Abstract
The calcium ion (Ca2+) is the main second messenger that helps to transmit depolarization status and synaptic activity to the biochemical machinery of a neuron. These features make Ca2+ regulation a critical process in neurons, which have developed extensive and intricate Ca2+ signaling pathways. High intensity Ca2+ signaling necessitates high ATP consumption to restore basal (low) intracellular Ca2+ levels after Ca2+ influx through plasma membrane receptor and voltage-dependent ion channels. Ca2+ influx may also lead to increased generation of mitochondrial reactive oxygen species (ROS). Impaired abilities of neurons to maintain cellular energy levels and to suppress ROS may impact Ca2+ signaling during aging and in neurodegenerative disease processes. This review focuses on mitochondrial and endoplasmic reticulum Ca2+ homeostasis and how they relate to synaptic Ca2+ signaling processes, neuronal energy metabolism, and ROS generation. Also, the contribution of altered Ca2+ signaling to neurodegeneration during aging will be considered. Advances in understanding the molecular regulation of Ca2+ homeostasis and how it is perturbed in neurological disorders may lead to therapeutic strategies that modulate neuronal Ca2+ signaling to enhance function and counteract disease processes. Antioxid. Redox Signal. 14, 1261–1273.
Introduction
The extracellular free calcium (Ca2+) concentration typically is 1.2 mM, whereas the typical resting cytosolic free Ca2+ concentration ([Ca2+]cyt) is approximately 100 nM. This 10,000-fold concentration gradient makes Ca2+ special when compared with the more abundant cations Na+ and K+, as it leads to a significant increase in [Ca2+]cyt after depolarization, whereas influx of Na+ and efflux of K+ significantly affect the membrane potential, but cause only relatively minor changes in cytosolic ion concentration. During evolution, a plethora of Ca2+-binding proteins and Ca2+-dependent signaling pathways have arisen, making Ca2+ a key element that can relay information about the plasma membrane potential to the biochemical and metabolic machinery of a cell. While Ca2+ regulation is essential to any cell, it is obvious that in excitable cells the connection between electrochemical ion gradients and biochemical regulatory pathways is of special importance. One prime example of this connection is in myocytes, where upon depolarization and increased [Ca2+]cyt the protein troponin C binds Ca2+ to initiate the protein conformation and protein–protein interaction changes that result in myocyte and muscle contraction (79). Coordinated contraction is the main function of muscles and the structure of myocytes (syncytial cells with triads of transverse tubules, sarcoplasmic reticulum, and contractile proteins) is clearly organized for excitation-contraction coupling. The main function of neurons is information processing (or integration) and transmission to effector cells that mediate behavioral responses (learning and memory, body movements, emotional responses, etc.). A typical neuron consists of complex dendritic arbors that receive electrochemical (synaptic) inputs from other neurons, a cell body (where genes are housed), and an axon that transfers signals to postsynaptic cells (neurons or muscle cells). In this (simplified) view of neurons, Ca2+ serves multiple complex and integrated functions, including the control of dendritic responses (morphological and functional) to neurotransmitters, signaling to the nucleus to regulate gene expression, and initiation of neurotransmitter release from presynaptic axon terminals.
Ca2+ influx into dendrites and the soma (cell body) is largely dependent on presynaptic neurotransmitter release and the membrane potential. The latter is mainly controlled by Na+ and K+ channels (37). Ca2+ is therefore a (second) messenger that transfers signals within the cell in response to membrane depolarization, thereby relaying information on neuronal activity status both locally (in a dendritic spine, for example) and globally (to initiate widespread changes in energy metabolism, for example) within the neuron. A major function of Ca2+ is therefore to regulate activity-dependent signaling. It is reasonable to assume that Ca2+ signaling helps a neuron adapt to its activity-dependent requirements and, with feed-back and feed-forward mechanisms, strengthens relevant synaptic connections, eliminates irrelevant connections, and avoids overexcitation. In these ways Ca2+ plays pivotal roles in controlling neuronal excitability. Ca2+ functions as a key regulator of electrochemical signaling, not only within individual neurons, but also among large populations of neurons that comprise neuronal networks.
The cost for extensive neuronal Ca2+ signaling is increased energy demand, as all Ca2+ that enters a neuron must be removed from the cytoplasm by ATP-dependent membrane Ca2+ “pumps” in order to maintain Ca2+ homeostasis. Therefore, Ca2+ signaling and excitability mutually regulate each other such that an optimal neuronal activity level is achieved for a given metabolic capacity. During aging and in pathological conditions, Ca2+ signaling and homeostasis will be affected and may thus change a neuron's excitability, which will in turn affect network activity and metabolism (47). As will be described below, neuronal Ca2+ homeostasis is altered during aging and in neurodegenerative conditions, and uncontrolled Ca2+ signaling may also actively contribute to neurodegenerative processes.
Because of the enormous database on the topic of Ca2+ in neuronal plasticity and disease, it is beyond the scope of any review to give an exhaustive overview on all Ca2+-dependent signaling cascades. Instead, in this article we focus on mitochondria and endoplasmic reticulum (ER) to illustrate how neuronal activity, energy metabolism, and Ca2+ homeostasis are kept in balance and how this balance may be affected during aging and in neurodegenerative processes.
Ca2+ and Mitochondria
The main function of mitochondria is to produce high energy intermediates (NADH and ATP) through the tricarboxylic acid (TCA) cycle (Krebs cycle) and oxidative phosphorylation. Mitochondria also function as a Ca2+ buffer and are the main source of reactive oxygen species (ROS) (77). Mitochondria perform many more functions, but here we focus on the interrelationships between mitochondrial Ca2+ homeostasis, energy metabolism, and oxidative stress in the contexts of synaptic plasticity and neurodegenerative disorders.
Ca2+ Signaling and Energy Homeostasis
The TCA cycle feeds the electron transport chain, which in turn converts oxygen (O2) to water and pumps protons from the mitochondrial matrix to the mitochondrial intermembrane space to generate the proton gradient. The proton gradient then is used by the F0F1-ATPase to generate ATP from ADP, and the adenosine nucleotide translocase (or transporter) (ANT) transports ATP from the matrix to the intermembrane space in exchange for ADP. Because proton pumping creates an inside-negative membrane potential, Ca2+ as a cation will tend to accumulate in the mitochondrial matrix (i.e., the Ca2+ electrochemical gradient is the main driving force of Ca2+ influx in to the mitochondrial matrix) (77). The conductance of the uniporter is increased by high [Ca2+]cyt and low ATP/ADP ratios (i.e., in situations of high energy demand) but is low in situations of low [Ca2+]cyt and high ATP/ADP ratios and may also depend on the mitochondrial membrane potential (1, 42, 46, 51, 56, 77). This mechanism ensures the unidirectional mode of transport. Increased mitochondrial matrix Ca2+ concentration ([Ca2+]mit) will increase activity of the three enzymes of the Krebs cycle: pyruvate dehydrogenase (indirectly via phosphatase activation), isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase (by binding directly to the enzyme), as well as the F1F0-ATPase, thus causing increased production of ATP and NADH (20, 21, 68). This is an elegant mechanism in which Ca2+ functions as an indicator of increased energy demand that signals to the mitochondria.
The following consideration outlines why mitochondrial buffering can be regarded as a mechanism that limits the maximal rate of O2 consumption required to maintain Ca2+ homeostasis. Pumping out one Ca2+ ion from the cytoplasm across the plasma membrane or the to the endoplasmic reticulum (ER) membrane costs one ATP molecule, either directly via activity of the plasma membrane Ca2+ ATPase (PMCA) or the sarcoplasmic endoplasmic reticulum Ca2+ ATPase (SERCA), or indirectly by activity of the Na+–Ca2+ exchanger (NCX) which exchanges three Na+ for one Ca2+ and then the three Na+ are pumped out by the Na+/K+ ATPase at the expense of one ATP. To restore the ATP level, three protons must be pumped by the ETC. If Ca2+ enters the mitochondrial matrix, this comes at the expense of only two protons (i.e., 0.67 ATP molecules). Thus, mitochondrial Ca2+ buffering requires fewer protons to be pumped and accordingly less O2 needs to be consumed. This is likely an important contribution to maintain energy homeostasis in neurons in situations of repeated axon potential generation, when cation influx and consequently ATP demand and O2 consumption reach high levels. Importantly, maintenance of high ATP levels and a high ATP\ADP ratio is critical for neurons. If compared with a scenario where all Ca2+ is extruded through Ca2+ ATPases, mitochondrial Ca2+ buffering blunts the peak rate of O2 that needs to be consumed in a situation of high ATP demand. This is important as neurons have high baseline O2 consumption rates and only a limited potential to increase their O2 consumption rate. While peak O2 consumption is reduced, total O2 consumption is in fact increased, as the extrusion of Ca2+ from the mitochondrial matrix to the cytosol, for example, by the mitochondrial sodium calcium exchanger (NCX) requires an extra proton to be pumped before Ca2+ can be extruded from the cytosol through Ca2+ ATPases. However, Ca2+ extrusion from mitochondria will occur at a timepoint when [Ca2+]cyt is back to baseline levels and ATP demand and O2 consumption are low.
