Abstract
The success of highly active antiretroviral therapy (HAART) among persons living with HIV is largely dependent on strict medication adherence. Recent research suggests that alcohol and other drug use (AOD) may be an important barrier to HAART adherence. In this study, we examined the impact of AOD on HAART adherence as well as the moderating effects of general and medication-specific social support. The data were collected as part of a longitudinal randomized control trial with 224 HIV-positive patients at an HIV primary care clinic in the northwestern United States. Findings indicated that AOD use was negatively associated with HAART adherence and that medication-specific (but not general) social support moderated the AOD-adherence association at 3 (but not at 6 or 9) months. Results indicate the importance of medication-specific social support to treat comorbid AOD use and HIV; implications for future research and intervention programs for HIV-positive AOD users are discussed.
Introduction
Highly active antiretroviral therapy (HAART) is now the standard of care for persons living with HIV/AIDS (PLWH) because of its demonstrated effectiveness at restraining viral replication, slowing clinical progression to AIDS, and increasing life expectancy.1,2 Indeed, HAART has transformed HIV/AIDS from an acute life-threatening illness to a chronic condition. The success of HAART, however, depends largely on optimal medication adherence.3,4 Although maintaining high levels of adherence appears to be important even for modern HAART regimens, adherence has been found to decrease significantly over time.5 Inadequate adherence continues to be one of the most frequent reasons for poor treatment outcomes and lack of sustained treatment benefits.6
Alcohol and other drug use (AOD) use is an important potential adherence barrier to examine because it is highly prevalent among PLWH. Indeed, large population surveys have indicated that 40% of HIV-positive persons have used illicit drugs in the previous year, and more than 12% screened positive for drug dependence.7 In addition, 8–10% of HIV patients report heavy alcohol use (defined as drinking 5 or more drinks 4 or more days per month),8–10 which is about twice that estimated in the general population (4.5%).11
Not only is AOD use common among PLWH, but there is convincing evidence that AOD use is associated with nonadherence to HAART.8,12–15 Specifically, alcohol use has been shown to correlate negatively with adherence in both cross-sectional16–18 and longitudinal studies.10,19,20 In a meta-analytic review, Hendershot et al.13 found that PLWH who used alcohol were about half as likely to be classified as adherent as those who abstained. In terms of drug use, some studies have found that adherence to HIV medication is lower among current drug users21 and that adherence declines in periods of relapse.22 A systematic review of 41 studies of adherence to HAART among drug users found that active substance use was associated with poor adherence.14
It should be noted that results from studies of AOD use and adherence association have been inconsistent.23,24 Indeed, some studies have found weak or no significant relationships between AOD use and poor adherence.25–28 Potential reasons for such discrepancies may include insufficient power in smaller samples and inconsistency in measurement approaches of AOD use and medication adherence.24
On the other hand, these discrepant findings could reflect the influence of moderating variables that have not yet been systematically evaluated.13 Examining moderators of the substance use-medication adherence relationship is crucial because it will help identify factors that may improve adherence and treatment effectiveness for this group and thus has implications for interventions targeting HIV-positive AOD users.
Social support is a likely moderator. It is one of the most consistent correlates of HAART adherence12,25,29 and, in a meta-analysis, DiMatteo and colleagues30 demonstrated that social support accounted for significant associations with treatment adherence across a variety of illnesses. Although not a specific measure of the relational aspects of social support, living with someone has also been found to positively associate with adherence.31,32 Consistent with these findings, studies have shown that underdeveloped partner, family, peer, and health care provider supports are associated with suboptimal adherence to dose instructions.15,18,33,34
Other findings specifically link social support to AOD use and adherence. For example, several studies have shown that drug use may adversely affect individuals' social support.25,35,36 In addition, in a study among active injection drug users, adjusted odds of viral suppression were at least three times higher among those with higher social support.37 Indeed, among a study of 130 crack cocaine users, adherence was found to improve with a more satisfying social network.33
In the present study, we examined whether social support may be an important factor to consider in attempts to understand and promote adherence among HIV-positive individuals who are AOD users. Specifically, we looked at the relationship between AOD and adherence and investigated whether social support, both general and medication-specific, moderated the relation between alcohol use and adherence and between drug use and adherence.
Method
Procedure
The data used in this analysis were collected as part of a National Institues of Health (NIH)-funded randomized control trial conducted in an outpatient HIV clinic in a large city in the Pacific Northwest to study the effect of pager reminders and peer support on HAART adherence. In order to be included in this study, participants needed to either be initiating HAART for the first time or changing HAART regimens, be 18 years of age or older, not have a psychotic disorder or dementia diagnosis, and live within range of the pagers. Participants (N = 224) were randomly assigned to one of four study arms: (1) pager intervention, (2) peer intervention, (3) both interventions, or (4) standard of care. Computer-assisted self-interviews were administered at baseline, 2 weeks, and 3, 6, and 9 months. The intervention involved the use of programmable two-way pagers and peers trained to provide social support during a 3-month intervention period. The pager intervention consisted of a customized pager system in which participants received reminder text messages particular to their medication regimen. In addition to dose reminders, educational and entertainment text messages also were sent. The peer intervention consisted of pairing participants with clinic patients who were HIV-positive and currently on HAART who provided medication-related social support to participants via bimonthly group gatherings and weekly phone calls. A full description of the study procedures and outcomes of the intervention are provided elsewhere (masked for review).37a
Participants
Of the 224 participants in the study, 47% self-identified as white, 30% as African American, 11% as Latino/Hispanic, 4% American Indian or Alaskan Native, and 8% as other or mixed race. The mean age was 40 years (standard deviation [SD] = 8; range, 19–60), and the majority of participants were male (76%), unemployed (81%), and low income (51% with yearly incomes below $552). The sample was fairly well educated: 71% had a high school degree or GED and 9% had a college degree.
