Abstract
ATP and NO are released from the urothelium in the bladder. Detrusor Overactivity (DO) following spinal cord injury results in higher ATP and lower NO release from the bladder urothelium. Our aim was to study the relationship between ATP and NO release in 1) early diabetic bladders, an overactive bladder model; and 2) in “diuretic” bladders, an underactive bladder model. To induce diabetes mellitus female rats received 65 mg/kg streptozocin (i.v.). To induce chronic diuresis rats were fed with 5% sucrose. At 28 days, in vivo open cystometry was performed. Bladder wash was collected to analyze the amount of ATP and NO released into the bladder lumen. For in vitro analysis of ATP and NO release, a Ussing chamber was utilized and hypoosmotic Krebs was perfused on the urothelial side of the chamber. ATP was analyzed with luminometry or HPLC-fluorometry while NO was measured with a Sievers NO-analyzer. In vivo ATP release was increased in diabetic bladders and unchanged in diuretic bladders. In vitro release from the urothelium followed the same pattern. NO release was unchanged both in vitro and in vivo in overactive bladders whereas it was enhanced in underactive bladders. We found that the ratio of ATP/NO, representing sensory transmission in the bladder, was high in overactive and low in underactive bladder dysfunction. In summary, ATP release has a positive correlation while NO release has a negative correlation with the bladder contraction frequency. The urinary ATP/NO ratio may be a clinically relevant biomarker to characterize the extent of bladder dysfunction.
Keywords: Diabetes, urinary bladder, urothelium, ATP release, NO release
1. Introduction
Prior studies demonstrate that activation of bladder afferent pathways is accompanied by release of sensory neurotransmitters from the urothelium, such as ATP or nitric oxide (NO) (Birder, 2006; Birder et al., 2002). In animal models of spinal cord injury or chronic inflammation, urothelial ATP release is greatly increased over normal values (Khera et al., 2004; Smith et al., 2005). In isolated tissues ATP can facilitate its own release, a phenomenon that has been described as cascade release (Sperlagh and Vizi, 1991). ATP instilled into the bladder lumen in vivo increases the frequency of bladder contractions (Pandita and Andersson, 2002) indicating that ATP exerts a facilitatory action in the bladder afferent system via urothelial purinergic receptors.
On the other hand NO is considered an inhibitory transmitter in various areas of the afferent and efferent neuronal system. In the inner hair cells of the cochlea that have sensory functions, NO inhibits Ca2+ influx by a negative feedback mechanism using the NO-cGMP – PKG pathway (Shen et al. 2005). Similarly, NO donors instilled into the bladder lumen reduce bladder contraction frequency “in vivo”, while instillation of the NOS inhibitor, L-NAME increases bladder contraction frequency (Ozawa et al., 1999). On the efferent side NO inhibits the reuptake of norepinephrine and dopamine and as such inhibits reverse transport in the CNS (Kiss et al., 1999).
It has been demonstrated that nNOS is colocalized with NMDA receptors in the CNS and P2X receptors in the periphery (Poole et al, 2002; Shen et al 2005). Both receptors are ionotropic channel receptors and after activation, they provide the necessary Ca2+ for nNOS to synthesize NO. Consequently, in the cochlea inner hair cell purinergic receptor antagonists inhibit NO synthesis (Shen et al, 2005).
Recent results from our laboratory have demonstrated that neurogenic detrusor hyperactivity in rats with chronic spinal cord injury show increased release of ATP while NO release was reduced below normal values. These results suggest that deviation of these transmitters from their normal release levels may contribute to an overly activated afferent system with resulting detrusor overactivity. Reducing bladder overactivity with BoNT-A treatment, decreased ATP release while NO release was increased to a level close to their normal values (Khera et al., 2005; Smith et al., 2008).