Ca2+ is extruded from the matrix through the mitochondrial NCX (3 Na+ for 1 Ca2+) (48, 77) and is also believed to leave the mitochondrial matrix through a mitochondrial Ca2+ proton exchanger (2 or more H+ for 1 Ca2+) (95), and also through transient opening of the mitochondrial permeability transition pore (mPTP) (41) (Fig. 1). Each mechanism will directly or indirectly reduce the proton gradient and thus come at the expense of ATP. One interesting aspect is that the long sought after molecular identities and compositions of all three efflux pathways and the Ca2+ uniporter remain unclear despite intense search. This is remarkable in the era of genomics and proteomics. It may therefore be possible that, for example, sodium–calcium exchange and proton–calcium exchange are not performed by single molecules specifically dedicated to this task, but rather by proteins or protein complexes that have other primary tasks but have additional, as yet unrecognized capabilities in Ca2+ transport. Of note is that the maximal transport kinetics of mitochondrial sodium–calcium and proton–calcium exchange are slow compared with the Ca2+ uniporter (96, 97). This would make mitochondria prone to Ca2+ overload. The existence of an additional high conductance Ca2+ efflux mechanism thus seems to be a physiological necessity. The mPTP fulfills all requirements for such an efflux route. Notably, mPTP opening is enhanced by high [Ca2+]mit. However, most of our knowledge of the mPTP stems from experiments with isolated mitochondria in pathological conditions (e.g., Ca2+ overload or apoptosis). Our knowledge of mPTP functions and modes under physiological conditions is sketchier. Since a permanent mPTP opening is incompatible with continued mitochondrial ATP production, under physiological conditions transient opening of the mPTP has been suggested. But most of the evidence for such mPTP flickering stems from experiments with pharmacological mPTP inhibitors such as cyclosporin A or bongkrekic acid and thus is indirect and prone to artifacts (6–8, 36, 80). Also, the classical models for the molecular composition of the mPTP have been called into question through recent studies. For example, genetic ablation of the proposed mPTP component voltage-dependent anion channel (VDAC) did not result in any observable deficit in mPTP formation or resistance to apoptotic stimuli (2). Genetic ablation of the mPTP regulator and molecular target of cyclosporine A, cyclophilin D, resulted in a reduced sensitivity of mitochondria to oxidative stress and Ca2+ overload but not to apoptotic stimuli (3, 4, 65, 82). Cyclophilin D, therefore, may regulate the mPTP also under physiological conditions in a Ca2+- and ROS-sensitive manner (see section on Ca2+ and ROS for details).
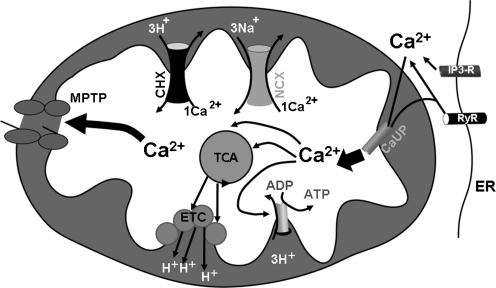
FIG. 1.
Mechansims of mitochondrial Ca2+ influx and efflux. Ca2+ enters the mitochondria through the Ca2+ uniporter (CaUP). Close apposition of ER Ca2+ release channels and Ca2+ uniporter are likely to result in enhanced mitochondrial Ca2+ uptake. Inside the mitochondrial matrix, Ca2+ can increase the activity of dehydrogenases of the tricarboxic acid cycle (TCA), leading to enhanced feeding of the electron transport chain (ETC) and increased transfer of protons to the intermembrane space. Mitochondrial Ca2+ also activates the F1F0 ATPase to produce more ATP. Ca2+ is extruded from the mitochondrial matrix through a sodium-dependent mechanism (sodium calcium exchanger, NCX) and a membrane potential-dependent mechanism (calcium proton exchanger, CHX), as well as the permeability transition pore (mPTP). Note that the molecular identities of CaUP, mitochondrial NCX and CHX are unclear.
It should be noted that mitochondrial Ca2+ influx in itself is not a detrimental event; rather it is an essential step to stimulate mitochondrial ATP production. Therefore (periodic) mitochondrial Ca2+ influx is desirable. If a time delay is assumed between mitochondrial Ca2+ influx and efflux and in the kinetics of Ca2+-dependent TCA cycle activation and deactivation, a model can be generated where periodic cytosolic Ca2+ influx will cause a periodic (but somewhat more protracted) mitochondrial Ca2+ influx, which in turn will lead to a nearly sustained enhanced activity of TCA cycle and oxidative phosphorylation and thus ensure high ATP and NADH levels (8, 35, 47) (Fig. 2). Such a model would be compatible with the observed beneficial effects of exercise or cognitive stimulation for mitochondrial and antioxidant function. Under pathological conditions with impaired mitochondrial capacity or low ATP levels, mitochondrial Ca2+ may pose more of a challenge to a neuron. But because Ca2+ is critical for activation of the TCA cycle and oxidative phosphorylation, inhibiting Ca2+ influx into mitochondria to reduce mitochondrial ROS generation will likely compromise energy production. Reducing [Ca2+]cyt or scavenging ROS seems a more sensible approach.
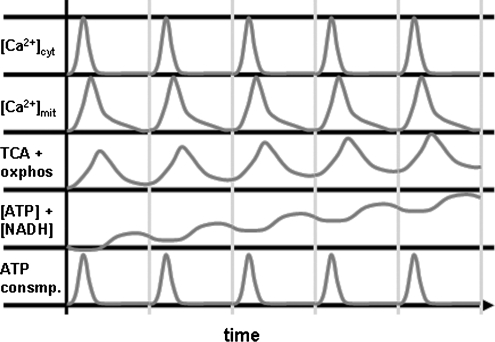
FIG. 2.
Model for kinetics of mitochondrial Ca2+ flux, metabolism and energy levels. If a kinetic delay is assumed between [Ca2+]i and [Ca2+]mit, then activity of the TCA cycle and oxidative phosphorylation will outlast the increase in [Ca2+]i and cytosolic ATP demand, resulting in slightly higher levels of ATP and NADH at the end of a [Ca2+]i transient. In this model, repeated [Ca2+]i transients with the right frequency can therefore result in higher levels of ATP and NADH.
