Summary
Antiepileptic drugs (AEDs) such as phenobarbital, phenytoin and valproic acid, when given in therapeutic doses to neonatal rats, cause pronounced neuronal apoptotic cell death. This effect is especially pronounced in the striatum and cortex during the second postnatal week; a period corresponding to the “brain growth spurt” (third trimester of gestation and early infancy) in humans. Of particular concern is the fact that phenobarbital is the most frequently used therapy for neonatal epilepsy. If AED-induced neuronal cell death leads to long-term functional impairment, then it becomes crucial to find therapies that avoid this neurotoxicity in the sensitive period. Here we examine short and long-term functional effects following exposure of neonatal rat pups to phenobarbital; the functions tested include striatal GABAergic synaptic responses and reflex development in pups, and fear conditioning, emotionality, and sensory-motor gating in adults. In all cases, phenobarbital exposure during the second postnatal week was sufficient to cause significant impairment. In contrast, adult animals exposed as pups to lamotrigine (given in a dose that does not cause apoptotic neuronal death) were not impaired on the tasks we examined. Our data suggest that treatments devoid of proapoptotic actions may be promising therapies for avoiding adverse outcomes after neonatal exposure. In addition, our findings identify early exposure to certain AEDs as an important potential risk factor contributing to psychiatric and neurological abnormalities later in life.
Keywords: phenobarbital, antiepileptic drugs, lamotrigine, GABA transmission, striatum, fear conditioning, elevated plus maze, prepulse inhibition, postnatal development, neurotoxicity
Introduction
Several antiepileptic drugs (AEDs), when given in therapeutically relevant doses to rats during the early postnatal period, cause pronounced apoptotic neuronal death in several brain regions (Bittigau, et al. 2002, Katz, et al. 2007, Kim, et al. 2007). This effect occurs during the highly vulnerable “brain growth spurt”, when programmed cell death accompanies synaptic proliferation, and neurons compete in a life-or-death campaign to establish functional connections. AED therapy can upset the survival/elimination balance during this sensitive period (corresponding to the third trimester of pregnancy through infancy in humans), and may impair CNS maturation and long-term functional outcomes. This is especially relevant to neonatal seizure management because phenobarbital, the drug most often used in this context, causes substantial neuronal death in the neonatal rat model.
As the first line treatment for neonatal seizures, phenobarbital is used in over 80% of cases (Bartha, et al. 2007, Blume, et al. 2009). With the especially high seizure incidence in neonates, as many as 25,000/year in the United States are affected (Hauser, et al. 1993), resulting in approximately 15,000–20,000 pre-term and full- term newborns who are likely to be treated with phenobarbital each year. Thus, there is a clear need to identify whether exposure to phenobarbital during specific stages of postnatal brain maturation leads to compromised CNS function in juveniles and adults.
In the clinical setting, neonatal seizures, AED exposure, and underlying neurological abnormalities are inextricably enmeshed making it impossible to isolate the contribution of any single variable. Although early seizures emerge as risk factors for later psychiatric, neurological, or cognitive deficits (Glass, et al. 2009, Tekgul, et al. 2006), the possibility that a portion of the risk is attributable to AED treatment cannot be ruled out. This confound has been explicitly acknowledged in the clinical literature (Glass, et al. 2009, Vestergaard, et al. 2005), but it can be experimentally addressed only in animal models.
In rodents, several AEDs have been examined for neurotoxicity during the first two postnatal weeks. Phenobarbital, phenytoin, and valproic acid increase apoptotic neuronal death when given acutely between postnatal day (P) 5 and 14 (Bittigau, et al. 2002, Katz, et al. 2007, Kim, et al. 2007). Moreover, AEDs such as lamotrigine and topiramate that do not cause neuronal apoptosis when given alone, exacerbate the neurotoxicity of phenytoin or phenobarbital (Katz, et al. 2007, Kim, et al. 2007). The neurotoxicity is especially severe within striatum, thalamus, and cortex, but it also occurs in amygdala, hippocampus, and cerebellum (Bittigau, et al. 2002, Kim, et al. 2007, Snyder, et al. 2008). However, we do not know if this cellular effect of AEDs contributes to adverse functional sequelae during development and in adulthood.