Measures
Demographic questions included items on age, gender, race/ethnicity, employment status (coded as unemployed or employed part- or full-time), and education (coded as less than high school or at least high school degree/GED).
Medication adherence
Medication adherence was assessed at 2 weeks and 3, 6, and 9 months with one item from the Simplified Medication Adherence Questionnaire (SMAQ; Knobel et al., 2002), in which the number of doses missed during the previous 7 days was reported on an ordinal scale (none of the time, 1–2 times, 3–5 times, 6–10 times, or more than 10 times). Since few participants (8–16%) reported missing more than 1–2 doses in the past week at any given time point, a dichotomous variable was created in which participants were classified as 100% adherent if they reported missing zero doses and nonadherent if they reported missing one or more doses in the last 7 days.
Alcohol use
Alcohol consumption, drinking behavior, and alcohol-related problems in the past year were measured at baseline using the 10-item Alcohol Use Disorder Identification Test (AUDIT).38 The AUDIT is more sensitive for hazardous or harmful drinking patterns and, therefore, is better suited than other measures of alcohol abuse or dependence to identify a wider spectrum of alcohol problems.39 Three items assess alcohol consumption (e.g., “How often do you have a drink containing alcohol?”); three assess drinking behavior (e.g., “How often did you find that you were not able to stop drinking once you had started?”); and four assess alcohol-related problems and adverse reactions (e.g., “How often during the last year have you been unable to remember what happened the night before because you had been drinking?”). Each item included 5 response options (e.g., never, less than monthly, monthly, weekly, daily or almost daily) and was scored from 0 to 4, with overall scores ranging from 0 to 40 and higher scores indicating more problematic alcohol use. In development and validation (N = 1888), 92% of those with hazardous or harmful alcohol use (judged by World Health Organization and the ICD-10 system) scored at least 8 points on the AUDIT scale.38 Therefore, a score of 8 or more is interpreted as indicative of a strong likelihood of hazardous or harmful alcohol consumption. In the current study, Cronbach α was 0.85.
Hard drug use
We used three items at baseline to assess the average weekly frequency of use of cocaine, heroin, and methamphetamine in the past year. Each item has 9 response options (e.g., never, less than once per week, 1, 2, 3, 4, 5, 6, or 7 days per week). Because multiple drug use is common and combining a range of substances used may be a more reliable predictor than individual drug use indicators,24 we computed an index of “hard drug” use as the average of the weekly frequency of cocaine, heroin, and methamphetamine use over the last year. We excluded marijuana from our indicator because it is especially likely to be used by individuals with HIV/AIDS for perceived relief of HIV-associated symptoms.40
General social support
We used the 19-item Medical Outcomes Study-Social Support survey (MOS-SS)41 at baseline to assess how participants perceive the availability of various types of support including companionship or assistance when they need it. The items (e.g., “Someone to confide in or talk to about yourself or your problems” or “Someone to do something enjoyable with”) are scored from 0 (none of the time) to 4 (all of the time). Items were averaged to form an index of general support ranging from 0 to 4, with higher scores reflecting more social support. Previous studies have demonstrated that the MOS-SS has high internal consistency (Cronbach α = 0.97) and sufficient test–retest reliability (α = 0.78).41 In the current study, Cronbach α was 0.98.
Medication social support
We created an 8-item survey of medication-specific social support to identify how often others may have helped participants with their antiretroviral therapy over a 3-month period (e.g., “Reminded you to take your medications”; “Helped you to believe you can take your medications as prescribed”; “Checked in with you about your medications”), scored from 0 (never) to 4 (very often). As over a third of the participants were initiating HAART at baseline, medication social support was not assessed until the 3-month assessment. Items were averaged to form a medication-specific index of support ranging from 0 to 4, with higher scores reflecting more medication social support. A confirmatory factor analysis found that the 8 items adequately measured a single latent dimension of medication-specific social support [χ2(18) = 28.49, p = 0.06; Normed Fit Index = 0.97; Comparative Fit Index = 0.99; RMSEA = 0.05]. In the current study, Cronbach α was 0.92.
Statistical methods
To assess for differences between participants with complete self-report data (82%), those who missed a single assessment (10%), and those who missed two or more assessments (8%), χ2 tests and one-way analyses of variance (ANOVAs) were conducted on categorical and continuous sociodemographic characteristics, respectively. No significant differences were found among these three groups on age, gender, race/ethnicity, employment, and education.
A multiple imputation using chained equations approach was utilized to address missing data.42 First, 10 complete datasets were generated by imputing the missing values in the original dataset. All subsequent analyses were replicated across each of the imputed datasets, with the final results calculated as a pooled average of the 10 analyses.
In descriptive analyses, we looked at means, standard deviations, and bivariate correlations of the demographic, medication adherence, AOD, and social support variables. Logistic regression was used to determine whether age, gender, race/ethnicity, employment, and education prospectively predicted 3-, 6-, or 9-month medication adherence.
