Abstract
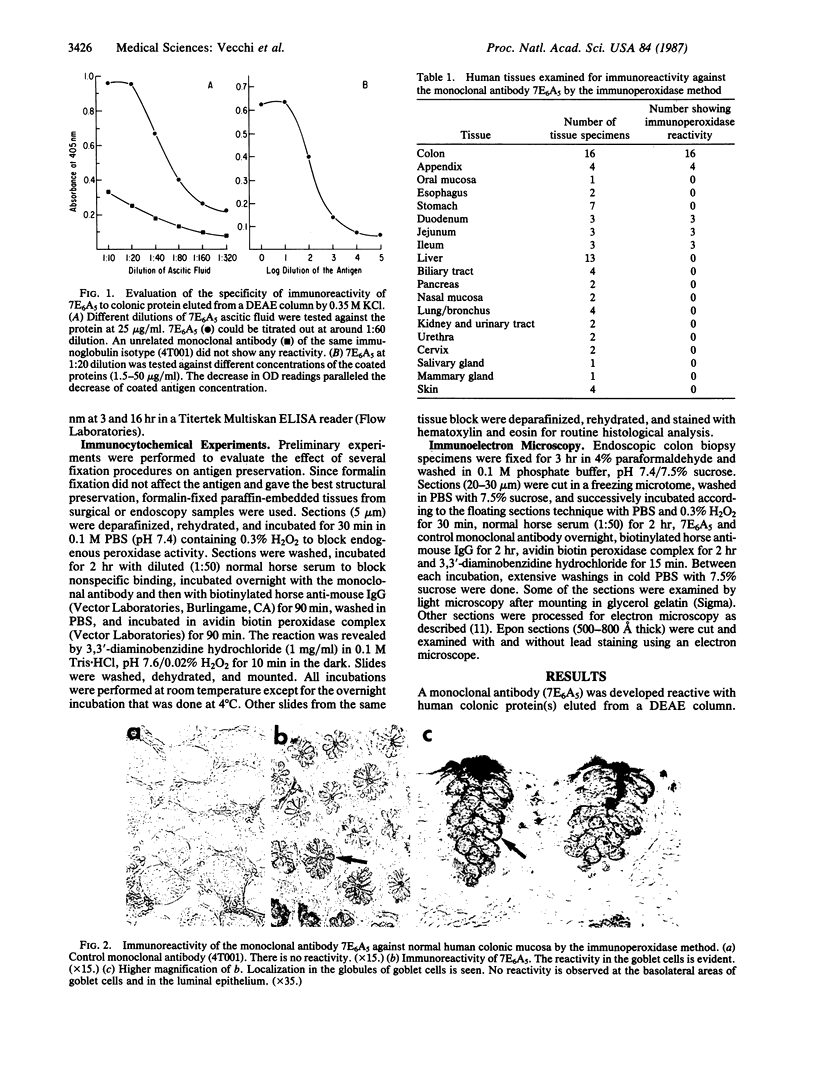
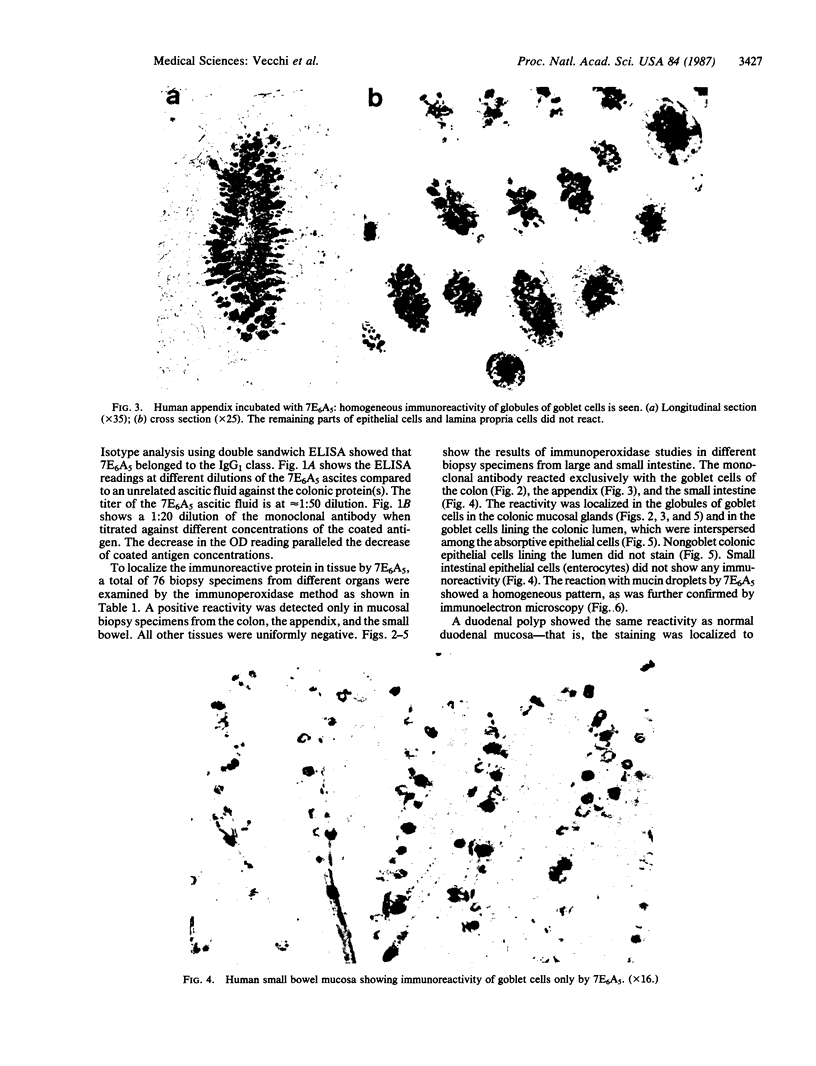
A mouse monoclonal antibody (7E6A5) of IgG isotype, reacting specifically with mucin-producing goblet cells of the human gastrointestinal tract, has been developed. 7E6A5 reacts by an ELISA with colonic protein eluted from a DEAE column. A screening by immunoperoxidase assay of 76 specimens from 19 different human tissues showed that the immunoreactivity of 7E6A5 was confined exclusively in the globules of goblet cells in the colon, the appendix, and the small intestine. Nongoblet small and large intestinal epithelial cells did not react. Immunoelectron microscopy demonstrated the reactivity with mucin droplets in a homogeneous granular pattern inside the globules of goblet cells. Mucus-secreting cells from remaining parts of the gastrointestinal tract and other mucus-secreting organs such as respiratory, genitourinary tracts, salivary and mammary glands did not show any reactivity to 7E6A5. These findings indicate that the antigen recognized by 7E6A5 is shared by the goblet cells of both the small and large intestines and is unique to them. The monoclonal antibody may be useful in the study of function of mucus-secreting goblet cells and may represent an important tool in the evaluation of diseases such as ulcerative colitis, colon cancer, and intestinal metaplasia in gastric mucosa that are associated with quantitative changes in goblet cell numbers or with qualitative differences in mucin secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier R. S., Boland C. R., Kim Y. S. Regional differences in normal and cancer-associated glycoconjugates of the human colon. J Natl Cancer Inst. 1985 Aug;75(2):249–260. [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Mucin histochemistry of the colon. Curr Top Pathol. 1976;63:143–178. doi: 10.1007/978-3-642-66481-6_5. [DOI] [PubMed] [Google Scholar]