In this study we wanted to address two important questions: 1) whether bladder overactivity during experimental diabetes mellitus (Daneshgari et al., 2006a) increases evoked ATP release and decreases NO release in a manner similar to responses we observed in spinal cord injured animals, and 2) whether bladder underactivity caused by increased diuresis in sucrose-fed rats (Liu and Daneshgari, 2006) would result in reduced ATP and increased NO release.
2. Experimental procedures
2.1 Animal preparation and treatments
Animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Acute diabetes was induced with a single intravenous injection of streptozotocin (STZ) prepared in 100 mM citrate buffer in female Sprague-Dawley rats (250-300 g). STZ (65 mg/kg) was injected into the lateral tail vein followed by a subcutaneous injection of glucose (1 g/kg). To mimic a diuretic condition some rats were fed ad libitum with 5% sucrose dissolved in the drinking water (Liu and Daneshgari, 2006). In all animals blood glucose was monitored with a One Touch Ultra Glucometer (LifeScan, Inc., Milpitas, CA). The experiments were carried out at 1, 2 or 4 weeks after the induction of experimental diabetes. As no significant changes were observed in bladder function after one or two weeks of treatment, we used four-week animals in these experiments together with age-matched saline- injected and sucrose-fed rats.
2.2 Cystometric studies and measurements of transmitters in the bladder lumen
To evaluate urinary bladder function a suprapubic catheter was implanted in the bladder dome of urethane-anesthetized control, diabetic or sucrose-fed animals. Saline solution was infused into the bladder at a rate of 0.125 ml/min. Intravesical pressure was recorded by a pressure transducer (WPI, Sarasota. FL). To distinguish between the voiding and non-voiding contractions the voided volume of saline was collected in a cup placed on the top of a force transducer (WPI, SarasotaFL). For analyzing the ATP and NO content in the bladder wash an eppendorf tube was placed under the meatus at the beginning of the voiding contraction and the excreted saline was collected until its volume reached at least 1 mL.
2.3 ATP and NO release from bladder urothelium
To evaluate the release of transmitters from the urothelial layer, normal, diabetic and sucrose-fed bladders were mounted in a Ussing chamber (WPI, Sarasota. FL) and hypoosmotic stimulation was performed as previously reported (Smith et al., 2005). Briefly, both hemi-chambers were perfused with iso-osmotic Krebs solution. After 30 min equilibration one min effluents were taken from the urothelial side of the bladder for 16 min. Stimulation with hypoosmotic solution (Krebs with 67.8 mM NaCl) was started from the 3rd min of perfusion and lasted for 3 min. Samples were stored at -80° C until ATP and NO assay were performed. Release of ATP and NO was expressed as percent of the initial baseline values.
2.4 ATP and NO assays
The amount of ATP released in vivo into the bladder lumen was measured with a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA) using a luciferine-luciferase assay kit. Briefly, 50 μL aliquots of the collected samples were injected into the system and the luminescence values were compared to those of standard ATP solutions. Standard curves for ATP were constructed at the beginning of every series of measurements. The evaluation of the samples' ATP content in the Ussing chamber experiments was performed using high pressure liquid chromatography (HPLC) connected to a fluorometric detector (Dynamex; Rainin, Woburn. MA) after precolumn derivatization with chloroacetyl aldehyde; 500 μL aliquots of the effluents obtained from the perfused Ussing chamber were derivatized with 10 μL 1M chloro-acetylaldehyde as previously described (Mihaylova-Todorova et al., 2001) and a 200 μL aliquot of the processed samples was injected into a semi preparative column (Nova-PakPhenyl 8×100mm Radial Pak; Waters, Milford, MA). The purine nucleotides (ATP, ADP, AMP and adenosine) were eluted from the column using a gradient system with 100 μM phosphate buffer as solvent A and 25% methanol phosphate buffer as solvent B using a pumping rate of 2 mL/min. The gradient was changed from 0% to 100% in a linear fashion from 4 to 16 min of the run. The total purine content of the samples was assayed with a Dynamex fluorometric detector (Rainin, Woburn, MA) at 230μm excitation and 410 um emission wavelength. The total purine release was calculated as the sum of ATP, ADP, AMP and adenosine. The hypoosmotic stimulation-evoked purine release was expressed as percent of the basal release measured at the beginning of the collection period. NO production was determined with a nitric oxide analyzer (NOA 280i; Sievers Instruments, Inc. Boulder, CO) using heated vanadium chloride as the reducing agent. At the beginning of every assay a nitrate standard curve was constructed, then 20 μL effluents were injected into the reaction chamber and the peak area was compared to that of the standard curve.
