Abstract
Photoinduced decarbonylation of 2,4-bis(spirocyclohexyl)-1,3-cyclobutanedione 1 in the crystalline solid state resulted in formation of deep blue transient with a λmax = 550 nm and a half-life of 42 min at 298 K identified as kinetically stabilized oxyallyl. Support for an open shell singlet species was obtained by spectroscopic analysis and (4/4) CASSCF calculations with the 6-31+G(d) basis set and multi-reference MP2 corrections. The electronic spectrum of the singlet biradical, confirmed by femtosecond pump-probe studies in solution, was matched by coupled cluster calculations with single and double corrections.
Oxyallyl (OA) is the oxygen analog of trimethylenemethane that is formally obtained by cleavage of the 2,3-bond of cyclopropanone. OA has been invoked in numerous reactions mechanisms, including the Nazarov cyclization,1 Favoroskii rearrangement,2 cycloaddition of cyclopropanones,3 photochemical reactions of cross-conjugated dienones,4 and prostaglandin biosynthesis5 (Scheme 1a). While many recent synthetic applications rely on the formulation of OA,6 in contrast to nearly all other reactive intermediates, up until now, it has resisted all attempts at direct detection and isolation.
Scheme 1.
Questions about OA abound: is it an intermediate or a transition state? A zwitterion or an open-shell biradical? If biradical, does it have singlet or triplet multiplicity in its lowest energy state? Some answers are available. Quantum mechanical calculations suggest that the electronic nature of OA depends on the substituent patterns.7 While early quantum mechanical calculations suggested that unsubstituted OA (Scheme 1a) could be a ground state triplet,8 subsequent refinements predict a singlet biradical.9,10 Experimental studies addressing the solvent polarity-dependence on the rates of reactions thought to proceed via OA, also supported by calculations,9 indicate that tetra-alkyl substitution favors a singlet biradical, which is 15–25 kcal/mol higher in energy than the corresponding cyclopropanone.11 The singlet biradical nature of unsubstituted OA was recently confirmed by photoelectron spectroscopy (PES) and by computational analysis with zero point energy corrections, both suggesting that closure occurs with no barrier!
Knowing that species trapped in the solid state tend to have longer lifetimes than their solution counterparts,12 and taking advantage of our experience on the generation of biradicals by the photoinduced decarbonylation of crystalline ketones,13 we decided to explore the generation and potential stabilization of a tetrasubstituted OA. Among several possible precursors, we selected 2,4-bis(spirocyclohexyl)-1,3-cyclobutanedione 1 as an ideal test system. Compound 1 is a relatively high melting solid (mp=159 °C) known to react photochemically in solution14 by a singlet state α-cleavage followed by fragmentation to cyclohexyl ketene 4, or by loss of CO to generate a transient cyclopropanone 3 through an intermediate OA 2 (Scheme 1b). While dicyclohexylidene 5 is the major product in inert solvents, reaction in furan leads to formation of adduct 6, in support of a trappable intermediate.
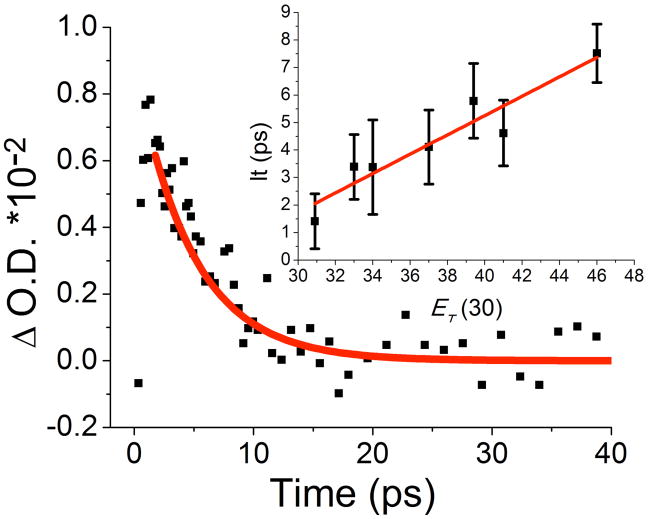
Irradiation of 1 with λ = 254 nm in the crystalline state led exclusively to alkene 5 by photodecarbonylation of the singlet state followed by a secondary photoreaction of cyclopropanone 3. Attempts to isolate 3 were unsuccessful, consistent with FTIR and 1H NMR observations indicating that it does not accumulate under the reaction conditions. While quantum yields the decomposition of 1 (ΦSol) in methylene chloride and in benzene were 0.34 and 0.31, respectively,14,15 the quantum yield of reaction (and formation of 5) in a nanocrystalline suspension using dicumyl ketone (Φ=0.2)16 as a chemical actinometer resulted in a value of only Φ≈0.001. In a key observation, we discovered that polycrystalline samples irradiated for ca. 30 min displayed an intense blue color that faded slowly over many hours (Figure 1). The diffuse-reflectance UV-Vis spectrum showed a relatively broad band with λmax= 580 nm and a half life of ca. 42 min at 298 K that was not affected by Ar or O2 atmospheres.
