase-1 [HDAC1]) within the nucleus. The Hsp72-facilitated potential recruitment of HDAC1 to HMGB1 may consequently prevent HMGB1 acetylation, cytoplasmic translocation, and subsequent release via the secretory lysosome pathway. Hsp70 inhibits apoptosis downstream of cytochrome c release and with LAMP-2A drives chaperone-mediated autophagy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Representing a universal response to diverse adverse stimuli, cells rapidly express stress-inducible HSPs such as Hsp90, Hsp70, Hsp60, and Hsp27 (75). As major stress-inducible proteins, the Hsp70 family consists of ubiquitous Hsp73 and Hsp72 inducible by heat shock, oxidative stress, and infection. Intracellular Hsp72 functions as a molecular chaperone to maintain cellular homeostasis (71, 75, 146). Nuclear Hsp72 confers a protective role against various environmental stressors (36, 50, 238, 248). Hsp70 inhibits apoptosis downstream of Cyt c release and upstream of caspase-3 (60, 115). Hsp70 with LAMP-2A mediates the so-called CMA (31, 37).
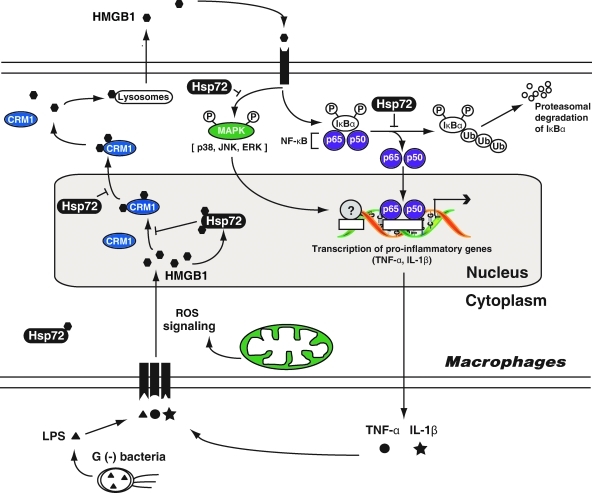
Notably, enhanced expression of Hsp72 (by gene transfection) renders murine macrophage cell lines resistant to H2O2-induced HMGB1 cytoplasmic translocation and release. In response to oxidative stress, cytoplasmic Hsp72 translocates to the nucleus, where it interacts with nuclear proteins, including HMGB1, and prevented oxidative stress-induced HMGB1 cytoplasmic translocation and release (216) (Fig. 8). Moreover, overexpression of Hsp72 inhibits CRM1 translocation and interaction between HMGB1 and CRM1 in macrophages after LPS or TNF-α treatment (217). In addition, overexpression of Hsp72 strongly inhibited HMGB1-induced cytokine expression and release, which correlates closely with (i) inhibition of the MAPKs (p38, c-jun N-terminal kinase, and ERK), and (ii) inhibition of the NF-κB pathway (Fig. 9). These findings suggest that Hsp72 plays an important role in the regulation of HMGB1 release and proinflammatory function in the setting of oxidative stress.
FIG. 9.
Hsp72 is an important intracellular protein to inhibition of HMGB1 release and proinflammatory function in macrophages (217). Hsp72 attenuates lipopolysaccharide (LPS)- or tumor necrosis factor (TNF)-α-induced HMGB1 release partly through inhibiting chromosome region maintenance 1 (CRM1)-dependent nuclear export pathway of HMGB1. Hsp72 also inhibits HMGB1-induced cytokine (i.e., TNF-α and interleukin [IL]-1β) expression and release potentially by inhibiting MAPKs and nuclear factor (NF)-κB activation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
TLR-4 Mediates HMGB1-Induced ROS Production
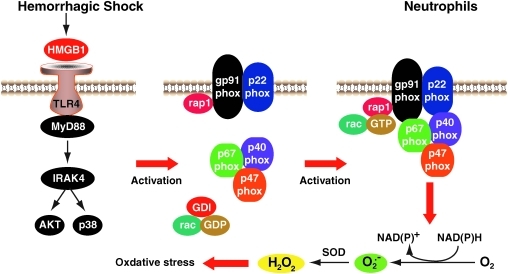
NADPH oxidase is a highly regulated membrane-bound enzyme complex that catalyzes the production of superoxide by the single electron reduction of oxygen, using NADPH as the electron donor. The core enzyme is comprised of both membrane-bound (i.e., gp91phox and p22phox) and cytosolic (i.e., p40phox, p47phox, p67phox, and rac-1/2) components. Upon stimulation, receptor-mediated activation of the oxidase complex leads to activation of secondary signaling intermediates, culminating in the phosphorylation and recruitment of the cytosolic components to the membrane-bound molecules to assemble the active oxidase (Fig. 10). HMGB1/TLR-4 signaling in the setting of hemorrhagic shock/resuscitation (HS/R) induces neutrophil NADPH oxidase activation. HS/R primes circulating neutrophils (PMN) NADPH oxidase activation in wild-type mice. This induction is diminished in TLR-4-mutant C3H/HeJ mice. Neutralizing Ab to HMGB1 prevents HS/R-induced activation of PMN NADPH oxidase. In addition, in vitro stimulation of PMN with recombinant HMGB1 causes TLR-4-dependent activation of NADPH oxidase as well as increased ROS production through both myeloid differentiation factor 88 (MyD88)-interleukin-1 receptor-associated kinase 4 (IRAK4)-p38 MAPK and MyD88-IRAK4-Akt signaling pathways (55). Thus p38 MAPK and Akt, as downstream components of HMGB1-TLR-4-MyD88-IRAK4 signaling, may act in a coordinate manner. Recent studies indicate that TLR-4-HMGB1 axis involves in the inflammatory basis of two very common disease processes, skin cancer and epilepsy (138, 148). However, whether or not oxidative stress involving in these processes remains to be elucidated.
FIG. 10.
TLR-4 mediates HMGB1-induced reactive oxygen species (ROS) production. The integral membrane of the phagocyte consists of two subunits: p22phox and gp91phox, which, respectively, produce the smaller and larger chain of the cytochrome-b558. Two cytosolic subunits (p67phox and p47phox), a p40phox accessory protein, and an Rac-GTP binding protein then translocate to the cell membrane upon cell activation to form the NADPH oxidase complex, which generates a respiratory burst. Hemorrhagic shock/resuscitation-induced HMGB1 release. HMGB1 increases neutrophil NADPH oxidase activation and subsequent ROS production by TLR-4-MyD88-IRAK4 signaling. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Redox of HMGB1 and DCs
The mammalian immune system discriminates between modes of cell death. Necrosis often results in inflammation and adaptive immunity, whereas apoptosis tends to be anti-inflammatory and promote immune tolerance. DCs are key components of innate and adaptive immune responses. The HMGB1 protein induces the migration and activation of human DCs (147, 255), and NK-DC cross-talk (141, 144). However, HMGB1 produced by colon cancer cells suppresses nodal DCs to promote anticancer immunity (111). HMGB1 interacts with TLR-4 on DCs, which are selectively involved in the cross-priming of antitumor T lymphocytes in vivo (7). NK cells trigger immature DCs to polarize and secrete IL-18. In turn, DCs activate NK cells that secrete HMGB1, and in turn induce DC maturation and promote DC survival (196). Moreover, RAGE and HMGB1 play a nonredundant role in DC homing to lymph nodes (135). HMGB1 and RAGE are required for the maturation of human plasmacytoid DCs (47). However, HMGB1 suppresses plasmacytoid DC cytokine secretion and maturation in response to TLR-9 agonists, including the hypomethylated oligodeoxynucleotide CpG- and DNA-containing viruses (165). Thus, HMGB1 coming from necrotic cells is an important activator of DCs (Fig. 11).
FIG. 11.
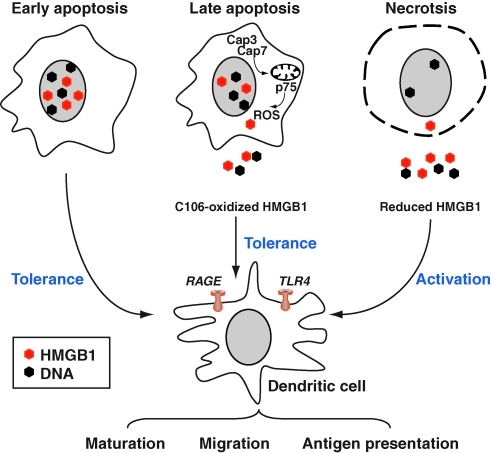
Redox of HMGB1 mediated its immunogenic activity. In necrotic cells, HMGB1 is released from dying cells possessing full immunogenic activity, including dendritic cell (DC). Binding of HMGB1 to RAGE and/or TLRs induces activation and/or maturation of DCs. In contrast, in cells undergoing apoptosis, activated caspase-3 and caspase-7 cleave the complex I component p75 NDUFS1 and thus halt the respiratory chain, leading to production of ROS, which oxidize cysteine 106 in HMGB1. Oxidized HMGB1 cannot fully activate DCs and has tolerogenic activities. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Initially, it was believed that the release of HMGB1 from the nucleus either to the cytosol or to the extracellular space was sufficient to distinguish necrotic from apoptotic cells (190). However, this idea has recently come into question for several reasons. First, nuclear DNA and associated proteins are released in a time-dependent manner during apoptosis (32) and that the binding of HMGB1 to DNA is increased during apoptosis, consistent with the notion that late-stage apoptotic cells can release both DNA and HMGB1 (15). In addition, apoptotic tumor cells release HMGB1 (15, 227). It was, therefore, postulated that a post-translational modification of HMGB1 is responsible for its various activities (18, 164, 236). Caspase activation targeted the mitochondria to produce ROS, which are critical to tolerance induction by apoptotic cells. Notably, ROS oxidized HMGB1 released from dying cells and thereby neutralized its stimulatory activity (100). Apoptotic cells failed to induce tolerance and instead stimulated immune responses by scavenging or by mutating a mitochondrial caspase target mitochondrial complex 1 protein p75 NDUFS1 when ROS activity was prohibited. Similarly, the oxidation of HMGB1 Cys106 alone was sufficient to block the immunogenic activity of HMGB1 for DCs (Fig. 11). Thus, although ROS are often associated with inflammatory conditions, other stimuli that are present in such settings are likely to override the effects on HMGB1 to promote immunity over tolerance during inflammation (100).