Most studies of behavioral effects of phenobarbital exposure in immature rodents have given the drug daily, starting during the first postnatal week and continuing for well over two weeks, in some cases beyond P30 (McBride, et al. 1985, Pick and Yanai 1985, Rogel-Fuchs, et al. 1992). These studies observed deficits in adults tested for spatial learning and memory in water maze, radial arm maze, and T-maze tasks. An important question is whether treatment limited to the second postnatal week, corresponding to the neonatal period in humans, is sufficient to cause later functional deficits.
A limited number of studies examined narrower periods of AED exposure, suggesting that even a single acute treatment during the period between P7–P14 may be sufficient to cause later behavioral impairment. A single treatment of mice at P14 with valproic acid caused regression in specific reflexes and deficits in later social behavior (Wagner, et al. 2006). A single treatment of rats with an anesthetic cocktail at P7 produced lasting deficits in radial arm and water maze performance, and in hippocampal long-term potentiation (Jevtovic-Todorovic, et al. 2003). Water maze performance was impaired in adult mice following treatment with phenytoin (35mg/kg) daily from P5–14 (Ogura, et al. 2002), and in adult rats following 4 treatments with phenobarbital (50 mg/kg) given on alternate days between P4 to P10 (Stefovska, et al. 2008). These results are consistent with the hypothesis that AED-induced neuronal cell death (especially pronounced during the period between P7–P14) is predictive of later functional impairment. If we can obtain further support for this hypothesis, then it will become crucial to identify drugs that are devoid of this neurotoxicity. The results we describe here will address two key initial questions: 1) what functional consequences of phenobarbital exposure are related to AED-induced developmental neuronal apoptosis? 2) are there AEDs that do not cause neurodevelopmental toxicity?
We compared the effects of acute (on P7 or P14) or chronic (daily treatment from P7 to P14) phenobarbital during the period between P7–P14. We present preliminary findings on striatal synaptic function, development of reflexes, and longer-term behaviors that may prove useful in detecting functional impairment associated with AED-induced neurotoxicity. In some experiments we included lamotrigine (an AED that does not cause neuronal cell death when given at therapeutic doses) in addition to phenobarbital.
GABAergic Synaptic Transmission in Striatal Slices
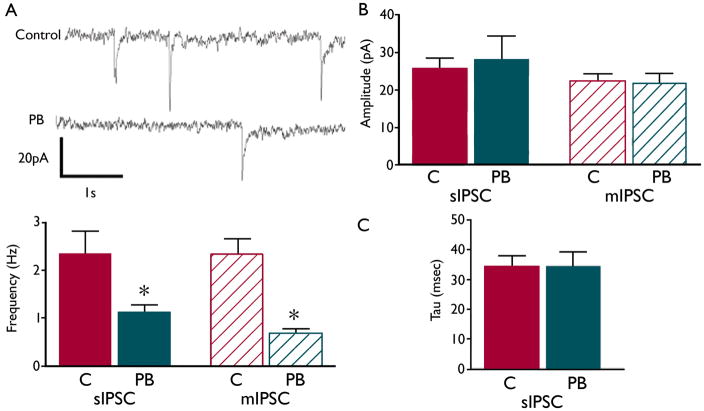
Sprague-Dawley rat pups received phenobarbital (75mg/kg, ip) on P7. Animals were sacrificed at P14, and whole-cell voltage-clamp recordings were made from striatal medium spiny neurons (MSNs) in slices as previously described (Ade, et al. 2008). MSNs from pups exposed to phenobarbital displayed a lower frequency of both miniature (t=2.769, df=23, p=0.0109 compared to controls) and spontaneous inhibitory post-synaptic currents (t=2.678, df=24, p=0.0131 compared to controls) (Figure 1a), indicating fewer GABAergic synapses. Decay constant (tau) and current amplitude did not differ between groups (Figure 1b).
Figure 1.
P7 phenobarbital (PB) exposure causes a decrease in striatal synaptic transmission at P14. Representative traces (1a) from recordings of MSNs in slices prepared from animals exposed to PB (grey) or C (saline, black) on P7 show a decrease in number of inhibitory post-synaptic events (IPSCs). Frequency of spontaneous (s)IPSCs (1a, bottom) is significantly decreased in PB (14 cells) as compared to C (12 cells). The frequency of miniature (m)IPSCs (1a, bottom) is also decreased in PB (14 cells) as compared to C (10 cells). Amplitude (1b) and decay (1c) were unaltered. * indicates significantly different than control, p>0.05. GABA currents were recorded using a KCl intracellular solution to facilitate detection of currents.