We initially considered evaluating adherence across time using a growth curve approach. We first conducted a preliminary growth curve analysis43 to assess variability across participants in the rate of change (i.e., linear slope) in 100% medication adherence (dichotomous) across the four time points. This unconditional model, which excluded all other predictors, estimated for each participant an intercept [β = 0.92, standard error (SE) = 0.14, p < 0.001] and a linear slope (β = −0.20, SE = 0.06, p = 0.002) and allowed both to randomly vary across participants. The variances of intercepts [variance = 0.95, χ2(223) = 250.32, p = 0.10] and slopes [variance = 0.002, χ2(223) = 181.36, p > 0.50] were nonsignificant, indicating that there was not sufficient variation in either the initial levels of medication adherence or the adherence trajectories from participant to participant to warrant a using a growth curve approach for the moderation analyses. Consequently, we proceeded with a more focused longitudinal analysis that evaluated the moderation hypotheses separately at each follow-up time point, controlling for baseline adherence.
Since the data came from an intervention study on adherence, we also controlled for intervention group. Specifically, logistic regression was used to investigate the association between each of the two AOD predictors and 100% medication adherence at 3, 6, and 9 months. In each of the 6 models, the adherence outcome was regressed on the AOD predictor, general social support, medication social support, AOD × general social support, and AOD × medication social support, with baseline adherence, race/ethnicity, age, prescribed doses, and intervention group included as covariates. All predictor variables were centered at their means, as recommended by Aiken and West.44
Where significant moderating effects were found, we used the simple slope technique44 to interpret the relationship between each AOD predictor and the adherence outcome. Since social support (the moderator) was assessed as a continuous variable, the relationship between AOD and adherence was depicted at three levels of social support. Specifically, the predicted probability of 100% adherence was evaluated separately at “high support” (+1 SD above the mean), “average support” (the mean), and “low support” (−1 SD below the mean) with all other covariates held at their means.
Results
Descriptive analyses
Means, standard deviations, and bivariate correlations of the demographic, medication adherence, AOD, and social support variables are presented in Table 1. Overall, the percentage of participants self-reporting 100% adherence in the past week declined over time (from 73% to 59%). With respect to patterns of adherence, 30% of participants reported 100% adherence at all assessment points, 43% were 100% adherent at 2 weeks and became non-adherent at follow-up, 20% were initially nonadherent and achieved 100% adherence at one or more follow-ups, and 6% reported being nonadherent at all assessment points.
Table 1.
Descriptive Data and Intercorrelations for Main Variables
| 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | Mean | SD | Rangea | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||||||
| 1. Age | 0.05 | −0.10 | 0.15b | 0.09 | −0.11 | −0.01 | 0.02 | −0.01 | −0.01 | −0.16b | 0.02 | 40.01 | 8.16 | 19–60 |
| 2. Genderc,d | −0.17b | −0.21b | 0.06 | −0.07 | −0.12d | −0.06 | 0.03 | −0.05 | 0.06 | 0.08 | 0.24 | 0.43 | 0–1 | |
| 3. Employmente,f | 0.06 | 0.13d | 0.08 | 0.08 | 0.04 | 0.03 | −0.13b | 0.06 | 0.06 | 0.19 | 0.39 | 0–1 | ||
| 4. Educationf,g | 0.12d | 0.08 | 0.04 | −0.06 | −0.10 | 0.04 | 0.05 | 0.07 | 0.79 | 0.41 | 0–1 | |||
| 100% Adherence | ||||||||||||||
| 5. 2 weeksf | 0.33b | 0.05 | 0.23b | −0.16b | −0.04 | 0.08 | −0.02 | 0.73 | 0.44 | 0–1 | ||||
| 6. 3 monthsf | 0.25b | 0.27b | −0.22b | −0.09 | 0.14d | 0.11 | 0.65 | 0.48 | 0–1 | |||||
| 7. 6 monthsf | 0.25b | 0.03 | −0.06 | −0.06 | 0.05 | 0.63 | 0.48 | 0–1 | ||||||
| 8. 9 monthsf | −0.08 | −0.12d | 0.15b | 0.03 | 0.59 | 0.49 | 0–1 | |||||||
| Substance use | ||||||||||||||
| 9. AUDIT | 0.32b | −0.02 | 0.07 | 5.62 | 7.60 | 0–38 | ||||||||
| 10. Hard drug | 0.01 | 0.01 | 0.62 | 1.02 | 0–7 | |||||||||
| Social support | ||||||||||||||
| 11. General | 0.23b | 2.47 | 1.10 | 0–4 | ||||||||||
| 12. Medication | 1.54 | 1.15 | 0–4 | |||||||||||
Range taken from the nonimputed data.
p < 0.05.
0 = male, 1 = female.
p < 0.10.
0 = unemployed, 1 = part-/full-time.
Mean interpreted as the proportion of participants coded as 1.
0 = less than high school, 1 = high school degree or GED.
SD, standard deviation.