2.5 Data analysis
The corresponding release values of ATP and NO in the in vivo experiments were normalized as pmol/voiding contraction, while in the Ussing chamber experiments the release was expressed as percent of the baseline release. For calculation of the ATP/NO ratios the ATP concentrations were divided by the corresponding NO values for the particular experiment and the ratio were multiplied by 1000. For statistical analysis we used one way-ANOVA followed by a Newman-Keuls multiple comparison posttest. Data are presented as mean ± S.E.M.; P values <0.05 were considered statistically significant. The correlation between in vivo ATP/NO ratio was analyzed by the non-parametric Spearman test. Prism (GraphPad, San Diego, CA) was used for all the statistical analyses and graphics.
2.6 Drugs used
Streptozotocin, sucrose, vanadium chloride, the luciferin-luciferase assay kit, and chloro-acetyl-aldehyde as well as all constituents of Krebs solution and HPLC buffers were purchased from Sigma (St Louis, MI).
3. Results
3.1 Changes in blood glucose, body weight and urinary bladder weight after streptozotocin or sucrose feeding
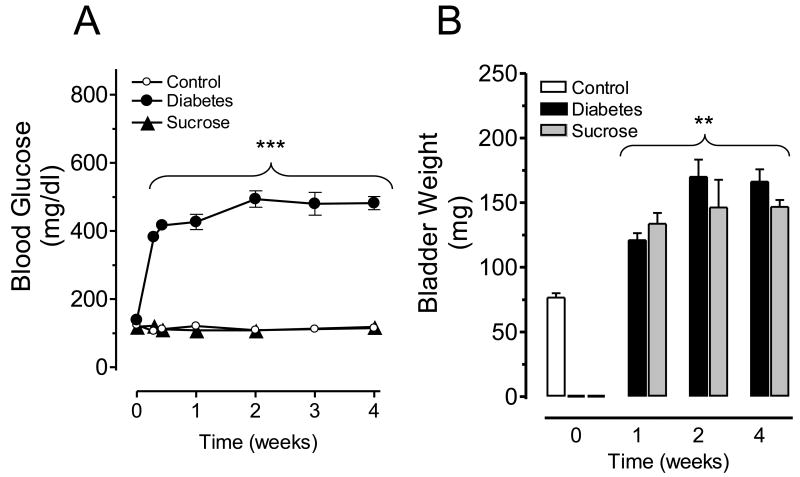
Streptozotocin treatment induced high blood glucose levels as soon as 24 hours post-injection and a hyperglycemic state was maintained over the entire evaluation period. Four weeks after treatment the mean+/-S.E.M. body weights were 280.0+/-7.8 in control, 246.0+/-8.2 in diabetic and 275.7+/-4.8 g in sucrose-fed rats (P<0.05 for diabetic vs. control/sucrose groups; N=6 animals per group). The corresponding blood glucose values were 114.8+/-3.8, 481.9 +/- 19.2 and 118.0+/-8.5 mg/dl for control, diabetic and sucrose fed rats, respectively (Figure 1A). In agreementwith findings by other investigators (Daneshgari et al., 2006b; Pitre et al., 2002), animal body weight decreased in diabetic rats (see above), whereas the bladder weight was significantly increased to 166.2+/-9.5 mg in diabetic and 146.8+/-5.2 mg in sucrose fed animals as compared to controls: 87.9+/-6.1 mg (Figure 1B).