Ca2+ and ROS
While the link between Ca2+ and energy homeostasis has been revealed in considerable molecular detail, the connection between ROS generation and Ca2+ is less well understood at the molecular level. The main (but not only) sources of ROS in the mitochondria are complexes I and III of the electron transport chain. The default reaction of these complexes is to transport electrons to the next link in the chain (e.g., ubiquinone for complex I). Occasionally, however, the electron will be transferred onto an O2 molecule, thereby generating one superoxide anion free radical (O2·-). O2·- is a reactive membrane-impermeable ROS; it can be converted to H2O2 spontaneously or by manganese superoxide dismutase (SOD) in the mitochondrial matrix and copper/zinc SOD in the cytosol. H2O2 can then be converted by catalase (to H2O and O2) or peroxidases (to H2O). Both SOD and catalase have a very high Vmax that is essentially diffusion rate limited, and they do not need electron donors (12, 25, 58). Thus, the location of these enzymes near the source of ROS generation is as important as total enzyme availability. Peroxidases can detoxify H2O2 and other peroxides (ROOR), making peroxidases important cytoprotective enzymes. They require electron donors, such as glutathione in the case of glutathione peroxidases (9, 60, 70). Oxidized glutathione in turn is reduced by glutathione reductase at the expense of NADPH and so peroxidase-mediated detoxification is energy dependent. The rate of aberrant electron transfer in mitochondria has been estimated to be approximately 0.4% (53), but this estimate is based on measurements made in isolated mitochondria or in non-neuronal cells. It is generally accepted that O2·- generation increases if the proton gradient is high and electron transport occurs against a high energy gradient (therefore uncoupling proteins may reduce O2·- generation). Increased O2 consumption by mitochondria naturally also will lead to more O2·- production (assuming a stable rate of 0.4% of aberrant electron transfer). At the same time increased TCA cycle activity will lead to increased NADH levels which in energized mitochondria can be used by nicotinamide nucleotide transhydrogenase to reduce NADP+ to NADPH. NADPH can then reduce oxidized glutathione, which in turn can be used by peroxidases (40). Another mechanism that links increased TCA activity with increased NADPH production is via mitochondrial NADP+-dependent isocitrate dehydrogenase (40). An increase in [Ca2+]mit will thus simultaneously increase O2·- generation and reducing equivalents. From a quantitative perspective the increase in reducing equivalents can compensate for the increased production of ROS. It can also be assumed that because of their high Vmax SOD and catalase have reserve capacities to deal with an increase in ROS generation.
ROS Accumulation in Aging
Some ROS that escape the antioxidant system may activate cellular signaling pathways involved in a range of functions including cell survival and even synaptic plasticity (45, 93). Importantly, ROS, through oxidation and (subsequent) glutathionylation of thiol groups, also modulate kinetics of key proteins in ER and mitochondrial Ca2+ homeostasis, for example, mPTP, SERCA, inositol-3-phosphate receptors (IP3-R), and ryanodine receptors (RyRs) (19). However, ROS may also react with lipids, proteins, or nucleic acids, leading in many instances to impaired biological function of the respective molecules. A large body of evidence has shown that such oxidative modifications of molecules (e.g., hydroxynonenal, carbonylated proteins, 8-oxoguanine) accumulate during aging in mice as well as human and nonhuman primates in most tissues, including the brain (53). Studies in transgenic mice also indicate that the levels of antioxidant enzymes correlate negatively with the amount of ROS damage (64), and an increase in molecular oxidative modifications is found in most neurodegenerative diseases (23, 75, 85). For neuronal mitochondria, oxidative damage means less efficient ATP and NADH production, a reduced maximal respiratory capacity (reserve capacity), and likely an increased rate of O2·- generation (particularly if complex I is damaged by oxidative stress). Because Ca2+ entry into the mitochondria is dependent on the proton gradient, it is likely that the mitochondrial capacity to buffer Ca2+ decreases with increased oxidative damage. Such a gradual increase in ROS-mediated mitochondrial damage in neurons may first result in compensatory mechanisms. Such mechanisms could involve, for example, prolonged increases in [Ca2+]cyt resulting in increased activation of calcineurin, which in turn dephosphorylates NMDA receptor proteins and thus causes a reduction in Ca2+ influx (55). The reduced ion influx would result in lower energy expenditure for ion homeostasis. The price for such a compensatory mechanism would be a reduction in information processing capacity, as maximal NMDA receptor activation would be impaired. In advanced stages of oxidative stress, ATP production may become so impaired that neurons become prone to excitotoxicity during periods of metabolic challenges (e.g., during hypoglycemia) or even just through repeated axon potential firing.
A scenario of life-long accumulation of oxidative modifications resulting in impaired biological function of molecules and tissues is plausible, and constitutes the free radical theory of aging. It should be noted, however, that current evidence suggests that (at least in mice) the free radical theory of aging applies mostly to pathological conditions, and may be less important in determining maximal life span if aging occurs in optimal conditions (78). Also, evidence that antioxidant therapy can in fact improve aging-related declines in cognitive function in mice or humans is sparse (75, 76). Nevertheless, the existing data of increased ROS-mediated alterations in brain cells during aging and in neurodegenerative diseases suggest that ROS-induced impaired mitochondrial function and consequently impaired metabolic efficiency eventually will lead to impaired brain functions. An interesting and yet unresolved question is how neuronal excitability is affected on a network level by compensatory mechanisms that adapt a neuron to its impaired mitochondrial function. A detailed understanding of such mechanisms may lead to interventional strategies that aim at improving network efficiency (31).
ROS in Acute Stress
Less clear than ROS-mediated impairment of mitochondrial function during aging is the question of whether an acute increase in [Ca2+]cyt or [Ca2+]mit causes a lethal increase of ROS. While there is little doubt that in situations such as stroke or seizures, acute dramatic influx of Ca2+ will cause increased ROS production, it is not easy to reliably differentiate between ATP depletion and ROS generation as the primary cause for cell death because reduced ATP levels, increased ROS levels, and increased [Ca2+]cyt occur concomitantly and amplify each other. One approach to address this problem is to measure neuronal O2 consumption. The rationale here is that, in a scenario of ATP depletion, neurons would be expected to maximally increase their O2 consumption, whereas in a scenario of mitochondrial impairment through ROS-mediated damage, mitochondrial ability to increase O2 consumption maximally would be impaired. Studies that have used this approach have shown that in glutamate toxicity (the prototypical in vitro paradigm of acute neuronal Ca2+ overload) O2 consumption increases maximally (2- to 2.5-fold over baseline), pointing to ATP depletion rather than ROS as a major cause of cell death (30, 69). Also, in the case of a purely ROS-mediated cell death, one would expect a low level of mitochondrial uncoupling to improve cell survival, as mitochondrial uncoupling lowers the proton gradient and thereby facilitates the flow of electrons through the electron transport chain (ETC), while it will increase TCA cycle activity and thus NADH production. Indeed, several studies have provided evidence that increased mitochondrial uncoupling, induced either pharmacologically or by overexpression of uncoupling proteins, can protect neurons under excitotoxic conditions (14, 50, 57). However, at least in some cases, low level mitochondrial uncoupling in fact accelerates cell death during glutamate toxicity, again pointing to ATP depletion as the more relevant contributor to cell death (43). Moreover, in single neuron measurements it was found that maximal O2 consumption is reached within 5 minutes after glutamate application (30). While it is not impossible, it would be surprising that in a healthy neuron a doubling of O2 consumption (and thus ETC activity) for 5 minutes would result in a lethal dose of ROS (assuming a constant rate of O2- generation, i.e., 0.4% of total O2 consumption) that is capable of overwhelming the entire antioxidant system. In vivo the situation is different though, especially in conditions of reduced energy availability as occurs in stroke or traumatic brain injury.
During ischemia or hypoxia, ATP levels drop while NADH accumulates together with acyl esters of coenzyme A and carnitine (66). Acyl coenzyme A and acyl carnitine are believed to impair the functional and structural integrity of mitochondria (66). During reperfusion or reoxygenation, some neurons will display NADH hyperoxidation, which is frequently accompanied by peri-infarct depolarizations in vivo or hypoxic spreading depression in vitro (i.e., prolonged depolarization of neurons starting in the brain regions close to the insult and spreading across the hemisphere) (26, 49). It is in this situation of structural mitochondrial damage combined with low NADH and high ATP demand in the reperfusion phase that the rate of O2·- generation will be higher than in physiological conditions. In addition, the antioxidant repair capacity is reduced due to a lack of reducing equivalents, as the transfer of hydrogen from NADH to NADP by nicotinamide nucleotide transferase is energetically not favored (40). In such a situation, it is more readily conceivable that acute ROS-mediated cell damage can contribute to neuronal cell death. Distinguishing between ATP depletion and ROS-induced cell damage is more difficult in vivo compared to cell culture glutamate toxicity models, because complex cellular interactions and vascular alterations occur in the intact brain. The role of reduced ATP levels, though, should not be underestimated or regarded as a mere end-stage epiphenomenon in any of the conditions that are associated with acute ROS stress. In this respect it is remarkable that the relative contributions of some basic metabolic pathways to neuronal ATP generation and antioxidant potential remain controversial. For example, the relative contribution of neuronal glycolysis and the glial lactate shuttle to ATP generation in neurons at rest and after depolarization are not clearly established (17, 73, 84). The same is true for NADPH production through the pentose phosphate cycle (which competes with glycolysis for glucose-6-phosphate) (10). Obtaining a detailed and quantitative understanding of the relative contribution of each of these pathways to a neuron's energy and redox balance may lead to a rational approach of, for example, dietary interventions that aim at supplying optimal substrates for the brain in physiological and pathological conditions. One such intervention, the ketogenic diet for epilepsy, is a reminder that such approaches are possible in principle, although the underlying mechanisms are poorly understood at the molecular level (59).