The baseline prevalence of past-year hazardous drinking was 27%, based on an AUDIT score of ≥8. Black [M = 7.25, SD = 9.33; t(215.5) = 2.63, p = 0.01] and other or mixed race [M = 7.52, SD = 9.01; t(216.7) = 2.07, p = 0.04] participants had higher AUDIT scores than White participants (M = 4.15, SD = 5.62). Over half (55%) of the sample reported any cocaine, heroin, or methamphetamine use in the past year, with unemployed participants (M = 0.69, SD = 1.07) reporting a higher weekly frequency of use compared with participants employed part- or full-time (M = 0.34, SD = 0.63), t(217.7) = −2.01, p = 0.05. Perceptions of general social support (M = 2.47, SD = 1.10) were higher than received medication-specific support (M = 1.54, SD = 1.15), t(766.5) = 9.72, p < 0.001, and inversely associated with age, r = −0.16, p = 0.01.
Logistic regression analyses assessed whether age, gender, race/ethnicity, employment, and education prospectively predicted 3-, 6-, or 9-month medication adherence, controlling for baseline adherence. In these models, the adherence outcome was regressed on baseline adherence and the demographic predictors. At 3 months, African American participants were less likely to report 100% adherence than White participants [odds ratio (OR) = 0.35, SE = 0.13, 95% confidence interval (CI) = 0.17–0.71, p < 0.01] and each additional year of age was associated with lower odds of adherence (OR = 0.96, SE = 0.02, 95% CI = 0.92–1.00, p = 0.04). On average, African American participants were more represented in the older ages (M = 43 years, SD = 7) than Caucasian (M = 39, SD = 9), Latino (M = 38 years, SD = 10), or other and mixed race participants (M = 39 years, SD = 7). When the prospective association between age and 3-month adherence was examined controlling for race, older age was no longer a statistically significant predictor of adherence (OR = 0.96, SE = 0.02, 95% CI = 0.92-1.01, p = 0.11). Sociodemographic factors were not significantly associated with adherence at 6 and 9 months. Since race/ethnicity and age were significantly related to at least one of the adherence outcomes, we controlled for these demographic factors statistically in further analyses.
Moderator analyses
A summary of the moderation findings across all time points is presented in Table 2.
Table 2.
Summary of Moderation Analyses
| |
Time point |
||
|---|---|---|---|
| 3 months OR (95% CI) | 6 months OR (95% CI) | 9 months OR (95% CI) | |
| Moderators of alcohol use | |||
| General social support | 0.99 (0.94–1.10) | 1.01 (0.97–1.06) | 1.00 (0.96–1.04) |
| Medication social support | 1.06 (1.01–1.12)a | 1.01 (0.97–1.05) | 1.00 (0.96–1.04) |
| Moderators of hard drug use | |||
| General social support | 1.23 (0.82–1.86) | 1.09 (0.78–1.53) | 1.09 (0.78–1.52) |
| Medication social support | 1.52 (1.10–2.09)a | 1.07 (0.77–1.48) | 1.01 (0.73–1.41) |
p < 0.05.
OR, odds ratio; CI, confidence interval.
Alcohol use
The interaction of AUDIT × general social support was non-significant (OR = 0.99, SE = 0.02, 95% CI = 0.94–1.03, p = 0.50), indicating that the association between initial AUDIT score and 3-month medication adherence was comparable across all levels of initial general social support. On the other hand, medication-specific social support moderated the effects of alcohol use (OR = 1.06, SE = 0.03, 95% CI = 1.01–1.12, p = 0.01), indicating that higher medication social support attenuated the association between initial alcohol use and medication adherence at 3 months. The buffering effect did not persist at the 6- and 9-month time points.
Hard drug use
Findings were similar with respect to hard drug use. The interaction of hard drug use and general social support was also non-significant (OR = 1.23, SE = 0.26, 95% CI = 0.82–1.86, p = 0.32), indicating that the association of initial hard drug use and 3-month medication adherence was similar at all levels of general social support. On the other hand, medication-specific social support moderated the effect of hard drug use (OR = 1.52, SE = 0.25, 95% CI = 1.10–2.09, p = 0.01), such that higher medication-specific social support reduced the association between initial hard drug use and medication adherence at 3 months. Once again, there were no buffering effects at 6 or 9 months.
Predicted probabilities of adherence
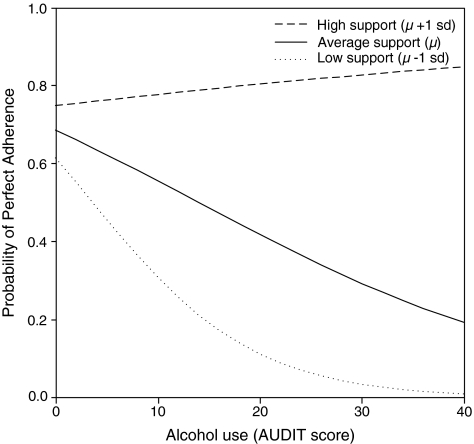
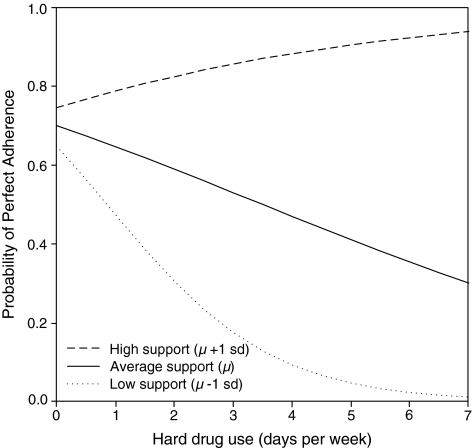
To interpret the degree to which medication social support buffered the effect of AOD use on adherence, the probability of 100% adherence at 3 months was plotted at each AUDIT score (Fig. 1) and average hard drug frequency (Fig. 2) at +1 SD, mean, and −1 SD levels of medication social support.