Behavioral Testing
Mid-Air Righting and Hanging Wire tests at P15–P21
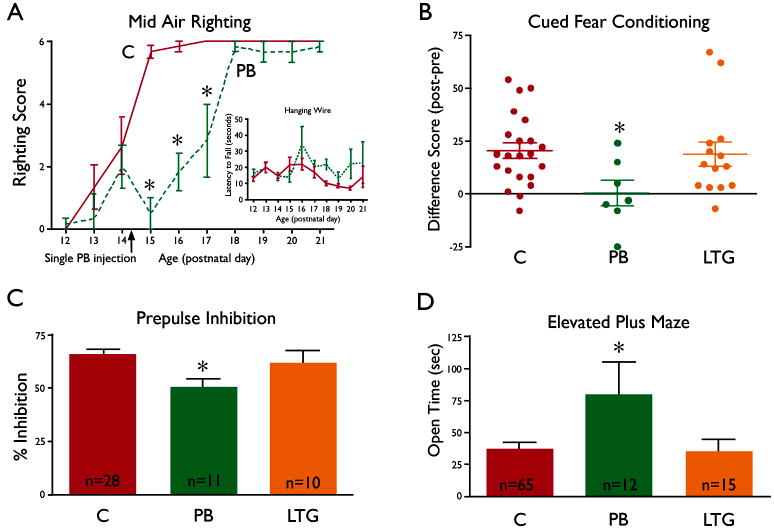
Rat pups were tested daily 3 times prior to a single phenobarbital treatment on P14, and then daily until P21 as previously described (Wagner, et al. 2006). Phenobarbital-treated animals performed significantly worse than controls on the mid-air righting task on the three test days following P14 treatment (Fig 2A), but showed no impairment in grip on a hanging wire (Fig 2A insert), suggesting a selective disruption of specific reflex development. Although the mid-air righting reflex recovered by P18 in the pups exposed to a single treatment, the possibility that chronic phenobarbital exposure might cause more prolonged effects remains to be determined.
Figure 2.
Effects of phenobarbital (PB) and lamotrigine (LTG) exposure on developmental reflexes (2A) and adult behaviors (2 B–D).
2A) Pups exposed to 75 mg/kg PB (broken line, n=6) as a single i.p. injection on P14 (indicated by the arrow on the x-axis) displayed a significant deficit (indicated by asterisks, p< 0.001, Bonferonni post-hoc following ANOVA) in mid-air righting as compared to saline-exposed controls (solid line, n=6). Mid-air righting was scored as 0 for no righting, 1 for incomplete righting, and 2 for full righting; the score for each day is the sum of scores from 3 tests (maximum score = 6). Inset shows the performance of the same animals on the hanging wire task; PB- exposed pups (broken line, n=6) were not different in latency to fall from the hanging wire as compared to controls (solid line, n=6) at any time point.
2B) Pups were exposed daily to either saline (C, controls, n=22), PB (n=7) or LTG (n=14) from P7 to P14 and tested as adults in a cued fear conditioning paradigm. The graph shows differences scores (% time freezing during tone presentation minus the % time freezing prior to tone presentation), with a score of zero indicates no conditioning. Control and LTG-treated animals had scores that were significantly above zero (p<0.01), indicating that learning had occurred. The scores of PB-exposed animals were significantly lower than controls (* = p<0.05, ANOVA with Dunnett post test) and not different from zero (1-sample t-test), indicating a lack of learning.
2C) In the PPI task, animals exposed to PB from P7 to P14 (n=11) and tested as adults had significantly lower % inhibition than did controls (n=28), indicating an impairment in sensory-motor gating. LTG-exposed animals (n=10) were not different from control. Asterisk indicates significantly different from control (p<0.05, Tukey HSD). There was a significant main effect of treatment (F2,46=4.502, p=0.016), no treatment by prepulse interaction, so data were collapsed across prepulse intensity for analysis).
2D) In the elevated plus maze, animals exposed to PB from P7 to P14 (n=12) and tested as adults spent significantly more time in the open arms of the elevated plus maze as compared either to control (n=65) or to animals exposed to LTG from P7 to P14 (n=15); this indicates that PB-exposed animals were less cautious and guarded than control animals. Asterisk indicates significantly different from control (p<0.05).