It should be kept in mind that outcomes of clinical trials using antioxidant strategies in stroke or cardiac ischemia have been consistently negative so far (22, 72, 83, 94). Reperfusion using the “clot-busting” drug TPA is an approved therapy for both conditions that is likely to increase ROS production. This may indicate that reperfusion-induced ROS generation reaches critical levels at approximately 6 and 3 hours after cardiac and cerebral ischemia, respectively. If this is the case, then it can be assumed that the highest potential of antioxidant therapies in these conditions lies in improving outcome of reperfusion therapy and extending its time window. However, the currently existing clinical trial data do not support this theory either (22, 83). A major challenge for any antioxidant therapy lies in the short half-life of ROS. Thus, in order to be effective, ROS scavengers must be lipid permeable to access ROS sources such as complex I of the ETC. Also, they need to be present at high concentrations because the most common lipid-permeable antioxidant compounds such as vitamin E are not catalysts of ROS conversion, but instead are scavengers that are consumed by free radicals and must therefore be replaced or converted into their reduced form. However, the latter process is typically impaired in situations like ischemia or reperfusion. However, if antioxidants are not efficient enough in scavenging ROS, then the question is, why do they improve outcomes in animal models of stroke and cardiac ischemia (including larger sized animals)? The animal models used may not adequately reflect the human pathological conditions and they certainly do not adequately predict outcomes of clinical trials. It may also be that preserving high ATP levels is a more powerful intervention than reducing ROS levels in human stroke or myocardial ischemia. Supporting such an interpretation is the fact that those therapies that are proven effective treatments in these conditions (reperfusion in both conditions as well as nitrates and ACE inhibitors in myocardial infarction [71, 100]) clearly will affect ATP supply or demand, but have no direct connection to ROS generation or scavenging. Conversely, those studies that looked specifically at ROS inhibitors or at reducing reperfusion injury had negative or mixed results (22).
ROS and mPTP
ROS are strongly implicated in sensitizing the mPTP (44, 54, 102), and ROS have been implicated in physiological cell signaling, rather than just cell damage (32, 39, 86). However, our understanding of the role of ROS in gating the mPTP and regulating Ca2+ homeostasis in physiological conditions is still rudimentary. One main reason is that our tools to study both the mPTP and ROS in intact cells (versus isolated mitochondria) are quite limited. From our current knowledge, one might predict that ROS generation would increase in the late phase after a Ca2+ challenge at a time point when [Ca2+]cyt has dropped, but [Ca2+]mit is still high, and O2 consumption and mitochondrial membrane potential are high. The high mitochondrial membrane potential would cause an increase in the rate of O2·- production that would then cause the transient opening of the mPTP in order to let Ca2+ leave the mitochondria in a situation where ATP availability is high. At the same time the mPTP-induced reduction in mitochondrial membrane potential would ensure that O2·- generation will decrease again, thus preventing excessive build-up of ROS. Cyclophilin D is a good candidate protein to have a modulating function in this model. This autoregulatory scenario for ROS-induced mPTP opening in physiological conditions, however attractive it may seem, is not supported by recent findings with a new mitochondrially-targeted O2--sensitive form of yellow fluorescent protein (YFP) (90). In the latter study, mitochondrial ROS production was found to occur in stochastic bursts that were independent of [Ca2+]mit. Also the O2- bursts (flashes) were caused by mPTP opening, rather than facilitating it and they were inhibited by complex I inhibitor rotenone. At the moment, these findings are not easily reconciled with all of our existing knowledge on mitochondrial ROS generation. They are a timely reminder that more sophisticated tools for the study of mitochondria in situ are needed and that the results of such in situ studies may well change the current models of mitochondrial function.
Ca2+ Homeostasis and the ER
The ER is a Ca2+ store within the cell with a typical intraluminal Ca2+ ([Ca2+]ER) of about 0.5 mM. Ca2+ is pumped into the ER by SERCA (subtype 1-3). The IP3-Rs (subtype 1–3), activated by IP3) and RyRs (subtype 1–3, activated by Ca2+) are the main channels through which Ca2+ leaves the ER. In addition, a small amount of Ca2+ leaves the ER also in the absence of any ligand-activated Ca2+ efflux (as evidenced by the increase in [Ca2+]cyt after SERCA inhibition) through the so-called leak channel. Recently, the presenilin 1 and 2 proteins were found to have ER leak channel properties (88). However, other reports found that presenilin 1 is not a leak channel, but rather enhances the outflow of Ca2+ from the ER through IP3-R (16) and RyR (15). These ER Ca2+-regulating functions of the presenilin protein were reported to be independent of its function as protease in the γ-secretase complex.
Stromal interaction molecules 1 and 2 (Stim1 and 2) have been identified recently as ER calcium sensors (101) that, by binding to the plasma membrane channels Orai (subtypes 1–3), induce storage-operated Ca2+ entry (SOCE) (24, 74, 98); these proteins mediate the signal of low [Ca2+]ER to Orai at the plasma membrane and induce the opening of Orai and thus induce Ca2+ influx from the plasma membrane. The name Orai was inspired by Greek mythology (24), where Orai are the keepers of heaven's gate. Synonyms are Ca2+ release-activated Ca2+ channel or Ca2+ release-activated Ca2+ modulator (CRACC or CRACM, respectively). Although the latter names better describe the protein function, it appears that the name Orai has been adopted by the majority of researchers and therefore will be used in this review.
Although the number of proteins involved in Ca2+ homeostasis in the ER is still relatively manageable, their elaborate kinetics and regulatory mechanisms make the regulation of Ca2+ homeostasis in the ER very complex. Nevertheless, there are two main aspects to ER Ca2+ homeostasis, namely, “calcium-induced calcium release” (CICR) and “storage-operated calcium entry” (SOCE). CICR is seen where a comparatively small increase in [Ca2+]cyt induces the opening of RyRs (RyR1 can also be activated by depolarizing membrane potential in skeletal muscle cells and possibly also in neurons), which will then release more Ca2+ from the ER. If the increase in [Ca2+]ER is accompanied by an increase in IP3 (typically via activation of phospholipase C) then IP3-Rs will also release Ca2+ from the ER (Fig. 3). SOCE describes a situation where the decrease in [Ca2+]ER (typically caused by opening of RyR or IP3-R) is sensed by Stim1, which will then bind and facilitate the opening of Orai at the plasma membrane, resulting in a further increase in [Ca2+]cyt. It is important to note that Stim is a true [Ca2+]ER sensor because the stimulus for SOCE is a decrease in [Ca2+]ER rather than an increase in [Ca2+]cyt. However, among the transient receptor potential channels (TRPCs), some are sensitive to [Ca2+]cyt and so can mediate increased Ca2+ influx from the plasma membrane in response to increased [Ca2+]cyt. But only if a reduction of [Ca2+]ER initiates the Ca2+ influx is it called SOCE. Additionally, interactions between TRPCs and Stim1 have also been described (13, 99). It is apparent that all these mechanisms amplify a cytosolic Ca2+ signal, but there are also multiple feedback mechanisms that exist to prevent Ca2+ overload. Indeed, the different RyR, IP3-R and SERCA isoforms have different kinetics and are differently regulated by [Ca2+]cyt and [Ca2+]ER. For example: IP3-R1 is activated by 300–400 nM [Ca2+]cyt, but is inactivated by higher or lower concentrations; IP3-R2 and 3 are not inactivated by higher [Ca2+]cyt concentrations; nanomolar concentrations of ryanodine typically activate RyRs and micromolar concentrations block RyR opening; and SERCAs are activated by low [Ca2+]ER and the different SERCAs have different kinetics. Accordingly, the precise RyR, IP3-R and SERCA isoform composition, and the subcellular localization of the ER, are important in shaping the magnitude and kinetics of a cell's CICR and SOCE responses.