HMGB1 and Ischemia Reperfusion Injury
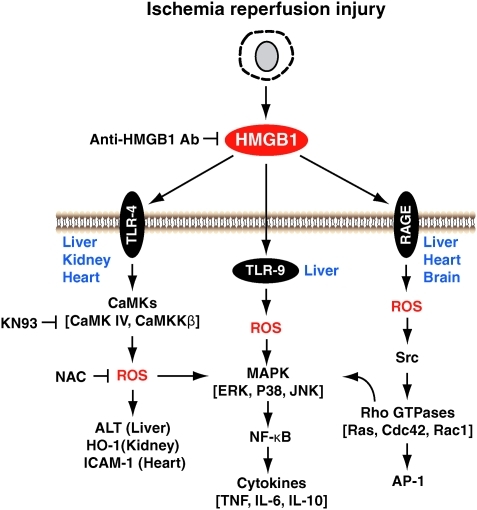
Ischemia reperfusion (I/R) injury is a pathophysiologic process whereby hypoxic organ damage is accentuated after return of blood flow and oxygen delivery. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function. Transient episodes of ischemia are encountered during solid organ transplantation, trauma, hypovolemic shock, and elective liver resection, when inflow occlusion or total vascular exclusion is used to minimize blood loss. HMGB1 levels were increased during mice liver I/R as early as 1 h after reperfusion and then increased in a time-dependent manner up to 24 h (232). Inhibition of HMGB1 activity with neutralizing antibody significantly decreased liver damage after I/R, whereas administration of recombinant HMGB1 worsened I/R injury. Moreover, HMGB1 is an early mediator of injury and inflammation in liver I/R (245) and kidney I/R (33, 252) and implicates TLR-4 (109, 232, 252), TLR-9 (10), RAGE (270) as receptors that is involved in the process.
HMGB1 is a marker of injury in human liver and kidney transplantation (80, 109, 267). Further, HMGB1 release induced by liver ischemia involves TLR-4-dependent ROS production and calcium-mediated signaling (231). HMGB1 release induced by oxidative stress was markedly reduced by inhibition of calcium/calmodulin-dependent kinases (231). Inhibition of HMGB1 activity by injection of neutralizing antibody partially abolished the increase in liver manganese SOD, a key mitochondrial antioxidant enzyme, after I/R (160). Pretreatment of mice with HMGB1 significantly decreased liver damage after I/R (85). The protection observed in mice pretreated with HMGB1 was associated with higher expression of IL-1R-associated kinase-M, a negative regulator of TLR-4 signaling, compared with controls (85). Moreover, HMGB1-TLR-4 pathway also plays an important role in the initiation of systemic inflammation and end-organ injury after isolated peripheral tissue injury (114). In the heart, HMGB1 plays a major role in the early events after I/R injury by binding to RAGE, resulting in the activation of proinflammatory pathways and enhanced myocardial I/R injury (5, 154). Src family kinases are necessary for cell migration induced by HMGB1-RAGE pathway (157). However, TLR-4 mediates inflammation signaling after cold I/R in the heart (92). HMGB1 is released and plays a cytokine-like function in the postischemic brain (72, 105) and ischemic injury from neurons (170). HMGB1 promotes metalloproteinase-9 upregulation through TLR-4 after cerebral ischemia (171). Moreover, HMBG1 mediates cerebral I/R injury by TIR-domain-containing adapter-inducing interferon-β (TRIF)-independent TLR-4 signaling (258). In rats, treatment with neutralizing anti-HMGB1 monoclonal antibody remarkably ameliorated brain infarction (114). Cannabidiol prevents a postischemic injury progressively induced by cerebral ischemia via a HMGB1-inhibiting mechanism (72). Taken together, HMGB1 is central to early activation of the innate immune response in the setting of organ I/R (Fig. 12).
FIG. 12.
Signaling pathway of HMGB1-mediated ischemia reperfusion injury. HMGB1 is an early mediator of injury and inflammation in liver, kidney, heart, and brain ischemia reperfusion injury and implicates TLR-4, TLR-9, and RAGE as receptors that are involved in the process. HMGB1's extracellular function partly through activation of MAPKs and NF-κB pathway. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
HMGB1 and Atherosclerosis
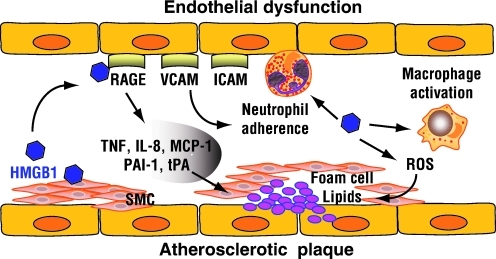
Atherosclerosis is the condition in which an artery wall thickens as the result of a build-up of fatty materials such as cholesterol. Atherosclerosis develops from low-density lipoprotein (LDL) molecules becoming oxidized (LDL-ox) by free radicals, particularly ROS. When LDL-ox comes in contact with an artery wall, a series of reactions occur to repair the damage to the artery wall caused by LDL-ox (22, 194, 240). Endothelial dysfunction is a key triggering event in atherosclerosis. HMGB1 activates vascular endothelial cells to express and the secretion of intercellular adhesion molecule 1, vascular cell adhesion molecule 1, RAGE, TNF-α, IL-8, monocyte chemotactic protein-1, plasminogen activator inhibitor 1, and tissue plasminogen activator (Fig. 13) (118, 230). Hyperglycemia-induced ROS production increases expression of RAGE and HMGB1 in human endothelial cells (259). Increased expression of the HMGB1 is observed in human atherosclerotic lesions (82, 93). Activated vascular smooth muscle cells are the source of HMGB1 in human advanced atherosclerotic lesions (82). HMGB1 directly stimulates the production of both C-reactive protein and matrix metalloproteinase through RAGE (82). Moreover, HMGB1 promotes smooth muscle cells in human atherosclerotic plaques to secrete a variety of vasoactive substances and to proliferate (166). HMGB1 induces phospholipase A2 and prostaglandin E2 production, which is the lipid mediators observed during atherogenesis (88). Simvastatin can alleviate the formation of the atherosclerotic plaques in the atherosclerotic rats, and decrease the protein and mRNA expression of HMGB1 (262). In a mouse model of atherosclerosis, apoE−/− mice, vascular and inflammatory stresses mediate atherosclerosis via RAGE and HMGB1 (69). Although diabetic apoE−/− mice have accelerated plaque accumulation, diabetic RAGE−/−apoE−/− mice had significantly reduced atherosclerotic plaque area to levels not significantly different from control apoE−/− mice (203). These beneficial effects on the vasculature were associated with attenuation of leukocyte recruitment and decreased expression of proinflammatory mediators, including the NF-κB subunit p65, vascular cell adhesion molecule 1, and monocyte chemotactic protein-1. Reduced oxidative stress, as reflected by staining for nitrotyrosine and reduced expression of various NADPH oxidase subunits, gp91phox, p47phox, and rac-1, is also noted. Both RAGE and RAGE ligands, including S100A8/A9, HMGB1, and the AGE carboxymethyllysine, are increased in plaques from diabetic apoE−/− mice. Further, the accumulation of AGEs, HMGB1, and other ligands for RAGE is reduced in diabetic RAGE−/−apoE−/− mice (203). Thus, RAGE and its ligand deficiency attenuate the development of atherosclerosis. Recent study shows that HMGB1 induced vascular endothelial activation by TLR-4/NF-κB signaling pathway (257).
FIG. 13.