FIG. 1.
Predicted probabilities of adherence for alcohol use by high, average, and low medication social support.
FIG. 2.
Predicted probabilities of adherence for hard drug use by high, average, and low medication social support.
AUDIT score
For an individual with high support (1 SD above the mean), the probability of 100% adherence was 75% (95% CI = 64–84%) with an AUDIT score of 0 and 77% (95% CI = 61–88%) with an AUDIT score at the clinical threshold of 8. For an individual with average support, the probability of 100% adherence was 68% (95% CI = 61–76%) with an AUDIT score of 0 versus 58% (95% CI = 45–71%) with an AUDIT score at the clinical threshold of 8. For an individual with low support, the probability of 100% adherence was 61% (95% CI = 49–73%) with an AUDIT score of 0 versus 37% (95% CI = 19–58%) with an AUDIT score at the clinical threshold of 8. Essentially, as medication-specific social support increased, the association between AUDIT scores and adherence was diminished and disappeared at high levels of support.
Hard drug frequency
Results were similar for hard drug use. For an individual with high support, the probability of 100% adherence was 74% (95% CI = 63–84%) with 0 days per week of hard drug use compared to 78% (95% CI = 64–89%) with 1 day per week of hard drug use. For an individual with average support, the probability of 100% adherence was 70% (95% CI = 62–77%) with 0 days per week of hard drug use compared to 65% (95% CI = 53–75%) with 1 day per week of hard drug use. For an individual with low support, the probability of 100% adherence was 65% (95% CI = 53–75%) with 0 days per week of hard drug use compared to 48% (95% CI = 30–65%) with 1 day per week of hard drug use. Similarly as in the alcohol use models, medication-specific social support buffered the effects of hard drug use on adherence, such that as support increased the effects of drug use on adherence diminished, disappearing at high levels of support.
Post hoc analyses
Post hoc analyses were conducted to evaluate three hypotheses for elucidating the presence of moderation effects at the 3-month time point, but not at 6 or 9 months. The first hypothesis was that social support was not sufficiently high at 6 or 9 months to buffer the effect of AOD use on adherence. We used linear growth curve modeling to evaluate the change in social support across time. In these analyses, each social support variable was regressed on the corresponding month of assessment. Mean levels of general support social support at baseline (M = 2.47, SD = 1.10); 3 (M = 2.54, SD = 1.10); 6 (M = 2.54, SD = 1.13); and 9 months (M = 2.55, SD = 1.18) were stable across time, β = 0.01, SE = 0.01, 95% CI = −0.01–0.02, p = 0.37. In contrast, mean levels of medication-specific social support dropped significantly between 3 (M = 1.54, SD = 1.15), 6 (M = 1.31, SD = 1.07), and 9 months (M = 1.21, SD = 1.17), β = −0.05, SE = 0.01, 95% CI = −0.08 to −0.03, p < 0.001. These results support the possibility that a floor effect with medication-specific social support accounted for the lack of moderation after 3 months when the intervention ended.
The second hypothesis was that only levels of social support concurrent with the outcome would moderate the relation between baseline AOD levels and medication adherence at 6 and 9 months. In these analyses, the 6- and 9-month measures of general and medication-specific social support were evaluated as the moderators for the 6- and 9-month analyses, respectively. Concurrent levels of general and medication-specific social support did not have a buffering effect on the AOD-adherence associations at 6 or 9 months, suggesting no support for this hypothesis.
The third hypothesis was that social support was a “short-term protective factor” that would only moderate the relation between baseline AOD levels and medication adherence within intervals no longer than 3 months. In these analyses, we modified the original moderation models to assess shorter longitudinal intervals (i.e., 9-month adherence controlling for 6-month AOD levels and 6-month adherence controlling for 3-month AOD levels). No additional findings emerged at the 6- and 9-month time points when the longitudinal intervals were narrowed, failing to support the “short-term protective factor” hypothesis.
Discussion
In a diverse sample of 224 men and women living with HIV in the Pacific Northwest, overall self-reported adherence to antiretroviral medications was moderate, declining over time from 73% to 59%. African Americans, in particular, reported lower adherence compared to white participants at 3 months, and older age was also associated with lower odds of adherence. The latter association appeared to be an artifact of there being more older African Americans in our sample. Indeed, while previous research has demonstrated that minority status is often associated with nonadherence, older age tends to be associated with higher rates of antiretroviral adherence.45 In addition, prevalence of AOD was high in the sample: one fourth of the sample met or exceeded the clinical threshold of the AUDIT, indicating a strong likelihood for hazardous or harmful alcohol consumption, while over half of the sample reported hard drug use.
Prospective analyses indicated that medication-specific social support moderated the association between both alcohol and hard drugs at baseline and adherence to antiretroviral medication three months later. Specifically, when alcohol problems increased from the lowest measured level to the clinical threshold, the probability of 100% medication adherence remained stable (75–77%) among individuals with high medication support. In contrast, the probability of 100% adherence decreased from 61% to 37% when medication support was low. Similarly, medication support buffered the effect of hard drug use on medication adherence. When weekly hard drug use increased from zero to 1 day the probability of 100% medication adherence among individuals with high medication support remained the same (74–78%). However, when medication support was low, the probability of 100% adherence decreased from 65% to 48%. Interestingly, there was no finding of a moderating role of general social support.