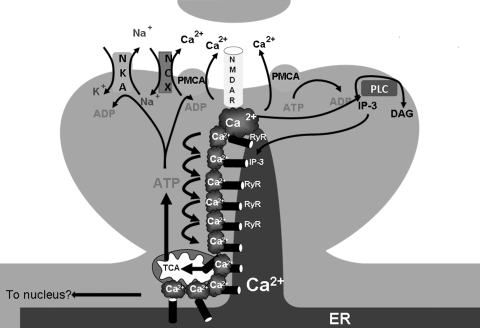
FIG. 3.
Ca2+-induced Ca2+ release (CICR). A postsynaptic spine is depicted. In CICR, Ca2+ enters the cytosol through channels in the plasma membrane. The increased [Ca2+]i will cause the opening of nearby ryanodine receptors (RyR) in the ER membrane. Additionally, increased [Ca2+]i levels result in increased IP3 levels through phospholipase C (PLC) activation. Opening of RyRs and IP3 receptors causes “hotspots” of Ca2+]i that may cause further opening of ER Ca2+ channels and also promote mitochondrial Ca2+ uptake.
The characteristic kinetics and feedback mechanisms of RyR and IP3-R subtypes have been reviewed previously (89), and will not be detailed here. The ability to act as an amplifier for [Ca2+]cyt elevation makes the ER an organelle that is, at least in theory, capable of generating Ca2+ transients in the absence of significant plasma membrane depolarization. Such Ca2+ transients have been described experimentally in neurons (18), but generally in neurons, ER Ca2+ release follows the membrane potential, as Ca2+ transients without plasma membrane changes may dissociate a neuron's Ca2+ homeostasis from synaptic input. Because CICR and SOCE have the potential for reciprocal stimulation, it is theoretically possible that such Ca2+ transients migrate along the ER and they may even continue through discontinuous ER membranes all the way to the nuclear envelope, where they may increase nuclear Ca2+ concentration (89). Obviously this is of great importance in nonexcitable cells (e.g., immune cells), as in these cells Ca2+ influx through VGCC is limited. It may also be important in neurons, because nuclear rises in [Ca2+] (but not [Ca2+]cyt) are essential for the phosphorylation and activation of the cAMP response element binding protein (CREB) transcription factor. Phospho-CREB will increase transcription of the brain-derived neurotrophic factor (BDNF) gene, among other genes. The importance of sustained CREB activity is illustrated by the neurodegenerative phenotype of old heterozygous CREB knockout mice (61). However, it is unclear whether in neurons, in addition to depolarizations associated with axon potential generation, CICR also contributes to relevant increases in nuclear [Ca2+] through Ca2+ transients in the absence of such depolarizations. Importantly, translational responses in a neuron can be restricted to a synaptic region. Therefore, in neurons a more obvious role for CICR lies in fine tuning the Ca2+ response to synaptic input (33, 89). This also includes the fine tuning of nuclear envelope Ca2+ release after full depolarization (33). The precise role of SOCE remains somewhat unclear (see Ref. 11 for a discussion on this latter issue). It has been shown that CICR modulates [Ca2+]cyt after potassium chloride-induced depolarization. Specifically, ER Ca2+ release accelerates the rise and slightly increases the amplitude in [Ca2+]cyt and in turn ER Ca2+ uptake accelerates the decline in [Ca2+]cyt. Such a kinetic profile with steep rise and decline contributes to a Ca2+ response that has all-or-nothing characteristics and thus will help to improve the signal to noise ratio in Ca2+ signaling.
At the synapse, another mechanism exists that shapes the Ca2+ signaling response in an all-or-nothing fashion; it is mediated by calpacitin family proteins, which includes neurogranin, growth associated protein 43, and pep 19 (also known as pcp4) (27, 28). These proteins scavenge calmodulin and prevent calmodulin from binding and activating other proteins such as Ca2+/calmodulin-dependent kinase. Calpacitins are Ca2+-binding proteins, but with a high Kd. Thus, only when [Ca2+]cyt reaches high (micromolar) concentrations will these proteins bind Ca2+ and through a conformational change release calmodulin to activate Ca2+/calmodulin dependent kinase and other binding partners. This sudden and massive release of calmodulin upon reaching a critical [Ca2+]cyt led to the name calpacitin, in analogy to capacitors in electricity. Obviously, if CICR modulates [Ca2+]cyt rises to have steep rise and decline kinetics it will further contribute to a clearly defined Ca2+ signal that is limited in duration and distribution within the neuron, thus enhancing the calpacitin-mediated Ca2+ response so that the neuron can appropriately translate synaptic activity to the biochemical apparatus. If aging or pathological processes lead to an alteration of CICR it will impact activity-dependent Ca2+ signaling in a neurons and thus change the adaptive response of the neuron to its activity status with potentially harmful consequences.
Another important aspect of ER Ca2+ release is that it helps to create micro-domains of high [Ca2+]cyt near RyRs and IP3-Rs. These micro-domains have been shown to be important for mitochondrial Ca2+ uptake, because the close opposition of ER calcium release sources and mitochondrial Ca2+ uniporter ensure that the uniporter becomes activated through high local [Ca2+]cyt that exceeds the average [Ca2+]cyt (77). The high uptake of Ca2+ into the mitochondrial matrix will induce increased production of ATP and NADH and thus ensure sufficient levels reducing equivalents and high energy phosphates. For the physical connection between ER and mitochondria (tethering), the protein mitofusin 2 that is expressed on both organelles is required (62) It is conceivable that in neurons CICR from the ER helps to transmit the synaptic input signal to mitochondria that may reside somewhat more distant from a synaptic spine and thus in the absence of CICR may not be exposed to the high micro-domain [Ca2+]cyt that is required for increased ATP generation (Fig. 3). If this is true, then ER trafficking is important in order to make the appropriate connections between ER, synaptic spine, and mitochondria. Similarly, mitochondrial motility is important. Mitochondrial motility has been shown to be controlled by the GTPases Miro 1 and 2, the adaptor protein Milton, and their interaction with kinesin in a Ca2+-dependent manner (81, 91). Notably, the molecular determinants of ER trafficking are not very well understood. ER membranes have been shown to reside in synaptic spines and upon NMDA receptor activation undergo rapid fission and fusion events (52). Also trafficking of ER patches in synaptic spines has been shown to be dependent on PKC, metalloproteinases, and gamma secretase activity (67). Taken together, these studies suggest that neuronal activity and endoproteolytic cleavage of ER resident transmembrane proteins by a sheddase (e.g., α-secretase) and γ-secretase are important in determining the subcellular localization of ER membranes (Fig. 4). The relevant substrates for α- and γ-secretase in these paradigms have not been established and it is of interest to see if either established (e.g., APP, ErbB, neuregulin) or yet undiscovered substrates (STIM1 or 2?) are involved. Thus, presenilins, apart from being implicated as ER leak channel proteins and modulators of IP3-R gating, via their protease activity in the γ-secretase complex, also regulate ER trafficking, thus being major players in ER Ca2+ homeostasis regulation in addition to their well-established role in Alzheimer's disease (AD). Obviously, this suggests a connection between AD and ER Ca2+ homeostasis.
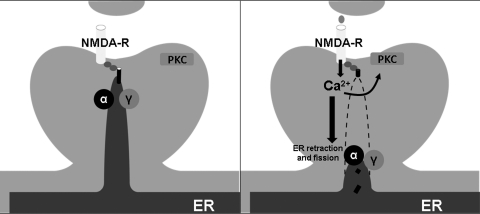
FIG. 4.
ER trafficking and Y-secretase. The ER can extend into synaptic spines. Upon activation of NMDA receptors the ER will retract and undergo fission events. Blockade of α- or Y-secretase prevents NMDA-induced retraction of ER membranes from synaptic spines. This suggests that certain, yet unidentified, single transmembrane-domain ER proteins that normally anchor the ER in the synaptic spine are substrates for α- and Y-secretase.