- Forstner G., Wesley A., Forstner J. Clinical aspects of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:199–224. doi: 10.1007/978-1-4615-9254-9_32. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Stockert R. J., Becker F. F., Yam A., Poruchynsky M. S., Levin W., Thomas P. E. Immunocytochemical localization of epoxide hydrase in hyperplastic nodules induced in rat liver by 2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5207–5211. doi: 10.1073/pnas.76.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Development of anti-human colonic mucin monoclonal antibodies. Characterization of multiple colonic mucin species. J Clin Invest. 1986 Apr;77(4):1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Shamsuddin A. M., Phelps P. C., Trump B. F. Human large intestinal epithelium: light microscopy, histochemistry, and ultrastructure. Hum Pathol. 1982 Sep;13(9):790–803. doi: 10.1016/s0046-8177(82)80075-0. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Das K. M. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J Clin Invest. 1985 Jul;76(1):311–318. doi: 10.1172/JCI111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley E. L., Murphy P., Mendelsohn G., Eggleston J. C. Distribution of blood group substances in normal human colon. Use of the unlabeled antibody (PAP) immunoperoxidase technic to identify A and B blood group substances. Am J Clin Pathol. 1981 Dec;76(6):806–809. doi: 10.1093/ajcp/76.6.806. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Nakamura T., Tanaka S., Sato E. Glycoconjugate with Ulex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst. 1982 Oct;69(4):777–785. [PubMed] [Google Scholar]