HMGB1 induces endothelial dysfunction and promotes atherosclerotic plaque formation. Activated vascular smooth muscle cells (SMCs) are the source of HMGB1 in human advanced atherosclerotic lesions. HMGB1 activates vascular endothelial cells to express and the secretion of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), RAGE, TNF-α, IL-8, monocyte chemotactic protein-1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1), and tissue plasminogen activator (tPA). Moreover, HMGB1 activates macrophagy, promotes neutrophil adherence, and increases ROS production. Atherosclerosis develops from low-density lipoprotein (LDL) molecules becoming oxidized by ROS in artery wall. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
HMGB1 and Aging
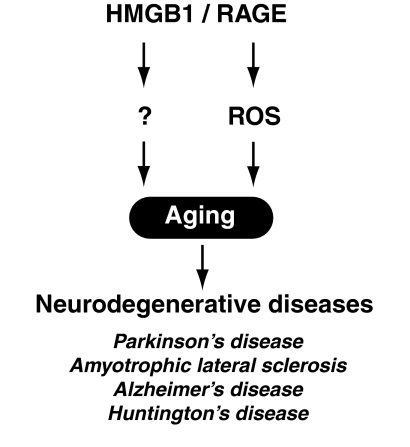
Aging is an inherently complex process that is manifested within an organism at genetic, molecular, cellular, tissue, and systemic levels. Aging is a major risk factor for several common neurodegenerative diseases, including Parkinson's disease, amyotrophic lateral sclerosis, Alzheimer's disease, and Hunting's disease (Fig. 14). Although the fundamental mechanisms are still poorly understood, a growing body of evidence points toward ROS as one of the primary determinants of aging (58, 108). Reduction of nuclear HMGB1 protein level within the nucleus is associated with DNA double-strand break (DDSB)-mediated neuronal damage in Huntington's disease (168). HMGB1 is localized in the nuclei of neurons and astrocytes in mouse, and the protein level changes in various brain regions age dependently (53). HMGB1 reduction is noted in neurons during aging, whereas it increases in astrocytes. In contrast, DDSB remarkably accumulates in neurons, but it does not change significantly in astrocytes during aging. These findings indicate that HMGB1 expression during aging is differentially regulated between neurons and astrocytes, and suggest that the reduction of nuclear HMGB1 might be causative for DDSB in neurons of the aged brain (53). HMGB1 binds preferentially to aggregated alpha-synuclein and is present in alpha-synuclein filament-containing Lewy bodies isolated from brain tissue affected with dementia or Parkinson's disease (124). Amyloid-β peptide is central to the pathology of Alzheimer's disease. It is neurotoxic, directly by inducing oxidant stress and indirectly by activating microglia. Increased expression of RAGE in Alzheimer's disease brain mediates the pathogenesis of neuronal dysfunction and death (253). sRAGE levels are significantly decreased in the serum of the patients with amyotrophic lateral sclerosis (81).
FIG. 14.
The HMGB1/RAGE pathway is involving in aging. Aging is a major risk factor for several common neurodegenerative diseases, including Parkinson's disease, amyotrophic lateral sclerosis, Alzheimer's disease, and Huntington's disease. ROS is one of the primary determinants of aging.
Potential Antioxidant Therapy Targeting HMGB1 Release
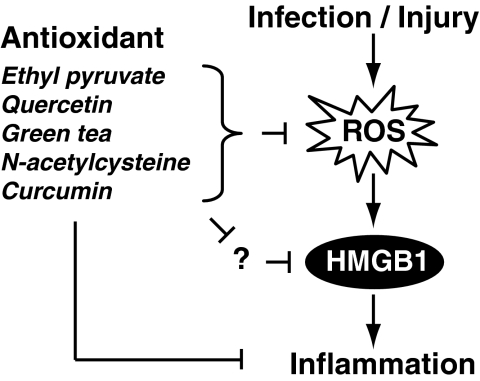
Several agents such as ethyl pyruvate, quercetin, green tea, N-acetylcysteine (NAC), and curcumin have not only antioxidative properties, but also antiinflammatory properties. They are protective in the setting of experimental inflammation, partly through attenuating systemic HMGB1 accumulation (Fig. 15).
FIG. 15.
Several agents such as ethyl pyruvate, quercetin, green tea, N-acetylcysteine, and curcumin have not only antioxidative properties, but also antiinflammatory properties. They are protective in the setting of experimental inflammation, partly through attenuating systemic HMGB1 accumulation.
Ethyl pyruvate
Ethyl pyruvate (CH3COCOOC2H5) is a simple derivative in Ca2+- and K+-containing balanced salt solutions of pyruvate originally designed to avoid the problems associated with the instability of pyruvate in solution. Treatment with ethyl pyruvate has been shown to improve survival and ameliorate organ dysfunction in a wide variety of preclinical models of critical illnesses, such as severe sepsis, acute respiratory distress syndrome, burn injury, acute pancreatitis, and stroke (56, 57). It ameliorates the effects of I/R injury in many organs (33, 98, 244, 265). Ethyl pyruvate is also an effective scavenger of oxygen radicals (39, 104, 237). Ethyl pyruvate was also found to be a pharmacological inhibitor of HMGB1 secretion (234). Ethyl pyruvate inhibits the release of TNF-α and HMGB1 from endotoxin-stimulated RAW 264.7 murine macrophages, as well as attenuates activation of both the p38 MAPKs and NF-κB signaling pathways. Ethyl pyruvate treatment of septic mice decreases circulating levels of HMGB1 (234). Pretreatment with ethyl pyruvate also prevents endotoxin lethality and inhibits the release of TNF-α and HMGB1 (234). Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis (40) and renal ischemia and reperfusion injury (33). Serum IL-6 and HMGB1 levels, which are elevated after tumor injection, are decreased significantly in ethyl pyruvate-treated animals (120).
Quercetin
As an antioxidant, quercetin (3,3′,4′,5,7-pentahydroxyflavone dehydrate, C15H10O7) has anti-inflammatory effects, regulating NO, IL-6, and TNF-α release (44, 136), thereby alleviating oxidative damage in the tissue (1, 44), and inhibiting the LPS-induced delay in spontaneous apoptosis and activation of neutrophils (126). Quercetin treatment significantly reduces circulating levels of HMGB1 in animals with established endotoxemia (218). In macrophage cultures, quercetin inhibits release as well as the cytokine activities of HMGB1, including limiting the activation of MAPKs and NF-κB, two signaling pathways that are critical for HMGB1-induced subsequent cytokine release. Quercetin and the autophagic inhibitor wortmannin inhibit LPS-induced type II microtubule-associated protein 1 light chain 3 production and aggregation as well as HMGB1 translocation and release (218).
Green tea
Green tea contains potent antioxidants called catechins. Tea extracts increase antioxidant activity in the blood (74). Accounting for 50% to 80% of the total catechin, epigallocatechin gallate (EGCG, C22H18O11) is effective in attenuating endotoxin-induced HMGB1 release by macrophage and monocytes (117). In addition, EGCG dose dependently inhibited HMGB1-induced release of TNF-A, IL-6, and NO in macrophage cultures (123). EGCG completely abrogated accumulation/clustering of exogenous HMGB1 on the macrophage cell surface (117), suggesting that EGCG inhibits HMGB1 cytokine activities by preventing its cell surface accumulation/clustering. Consistently, delayed administration of EGCG significantly attenuated circulating levels of HMGB1, as well as surrogate markers of experimental sepsis (such as IL-6) (117). Considered together, these experimental data indicate that EGCG protects mice after otherwise lethal sepsis partly by attenuating systemic HMGB1 accumulation and partly by inhibiting the HMGB1-mediated inflammatory response.
N-acetylcysteine
NAC, HSCH2CH(NHCOCH3)CO2H, is a potent antioxidant that has been used to investigate the role of ROS in numerous biological and pathological processes (268). NAC decreases the expression of TNF-α, IL-1, IL-6, IL-12p40, and MIP-1α in the setting of endotoxin-induced lung inflammation (178). NAC has the potential to counter the intertwined redox and inflammatory imbalances in cystic fibrosis (228). In vitro, NAC inhibited hypoxia- or H2O2-mediated HMGB1 release in hepatocytes (231). NAC reduces I/R liver injury in wild-type mice to the level observed in TLR-4 mutant mice but fails to reduce the injury in TLR-4 mutant mice. In vivo, NAC treatment inhibits the expression of TNF-α and IL-6 mRNA, and serum HMGB1 levels after I/R liver injury (231). These results indicate that HMGB1 release during I/R is mediated, in part, by TLR-4 signaling and parallels the extent of oxidant production.
Curcumin
Curcumin ([HOC6H3(OCH3)CH = CHCO]2CH2) is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family. Curcumin acts as a free radical scavenger and antioxidant, inhibiting lipid peroxidation and oxidative DNA damage (200). Curcumin inhibited PMA-mediated activation of ERK and NF-κB transcriptional activity (121). In rats, curcumin decreased oxidative stress, inhibited NF-κB activation, and ameliorated liver pathologic changes in the setting of ethanol-induced liver injury (186). Uric acid, a final metabolite of purine metabolism in mammals but not birds, triggers the release of HMGB1 in a time- and dose-dependant fashion in mouse macrophage cells (RAW 264.7), human leukemic promonocytes (THP-1 cells), as well as in macrophages, but not in fibroblasts obtained from synovial fluid of patients with rheumatoid arthritis. Curcumin significantly suppressed HMGB1 release in response to uric acid (19).
Conclusion
ROS intermediates are indeed signaling molecules in various pathways regulating both cell survival and cell death. HMGB1 is both a nuclear factor and a secreted protein. In the cell nucleus it acts as a DNA chaperone. Outside the cell, it serves as a DAMP signal. The findings discussed here support the notion that HMGB1 is integral to the response to oxidative stress. The precise mechanisms promoting the release of HMGB1 in the setting of oxidative stress and the signaling pathways it activates remain to be completely elucidated. Understanding HMGB1 and its complex effects in the setting of oxidative stress may lead to the development of novel strategies to attenuate oxidative injury in various clinical states, particularly those associated with chronic inflammation, including cancer.