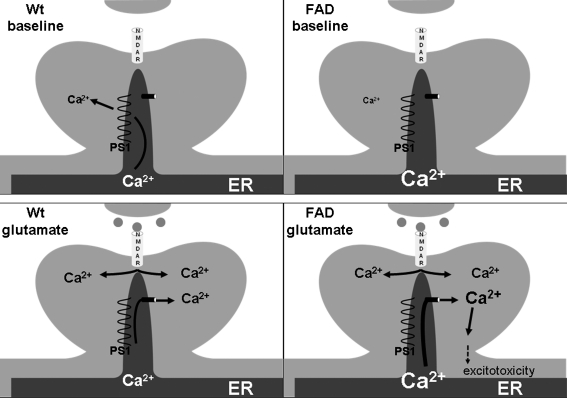
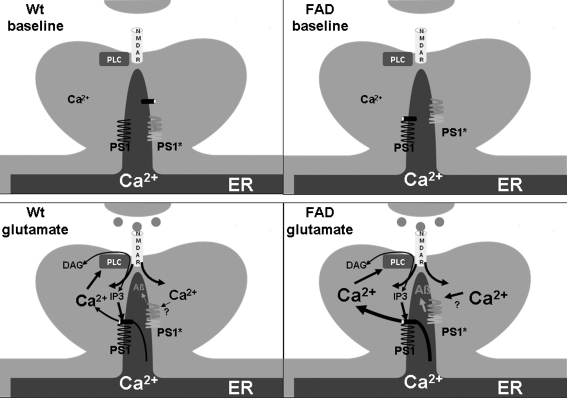
The uncleaved presenilin 1 and 2 holoproteins, when recombinantly expressed and added to artificial lipid bilayer membranes, were found to have Ca2+ channel properties (88). Importantly, this property was lost in PS1 with mutations linked to AD. From these studies, PS 1 and 2 were implicated as the ER leak channels and the AD-linked mutations were found to increase [Ca2+]ER and CICR from ER. The latter aspect was thought to contribute to neuronal vulnerability in excitotoxicity in AD, a finding that had been observed much earlier in neuronal cell lines expressing mutated PS1 (34, 63). This study implicates that in absence of the ER leak channel the remaining SERCA activity (which is negatively correlated with [Ca2+]ER) will continue to pump Ca2+ into the ER. Upon opening of RyR or IP3-R, the increased driving force will then result in increased [Ca2+]cyt (Fig. 5). In a second publication (16), ER membranes from nuclear envelopes of PS1 infected cells were tested electrophysiologically. Here PS1 was not found to have leak channels properties, but to modulate IP3-R gating. Specifically, wild-type PS1 was found to slightly increase the duration of the IP3-R open status, but not to increase the frequency of opening, whereas AD PS1 mutations increased both the channel open probability and the duration of the open time, resulting in IP3-R being “locked” in the open conformation (Fig. 6). Accordingly, IP3-mediated increases in [Ca2+]cyt were amplified in cells expressing mutant PS1, in AD patient fibroblasts (29), and in neurons in a mouse model that expresses mutant presenilin 1 (87). In both publications, the effect on ER Ca2+ homeostasis was mediated by the uncleaved PS1 holoprotein and thus independent of γ-secretase activity (which requires PS1 endoproteolytic cleavage). However, in the second publication mutant PS1-mediated enhancement of IP3-R gating resulted in increased γ-secretase activity and increased β-amyloid (Aβ) production (Fig. 6). This raises the possibility that, apart from directly affecting the proteolytic activity of γ-secretase (5), AD PS1 mutations may also act indirectly through enhanced ER Ca2+ release.
FIG. 5.
Presenilin as an ER leak channel. In this model, uncleaved presenilin 1 (PS1) acts as a Ca2+ leak channel in the ER membrane. PS1 mutations causing familial Alzheimer's disease (FAD) result in a loss of function of the ER leak channel properties of PS1. This results in increased [Ca2+]ER and increased CICR. Increased CICR can lead to [Ca2+]i levels that promote excitotoxicity and neuronal cell death.
FIG. 6.
Presenilin as an IP3R modulator. In this model, Presenilin 1 (PS1) modulates the gating of the IP3R in the ER. Wild-type PS1 will slightly prolong the time that the IP3R is in the open confirmation in situation of CICR. PS1 mutations causing familial Alzheimer's disease (FAD) cause PS1 to “lock” the IP3R in the open conformation and also increase the flux of Ca2+ through the IP3R, resulting in increased [Ca2+]i in a situation of CICR. Depolarization and CICR also lead to increased activity of auto cleaved PS1 in the γ-secretase complex.
It is unclear if IP3-R-mediated ER Ca2+ release enhances endoproteolytic cleavage of PS1 or enhances activity of pre-existing γ-secretase complexes. While the literature so far is unclear about the mechanism, it unambiguously concludes that mutant PS1 causes increased CICR responses. Apart from contributing to direct cell death through excitotoxicity, such an alteration may affect synaptic plasticity in more subtle ways. For example, it was found that in mutant PS1 knock-in mice LTP was not impaired, but the effect of cholinergic transmission was reversed (i.e., instead of enhancing LTP, cholinesterase inhibitors caused LTP reduction in these mice). It was found that NMDA receptor currents in these mice were reduced but could be restored to normal levels by intraneuronal Ca2+ chelation (92). These results suggest that increased ER Ca2+ release in PS1 knock-in mice results in (a compensatory) inactivation/desensitization of NMDA receptors. Similar results were found in neurons of so-called triple transgenic mice (that in addition to the PS1 mutant knock-in express APP and tau protein mutations) (92). Interestingly, Aβ also has been found to inhibit glutamatergic synaptic transmission (through NMDA receptor stimulation and possibly desensitization). Thus, PS1 mutations may cause a reduction of NMDA receptor synaptic transmission by two different mechanisms that may contribute to the cognitive deficits seen in patients with such mutations.
If CICR in patients with PS1 mutations contributes to Aβ production and/or cell death, then apart from Aβ reduction, inhibition of CICR may be a therapeutic option in such cases. Importantly, in humans PS1 mutations are strongly associated with intractable epileptic seizures (much more so than sporadic AD), indicating that these mutations have a profound effect on overall network excitability. While the connection between ER Ca2+ release and AD is obvious in familial cases with PS1 mutations, it is less clear in sporadic AD. The data from PS1 knock-in mice suggest a connection between ER Ca2+ release and strength of NMDA currents. If this connection is reciprocal, then it is conceivable that a decline in NMDA-mediated currents during aging will result in a compensatory increase in ER-mediated CICR, which could then cause increased Aβ production through γ-secretase, resulting in further NMDA current reduction by Aβ.
The dual function of presenilin in ER Ca2+ homeostasis and its established role in APP processing reveals a link that connects the Ca2+ theory of aging with the Aβ theory of AD. Given the connection between ER and mitochondria and the fact that Ca2+ cycling through the ER is dependent on ATP, it is also apparent how ROS-mediated mitochondrial alterations may impact ER Ca2+ homeostasis. Again, it should be emphasized that while mitochondrial dysfunction, ROS stress, and impaired Ca2+ homeostasis feed each other and ultimately will lead to neuronal cell death, in early stages of aging or cognitive impairment, neuronal cell death is unlikely to play a substantive role. On the other hand, it is in the early stages of aging and neurodegenerative disorders that the therapeutic potential is highest. In these early stages the effect of ROS and mitochondrial dysfunction is likely to result in compensatory changes of neuronal transmission that adjust for reduced ATP generation and Ca2+ buffering capacity. A precise understanding of these compensatory adjustments is likely to provide us insight into altered synaptic processing and network activity. This may lead to the discovery of new strategies to prevent cognitive decline during aging and in AD.