Figure 1.
Diffuse reflectance spectrum of cyclobutanedione 1 before (black) and after 10, 20 and 30 min of UV irradiation (blue) at 298 K. Inset: powder before and after irradiation. The blue color faded out after several hours, and its bleaching is accelerated by irradiation at λ=530 nm.
Given the saturated nature of the dispirodiketone and its known photoproducts, we realized that OA could be associated with the blue color17 and we set out to test our hypothesis. In fact, while the color disappears upon dissolution in most solvents and chemical analysis reveals the formation of 5, exposure to neat furan resulted in the exclusive formation of small amounts of 6 (ca. 6%), consistent an interfacial (surface-solution) cycloaddition between furan and OA 2 (Figure S3).18 The blue intermediate showed no fluorescence in the solid, and it was later shown to be EPR silent between 77–298 K, indicative of a singlet state.19
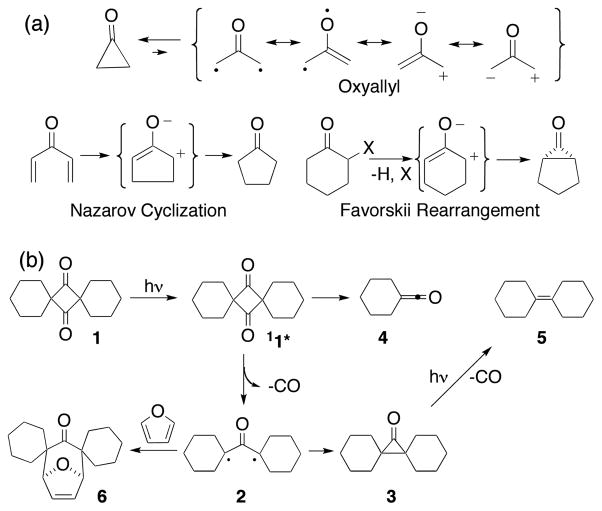
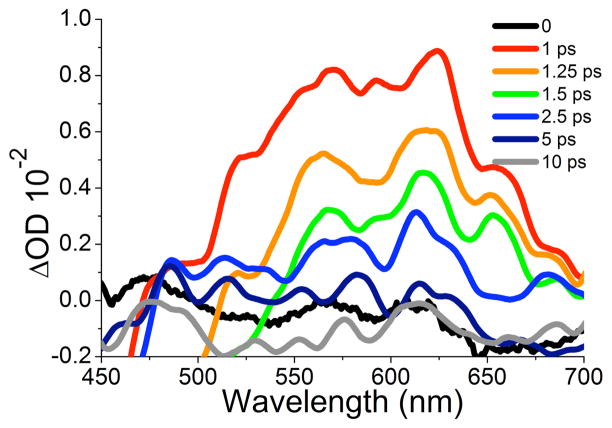
With guidance provided by the UV-Vis diffuse reflectance spectrum, we set out to look for the formation of a similar transient in solution. Consistent with reports by Neckers,3c,20 there were no transients that could be assigned to OA in the nanosecond time scale. Excitation at 258 nm with 150 fs pulses in dichloroethane showed stimulated emission from 1 below 475 nm and only one transient in the region of 500–700 nm with a lifetime of 3.3 ps, with an apparent vibrational structure displaying several maxima (at ca. 525, 555, 570, 590, 625, 650 and 690 nm, Figure 2). A comparison of the transients observed in solution and in the solid state shows that they are very similar, with the solution spectrum displaying a bathochromic shift of ca. 40 nm and some vibrational resolution (S7). Measurements in ethers, nitriles, hydrocarbons, and halocarbons showed identical transients with lifetimes between 1.0–8.0 ps depending on the polarity of the solvent (S9). A plot of the lifetime vs. the ET(30). polarity parameter produced a straight line (Figure 3, inset) with a modest polarity dependence. As analogous plots for reactions with zwitterionic intermediates have lifetime changes of ca. 102–104,21 our results are consistent with a neutral species. The rapid formation of the transient indicates a reaction from the excited singlet state, and a lifetime of ca. 5 ps in solution is consistent with the need for high concentrations of trapping species, even with reactions that should be diffusion controlled.22
Figure 2.