Abbreviations Used
- AA
amino acid
- AGE
advanced glycation end product
- APE1
apurinic/apyrimidinic endonuclease 1/redox factor-1
- ATG
autophagy-related gene
- CMA
chaperone-mediated autophagy
- CRM1
chromosome region maintenance 1
- Cys
cysteines
- Cyt c
cytochrome c
- DAMP
damage-associated molecular pattern molecule
- DCs
dendritic cells
- DDSB
DNA double-strand break
- EGCG
epigallocatechin gallate
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- HDAC1
histone deacetylase-1
- HMGB1
high-mobility group box 1 protein
- H2O2
hydrogen peroxide
- HSP
heat shock protein
- HS/R
hemorrhagic shock/resuscitation
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- I/R
ischemia reperfusion
- IRAK4
interleukin-1 receptor-associated kinase 4
- LAMP-2A
lysosome-associated membrane protein type-2A
- LDL
low-density lipoprotein
- LDL-ox
oxidized low-density lipoprotein
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- MCP-1
monocyte chemotactic protein-1
- METC
mitochondrial electron transport chain
- MPT
mitochondrial permeability transition
- MyD88
myeloid differentiation factor 88
- NAC
N-acetylcysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- Nix
nip3-like protein X
- NK
natural killer
- NO
nitric oxide
- O2•−
superoxide anion
- 1O2
singlet oxygen
- •OH
hydroxyl radical
- ONOO•
peroxynitrite
- PAI-1
plasminogen activator inhibitor 1
- PKC
protein kinase C
- PMN
primes circulating neutrophils
- PTKs
tyrosine kinases
- PTPs
protein tyrosine phosphatases
- PUMA
p53-upregulated mediator of apoptosis
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- R-SNO
S-nitrosothiol
- R-SOH
sulphenic acid
- R-S-S-R
disulphide
- -SH
thiol side-chain
- SMC
smooth muscle cells
- SOD
superoxide dismutase
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- tPA
tissue plasminogen activator
- TREM-1
triggering receptor expressed on myeloid cells-1
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- Trx1
thiol-disulfide oxidoreductase thioredoxin-1
- VCAM-1
vascular cell adhesion molecule 1
References
- 1.Abd El-Gawad HM. Khalifa AE. Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res. 2001;43:257–263. doi: 10.1006/phrs.2000.0781. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E. Arcaroli J. Carmody A. Wang H. Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 3.Agresti A. Lupo R. Bianchi ME. Muller S. HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun. 2003;302:421–426. doi: 10.1016/s0006-291x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 4.Andersson U. Wang H. Palmblad K. Aveberger AC. Bloom O. Erlandsson-Harris H. Janson A. Kokkola R. Zhang M. Yang H. Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrassy M. Volz HC. Igwe JC. Funke B. Eichberger SN. Kaya Z. Buss S. Autschbach F. Pleger ST. Lukic IK. Bea F. Hardt SE. Humpert PM. Bianchi ME. Mairbaurl H. Nawroth PP. Remppis A. Katus HA. Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 6.Apel K. Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 7.Apetoh L. Ghiringhelli F. Tesniere A. Criollo A. Ortiz C. Lidereau R. Mariette C. Chaput N. Mira JP. Delaloge S. Andre F. Tursz T. Kroemer G. Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 8.Aragones J. Fraisl P. Baes M. Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo D. Tacnet F. Delaunay A. Rodrigues-Pousada C. Toledano MB. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic Biol Med. 2003;35:889–900. doi: 10.1016/s0891-5849(03)00434-9. [DOI] [PubMed] [Google Scholar]
- 10.Bamboat ZM. Balachandran VP. Ocuin LM. Obaid H. Plitas G. Dematteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee S. Friggeri A. Liu G. Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol. 2010;88:973–979. doi: 10.1189/jlb.0510262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee S. Kundu TK. The acidic C-terminal domain and A-box of HMGB-1 regulates p53-mediated transcription. Nucleic Acids Res. 2003;31:3236–3247. doi: 10.1093/nar/gkg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH. Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 14.Barnham KJ. Masters CL. Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 15.Bell CW. Jiang W. Reich CF., 3rd Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 16.Benz CC. Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi ME. Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 19.Biswas KK. Oyama Y. Abeyama K. Hashiguchi T. Maruyama I. Uric acid induces high mobility group box1 protein release in monocytes/macrophages through P38 MAPK, ERK1/2, JNK and AP-1 activation. ASH Annu Meet Abstr. 2004;104:1464. [Google Scholar]
- 20.Blankson H. Holen I. Seglen PO. Disruption of the cytokeratin cytoskeleton and inhibition of hepatocytic autophagy by okadaic acid. Exp Cell Res. 1995;218:522–530. doi: 10.1006/excr.1995.1187. [DOI] [PubMed] [Google Scholar]
- 21.Bonaldi T. Talamo F. Scaffidi P. Ferrera D. Porto A. Bachi A. Rubartelli A. Agresti A. Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonomini F. Tengattini S. Fabiano A. Bianchi R. Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol. 2008;23:381–390. doi: 10.14670/HH-23.381. [DOI] [PubMed] [Google Scholar]
- 23.Brickman JM. Adam M. Ptashne M. Interactions between an HMG-1 protein and members of the Rel family. Proc Natl Acad Sci U S A. 1999;96:10679–10683. doi: 10.1073/pnas.96.19.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brigelius-Flohe R. Banning A. Kny M. Bol GF. Redox events in interleukin-1 signaling. Arch Biochem Biophys. 2004;423:66–73. doi: 10.1016/j.abb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24:1028–1032. doi: 10.1042/bst0241028. [DOI] [PubMed] [Google Scholar]
- 26.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci. 2001;26:152–153. doi: 10.1016/s0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- 27.Calogero S. Grassi F. Aguzzi A. Voigtlander T. Ferrier P. Ferrari S. Bianchi ME. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–280. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 28.Carta S. Castellani P. Delfino L. Tassi S. Vene R. Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 29.Case J. Ingram DA. Haneline LS. Oxidative stress impairs endothelial progenitor cell function. Antioxid Redox Signal. 2008;10:1895–1907. doi: 10.1089/ars.2008.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen GY. Tang J. Zheng P. Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang HL. Terlecky SR. Plant CP. Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 32.Choi JJ. Reich CF., 3rd Pisetsky DS. Release of DNA from dead and dying lymphocyte and monocyte cell lines in vitro. Scand J Immunol. 2004;60:159–166. doi: 10.1111/j.0300-9475.2004.01470.x. [DOI] [PubMed] [Google Scholar]
- 33.Chung KY. Park JJ. Kim YS. The role of high-mobility group box-1 in renal ischemia and reperfusion injury and the effect of ethyl pyruvate. Transplant Proc. 2008;40:2136–2138. doi: 10.1016/j.transproceed.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Connor KM. Subbaram S. Regan KJ. Nelson KK. Mazurkiewicz JE. Bartholomew PJ. Aplin AE. Tai YT. Aguirre-Ghiso J. Flores SC. Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 35.Coughlan MT. Cooper ME. Forbes JM. Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxid Redox Signal. 2007;9:331–342. doi: 10.1089/ars.2006.1469. [DOI] [PubMed] [Google Scholar]
- 36.Cowan KJ. Diamond MI. Welch WJ. Polyglutamine protein aggregation and toxicity are linked to the cellular stress response. Hum Mol Genet. 2003;12:1377–1391. doi: 10.1093/hmg/ddg151. [DOI] [PubMed] [Google Scholar]
- 37.Cuervo AM. Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 38.Das D. Peterson RC. Scovell WM. High mobility group B proteins facilitate strong estrogen receptor binding to classical and half-site estrogen response elements and relax binding selectivity. Mol Endocrinol. 2004;18:2616–2632. doi: 10.1210/me.2004-0125. [DOI] [PubMed] [Google Scholar]
- 39.Das UN. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med Sci Monit. 2006;12:RA79–RA84. [PubMed] [Google Scholar]
- 40.Dave SH. Tilstra JS. Matsuoka K. Li F. DeMarco RA. Beer-Stolz D. Sepulveda AR. Fink MP. Lotze MT. Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Degryse B. Bonaldi T. Scaffidi P. Muller S. Resnati M. Sanvito F. Arrigoni G. Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Mori R. Straino S. Di Carlo A. Mangoni A. Pompilio G. Palumbo R. Bianchi ME. Capogrossi MC. Germani A. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2377–2383. doi: 10.1161/ATVBAHA.107.153429. [DOI] [PubMed] [Google Scholar]
- 43.Demple B. Amabile-Cuevas CF. Redox redux: the control of oxidative stress responses. Cell. 1991;67:837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- 44.Dias AS. Porawski M. Alonso M. Marroni N. Collado PS. Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–2304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 45.Dintilhac A. Bernues J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences. J Biol Chem. 2002;277:7021–7028. doi: 10.1074/jbc.M108417200. [DOI] [PubMed] [Google Scholar]
- 46.Dong XD. Ito N. Lotze MT. Demarco RA. Popovic P. Shand SH. Watkins S. Winikoff S. Brown CK. Bartlett DL. Zeh HJ., 3rd High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 47.Dumitriu IE. Baruah P. Bianchi ME. Manfredi AA. Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2184–2190. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- 48.Dumitriu IE. Bianchi ME. Bacci M. Manfredi AA. Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 49.Ellerman JE. Brown CK. de Vera M. Zeh HJ. Billiar T. Rubartelli A. Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 50.Ellis S. Killender M. Anderson RL. Heat-induced alterations in the localization of HSP72 and HSP73 as measured by indirect immunohistochemistry and immunogold electron microscopy. J Histochem Cytochem. 2000;48:321–332. doi: 10.1177/002215540004800302. [DOI] [PubMed] [Google Scholar]