Abbreviations Used
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ADP
adenosine-diphosphate
- AMP
adenosine-monophosphate
- APP
amyloid beta precursor protein
- ATP
adenosine-triphosphate
- BDNF
brain-derived neurotrophic factor
- Ca2+
calcium
- [Ca2+]cy
cytosolic calcium concentration
- [Ca2+]mit
mitochondrial calcium concentration
- cAMP
cyclic AMP
- CICR
calcium-induced calcium release
- CREB
cAMP response element binding protein
- ER
endoplasmic reticulum
- ETC
electron transport chain
- IP3-R
inositol-triphosphate receptor
- K+
potassium
- mPTP
mitochondrial permeability transition pore
- Na+
sodium
- NAD+/NADH
nicotinamide adenine dinucleotide (oxidized/reduced form)
- NADP+/NADPH
nicotinamide adenine dinucleotide phosphate (oxidized/reduced)
- NCX
sodium calcium exchanger
- NMDA
N-methyl-D-aspartate
- O2
oxygen
- PMCA
plasma membrane calcium ATPase
- PS1
presenilin 1
- ROOR
peroxide, not further specified
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmic and endoplasmic reticulum ATPase
- SOCE
storage operated calcium entry
- SOD
superoxide dismutase
- Stim1/2
stromal interaction molecule 1/2
- TCA
tricarboxylic acid cylce
- VGCC
voltage gated calcium channels
- Vmax
maximal enzymatic reaction velocity
- YFP
yellow fluorescent protein
Acknowledgments
This research was entirely supported by the Intramural Research Program NIH, National Institute on Aging. We thank K.C. Alexander for help with the figures.
References
- 1.Altschuld RA. Hohl CM. Castillo LC. Garleb AA. Starling RC. Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. Am J Physiol. 1992;262:H1699–704. doi: 10.1152/ajpheart.1992.262.6.H1699. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP. Kaiser RA. Sheiko T. Craigen WJ. Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines CP. Kaiser RA. Purcell NH. Blair NS. Osinska H. Hambleton MA. Brunskill EW. Sayen MR. Gottlieb RA. Dorn GW. Robbins J. Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 4.Basso E. Fante L. Fowlkes J. Petronilli V. Forte MA. Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 5.Berezovska O. Lleo A. Herl LD. Frosch MP. Stern EA. Bacskai BJ. Hyman BT. Familial Alzheimer's disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J Neurosci. 2005;25:3009–3017. doi: 10.1523/JNEUROSCI.0364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernardi P. Mitochondrial transport of cations: Channels, exchangers and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi P. Petronilli V. The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomemb. 1996;28:131–138. doi: 10.1007/BF02110643. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi P. Rasola A. Calcium and cell death: The mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 9.Bindoli A. Fukuto JM. Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolaños JP. Almeida A. The pentose-phosphate pathway in neuronal survival against nitrosative stress. IUBMB Life. 2009;62:14–18. doi: 10.1002/iub.280. [DOI] [PubMed] [Google Scholar]
- 11.Bolotina VM. Orai, STIM1 and iPLA2beta: A view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull C. Niederhoffer EC. Yoshida T. Fee JA. Kinetic studies of superoxide dismutases properties of the manganese-containing protein from thermus-thermophilus. J Am Chem Soc. 1991;113:4069–4076. [Google Scholar]
- 13.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SL. Liu D. Kyriazis GA. Bagsiyao P. Ouyang X. Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J Biol Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- 15.Chan SL. Mayne M. Holden CP. Geiger JD. Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 16.Cheung KH. Shineman D. Müller M. Cárdenas C. Mei L. Yang J. Tomita T. Iwatsubo T. Lee VM. Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chih CP. Lipton P. Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- 18.Collin T. Franconville R. Ehrlich BE. Llano I. Activation of metabotropic glutamate receptors induces periodic burst firing and concomitant cytosolic Ca2+ oscillations in cerebellar interneurons. J Neurosci. 2009;29:9281–9291. doi: 10.1523/JNEUROSCI.1865-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csordas G. Hajnoczky G. SR/ER-mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das AM. Harris DA. Control of mitochondrial ATP synthase in heart cells: Inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res. 1990;24:411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 21.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Dirksen MT. Laarman GJ. Simoons ML. Duncker DJ. Reperfusion injury in humans: A review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc Res. 2007;74:343–355. doi: 10.1016/j.cardiores.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer I. Early involvement of the cerebral cortex in Parkinson's disease: Convergence of multiple metabolic defects. Prog Neurobiol. 2009;88:89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Feske S. Gwack Y. Prakriya M. Srikanth S. Puppel SH. Tanasa B. Hogan PG. Lewis RS. Daly M. Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 25.Fielden EM. Roberts PB. Bray RC. Lowe DJ. Mautner GN. Rotilio G. Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974;139:49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster KA. Margraf RR. Turner DA. NADH hyperoxidation correlates with enhanced susceptibility of aged rats to hypoxia. Neurobiol Aging. 2008;29:598–613. doi: 10.1016/j.neurobiolaging.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerendasy D. Homeostatic tuning of Ca2+ signal transduction by members of the calpacitin protein family. J Neurosci Res. 1999;58:107–119. [PubMed] [Google Scholar]
- 28.Gerendasy DD. Sutcliffe JG. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Mol Neurobiol. 1997;15:131–163. doi: 10.1007/BF02740632. [DOI] [PubMed] [Google Scholar]
- 29.Gibson G. Martins R. Blass J. Gandy S. Altered oxidation and signal transduction systems in fibroblasts from Alzheimer patients. Life Sci. 1996;59:477–489. doi: 10.1016/0024-3205(96)00327-x. [DOI] [PubMed] [Google Scholar]
- 30.Gleichmann M. Collis LP. Smith PJ. Mattson MP. Simultaneous single neuron recording of O2 consumption, [Ca2+]i and mitochondrial membrane potential in glutamate toxicity. J Neurochem. 2009;109:644–655. doi: 10.1111/j.1471-4159.2009.05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleichmann M. Mattson MP. Alzheimer's disease and neuronal network activity. Neuromolecular Med. 2010;12:44–47. doi: 10.1007/s12017-009-8100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldhaber JI. Qayyum MS. Oxygen free radicals and excitation-contraction coupling. Antioxid Redox Signal. 2000;2:55–64. doi: 10.1089/ars.2000.2.1-55. [DOI] [PubMed] [Google Scholar]
- 33.Gruol DL. Netzeband JG. Nelson TE. Somatic Ca2+ signaling in cerebellar Purkinje neurons. J Neurosci Res. 2010;88:275–289. doi: 10.1002/jnr.22204. [DOI] [PubMed] [Google Scholar]
- 34.Guo Q. Sopher BL. Furukawa K. Pham DG. Robinson N. Martin GM. Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajnóczky G. Robb–Gaspers LD. Seitz MB. Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 36.Hausenloy D. Wynne A. Duchen M. Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 37.Häusser M. The Hodgkin–Huxley theory of the action potential. Nat Neurosci. 2000;3(Suppl):1165. doi: 10.1038/81426. [DOI] [PubMed] [Google Scholar]
- 38.Hayat LH. Crompton M. Evidence for the existence of regulatory sites for Ca2+ on the Na+/Ca2+ carrier of cardiac mitochondria. Biochem J. 1982;202:509–518. doi: 10.1042/bj2020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hidalgo C. Donoso P. Crosstalk between calcium and redox signaling: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 40.Hoek JB. Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J. 1988;254:1–10. doi: 10.1042/bj2540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hüser J. Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343:311–317. [PMC free article] [PubMed] [Google Scholar]
- 42.Igbavboa U. Pfeiffer DR. EGTA inhibits reverse uniport-dependent Ca2+ release from uncoupled mitochondria. Possible regulation of the Ca2+ uniporter by a Ca2+ binding site on the cytoplasmic side of the inner membrane. J Biol Chem. 1988;263:1405–1412. [PubMed] [Google Scholar]
- 43.Johnson–Cadwell LI. Jekabsons MB. Wang A. Polster BM. Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 44.Juhaszova M. Zorov DB. Yaniv Y. Nuss HB. Wang S. Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamsler A. Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 46.Kapùs A. Szászi K. Káldi K. Ligeti E. Fonyó A. Is the mitochondrial Ca2+ uniporter a voltage-modulated transport pathway? FEBS Lett. 1991;282:61–64. doi: 10.1016/0014-5793(91)80444-8. [DOI] [PubMed] [Google Scholar]