Cued Fear Conditioning
Adult male rats treated from P7 to P14 with phenobarbital (75mg/kg), exhibited impaired cued fear conditioning (assayed by measuring percent time freezing in response to a tone, as previously described by Ferreira, et al. 2008). Difference scores (Fig 2B) were calculated for each animal by subtracting the time spent freezing before conditioning from the time spent freezing after conditioning. Both control and lamotrigine-exposed animals showed significant conditioning, while phenobarbital-exposed animals did not.
PPI test for Sensory-Motor Gating
PPI refers to the normal reduction in startle response produced by the presentation of a weak startling stimulus prior to presenting the test stimulus; this measure of ‘sensorimotor gating’ is often abnormal in a number of psychiatric disorders (Swerdlow, et al. 2001). Testing of adult male rats was performed as previously described (Kodsi and Swerdlow 1995). Rats exposed as pups (P7–P14 to phenobarbital (75 mg/kg), showed a reduction in PPI as compared to controls (Fig 2C). Lamotrigine-exposed animals were not different from controls.
Elevated Plus Maze
The elevated plus maze is used to evaluate amygdala-related fear/anxiety by examining the amount of time an animal spends exploring arms of the maze that are exposed to light and have no protective walls or edges (“open” arms). Adult male rats that had been treated from with phenobarbital from P7 to P14 were allowed to explore the elevated plus maze; their pattern of behavior was evaluated based on number of entries and time spent in the open and closed arms during a five minute test. Phenobarbital-exposed rats showed a significant increase in time spent in the open arms of the maze (Fig 2D), indicating reduced apprehensiveness. Rats exposed to lamotrigine exposure were not different from controls. Total arm entries did not differ between groups (data not shown), indicating that general activity was not altered by the treatments.
Functional implications for AED use in infancy
The results described above demonstrate that brief exposure of rat pups to phenobarbital during the second postnatal week is sufficient to cause impaired cellular and behavioral function during later maturation and in adulthood. A single treatment with phenobarbital at P7 resulted in a deficit in GABAergic synaptic function in striatal MSNs measure a week later, while a single treatment at P14 caused a markedly delayed maturation of the mid-air righting reflex. Adult animals that had been exposed to phenobarbital as pups between P7 and P14 exhibited impaired fear conditioning, impaired sensory-motor gating, and altered anxiety-like behavior. In contrast, lamotrigine, given between P7 and P14 at a dose that does not cause apoptotic neuronal death in the developing brain (Katz, et al. 2007) did not cause long-term behavioral impairment in these tasks. The contrast we have observed between the effects of phenobarbital and lamotrigine supports the hypothesis that AED-induced neuronal cell death during the second postnatal week in rats is predictive of later functional impairment. Further testing of this hypothesis will come from evaluating the behavioral consequences and effects on synaptic maturation of early exposure to other AEDs that are known to cause developmental neuronal apoptosis (phenytoin and valproic acid), and a comparison to additional AEDs that do not have this neurotoxic action (levetiracetam and carbamazepine, as described by Kim, et al. 2007).
A region that is especially sensitive to the pro-apoptotic actions of AEDs during the second postnatal week is the striatum (Bittigau, et al. 2002, Kim, et al. 2007). This is why we selected this region for evaluating the maturation of GABAergic transmission after phenobarbital exposure. The abnormalities we observed in GABAergic transmission in this region one week after a single treatment with phenobarbital may or may not be a direct effect of the neuronal loss. On the one hand, the neurons that die may deprive neighboring neurons of synaptic input, thereby compromising their function. Alternatively, the drug toxicity may manifest as a spectrum, with the death of the most vulnerable neurons at one extreme, and compromised synaptic function at the other. Our observations in striatum also suggest that compromised synaptic function may occur in other brain areas in which drug-induced apoptosis has been documented, and possibly also in regions which receive inputs from the injured neuronal populations. Likewise, although some of the behavioral tasks we have used (i.e. PPI and fear conditioning) are sensitive to striatal damage (Ferreira, et al. 2008, Kodsi and Swerdlow 1995), they are also sensitive to damage in other regions in which AED-induced apoptosis occurs, such as prefrontal cortex and amygdala (Kim and Jung 2006, Swerdlow, et al. 2001).
Our behavioral results with phenobarbital extend previous data showing deficits in spatial learning and memory in adults animals exposed to AEDs during early postnatal development (Jevtovic-Todorovic, et al. 2003, McBride, et al. 1985, Pereira de Vasconcelos, et al. 1990, Pick and Yanai 1985, Rogel-Fuchs, et al. 1992, Stefovska, et al. 2008). Taken together, the findings highlight the importance of drug treatment in infancy as a risk factor for neurological, psychiatric, and cognitive abnormalities in adolescence and adulthood (Glass, et al. 2009, Tekgul, et al. 2006, Vestergaard, et al. 2005). These findings also underscore the importance of identifying AED treatments for infants that are devoid of adverse long-term consequences, and avoiding the clinical use of developmentally neurotoxic drugs.