- 51.El Marzouk S. Gahattamaneni R. Joshi SR. Scovell WM. The plasticity of estrogen receptor-DNA complexes: binding affinity and specificity of estrogen receptors to estrogen response element half-sites separated by variant spacers. J Steroid Biochem Mol Biol. 2008;110:186–195. doi: 10.1016/j.jsbmb.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 52.El Mezayen R. El Gazzar M. Seeds MC. McCall CE. Dreskin SC. Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enokido Y. Yoshitake A. Ito H. Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376:128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- 54.Fages C. Nolo R. Huttunen HJ. Eskelinen E. Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113(Pt 4):611–620. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- 55.Fan J. Li Y. Levy RM. Fan JJ. Hackam DJ. Vodovotz Y. Yang H. Tracey KJ. Billiar TR. Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 56.Fink MP. Ethyl pyruvate. Curr Opin Anaesthesiol. 2008;21:160–167. doi: 10.1097/ACO.0b013e3282f63c2e. [DOI] [PubMed] [Google Scholar]
- 57.Fink MP. Ethyl pyruvate: a novel treatment for sepsis. Novartis Found Symp. 2007;280:147–156. discussion 156–164. [PubMed] [Google Scholar]
- 58.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 59.Gardella S. Andrei C. Ferrera D. Lotti LV. Torrisi MR. Bianchi ME. Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrido C. Schmitt E. Cande C. Vahsen N. Parcellier A. Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–584. [PubMed] [Google Scholar]
- 61.Germani A. Limana F. Capogrossi MC. Pivotal advances: high-mobility group box 1 protein—a cytokine with a role in cardiac repair. J Leukoc Biol. 2007;81:41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 62.Goodwin GH. Johns EW. The isolation and purification of the high mobility group (HMG) nonhistone chromosomal proteins. Methods Cell Biol. 1977;16:257–267. doi: 10.1016/s0091-679x(08)60104-1. [DOI] [PubMed] [Google Scholar]
- 63.Goula AV. Berquist BR. Wilson DM., 3rd Wheeler VC. Trottier Y. Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS Genet. 2009;5:e1000749. doi: 10.1371/journal.pgen.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gourlay CW. Carpp LN. Timpson P. Winder SJ. Ayscough KR. A role for the actin cytoskeleton in cell death and aging in yeast. J Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green J. Paget MS. Bacterial redox sensors. Nat Rev Microbiol. 2004;2:954–966. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- 66.Griendling KK. Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- 67.Grover A. Taylor J. Troudt J. Keyser A. Sommersted K. Schenkel A. Izzo AA. Mycobacterial infection induces the secretion of high-mobility group box 1 protein. Cell Microbiol. 2008;10:1390–1404. doi: 10.1111/j.1462-5822.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 68.Hanspal M. Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84:3494–3504. [PubMed] [Google Scholar]
- 69.Harja E. Bu DX. Hudson BI. Chang JS. Shen X. Hallam K. Kalea AZ. Lu Y. Rosario RH. Oruganti S. Nikolla Z. Belov D. Lalla E. Ramasamy R. Yan SF. Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison CA. Raftery MJ. Walsh J. Alewood P. Iismaa SE. Thliveris S. Geczy CL. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J Biol Chem. 1999;274:8561–8569. doi: 10.1074/jbc.274.13.8561. [DOI] [PubMed] [Google Scholar]
- 71.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 72.Hayakawa K. Mishima K. Irie K. Hazekawa M. Mishima S. Fujioka M. Orito K. Egashira N. Katsurabayashi S. Takasaki K. Iwasaki K. Fujiwara M. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008;55:1280–1286. doi: 10.1016/j.neuropharm.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Hidalgo C. Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 74.Higdon JV. Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 75.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 76.Hofner P. Seprenyi G. Miczak A. Buzas K. Gyulai Z. Medzihradszky KF. Rouhiainen A. Rauvala H. Mandi Y. High mobility group box 1 protein induction by Mycobacterium bovis BCG. Mediators Inflamm. 2007;2007:53805. doi: 10.1155/2007/53805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoppe G. Talcott KE. Bhattacharya SK. Crabb JW. Sears JE. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res. 2006;312:3526–3538. doi: 10.1016/j.yexcr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 78.Hu J. Dong L. Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huttunen HJ. Fages C. Kuja-Panula J. Ridley AJ. Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 80.Ilmakunnas M. Tukiainen EM. Rouhiainen A. Rauvala H. Arola J. Nordin A. Makisalo H. Hockerstedt K. Isoniemi H. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517–1525. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- 81.Ilzecka J. Serum-soluble receptor for advanced glycation end product levels in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;120:119–122. doi: 10.1111/j.1600-0404.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 82.Inoue K. Kawahara K. Biswas KK. Ando K. Mitsudo K. Nobuyoshi M. Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16:136–143. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Ito N. Demarco RA. Mailliard RB. Han J. Rabinowich H. Kalinski P. Stolz DB. Zeh HJ., 3rd Lotze MT. Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol. 2007;81:75–83. doi: 10.1189/jlb.0306169. [DOI] [PubMed] [Google Scholar]
- 84.Ivanov S. Dragoi AM. Wang X. Dallacosta C. Louten J. Musco G. Sitia G. Yap GS. Wan Y. Biron CA. Bianchi ME. Wang H. Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izuishi K. Tsung A. Jeyabalan G. Critchlow ND. Li J. Tracey KJ. Demarco RA. Lotze MT. Fink MP. Geller DA. Billiar TR. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006;176:7154–7158. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- 86.Jalilian C. Gallant EM. Board PG. Dulhunty AF. Redox potential and the response of cardiac ryanodine receptors to CLIC-2, a member of the glutathione S-transferase structural family. Antioxid Redox Signal. 2008;10:1675–1686. doi: 10.1089/ars.2007.1994. [DOI] [PubMed] [Google Scholar]
- 87.Jantzen HM. Admon A. Bell SP. Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990;344:830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- 88.Jaulmes A. Thierry S. Janvier B. Raymondjean M. Marechal V. Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. FASEB J. 2006;20:1727–1729. doi: 10.1096/fj.05-5514fje. [DOI] [PubMed] [Google Scholar]
- 89.Jayaraman L. Moorthy NC. Murthy KG. Manley JL. Bustin M. Prives C. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 1998;12:462–472. doi: 10.1101/gad.12.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiao Y. Wang HC. Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28:1957–1967. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 91.Jin X. Wang L. Wu HS. Zhang L. Wang CY. Tian Y. Zhang JH. N-acetylcysteine inhibits activation of toll-like receptor 2 and 4 gene expression in the liver and lung after partial hepatic ischemia-reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2007;6:284–289. [PubMed] [Google Scholar]
- 92.Kaczorowski DJ. Nakao A. Vallabhaneni R. Mollen KP. Sugimoto R. Kohmoto J. Zuckerbraun BS. McCurry KR. Billiar TR. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;87:1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalinina N. Agrotis A. Antropova Y. DiVitto G. Kanellakis P. Kostolias G. Ilyinskaya O. Tararak E. Bobik A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24:2320–2325. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- 94.Kaneto H. Katakami N. Kawamori D. Miyatsuka T. Sakamoto K. Matsuoka TA. Matsuhisa M. Yamasaki Y. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal. 2007;9:355–366. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- 95.Kang R. Tang D. Schapiro NE. Livesey KM. Farkas A. Loughran P. Bierhaus A. Lotze MT. Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang R. Tang D. Yu Y. Wang Z. Hu T. Wang H. Cao L. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia. 2010;24:177–186. doi: 10.1038/leu.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanki T. Wang K. Cao Y. Baba M. Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karabeyoglu M. Unal B. Bozkurt B. Dolapci I. Bilgihan A. Karabeyoglu I. Cengiz O. The effect of ethyl pyruvate on oxidative stress in intestine and bacterial translocation after thermal injury. J Surg Res. 2008;144:59–63. doi: 10.1016/j.jss.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 99.Kaushik S. Cuervo AM. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med. 2006;27:444–454. doi: 10.1016/j.mam.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kazama H. Ricci JE. Herndon JM. Hoppe G. Green DR. Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khan BV. Harrison DG. Olbrych MT. Alexander RW. Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiffin R. Bandyopadhyay U. Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 103.Kiffin R. Christian C. Knecht E. Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim HS. Cho IH. Kim JE. Shin YJ. Jeon JH. Kim Y. Yang YM. Lee KH. Lee JW. Lee WJ. Ye SK. Chung MH. Ethyl pyruvate has an anti-inflammatory effect by inhibiting ROS-dependent STAT signaling in activated microglia. Free Radic Biol Med. 2008;45:950–963. doi: 10.1016/j.freeradbiomed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Kim JB. Lim CM. Yu YM. Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86:1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- 106.Klionsky DJ. Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knapp S. Muller S. Digilio G. Bonaldi T. Bianchi ME. Musco G. The long acidic tail of high mobility group box 1 (HMGB1) protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes. Biochemistry. 2004;43:11992–11997. doi: 10.1021/bi049364k. [DOI] [PubMed] [Google Scholar]
