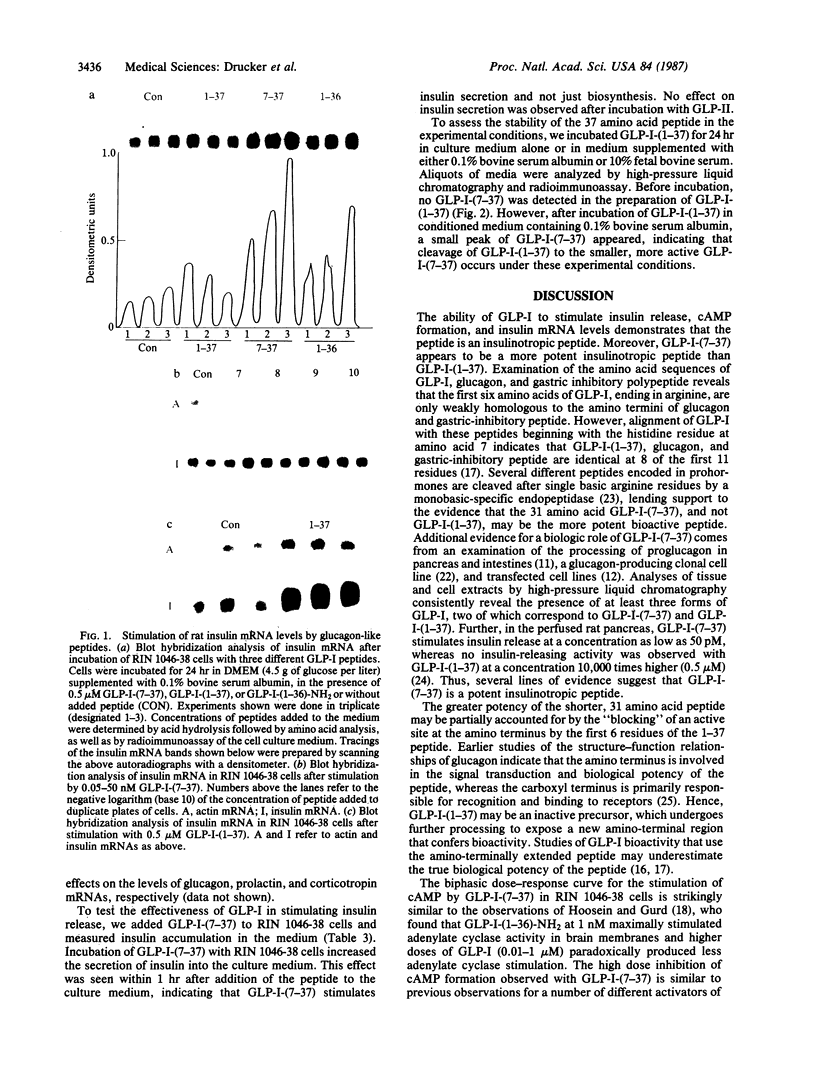
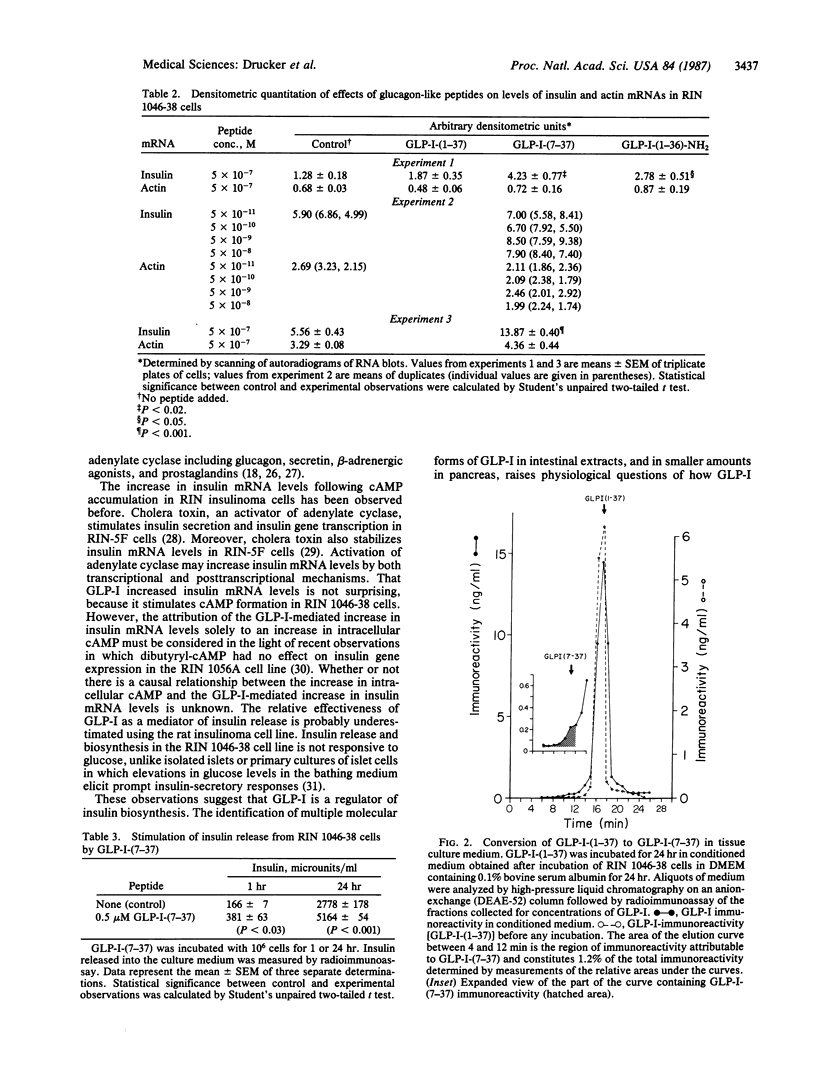
Abstract
Insulin secretion is controlled by a complex set of factors. Although blood glucose levels serve as the major stimulus of insulin secretion in mammals, insulin release is also modulated by amino acids, catecholamines, glucagon, and other, intestinal hormones. The identification of factors that modulate insulin production has engendered much interest because of their potential importance in the altered dynamics of insulin secretion in response to glucose characteristic of maturity-onset diabetes mellitus. Decoding of the glucagon gene has uncovered two additional glucagon-like peptides encoded in proglucagon, the polypeptide precursor of glucagon. One of these peptides, glucagon-like peptide I, is processed from proglucagon in two forms, of 31 and 37 amino acids. We report that the smaller of the two glucagon-like peptides potently increases cAMP levels, insulin mRNA transcripts, and insulin release in cultured rat insulinoma cells. These results indicate that glucagon-like peptide I may be a physiologic modulator of insulin gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Santerre R. F., Mullenbach G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983 Apr 21;302(5910):716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Drucker D. J., Mojsov S., Habener J. F. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J Biol Chem. 1986 Jul 25;261(21):9637–9643. [PubMed] [Google Scholar]
- England R. D., Jenkins W. T., Flanders K. C., Gurd R. S. Noncooperative receptor interactions of glucagon and eleven analogues: inhibition of adenylate cyclase. Biochemistry. 1983 Mar 29;22(7):1722–1728. doi: 10.1021/bi00276a031. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. K., Uttenthal L. O., Ghiglione M., Bloom S. R. Molecular forms of glucagon-like peptides in man. FEBS Lett. 1985 Nov 18;192(2):275–278. doi: 10.1016/0014-5793(85)80124-1. [DOI] [PubMed] [Google Scholar]
- Ghiglione M., Uttenthal L. O., George S. K., Bloom S. R. How glucagon-like is glucagon-like peptide-1? Diabetologia. 1984 Dec;27(6):599–600. doi: 10.1007/BF00276976. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Gros P., Habener J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J Biol Chem. 1984 Nov 25;259(22):14082–14087. [PubMed] [Google Scholar]
- Heinrich G., Gros P., Lund P. K., Bentley R. C., Habener J. F. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology. 1984 Dec;115(6):2176–2181. doi: 10.1210/endo-115-6-2176. [DOI] [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Gurd R. S. Human glucagon-like peptides 1 and 2 activate rat brain adenylate cyclase. FEBS Lett. 1984 Dec 3;178(1):83–86. doi: 10.1016/0014-5793(84)81245-4. [DOI] [PubMed] [Google Scholar]