While these findings should be viewed as preliminary, they offer an explanation to the stubbornly inconsistent findings in the literature regarding associations between AOD use and antiretroviral adherence. Although there have been calls to investigate factors that might explain the inconsistencies, few researchers have systematically examined potential moderators of this relationship. Given the consistently positive association between social support and adherence and its applicability in interventions, social support was targeted as a moderator. Our findings showed that medication-specific but not general social support moderated the association between AOD use and adherence. Perhaps this is because general social support has been shown to promote well-being, while specific social support is tied to particular functions.46 Optimal adherence to antiretroviral medication requires fastidious attention to daily scheduling, the ability to plan ahead for possible disruptions, and forbearance in the face of treatment fatigue and environmental obstacles. Social support that specifically addresses medication adherence would more likely target these antecedents of adherence. Indeed, in a systematic review of the research literature on patient support and education interventions intended to improve adherence, findings showed that interventions targeting practical medication management skills were associated with improved adherence outcomes.47 Alternatively, another potential explanation for the differential findings for medication-specific versus general support is that the two measures assessed different dimensions of support: the general social support items tapped perceptions of available support while the medication-specific social support items assessed actual receipt of support. Future research might attempt to disentangle the specificity and type of support.
Our results suggest that even if PLWH use drugs or alcohol, there may be factors that help them achieve optimal adherence. Thus, if abstinence is an unrealistic goal (which seems likely among many of the chronic users among HIV populations), a harm reduction approach that stresses consistent adherence bolstered with support specifically targeted to medication taking even during times of AOD use might be adopted. Some have argued that this is not ideal, as at least with alcohol use, abstinence has been associated with better adherence compared with at-risk usage or moderate-usage.10 Nonetheless, our findings showed that there are protective factors (i.e., medication-specific social support) that may help individuals with their medication regimens when abstinence does not appear obtainable. This offers preliminary support for practice and policy guidelines for HIV treatment and care.
There may be intervention components beyond medication-specific social support that could help HIV-positive AOD users achieve better adherence. For example, a motivational interviewing and cognitive-behavioral intervention with PLWH who met criteria for hazardous drinking showed that while there were no significant intervention effects for alcohol use, participants showed improvement in adherence at 3 month follow-up.48 Supplemental education about interactions may also be helpful; while controlled intervention studies are largely not available, existing data suggest that interactions between medications commonly prescribed for patients with HIV and illicit drugs can occur, and health care professionals should encourage open dialogue with their patients on this issue.49
Enhancing social support services in general for HIV-positive AOD users, which may include promoting a stable routine and structure that also provides medication-specific support, is another type of intervention that may prove useful. Indeed, although studies have generally shown that adherence is lower among current drug users,14,21 other studies have found that HIV-positive drug users who have access to substance abuse and mental health treatment achieve similar levels of adherence as HIV-positive non-illicit drug users.14,50 Referrals and treatment for mental health problems may be particularly beneficial for this population as AOD is often associated with depression,51 a consistent barrier to adherence and HIV viral suppression.30,52 Finally, other studies have highlighted the importance of the patient-provider relationship and communication supports, especially social support and ancillary services, in increasing adherence among marginalized populations.14,37 Health care professionals may be a rich resource for providing patients with medication-specific social support. Provider education around these issues may thus be an important intervention component to ensure that opportunities to provide medication support are taken, created, and maintained.
Taken together, our results suggest the importance of medication-specific social support to treat comorbid HIV and AOD use. Indeed, researchers have called for multifaceted interventions that are able to address a matrix of factors to promote adherence.53,54 Medication-specific social support may be one such important factor.
Despite this optimistic view, however, findings only showed medication-specific social support moderating the AOD-adherence relationship at 3 months, but not at 6 or 9 months. Our post hoc analyses indicated that one possibility for this was a progressive drop in medication-specific social support at 6 and 9 months, which may have negated any benefit that initial elevations in medication social support would have afforded. Indeed, our results indicate that the conclusion of the peer intervention at 3 months may have accounted for this drop. Our findings are modest given that the moderation effects did not replicate at 6 or 9 months; however, the effect at 3 months was robust and consistent with respect to both measures of AOD. Clearly, these results need to be replicated to further clarify the role and impact of medication-specific social support. One possibility is that perhaps ongoing medication-specific social support needs to be developed in programs for HIV-positive alcohol and substance users in order to maintain adherence gains.
Given that the buffering effect was moderate in size and noted only at the 3-month assessment, our results should be considered preliminary, and there are certainly other important factors that impact adherence among AOD users, many of which may be beyond the scope of social support to buffer. Persons with heavy AOD use are at increased risk for homelessness, unemployment, and incarceration. Lack of stable housing and changes in routines affect ability to track dose intake and maintain adherence.55 For example, research has shown that alcohol reduced patient abilities to maintain a consistent pill-taking schedule.15 Another potential mechanism is that patients who use AOD may intentionally skip HAART doses because of beliefs about negative interactions between AOD and HAART.56 Indeed, a recent study demonstrated that beliefs that alcohol and HIV medications should not be mixed were common among PLWH, and that stopping antiretroviral therapy when drinking was associated with treatment nonadherence over and above problem drinking.57 Alternatively, it may be that AOD use results in cognitive impairments that make adherence more difficult.58–60 These factors need to be considered as well in any comprehensive adherence-promotion intervention. There were some methodological limitations to our investigation. First, our estimates of adherence relied solely on self-report, which is known to overestimate adherence and is subject to recall bias and social desirability.54 However, it is generally considered a valid assessment technique that correlates consistently (if moderately) with biological markers of treatment success such as viral load and CD4 count.61,62 Second, we only found the effects for one time point, suggesting the preliminary nature of our findings and the potential tenuousness of the effect. Finally, the effect sizes were moderate,63 suggesting that perhaps the inclusion of other moderating factors are needed to more fully explain the effects on adherence.