- 108.Kregel KC. Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 109.Kruger B. Krick S. Dhillon N. Lerner SM. Ames S. Bromberg JS. Lin M. Walsh L. Vella J. Fischereder M. Kramer BK. Colvin RB. Heeger PS. Murphy BT. Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krynetski EY. Krynetskaia NF. Bianchi ME. Evans WE. A nuclear protein complex containing high mobility group proteins B1 and B2, heat shock cognate protein 70, ERp60, and glyceraldehyde-3-phosphate dehydrogenase is involved in the cytotoxic response to DNA modified by incorporation of anticancer nucleoside analogues. Cancer Res. 2003;63:100–106. [PubMed] [Google Scholar]
- 111.Kusume A. Sasahira T. Luo Y. Isobe M. Nakagawa N. Tatsumoto N. Fujii K. Ohmori H. Kuniyasu H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76:155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- 112.Lange SS. Mitchell DL. Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105:10320–10325. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lange SS. Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog. 2009;48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levy RM. Mollen KP. Prince JM. Kaczorowski DJ. Vallabhaneni R. Liu S. Tracey KJ. Lotze MT. Hackam DJ. Fink MP. Vodovotz Y. Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 115.Li CY. Lee JS. Ko YG. Kim JI. Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 116.Li J. Kokkola R. Tabibzadeh S. Yang R. Ochani M. Qiang X. Harris HE. Czura CJ. Wang H. Ulloa L. Warren HS. Moldawer LL. Fink MP. Andersson U. Tracey KJ. Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 117.Li W. Ashok M. Li J. Yang H. Sama AE. Wang H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS ONE. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li W. Febbraio M. Reddy SP. Yu DY. Yamamoto M. Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li W. Sama AE. Wang H. Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol. 2006;6:130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liang X. Chavez AR. Schapiro NE. Loughran P. Thorne SH. Amoscato AA. Zeh HJ. Beer-Stolz D. Lotze MT. de Vera ME. Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol. 2009;86:599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 121.Lim JH. Kwon TK. Curcumin inhibits phorbol myristate acetate (PMA)-induced MCP-1 expression by inhibiting ERK and NF-kappaB transcriptional activity. Food Chem Toxicol. 2010;48:47–52. doi: 10.1016/j.fct.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 122.Limana F. Germani A. Zacheo A. Kajstura J. Di Carlo A. Borsellino G. Leoni O. Palumbo R. Battistini L. Rastaldo R. Muller S. Pompilio G. Anversa P. Bianchi ME. Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 123.Lin MT. Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 124.Lindersson EK. Hojrup P. Gai WP. Locker D. Martin D. Jensen PH. Alpha-Synuclein filaments bind the transcriptional regulator HMGB-1. Neuroreport. 2004;15:2735–2739. [PubMed] [Google Scholar]
- 125.Liu G. Wang J. Park YJ. Tsuruta Y. Lorne EF. Zhao X. Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu JJ. Song CW. Yue Y. Duan CG. Yang J. He T. He YZ. Quercetin inhibits LPS-induced delay in spontaneous apoptosis and activation of neutrophils. Inflamm Res. 2005;54:500–507. doi: 10.1007/s00011-005-1385-2. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y. Prasad R. Beard WA. Hou EW. Horton JK. McMurray CT. Wilson SH. Coordination between polymerase beta and FEN1 can modulate CAG repeat expansion. J Biol Chem. 2009;284:28352–28366. doi: 10.1074/jbc.M109.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Y. Prasad R. Wilson SH. HMGB1: roles in base excision repair and related function. Biochim Biophys Acta. 2010;1799:119–130. doi: 10.1016/j.bbagrm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Livesey KM. Tang D. Zeh HJ. Lotze MT. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs. 2009;10:1269–1279. [PubMed] [Google Scholar]
- 130.Livesey KM. Tang D. Zeh HJ. Lotze MT. Not just nuclear proteins: “novel” autophagy cancer treatment targets—p53 and HMGB1. Curr Opin Investig Drugs. 2008;9:1259–1263. [PubMed] [Google Scholar]
- 131.Lotze MT. Deisseroth A. Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. doi: 10.1016/j.clim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lotze MT. DeMarco RA. Dealing with death: HMGB1 as a novel target for cancer therapy. Curr Opin Investig Drugs. 2003;4:1405–1409. [PubMed] [Google Scholar]
- 133.Lotze MT. Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 134.Lotze MT. Zeh HJ. Rubartelli A. Sparvero LJ. Amoscato AA. Washburn NR. Devera ME. Liang X. Tor M. Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 135.Manfredi AA. Capobianco A. Esposito A. De Cobelli F. Canu T. Monno A. Raucci A. Sanvito F. Doglioni C. Nawroth PP. Bierhaus A. Bianchi ME. Rovere-Querini P. Del Maschio A. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol. 2008;180:2270–2275. doi: 10.4049/jimmunol.180.4.2270. [DOI] [PubMed] [Google Scholar]
- 136.Manjeet KR. Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol. 1999;21:435–443. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 137.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid Redox Signal. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 138.Maroso M. Balosso S. Ravizza T. Liu J. Aronica E. Iyer AM. Rossetti C. Molteni M. Casalgrandi M. Manfredi AA. Bianchi ME. Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 139.Massullo P. Druhan LJ. Bunnell BA. Hunter MG. Robinson JM. Marsh CB. Avalos BR. Aberrant subcellular targeting of the G185R neutrophil elastase mutant associated with severe congenital neutropenia induces premature apoptosis of differentiating promyelocytes. Blood. 2005;105:3397–3404. doi: 10.1182/blood-2004-07-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Massullo P. Sumoza-Toledo A. Bhagat H. Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–666. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 141.Matsuoka N. Itoh T. Watarai H. Sekine-Kondo E. Nagata N. Okamoto K. Mera T. Yamamoto H. Yamada S. Maruyama I. Taniguchi M. Yasunami Y. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McKinney K. Prives C. Efficient specific DNA binding by p53 requires both its central and C-terminal domains as revealed by studies with high-mobility group 1 protein. Mol Cell Biol. 2002;22:6797–6808. doi: 10.1128/MCB.22.19.6797-6808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meissner F. Molawi K. Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 144.Melki MT. Saidi H. Dufour A. Olivo-Marin JC. Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk—a pivotal role of HMGB1. PLoS Pathog. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melloni E. Sparatore B. Patrone M. Pessino A. Passalacqua M. Pontremoli S. Extracellular release of the “differentiation enhancing factor,” a HMG1 protein type, is an early step in murine erythroleukemia cell differentiation. FEBS Lett. 1995;368:466–470. doi: 10.1016/0014-5793(95)00716-m. [DOI] [PubMed] [Google Scholar]
- 146.Meng X. Harken AH. The interaction between Hsp70 and TNF-alpha expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17:345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 147.Messmer D. Yang H. Telusma G. Knoll F. Li J. Messmer B. Tracey KJ. Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 148.Mittal D. Saccheri F. Venereau E. Pusterla T. Bianchi ME. Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Monastyrska I. Rieter E. Klionsky DJ. Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc. 2009;84:431–448. doi: 10.1111/j.1469-185X.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muller S. Scaffidi P. Degryse B. Bonaldi T. Ronfani L. Agresti A. Beltrame M. Bianchi ME. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nicholas SA. Sumbayev VV. The role of redox-dependent mechanisms in the downregulation of ligand-induced Toll-like receptors 7, 8 and 4-mediated HIF-1alpha prolyl hydroxylation. Immunol Cell Biol. 2010;88:180–186. doi: 10.1038/icb.2009.76. [DOI] [PubMed] [Google Scholar]
- 152.Noble CG. Dong JM. Manser E. Song H. Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1. J Biol Chem. 2008;283:26274–26282. doi: 10.1074/jbc.M804723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohndorf UM. Rould MA. He Q. Pabo CO. Lippard SJ. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature. 1999;399:708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- 154.Oozawa S. Mori S. Kanke T. Takahashi H. Liu K. Tomono Y. Asanuma M. Miyazaki I. Nishibori M. Sano S. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J. 2008;72:1178–1184. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- 155.Orlova VV. Choi EY. Xie C. Chavakis E. Bierhaus A. Ihanus E. Ballantyne CM. Gahmberg CG. Bianchi ME. Nawroth PP. Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Outten FW. Theil EC. Iron-based redox switches in biology. Antioxid Redox Signal. 2009;11:1029–1046. doi: 10.1089/ars.2008.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palumbo R. De Marchis F. Pusterla T. Conti A. Alessio M. Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol. 2009;86:617–623. doi: 10.1189/jlb.0908581. [DOI] [PubMed] [Google Scholar]
- 158.Palumbo R. Galvez BG. Pusterla T. De Marchis F. Cossu G. Marcu KB. Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-{kappa}B activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Palumbo R. Sampaolesi M. De Marchis F. Tonlorenzi R. Colombetti S. Mondino A. Cossu G. Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pardo M. Budick-Harmelin N. Tirosh B. Tirosh O. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic Biol Med. 2008;45:1073–1083. doi: 10.1016/j.freeradbiomed.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 161.Park HS. Kim SR. Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 162.Passalacqua M. Patrone M. Picotti GB. Del Rio M. Sparatore B. Melloni E. Pontremoli S. Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience. 1998;82:1021–1028. doi: 10.1016/s0306-4522(97)00352-7. [DOI] [PubMed] [Google Scholar]