- Horrobin D. F. Interactions between prostaglandins and calcium: the importance of bell-shaped dose-response curves. Prostaglandins. 1977 Oct;14(4):667–677. doi: 10.1016/0090-6980(77)90194-0. [DOI] [PubMed] [Google Scholar]
- Korman L. Y., Bhathena S. J., Voyles N. R., Oie H. K., Recant L. Characteristics of the interaction of the glucagon receptor, cAMP, and insulin secretion in parent cells and clone 5F of a cultured rat insulinoma. Diabetes. 1985 Aug;34(8):717–722. doi: 10.2337/diab.34.8.717. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Frazier M. L., Su C. J., Kumar A., Saunders G. F. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Montminy M. R., Dee P. C., Habener J. F. Anglerfish islet pre-proglucagon II. Nucleotide and corresponding amino acid sequence of the cDNA. J Biol Chem. 1983 Mar 10;258(5):3280–3284. [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Mojsov S., Weir G. C., Habener J. F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987 Feb;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. A., Welsh M., Casadaban M. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J Biol Chem. 1985 Nov 5;260(25):13585–13589. [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Habener J. F. Glucagon gene transcription in an islet cell line is regulated via a protein kinase C-activated pathway. J Biol Chem. 1987 Feb 5;262(4):1823–1828. [PubMed] [Google Scholar]
- Philippe J., Mojsov S., Drucker D. J., Habener J. F. Proglucagon processing in a rat islet cell line resembles phenotype of intestine rather than pancreas. Endocrinology. 1986 Dec;119(6):2833–2839. doi: 10.1210/endo-119-6-2833. [DOI] [PubMed] [Google Scholar]
- Praz G. A., Halban P. A., Wollheim C. B., Blondel B., Strauss A. J., Renold A. E. Regulation of immunoreactive-insulin release from a rat cell line (RINm5F). Biochem J. 1983 Feb 15;210(2):345–352. doi: 10.1042/bj2100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebalin M., Said S. I., Makhlouf G. M. Stimulation of insulin and glucagon secretion by vasoactive intestinal peptide. Am J Physiol. 1977 Feb;232(2):E197–E200. doi: 10.1152/ajpendo.1977.232.2.E197. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Siegel E. G., Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985 Sep;28(9):704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W. The processing of peptide precursors. 'Proline-directed arginyl cleavage' and other monobasic processing mechanisms. FEBS Lett. 1986 May 5;200(1):1–10. doi: 10.1016/0014-5793(86)80500-2. [DOI] [PubMed] [Google Scholar]
- Welsh M., Nielsen D. A., MacKrell A. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985 Nov 5;260(25):13590–13594. [PubMed] [Google Scholar]