Indeed, future studies that examine potential moderators and mediators of the relation between AOD and adherence are crucial as they have important treatment implications for this high-risk and marginalized group. A few studies have begun to examine these relationships; for example, positive affect and self-efficacy have been shown to be associated with higher levels of adherence among drug users.33, 64 Our findings suggest that medication-specific social support may be another protective factor, potentially assisting with the development of innovative psychological treatments designed to meet the needs of HIV-positive AOD users.
Acknowledgment
This research was supported by National Institute on Mental Health (NIMH) grant (R01 MH58986) award to Jane M. Simoni.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Crum NF. Riffenburgh RH. Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Jones J. Taylor B. Wilkin TJ. Hammer SM. Advances in antiretroviral therapy. Top HIV Med. 2007;15:48–82. [PubMed] [Google Scholar]
- 3.Gardner EM. Sharma S. Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22:75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggiolo F. Airoldi M. Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8:282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 5.Lima VD. Harrigan R. Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway B. The role of adherence to antiretroviral therapy in the management of HIV infection. J Acquir Immune Defic Syndr. 2007;45(Suppl 1):S14–18. doi: 10.1097/QAI.0b013e3180600766. [DOI] [PubMed] [Google Scholar]
- 7.Bing EG. Burnam MA. Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 8.Cook RL. Sereika SM. Hunt SC. Woodward WC. Erlen JA. Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvan FH. Bing EG. Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 10.Samet JH. Horton NJ. Meli S. Freedberg KA. Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield TK. Midanik LT. Rogers JD. A 10-year national trend study of alcohol consumption, 1984–1995: Is the period of declining drinking over? Am J Public Health. 2000;90:47–52. doi: 10.2105/ajph.90.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordillo V. del Amo J. Soriano V. González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 13.Hendershot CS. Stoner SA. Pantalone DW. Simoni JM. Alcohol use and antiretroviral adherence: Review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malta M. Strathdee SA. Magnanini MM. Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction. 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 15.Murphy DA. Marelich WD. Hoffman D. Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004;16:471–484. doi: 10.1080/09540120410001683402. [DOI] [PubMed] [Google Scholar]
- 16.Holmes WC. Bilker WB. Wang H. Chapman J. Gross R. HIV/AIDS-specific quality of life and adherence to antiretroviral therapy over time. J Acquir Immune Defic Syndr. 2007;46:323–327. doi: 10.1097/QAI.0b013e31815724fe. [DOI] [PubMed] [Google Scholar]
- 17.Murphy DA. Greenwell L. Hoffman D. Factors associated with antiretroviral adherence among HIV-infected women with children. Women Health. 2002;36:97–111. doi: 10.1300/J013v36n01_07. [DOI] [PubMed] [Google Scholar]
- 18.Power R. Koopman C. Volk J, et al. Social support, substance use, and denial in relationship to antiretroviral treatment adherence among HIV-infected persons. AIDS Patient Care STDs. 2003;17:245–252. doi: 10.1089/108729103321655890. [DOI] [PubMed] [Google Scholar]
- 19.Bryson CL. Au DH. Sun H. Williams EC. Kivlahan DR. Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149:795–804. doi: 10.7326/0003-4819-149-11-200812020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Chander G. Lau B. Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celentano DD. Vlahov D. Cohn S. Shadle VM. Obasanjo O. Moore RD. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280:544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 22.Lucas GM. Gebo KA. Chaisson RE. Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 23.Ammassari A. Murri R. Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Peretti-Watel P. Spire B. Lert F. Obadia Y. Group V. Drug use patterns and adherence to treatment among HIV-positive patients: Evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003) Drug Alcohol Depend. 2006;82(Suppl 1):S71–79. doi: 10.1016/s0376-8716(06)80012-8. [DOI] [PubMed] [Google Scholar]
- 25.Catz SL. Kelly JA. Bogart LM. Benotsch EG. McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 26.Kalichman SC. Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect. 2003;79:59–61. doi: 10.1136/sti.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini M. Recchia E. Nasta P, et al. Illicit drug use: can it predict adherence to antiretroviral therapy? Eur J Epidemiol. 2004;19:585–587. doi: 10.1023/b:ejep.0000032353.03967.ef. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed H. Kieltyka L. Richardson-Alston G, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care STDs. 2004;18:289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- 29.Simoni JM. Frick PA. Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25:74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMatteo MR. Lepper HS. Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 31.Godin G. Côté J. Naccache H. Lambert LD. Trottier S. Prediction of adherence to antiretroviral therapy: a one-year longitudinal study. AIDS Care. 2005;17:493–504. doi: 10.1080/09540120412331291715. [DOI] [PubMed] [Google Scholar]