- 163.Pattingre S. Tassa A. Qu X. Garuti R. Liang XH. Mizushima N. Packer M. Schneider MD. Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 164.Peter ME. ROS eliminate danger. Immunity. 2008;29:1–2. doi: 10.1016/j.immuni.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 165.Popovic PJ. DeMarco R. Lotze MT. Winikoff SE. Bartlett DL. Krieg AM. Guo ZS. Brown CK. Tracey KJ. Zeh HJ., 3rd High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol. 2006;177:8701–8707. doi: 10.4049/jimmunol.177.12.8701. [DOI] [PubMed] [Google Scholar]
- 166.Porto A. Palumbo R. Pieroni M. Aprigliano G. Chiesa R. Sanvito F. Maseri A. Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–2566. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 167.Prasad R. Liu Y. Deterding LJ. Poltoratsky VP. Kedar PS. Horton JK. Kanno S. Asagoshi K. Hou EW. Khodyreva SN. Lavrik OI. Tomer KB. Yasui A. Wilson SH. HMGB1 is a cofactor in mammalian base excision repair. Mol Cell. 2007;27:829–841. doi: 10.1016/j.molcel.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qi ML. Tagawa K. Enokido Y. Yoshimura N. Wada Y. Watase K. Ishiura S. Kanazawa I. Botas J. Saitoe M. Wanker EE. Okazawa H. Proteome analysis of soluble nuclear proteins reveals that HMGB1/2 suppress genotoxic stress in polyglutamine diseases. Nat Cell Biol. 2007;9:402–414. doi: 10.1038/ncb1553. [DOI] [PubMed] [Google Scholar]
- 169.Qin S. Wang H. Yuan R. Li H. Ochani M. Ochani K. Rosas-Ballina M. Czura CJ. Huston JM. Miller E. Lin X. Sherry B. Kumar A. Larosa G. Newman W. Tracey KJ. Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiu J. Nishimura M. Wang Y. Sims JR. Qiu S. Savitz SI. Salomone S. Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 171.Qiu J. Xu J. Zheng Y. Wei Y. Zhu X. Lo EH. Moskowitz MA. Sims JR. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41:2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raucci A. Palumbo R. Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity. 2007;40:285–289. doi: 10.1080/08916930701356978. [DOI] [PubMed] [Google Scholar]
- 173.Rauvala H. Rouhiainen A. RAGE as a receptor of HMGB1 (Amphoterin): roles in health and disease. Curr Mol Med. 2007;7:725–734. doi: 10.2174/156652407783220750. [DOI] [PubMed] [Google Scholar]
- 174.Reggiori F. Monastyrska I. Shintani T. Klionsky DJ. The actin cytoskeleton is required for selective types of autophagy, but not nonspecific autophagy, in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2005;16:5843–5856. doi: 10.1091/mbc.E05-07-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rendon-Mitchell B. Ochani M. Li J. Han J. Wang H. Yang H. Susarla S. Czura C. Mitchell RA. Chen G. Sama AE. Tracey KJ. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 176.Riuzzi F. Sorci G. Donato R. The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo. J Biol Chem. 2006;281:8242–8253. doi: 10.1074/jbc.M509436200. [DOI] [PubMed] [Google Scholar]
- 177.Riuzzi F. Sorci G. Donato R. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am J Pathol. 2007;171:947–961. doi: 10.2353/ajpath.2007.070049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rocksen D. Lilliehook B. Larsson R. Johansson T. Bucht A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122:249–256. doi: 10.1046/j.1365-2249.2000.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rong Y. Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 180.Rossini A. Zacheo A. Mocini D. Totta P. Facchiano A. Castoldi R. Sordini P. Pompilio G. Abeni D. Capogrossi MC. Germani A. HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol. 2008;44:683–693. doi: 10.1016/j.yjmcc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 181.Rouhiainen A. Kuja-Panula J. Wilkman E. Pakkanen J. Stenfors J. Tuominen RK. Lepantalo M. Carpen O. Parkkinen J. Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104:1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 182.Rovere-Querini P. Capobianco A. Scaffidi P. Valentinis B. Catalanotti F. Giazzon M. Dumitriu IE. Muller S. Iannacone M. Traversari C. Bianchi ME. Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rubartelli A. Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 184.Rubartelli A. Sitia R. Stress as an intercellular signal: the emergence of stress associated molecular patterns (SAMP) Antioxid Redox Signal. 2009;11:2621–2629. doi: 10.1089/ars.2009.2377. [DOI] [PubMed] [Google Scholar]
- 185.Sahu D. Debnath P. Takayama Y. Iwahara J. Redox properties of the A-domain of the HMGB1 protein. FEBS Lett. 2008;582:3973–3978. doi: 10.1016/j.febslet.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Samuhasaneeto S. Thong-Ngam D. Kulaputana O. Suyasunanont D. Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J Biomed Biotechnol. 2009;2009:981963. doi: 10.1155/2009/981963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sandoval H. Thiagarajan P. Dasgupta SK. Schumacher A. Prchal JT. Chen M. Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sappington PL. Yang R. Yang H. Tracey KJ. Delude RL. Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 189.Sarsour EH. Kumar MG. Chaudhuri L. Kalen AL. Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Scaffidi P. Misteli T. Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 191.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 192.Scherz-Shouval R. Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 193.Scherz-Shouval R. Shvets E. Fass E. Shorer H. Gil L. Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schulze PC. Lee RT. Oxidative stress and atherosclerosis. Curr Atheroscler Rep. 2005;7:242–248. doi: 10.1007/s11883-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 195.Schwertassek U. Balmer Y. Gutscher M. Weingarten L. Preuss M. Engelhard J. Winkler M. Dick TP. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Semino C. Angelini G. Poggi A. Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 197.Sevier CS. Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 198.Shaikhali J. Heiber I. Seidel T. Stroher E. Hiltscher H. Birkmann S. Dietz KJ. Baier M. The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 2008;8:48. doi: 10.1186/1471-2229-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sharma P. Chakraborty R. Wang L. Min B. Tremblay ML. Kawahara T. Lambeth JD. Haque SJ. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shukla PK. Khanna VK. Khan MY. Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003;22:653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 201.Simon HU. Haj-Yehia A. Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 202.Soderberg A. Barral AM. Soderstrom M. Sander B. Rosen A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med. 2007;43:90–99. doi: 10.1016/j.freeradbiomed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 203.Soro-Paavonen A. Watson AM. Li J. Paavonen K. Koitka A. Calkin AC. Barit D. Coughlan MT. Drew BG. Lancaster GI. Thomas M. Forbes JM. Nawroth PP. Bierhaus A. Cooper ME. Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sparatore B. Patrone M. Passalacqua M. Pedrazzi M. Gaggero D. Pontremoli S. Melloni E. Extracellular processing of amphoterin generates a peptide active on erythroleukaemia cell differentiation. Biochem J. 2001;357:569–574. doi: 10.1042/0264-6021:3570569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sparatore B. Pedrazzi M. Passalacqua M. Gaggero D. Patrone M. Pontremoli S. Melloni E. Stimulation of erythroleukaemia cell differentiation by extracellular high-mobility group-box protein 1 is independent of the receptor for advanced glycation end-products. Biochem J. 2002;363:529–535. doi: 10.1042/0264-6021:3630529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sparvero LJ. Asafu-Adjei D. Kang R. Tang D. Amin N. Im J. Rutledge R. Lin B. Amoscato AA. Zeh HJ. Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stott K. Watson M. Howe FS. Grossmann JG. Thomas JO. Tail-mediated collapse of HMGB1 is dynamic and occurs via differential binding of the acidic tail to the A and B domains. J Mol Biol. 2010;403:706–722. doi: 10.1016/j.jmb.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 208.Stros M. Muselikova-Polanska E. Pospisilova S. Strauss F. High-affinity binding of tumor-suppressor protein p53 and HMGB1 to hemicatenated DNA loops. Biochemistry. 2004;43:7215–7225. doi: 10.1021/bi049928k. [DOI] [PubMed] [Google Scholar]
- 209.Stros M. Ozaki T. Bacikova A. Kageyama H. Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem. 2002;277:7157–7164. doi: 10.1074/jbc.M110233200. [DOI] [PubMed] [Google Scholar]
- 210.Stumbo AC. Cortez E. Rodrigues CA. Henriques MG. Porto LC. Barbosa HS. Carvalho L. Mitochondrial localization of non-histone protein HMGB1 during human endothelial cell-Toxoplasma gondii infection. Cell Biol Int. 2008;32:235–238. doi: 10.1016/j.cellbi.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 211.Sundberg E. Fasth AE. Palmblad K. Harris HE. Andersson U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology. 2009;214:303–309. doi: 10.1016/j.imbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 212.Surh YJ. Kundu JK. Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 213.Tang D. Kang R. Cao L. Zhang G. Yu Y. Xiao W. Wang H. Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med. 2008;36:291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- 214.Tang D. Kang R. Cheh CW. Livesey KM. Liang X. Schapiro NE. Benschop R. Sparvero LJ. Amoscato AA. Tracey KJ. Zeh HJ. Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tang D. Kang R. Livesey KM. Cheh C. Adam Farkas A. Loughran P. Hoppe G. Bianchi ME. Tracey KJ. Zeh HJ. Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tang D. Kang R. Xiao W. Jiang L. Liu M. Shi Y. Wang K. Wang H. Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178:7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tang D. Kang R. Xiao W. Wang H. Calderwood SK. Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tang D. Kang R. Xiao W. Zhang H. Lotze MT. Wang H. Xiao X. Quercetin prevents lipopolysaccharide-induced HMGB1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang D. Kang R. Zeh HJ., 3rd Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tang D. Lotze MT. Zeh HJ., 3rd Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010;6:1181–1183. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- 221.Tang D. Shi Y. Jang L. Wang K. Xiao W. Xiao X. Heat shock response inhibits release of high mobility group box 1 protein induced by endotoxin in murine macrophages. Shock. 2005;23:434–440. doi: 10.1097/01.shk.0000159556.95285.df. [DOI] [PubMed] [Google Scholar]