Figure 1.
Blood glucose levels (A) in control, diabetic or sucrose-fed rats during diabetes progression. Changes in urinary bladder weight (B) in control, diabetic or sucrose-fed rats during the early stages of diabetes. ***P<0.001, **P<0.01 vs. control group. Data represent means +/- S.E.M. from control (n=8), diabetic (n=10) and sucrose-fed (n=10) rats.
3.2 Cystometric Studies and ATP and NO release from bladder urothelium in vivo
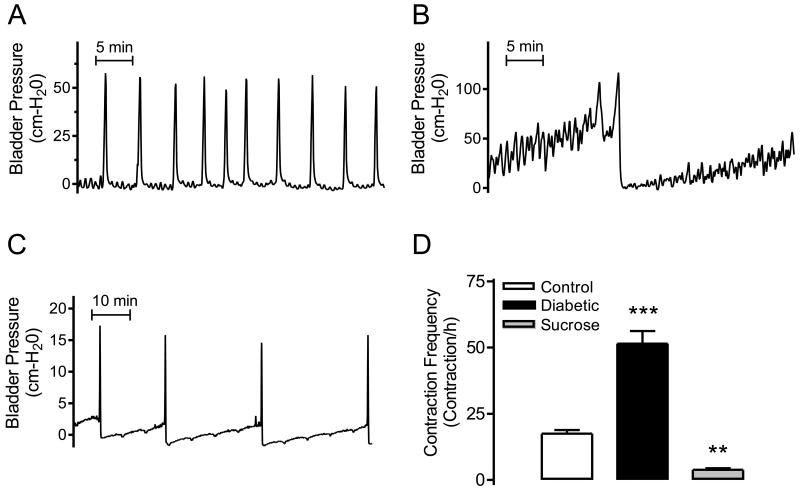
To evaluate the in vivo release rate of ATP and NO from the urothelial side of the bladder, we performed open cystometry and collected the voided saline solution (bladder wash) at the time of a voiding contraction. The cystometric studies indicated that control rats have a rhythmic and well-timed frequency of voiding contractions (Figure 2A); whereas, in diabetic animals the frequency of voiding and non-voiding bladder contractions were significantly increased (Figure 2B and D). In contrast, the bladder contraction frequency was significantly reduced in sucrose-fed animals (Figure 2C and D).
Figure 2.
Representative cystometrograms are shown for control (A), diabetic (B) and sucrose-fed rats (C) together with contraction frequency analysis (D). ***P<0.001 and **P<0.01 for diabetic/sucrose vs. control group.
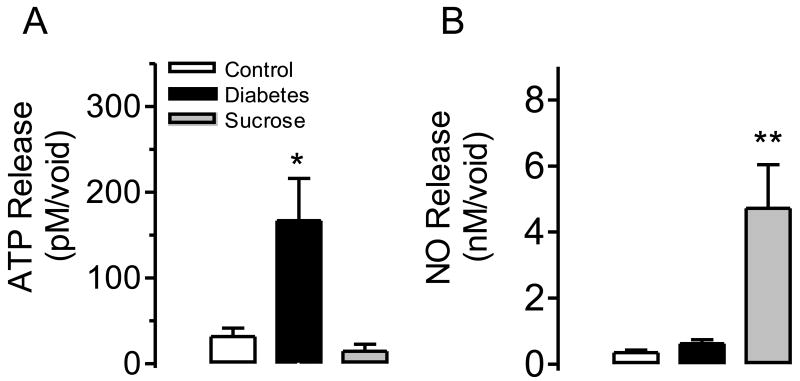
The released amount of ATP in the bladder wash from diabetic rats was much higher than in control animals while ATP release did not change significantly in sucrose-fed rats (Figure 3A). On the other hand, the release of NO in diabetic animals did not change, while it was significantly enhanced in sucrose- fed rats (Figure 3B).
Figure 3.