The contrast between lamotrigine and phenobarbital presented here raise the possibility, that like the proapoptotic actions of AEDs, the cellular and behavioral neurotoxicity of the drugs is not an inextricable feature of AED action, but rather a feature of specific drugs (and in some cases, doses) (Katz, et al. 2007). This indicates that a careful selection of AED treatment during certain sensitive periods in development can avoid disruptions in CNS maturation and the associated long-term functional consequences. This goal should be a high priority for the management of seizures in neonates.
Acknowledgments
This study was supported by a Predoctoral Fellowship from the Epilepsy Foundation and F31NS066822, a research grant from GlaxoSmithKline, NIH research grants R21MH079991 and R01NS047700, and NIH training grant T32DA007291. We thank Charles Snyder, Ryan Kozlowski and Sam Paskewitz for their assistance in treating and testing animals.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero D, Ment LR, Silverstein F. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J Child Neurol. 2009;24:148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- Ferreira TL, Shammah-Lagnado SJ, Bueno OF, Moreira KM, Fornari RV, Oliveira MG. The indirect amygdala-dorsal striatum pathway mediates conditioned freezing: insights on emotional memory networks. Neuroscience. 2008;153:84–94. doi: 10.1016/j.neuroscience.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz I, Kim J, Gale K, Kondratyev A. Effects of Lamotrigine Alone and in Combination with MK-801, Phenobarbital, or Phenytoin on Cell Death in the Neonatal Rat Brain. J Pharmacol Exp Ther. 2007;322:494–500. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- Kim J, Kondratyev A, Gale K. Antiepileptic Drug-Induced Neuronal Cell Death in the Immature Brain: Effects of Carbamazepine, Topiramate, and Levetiracetam as Monotherapy versus Polytherapy. J Pharmacol Exp Ther. 2007;323:165–173. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Prepulse inhibition in the rat is regulated by ventral and caudodorsal striato-pallidal circuitry. Behav Neurosci. 1995;109:912–928. doi: 10.1037//0735-7044.109.5.912. [DOI] [PubMed] [Google Scholar]
- McBride MC, Rosman NP, Davidson SJ, Oppenheimer EY. Long-term behavioral effects of phenobarbital in suckling rats. Exp Neurol. 1985;89:59–70. doi: 10.1016/0014-4886(85)90265-1. [DOI] [PubMed] [Google Scholar]
- Ogura H, Yasuda M, Nakamura S, Yamashita H, Mikoshiba K, Ohmori H. Neurotoxic damage of granule cells in the dentate gyrus and the cerebellum and cognitive deficit following neonatal administration of phenytoin in mice. J Neuropathol Exp Neurol. 2002;61:956–967. doi: 10.1093/jnen/61.11.956. [DOI] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Colin C, Desor D, Divry M, Nehlig A. Influence of early neonatal phenobarbital exposure on cerebral energy metabolism and behavior. Exp Neurol. 1990;108:176–187. doi: 10.1016/0014-4886(90)90025-n. [DOI] [PubMed] [Google Scholar]
- Pick C, Yanai J. Long term reduction in eight arm maze performance after early exposure to phenobarbital. Int J Dev Neurosci. 1985;3:223–227. doi: 10.1016/0736-5748(85)90027-9. [DOI] [PubMed] [Google Scholar]
- Rogel-Fuchs Y, Newman ME, Trombka D, Zahalka EA, Yanai J. Hippocampal cholinergic alterations and related behavioral deficits after early exposure to phenobarbital. Brain Res Bull. 1992;29:1–6. doi: 10.1016/0361-9230(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Snyder C, Forcelli P, Goyal S, Ritter J, Kim J, Kondratyev A, Gale K. Antiepileptic drug- induced cell death in limbic regions of the neonatal rat brain. Epilepsia. 2008;49:95. doi: 10.1111/j.1528-1167.2011.03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Pedersen C, Christensen J, Madsen K, Olsen J, Mortensen P. Febrile seizures and risk of schizophrenia. Schizophrenia Research. 2005;73:343–349. doi: 10.1016/j.schres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36:779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]