- 222.Tang D. Shi Y. Kang R. Li T. Xiao W. Wang H. Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Taniguchi N. Kawahara K. Yone K. Hashiguchi T. Yamakuchi M. Goto M. Inoue K. Yamada S. Ijiri K. Matsunaga S. Nakajima T. Komiya S. Maruyama I. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–981. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 224.Tatsuta T. Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tesniere A. Panaretakis T. Kepp O. Apetoh L. Ghiringhelli F. Zitvogel L. Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 226.Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001;29:395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- 227.Tian J. Avalos AM. Mao SY. Chen B. Senthil K. Wu H. Parroche P. Drabic S. Golenbock D. Sirois C. Hua J. An LL. Audoly L. La Rosa G. Bierhaus A. Naworth P. Marshak-Rothstein A. Crow MK. Fitzgerald KA. Latz E. Kiener PA. Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 228.Tirouvanziam R. Conrad CK. Bottiglieri T. Herzenberg LA. Moss RB. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci U S A. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Topalova D. Ugrinova I. Pashev IG. Pasheva EA. HMGB1 protein inhibits DNA replication in vitro: a role of the acetylation and the acidic tail. Int J Biochem Cell Biol. 2008;40:1536–1542. doi: 10.1016/j.biocel.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 230.Treutiger CJ. Mullins GE. Johansson AS. Rouhiainen A. Rauvala HM. Erlandsson-Harris H. Andersson U. Yang H. Tracey KJ. Andersson J. Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 231.Tsung A. Klune JR. Zhang X. Jeyabalan G. Cao Z. Peng X. Stolz DB. Geller DA. Rosengart MR. Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tsung A. Sahai R. Tanaka H. Nakao A. Fink MP. Lotze MT. Yang H. Li J. Tracey KJ. Geller DA. Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ulloa L. Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 234.Ulloa L. Ochani M. Yang H. Tanovic M. Halperin D. Yang R. Czura CJ. Fink MP. Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Uramoto H. Izumi H. Nagatani G. Ohmori H. Nagasue N. Ise T. Yoshida T. Yasumoto K. Kohno K. Physical interaction of tumour suppressor p53/p73 with CCAAT-binding transcription factor 2 (CTF2) and differential regulation of human high-mobility group 1 (HMG1) gene expression. Biochem J. 2003;371:301–310. doi: 10.1042/BJ20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Urbonaviciute V. Meister S. Furnrohr BG. Frey B. Guckel E. Schett G. Herrmann M. Voll RE. Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis. Autoimmunity. 2009;42:305–307. doi: 10.1080/08916930902831803. [DOI] [PubMed] [Google Scholar]
- 237.Varma SD. Devamanoharan PS. Ali AH. Prevention of intracellular oxidative stress to lens by pyruvate and its ester. Free Radic Res. 1998;28:131–135. doi: 10.3109/10715769809065799. [DOI] [PubMed] [Google Scholar]
- 238.Velazquez JM. Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984;36:655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- 239.Verrier CS. Roodi N. Yee CJ. Bailey LR. Jensen RA. Bustin M. Parl FF. High-mobility group (HMG) protein HMG-1 and TATA-binding protein-associated factor TAF(II)30 affect estrogen receptor-mediated transcriptional activation. Mol Endocrinol. 1997;11:1009–1019. doi: 10.1210/mend.11.8.9962. [DOI] [PubMed] [Google Scholar]
- 240.Victor VM. Rocha M. Sola E. Banuls C. Garcia-Malpartida K. Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 241.Wang H. Bloom O. Zhang M. Vishnubhakat JM. Ombrellino M. Che J. Frazier A. Yang H. Ivanova S. Borovikova L. Manogue KR. Faist E. Abraham E. Andersson J. Andersson U. Molina PE. Abumrad NN. Sama A. Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 242.Wang H. Ward MF. Sama AE. Novel Hmgb1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang H. Yang H. Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 244.Wang Q. Ding Q. Zhou Y. Gou X. Hou L. Chen S. Zhu Z. Xiong L. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology. 2009;110:1279–1286. doi: 10.1097/ALN.0b013e3181a160d6. [DOI] [PubMed] [Google Scholar]
- 245.Watanabe T. Kubota S. Nagaya M. Ozaki S. Nagafuchi H. Akashi K. Taira Y. Tsukikawa S. Oowada S. Nakano S. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124:59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 246.Webb C. Twedt D. Oxidative stress and liver disease. Vet Clin North Am Small Anim Pract. 2008;38:125–135. doi: 10.1016/j.cvsm.2007.10.001. v, [DOI] [PubMed] [Google Scholar]
- 247.Wegerich F. Turano P. Allegrozzi M. Mohwald H. Lisdat F. Cytochrome C mutants for superoxide biosensors. Anal Chem. 2009;81:2976–2984. doi: 10.1021/ac802571h. [DOI] [PubMed] [Google Scholar]
- 248.Welch WJ. Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984;259:4501–4513. [PubMed] [Google Scholar]
- 249.Wells PG. McCallum GP. Chen CS. Henderson JT. Lee CJ. Perstin J. Preston TJ. Wiley MJ. Wong AW. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- 250.Wilmanski J. Siddiqi M. Deitch EA. Spolarics Z. Augmented IL-10 production and redox-dependent signaling pathways in glucose-6-phosphate dehydrogenase-deficient mouse peritoneal macrophages. J Leukoc Biol. 2005;78:85–94. doi: 10.1189/jlb.0105010. [DOI] [PubMed] [Google Scholar]
- 251.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 252.Wu H. Chen G. Wyburn KR. Yin J. Bertolino P. Eris JM. Alexander SI. Sharland AF. Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yan SD. Chen X. Fu J. Chen M. Zhu H. Roher A. Slattery T. Zhao L. Nagashima M. Morser J. Migheli A. Nawroth P. Stern D. Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 254.Yanai H. Ban T. Wang Z. Choi MK. Kawamura T. Negishi H. Nakasato M. Lu Y. Hangai S. Koshiba R. Savitsky D. Ronfani L. Akira S. Bianchi ME. Honda K. Tamura T. Kodama T. Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 255.Yang D. Chen Q. Yang H. Tracey KJ. Bustin M. Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 256.Yang H. Hreggvidsdottir HS. Palmblad K. Wang H. Ochani M. Li J. Lu B. Chavan S. Rosas-Ballina M. Al-Abed Y. Akira S. Bierhaus A. Erlandsson-Harris H. Andersson U. Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang J. Huang C. Jiang H. Ding J. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation: a key role for TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2010;345:189–195. doi: 10.1007/s11010-010-0572-9. [DOI] [PubMed] [Google Scholar]
- 258.Yang QW. Lu FL. Zhou Y. Wang L. Zhong Q. Lin S. Xiang J. Li JC. Fang CQ. Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2010 Aug 11; doi: 10.1038/jcbfm.2010.129. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yao D. Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of RAGE and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yee KS. Wilkinson S. James J. Ryan KM. Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135–1145. doi: 10.1038/cdd.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yen WL. Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 2008;23:248–262. doi: 10.1152/physiol.00013.2008. [DOI] [PubMed] [Google Scholar]
- 262.Yin YX. Yao YM. Liu RM. Zhai HX. Li L. Zhang JJ. Chen HW. Wang L. Li N. Xia YF. [The effect of simvastatin on the expression of high mobility group box-1 protein in atherosclerotic rats] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22:306–308. [PubMed] [Google Scholar]
- 263.Youn JH. Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 264.Yu M. Wang H. Ding A. Golenbock DT. Latz E. Czura CJ. Fenton MJ. Tracey KJ. Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 265.Yu YM. Kim JB. Lee KW. Kim SY. Han PL. Lee JK. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- 266.Yuk JM. Yang CS. Shin DM. Kim KK. Lee SK. Song YJ. Lee HM. Cho CH. Jeon BH. Jo EK. A dual regulatory role of apurinic/apyrimidinic endonuclease 1/redox factor-1 in HMGB1-induced inflammatory responses. Antioxid Redox Signal. 2009;11:575–588. doi: 10.1089/ars.2008.2196. [DOI] [PubMed] [Google Scholar]
- 267.Yun N. Eum HA. Lee SM. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid Redox Signal. 2010;13:1503–1512. doi: 10.1089/ars.2009.2873. [DOI] [PubMed] [Google Scholar]
- 268.Zafarullah M. Li WQ. Sylvester J. Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zeh HJ., 3rd Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 270.Zeng S. Dun H. Ippagunta N. Rosario R. Zhang QY. Lefkowitch J. Yan SF. Schmidt AM. Emond JC. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J Hepatol. 2009;50:929–936. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 271.Zhang CC. Krieg S. Shapiro DJ. HMG-1 stimulates estrogen response element binding by estrogen receptor from stably transfected HeLa cells. Mol Endocrinol. 1999;13:632–643. doi: 10.1210/mend.13.4.0264. [DOI] [PubMed] [Google Scholar]
- 272.Zhang X. Wheeler D. Tang Y. Guo L. Shapiro RA. Ribar TJ. Means AR. Billiar TR. Angus DC. Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]