In vivo ATP release (A) and NO production (B) in the bladder wash from control (n=5), diabetic (n=5), or sucrose-fed rats (n=5) at the time of a voiding contraction. In panel (A) *P<0.05 for control vs. diabetic rats. In panel (B) **P<0.01 for control vs. sucrose fed rats.
3.3 Urothelial ATP and NO release by hypoosmotic stimulation
To evaluate the contribution of the urothelium to the release of ATP and NO “in vitro” we mounted the bladders in a Ussing chamber and applied hypoosmotic stimulation on the urothelial side of the chamber. Hypoosmotic stimulation mimics the activation of mechano-receptors and evokes release of ATP (Birder et al., 2002; Khera et al., 2004) as well as NO (Smith et al., 2008) from the urothelium. In the urothelium of control bladders, hypoosmotic stimulation enhanced both ATP and NO release (Figure 4 A and B). However the ATP release was significantly higher in diabetic rats as compared to controls but remained unchanged in sucrose fed rats (Figure 4A). On the other hand, NO production was significantly higher in sucrose fed but was significantly reduced in diabetic animals (Figure 4B).
Figure 4.
Purine release (A) and NO production (B) expressed as percent of baseline from the urothelial side of bladders following hypoosmotic stimulation. Before stimulation the basal purines release was 22.1±4.9, 38.9+/-13.6 and 19.95±4.7 fmol/mg, whereas the baseline NO release was 3.4±0.6, 3.1+/-0.37 and 5.5±1.45 nmol/mg in normal, diabetic and sucrose fed rats respectively (P>0.1 one way ANOVA). The number of experiments was: control n=5, diabetic n=6 and sucrose fed rats n=7. In panel (A) *P<0.05 for normal vs. diabetes group. In panel (B) *P<0.05 for normal vs. diabetes/sucrose groups and **P<0.01 for diabetic vs. sucrose group.
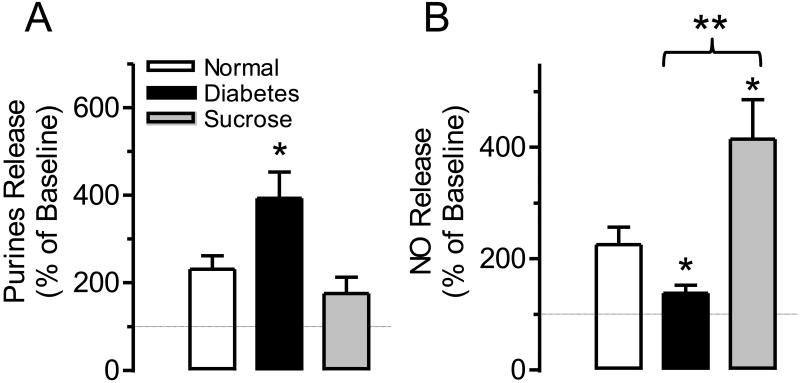
3.4. Change in ATP/NO ratio in sucrose fed and diabetic animals
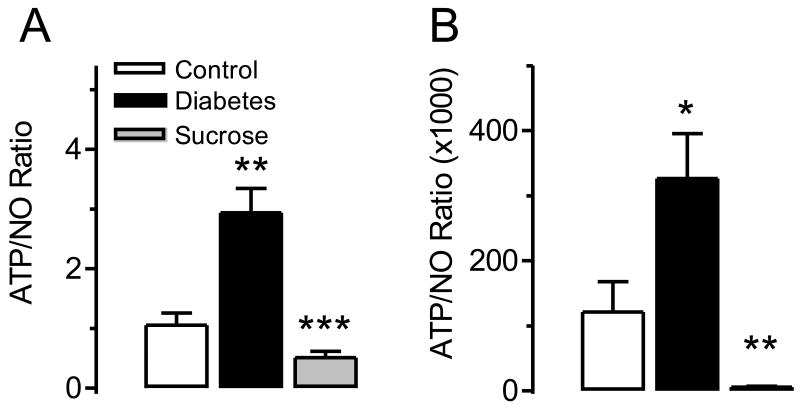
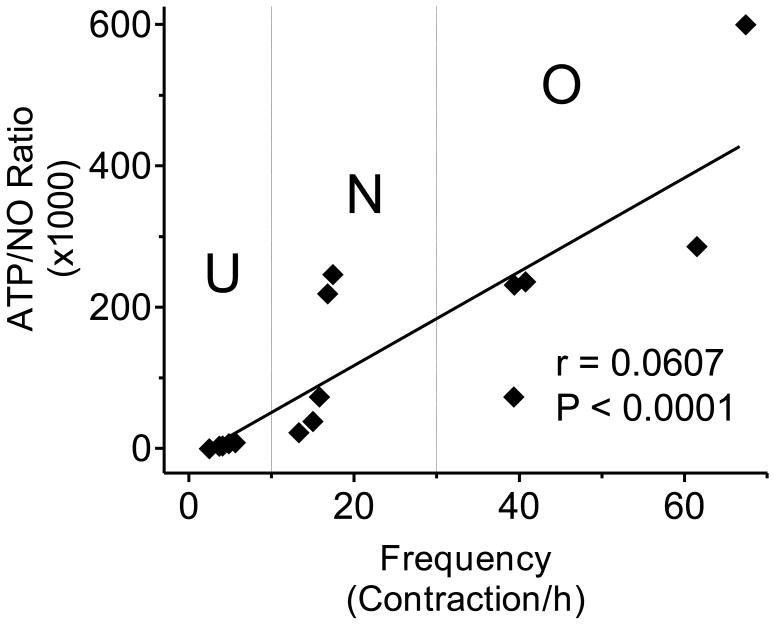