- 32.Pratt RJ. Robinson N. Loveday HP, et al. Adherence to antiretroviral therapy: Appropriate use of self-reporting in clinical practice. HIV Clin Trials. 2001;2:146–159. doi: 10.1310/89E2-XNJL-W107-R2GL. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson JS. Schönnesson LN. Williams ML. Timpson SC. Associations among correlates of schedule adherence to antiretroviral therapy (ART): A path analysis of a sample of crack cocaine using sexually active African-Americans with HIV infection. AIDS Care. 2008;20:253–262. doi: 10.1080/09540120701506788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication Adherence among HIV-infected adults attending a public health clinic. AIDS Patient Care STDs. 2002;16:43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- 35.Broadhead RS. Heckathorn DD. Altice FL, et al. Increasing drug users' adherence to HIV treatment: Results of a peer-driven intervention feasibility study. Soc Sci Med. 2002;55:235–246. doi: 10.1016/s0277-9536(01)00167-8. [DOI] [PubMed] [Google Scholar]
- 36.Carrieri MP. Chesney MA. Spire B, et al. Failure to maintain adherence to HAART in a cohort of French HIV-positive injecting drug users. Int J Behav Med. 2003;10:1–14. doi: 10.1207/s15327558ijbm1001_01. [DOI] [PubMed] [Google Scholar]
- 37.Knowlton A. Arnsten J. Eldred L, et al. Individual, interpersonal, and structural correlates of effective HAART use among urban active injection drug users. J Acquir Immune Defic Syndr. 2006;41:486–492. doi: 10.1097/01.qai.0000186392.26334.e3. [DOI] [PubMed] [Google Scholar]
- 37a.Simoni JM. Huh D. Frick PA. Pearson CR. Andrasik MP. Dunbar PJ. Hooton TM. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52:465–473. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders JB. Aasland OG. Babor TF. de la Fuente JR. Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 39.Fiellin DA. Reid MC. O'Connor PG. Screening for alcohol problems in primary care: A systematic review. Arch Intern Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- 40.Prentiss D. Power R. Balmas G. Tzuang G. Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr. 2004;35:38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Sherbourne CD. Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 42.van Buuren S. Brands JPL. Groothuis-Oudshoorn CGM. Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049–1064. [Google Scholar]
- 43.Raudenbush SW. Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage. 2002 [Google Scholar]
- 44.Aiken L. West S. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 45.Fogarty L. Roter D. Larson S. Burke J. Gillespie J. Levy R. Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- 46.Darbes LA. Lewis MA. HIV-specific social support predicts less sexual risk behavior in gay male couples. Health Psychol. 2005;24:617–622. doi: 10.1037/0278-6133.24.6.617. [DOI] [PubMed] [Google Scholar]
- 47.Rueda S. Park-Wyllie LY. Bayoumi AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsons JT. Golub SA. Rosof E. Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoniou T. Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36:1598–1613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- 50.Crystal S. Sambamoorthi U. Moynihan PJ. McSpiritt E. Initiation and continuation of newer antiretroviral treatments among medicaid recipients with AIDS. J Gen Intern Med. 2001;16:850–859. doi: 10.1111/j.1525-1497.2001.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tegger MK. Crane HM. Tapia KA. Uldall KK. Holte SE. Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDs. 2008;22:233–243. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- 52.Shacham E. Nurutdinova D. Onen N. Stamm K. Overton ET. The interplay of sociodemographic factors on virologic suppression among a U.S. outpatient HIV clinic population. AIDS Patient Care STDs. 2010;24:229–235. doi: 10.1089/apc.2009.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell-Cope GM. White J. Henkelman EJ. Turner BJ. Qualitative and quantitative assessments of HAART adherence of substance-abusing women. AIDS Care. 2003;15:239–249. doi: 10.1080/0954012031000068380. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds NR. Adherence to antiretroviral therapies: state of the science. Curr HIV Res. 2004;2:207–214. doi: 10.2174/1570162043351309. [DOI] [PubMed] [Google Scholar]
- 55.Bouhnik AD. Chesney M. Carrieri P, et al. Nonadherence among HIV-infected injecting drug users: The impact of social instability. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S149–153. doi: 10.1097/00126334-200212153-00013. [DOI] [PubMed] [Google Scholar]
- 56.Sankar A. Wunderlich T. Neufeld S. Luborsky M. Sero-positive African Americans' beliefs about alcohol and their impact on anti-retroviral adherence. AIDS Behav. 2007;11:195–203. doi: 10.1007/s10461-006-9144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalichman SC. Amaral CM. White D, et al. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care STDs. 2009;23:449–454. doi: 10.1089/apc.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinkin CH. Hardy DJ. Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parsons JT. Rosof E. Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007;12:357–370. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothlind JC. Greenfield TM. Bruce AV, et al. Heavy alcohol consumption in individuals with HIV infection: Effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearson CR. Simoni JM. Hoff P. Kurth AE. Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self-report: An examination of key methodological issues. AIDS Behav. 2007;11:161–173. doi: 10.1007/s10461-006-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simoni JM. Kurth AE. Pearson CR. Pantalone DW. Merrill JO. Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenthal JA. Qualitative descriptors of strength of association and effect size. J Soc Service Res. 1996;21:37. [Google Scholar]
- 64.Carrico AW. Johnson MO. Colfax GN. Moskowitz JT. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav. 2010;14:769–777. doi: 10.1007/s10461-008-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]