- 47.Khachaturian ZS. The role of calcium regulation in brain aging: Reexamination of a hypothesis. Aging (Milano) 1989;1:17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 48.Kim B. Matsuoka S. Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+-Ca2+ exchange. J Physiol. 2008;586:1683–1697. doi: 10.1113/jphysiol.2007.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kogure K. Busto R. Schwartzman RJ. Scheinberg P. The dissociation of cerebral blood flow, metabolism, and function in the early stages of developing cerebral infarction. Ann Neurol. 1980;8:278–290. doi: 10.1002/ana.410080310. [DOI] [PubMed] [Google Scholar]
- 50.Korde AS. Sullivan PG. Maragos WF. The uncoupling agent 2,4-dinitrophenol improves mitochondrial homeostasis following striatal quinolinic acid injections. J Neurotrauma. 2005;22:1142–1149. doi: 10.1089/neu.2005.22.1142. [DOI] [PubMed] [Google Scholar]
- 51.Kröner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys. 1986;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- 52.Kucharz K. Krogh M. Ng AN. Toresson H. NMDA receptor stimulation induces reversible fission of the neuronal endoplasmic reticulum. PLoS One. 2009;4:e5250. doi: 10.1371/journal.pone.0005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ledenev AN. Konstantinov AA. Popova E. Ruuge EK. A simple assay of the superoxide generation rate with Tiron as an EPR-visible radical scavenger. Biochem Int. 1986;12:785–793. [PubMed] [Google Scholar]
- 54.Lemasters JJ. Theruvath TP. Zhong Z. Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lieberman DN. Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 56.Litsky ML. Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: The uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 57.Liu D. Chan SL. de Souza–Pinto NC. Slevin JR. Wersto RP. Zhan M. Mustafa K. de Cabo R. Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 58.Ludwig ML. Metzger AL. Pattridge KA. Stallings WC. Manganese superoxide dismutase from thermus thermophilus. A structural model and refined at 1.8 A resolution. J Mol Biol. 1991;219:335–358. doi: 10.1016/0022-2836(91)90569-r. [DOI] [PubMed] [Google Scholar]
- 59.Maalouf M. Rho JM. Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maher P. Redox control of neural function: Background, mechanisms, and significance. Antioxid Redox Signal. 2006;8:1941–1970. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- 61.Mantamadiotis T. Lemberger T. Bleckmann SC. Kern H. Kretz O. Martin Villalba A. Tronche F. Kellendonk C. Gau D. Kapfhammer J. Otto C. Schmid W. Schütz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- 62.Martins de Brito O. Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–609. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 63.Mattson MP. Guo Q. Furukawa K. Pedersen WA. Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. J Neurochem. 1998;70:1–14. doi: 10.1046/j.1471-4159.1998.70010001.x. [DOI] [PubMed] [Google Scholar]
- 64.Muller FL. Lustgarten MS. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa T. Shimizu S. Watanabe T. Yamaguchi O. Otsu K. Yamagata H. Inohara H. Kubo T. Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 66.Neely JR. Feuvray D. Metabolic products and myocardial ischemia. Am J Pathol. 1981;102:282–291. [PMC free article] [PubMed] [Google Scholar]
- 67.Ng AN. Toresson H. Gamma-secretase and metalloproteinase activity regulate the distribution of endoplasmic reticulum to hippocampal neuron dendritic spines. FASEB J. 2008;22:2832–2842. doi: 10.1096/fj.07-103903. [DOI] [PubMed] [Google Scholar]
- 68.Nichols BJ. Denton RM. Towards the molecular basis for the regulation of mitochondrial dehydrogenases by calcium ions. Mol Cell Biochem. 1995;149–150:203–212. doi: 10.1007/BF01076578. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann NY Acad Sci. 2008;1147:53–60. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 70.Oktyabrsky ON. Smirnova GV. Redox regulation of cellular functions. Biochemistry (Mosc) 2007;72:132–145. doi: 10.1134/s0006297907020022. [DOI] [PubMed] [Google Scholar]
- 71.Opie LH. The new trials: AIRE, ISIS-4, and GISSI-3. Is the dossier on ACE inhibitors and myocardial infarction now complete? Cardiovasc Drugs Ther. 1994;8:469–472. doi: 10.1007/BF00877924. [DOI] [PubMed] [Google Scholar]
- 72.Pearce KA. Boosalis MG. Yeager B. Update on vitamin supplements for the prevention of coronary disease and stroke. Am Fam Physician. 2000;62:1359–1366. [PubMed] [Google Scholar]
- 73.Pellerin L. Bouzier–Sore AK. Aubert A. Serres S. Merle M. Costalat R. Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 74.Prakriya M. Feske S. Gwack Y. Srikanth S. Rao A. Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 75.Praticò D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: Lights and shadows. Ann NY Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 76.Quick KL. Ali SS. Arch R. Xiong C. Wozniak D. Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Rizzuto R. Bernardi P. Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salmon AB. Richardson A. Pérez VI. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;25:176–201. [PMC free article] [PubMed] [Google Scholar]
- 80.Saotome M. Katoh H. Yaguchi Y. Tanaka T. Urushida T. Satoh H. Hayashi H. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2009;296:H1125–1132. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- 81.Saotome M. Safiulina D. Szabadkai G. Das S. Fransson A. Aspenstrom P. Rizzuto R. Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schinzel AC. Takeuchi O. Huang Z. Fisher JK. Zhou Z. Rubens J. Hetz C. Danial NN. Moskowitz MA. Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shuaib A. Lees KR. Lyden P. Grotta J. Davalos A. Davis SM. Diener HC. Ashwood T. Wasiewski WW. Emeribe U. SAINT II Trial Investigators. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 84.Simpson IA. Carruthers A. Vannucci SJ. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith CD. Carney JM. Starke–Reed PE. Oliver CN. Stadtman ER. Floyd RA. Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stowe DF. Camara AK. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stutzmann GE. Caccamo A. LaFerla FM. Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tu H. Nelson O. Bezprozvanny A. Wang Z. Lee SF. Hao YH. Serneels L. De Strooper B. Yu G. Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 90.Wang W. Fang H. Groom L. Cheng A. Zhang W. Liu J. Wang X. Li K. Han P. Zheng M. Yin J. Wang W. Mattson MP. Kao JP. Lakatta EG. Sheu SS. Ouyang K. Chen J. Dirksen RT. Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X. Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y. Greig NH. Yu QS. Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging. 2009;30:1061–1068. doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watson JB. Khorasani H. Persson A. Huang KP. Huang FL. O'Dell TJ. Age-related deficits in long-term potentiation are insensitive to hydrogen peroxide: coincidence with enhanced autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Neurosci Res. 2002;70:298–308. doi: 10.1002/jnr.10427. [DOI] [PubMed] [Google Scholar]
- 94.Willcox BJ. Curb JD. Rodriguez BL. Antioxidants in cardiovascular health and disease: Key lessons from epidemiologic studies. Am J Cardiol. 2008;101:75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 95.Williams AJ. Fry CH. Calcium–proton exchange in cardiac and liver mitochondria. FEBS Lett. 1979;97:288–292. doi: 10.1016/0014-5793(79)80104-0. [DOI] [PubMed] [Google Scholar]
- 96.Wingrove DE. Gunter TE. Kinetics of mitochondrial calcium transport. I. Characteristics of the sodium-independent calcium efflux mechanism of liver mitochondria. J Biol Chem. 1986;261:15159–15165. [PubMed] [Google Scholar]
- 97.Wingrove DE. Gunter TE. Kinetics of mitochondrial calcium transport. II. A kinetic description of the sodium-dependent calcium efflux mechanism of liver mitochondria and inhibition by ruthenium red and by tetraphenylphosphonium. J Biol Chem. 1986;261:15166–15171. [PubMed] [Google Scholar]
- 98.Yeromin AV. Zhang SL. Jiang W. Yu Y. Safrina O. Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan JP. Kim MS. Zeng W. Shin DM. Huang G. Worley PF. Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 2009;3:221–225. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 100.Yusuf S. Collins R. MacMahon S. Peto R. Effect of intravenous nitrates on mortality in acute myocardial infarction: An overview of the randomised trials. Lancet. 1988;1:1088–1092. doi: 10.1016/s0140-6736(88)91906-x. [DOI] [PubMed] [Google Scholar]
- 101.Zhang SL. Yu Y. Roos J. Kozak JA. Deerinck TJ. Ellisman MH. Stauderman KA. Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zorov DB. Juhaszova M. Sollott SJ. Mitochondrial ROS-induced ROS release: An update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]