Because ATP is a facilitatory transmitter while NO is an inhibitory transmitter in the bladder sensory pathways and because we found that they exhibited a tendency to change in opposite directions in overactive and underactive bladders we suggest that the release ratio of the two transmitters (ATP/NO ratio) most adequately describes the change in bladder afferents under pathological conditions. Figure 5 shows the ATP/NO ratio calculated for both the in vitro and in vivo experiments. Although the calculated values of the ratios cannot be compared directly because of the differences in calculating the ATP and NO release values, it is obvious that the tendencies of the changes are predictive; the ratio is highly increased in overactive (diabetes) and significantly decreased in underactive (diuretic) bladder conditions (Figure 5 A and B). The results clearly show that the ATP/NO ratio became more prominent when it was measured in the bladder wash (Figure 5B). In addition, when analyzing our in vivo data we found a highly significant correlation between the ATP/NO ratio and bladder contraction frequency (P<0.001) with a correlation coefficient of 0.9607 (Figure 6).
Figure 5.
Comparison of the ATP/NO ratios in normal, overactive and underactive bladder conditions. Hypoosmotic stimulation of the urothelial side of the Ussing chamber during in vitro studies (A) and in vivo evaluation of bladder wash at the time of a voiding contraction (B) in control n=5, diabetic n=5 and sucrose fed n=5 rats. In panel (A) **P<0.01 for control vs. diabetic group and ***P<0.001 for diabetic vs. sucrose group. In panel (B) *P<0.05 for control vs. diabetic group and **P<0.01 for diabetic vs. sucrose group.
Figure 6.
Correlation analysis between the in vivo ATP/NO ratio and the contractile frequency analyzed by a non-parametric Spearman test (correlation coefficient: 0.9607; P<0.001). U= underactive bladder; N= normal bladder; O= overactive bladder.
4. Discussion
The main observation arising from this study is that detrusor overactivity in diabetic bladders is accompanied by elevated release of ATP while NO release remains at the control levels. On the other hand, diuretic bladders, induced by high chronic sugar intake, manifest as underactive bladders in vivo which results in enhanced NO release while release of ATP remains at control levels. This inverse shift in the release of ATP and NO is confirmed during both in vivo and in vitro experiments. The shift of ATP release to higher values has previously been shown in another model of bladder overactivity (i.e. spinal cord injury, SCI) (Khera et al., 2005). In SCI rats application of hypoosmotic stimulation to the urothelium simultaneously released ATP and NO. While NO release was decreased, ATP release was enhanced as compared to release in neurally intact rats (Birder et al., 2002; Smith et al., 2005).
ATP and NO are released in vivo into the bladder wash proving that 1) urothelium is the source of both transmitters and 2) bladder wash or urine can serve as a source for monitoring ATP and NO release under physiological or pathological conditions. These experiments revealed that ATP and NO release are correlated to the frequency of bladder contractions. In overactive bladders ATP but not NO release was enhanced while in diuretic underactive bladders, where the contraction frequency was significantly reduced (Daneshgari et al., 2006b) NO but not ATP release was enhanced. Thus, bladder contraction frequency positively correlated with the intraluminal ATP release and negatively correlated with NO release.
The above findings have been confirmed by utilizing “in vitro” experiments. The urothelial side of the bladder was stimulated by hypoosmotic shock serving as a mechanosensitive stimulus. In concert with the in vivo results the release of ATP was enhanced while NO release was diminished on the urothelial side of diabetic overactive bladders. These observations are in agreement with results of r previous studies in which we found that in overactive bladders after spinal cord injury, ATP release was significantly enhanced on the urothelial side while NO release was reduced (Khera et al., 2004; Smith et al., 2008). Our present findings suggest that increased ATP release is a common feature of overactive bladders irrespective of the underlying pathophysiology. The fact that the in vitro findings mirror in vivo results suggests that changes in ATP and NO release occur at the urothelial level without significant contributions from afferent and efferent pathways of the micturition reflex.
Since ATP is a facilitatory transmitter in sensory nerve terminals (Pandita and Andersson, 2002) while NO is inhibitory (Page et al., 2009) their relative potency in the sensory pathways will determine the frequency of the bladder contractions. Thus, the ratio of the released amounts of ATP/NO can be used as a measure of bladder dysfunction. Figure 5 shows the ratios calculated for the “in vivo” and “in vitro” experiments. The ATP/NO ratio correlates well with the sensory response of the bladder; it is high in overactive conditions and it is greatly reduced in underactive bladder conditions. In other words, it positively correlates with the bladder contraction frequency (Figure 6). We suggest that the ATP/NO ratio is a sensitive marker of bladder overactivity or underactivity and that it can be a useful means to monitor pathological bladder activity and to objectively measure clinical improvements resulting from drug therapy.
The question arises as to why ATP and NO change in opposite directions? It has been demonstrated that NOS activation can be triggered by activation of ionotropic channel receptors like the P2X receptors that eventually increase intracellular Ca2+ levels in the vicinity of nNOS (Garthwaite and Boulton, 1995). Since the ionotropic P2X3 receptors are co-localized with NOS in afferent neurons it is conceivable that activation of the purinergic receptors is providing the essential Ca2+ for NO synthesis (Poole et al., 2002). Thus, release of ATP and NO, under normal conditions, seems to be well balanced when P2X3 receptors are activated by the released ATP while at the same time providing Ca2+ for nNOS to synthesize NO. On the other hand, the released NO negatively affects further ATP release and consequently keeps bladder activity under control (Shen et al, 2005). Our results indicate that in overactive diabetic bladders the purinergic and nitrergic systems are uncoupled (i.e. while the increased ATP release enhances P2X3 receptor activity and consequently increases Ca2+ influx, NO release remains unchanged). This means that the extra amount of Ca2+ is incapable of augmenting NO synthesis andsuggests that changes may occur downstream from the P2X3 receptors. It is tempting to speculate that in overactive bladder conditions nNOS is downregulated and thus the increased Ca2+ influx can activate only a smaller amounts of enzymes. Conversely, in underactive bladders where the P2X3 receptor activity is diminished with lowered Ca2+ influx we hypothesize that nNOS is upregulated and synthesizes greater amounts of NO. The higher level of NO may negatively feedback on afferent terminals to keep released amounts of ATP at a low level.
Our present results may have important clinical significance. Clinicians need objective measurements to guide therapeutic decision making in overactive bladder symptoms. One way is doing that is to find a reliable biomarker that value 1) correlates with the severity of symptoms, and 2) indicates the success of any therapeutical intervention. Recent reports using urinary nerve growth factor levels to track symptom severity and response to antimuscarininc therapy in OAB patients has been encouraging (Liu, et al, 2009) in as much as they found a decrease of urinary nerve growth factor levels after antimuscarinic therapy. Similar testing, using the ATP/NO ratio in urine samples, may provide important complementary information on the sensory nature of OAB symptoms providing a reliable biomarker for therapy.
In summary, we demonstrated that the release ratio of ATP and NO (ATP/NO ratio) positively correlates with the bladder contraction frequency. We suggest that the ATP/NO ratio in overactive bladders, irrespective of the etiology (i.e. diabetes or SCI), is enhanced. On the other hand, the ATP/NO ratio is significantly diminished in underactive bladders. We conclude that ATP and NO release are important biomarkers for bladder contraction frequency and both can be easily obtained and measured in the bladder wash and/or urine. Moreover the ratio of ATP/NO is an objective measure of the bladder afferent system and as such can be used for monitoring changes in bladder activity during drug therapy.
Acknowledgments
The authors are grateful to Dr. Zaneta Romain for helping perform some of the preliminary experiments. This work was supported by the National Institutes of Health (RO1-DK-069988 to GTS).
Footnotes
Competing interests: The authors declare that they don't have competing interest.
Authors' contributions: AM performed experiments and calculations. AM, CPS, TBB and GTS contributed in planning the experiments, calculating the statistical values, as well as in writing the manuscript. GTS is the Principal Investigator, he supervised the study and wrote the paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alvaro Munoz, Email: amunoz@bcm.edu.
Christopher P. Smith, Email: cps@bcm.edu.
Timothy B. Boone, Email: TBoone3@tmhs.org.
George T. Somogyi, Email: gsomogyi@bcm.edu.
References
- Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol. 2006;45:221–226. doi: 10.1016/j.vph.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006a;176:380–386. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006b;176:380–386. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Ann Rev Physiol. 1995b;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–993. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology. 2005;66:208–212. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Hennings EC, Zsilla G, Vizi ES. A possible role of nitric oxide in the regulation of dopamine transporter function in the striatum. Neurochem Int. 1999;34:345–350. doi: 10.1016/s0197-0186(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837–843. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level isincreased in patients with interstitial cystitis/bladder pain syndrome anddecreased in responders to treatment. BJU Int. 2009;104:1476–81. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- Mihaylova-Todorova S, Todorov LD, Westfall DP. Correlation between the release of the sympathetic neurotransmitter ATP and soluble nucleotidases from the guinea pig vas deferens. J Pharmacol Exp Ther. 2001;296:64–70. [PubMed] [Google Scholar]
- Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J Neurosci. 2009;29:7246–7255. doi: 10.1523/JNEUROSCI.6099-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- Pitre DA, Ma T, Wallace LJ, Bauer JA. Time-dependent urinary bladder remodeling in the streptozotocin-induced diabetic rat model. Acta Diabetol. 2002;39:23–27. doi: 10.1007/s005920200008. [DOI] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/s1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Smith CP, Gangitano DA, Munoz A, Salas NA, Boone TB, Aoki KR, Francis J, Somogyi GT. Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neuroch Int. 2008;52:1068–1075. doi: 10.1016/j.neuint.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int. 2005;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES. Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem. 1991;56:1466–1470. doi: 10.1111/j.1471-4159.1991.tb02039.x. [DOI] [PubMed] [Google Scholar]