Transient absorption spectra upon excitation of cyclobutanedione 1 in dichloroethane by excitation at 258 nm with 150 fs laser pulses. No other transients were seen at delay times up to 3 ns.
Figure 3.
Picosecond decay measured at 600 nm of the OA transient generated by excitation cyclobutanedione 1 in dichloroethane. Inset: Changes in the lifetime of OA as a function of the solvent polarity parameter ET(30).
To gain further evidence for or against our assignment we carried out complete active space self-consistent field (CASSCF)23,24,25 calculations with the 6-31+G(d) basis set. Multi-reference Møller-Plesset second-order perturbation theory (MRMP2)26 corrections were also implemented to account for the effects of dynamic correlation. The lowest energy singlet biradical structure shows a nearly coplanar geometry of the carbonyl and α-substituents, with most of the spin density on the α-carbons (Figure 4). The corresponding triplet biradical was calculated to be 1.5 kcal/mol higher in energy. Equation of motion coupled cluster theory with single and double corrections27 were used to predict the excitation energies. Comparison of the experimental result with the predicted excitation energy of the singlet biradical (1BR) showed a reasonable match with a broad absorption between 400 and 800 nm with λmax(1BR) = 530 nm or 1.89 eV. Given the short lifetime in solution and the position of the spectrum, we conclude that the transient is unlikely to arise from a photoproduct. Tetraalkylcyclopropanone transients have lifetimes longer than microseconds,28 and dialkyl ketenes as well, as acyl radicals absorb, with λ < 450 nm.29 In addition, the long lifetime of the blue transient in the crystal is not compatible with an excited state species. The ring-closure of 1BR to cyclopropanone 3 is calculated to be exothermic by 14.8 kcal/mol, with a barrier of only 2.6 kcal/mol,30 which is consistent with a lifetime of a few picoseconds in solution.
Figure 4.
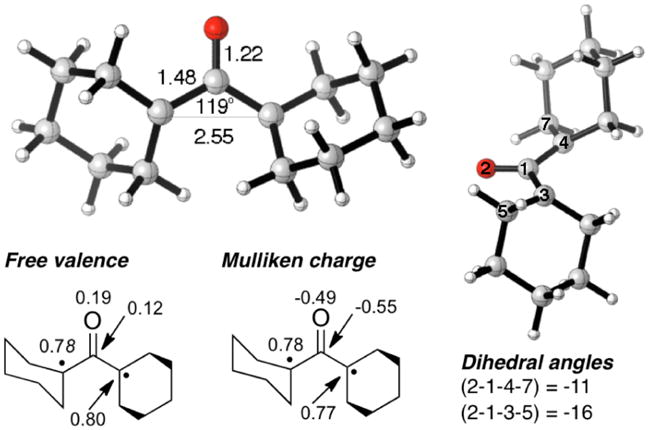
CASSCF/6-31+G(d) geometrical parameters, free valence, and charges of the singlet biradical 1BR.
The large size of the system under study precluded a vibrational frequency analysis at the CASSCF level. However, vibrational analysis of the analogous tetramethyloxyallyl gives a scaled CO stretch of 1758 cm−1 for the singlet and 1555 cm−1 for the pure triplet,31 which are similar to those recently reported for the parent structure.32 Unfortunately, FTIR measurements in the solid state using attenuated total reflection (ATR) and grazing angle reflectance spectroscopies showed insignificant changes in the region of the triplet while the starting material absorbs very strongly in the region of the singlet, making the IR assignment of OA impossible despite the deep blue color.33 Only a weak band assigned to ketene 4 was observed at 2111 cm−1, with the carbonyl signal of cyclopropanone 3 at ca. 1813–1850 cm−1 also absent.34
With the blue transient assigned as the singlet 1,3-biradical, it is remarkable that its lifetime in crystals is extended by 14 orders of magnitude with respect to the lifetime in solution! We attribute this to the kinetic stabilization provided by the rigidity of the crystal lattice. A single crystal X-ray structure of 135 solved in the orthorhombic space group Cmca supports this view. The adjacent molecules severely hinder the ca. 0.33 Å displacement between the two radical carbons and the concomitant bending of the two cyclohexane mean planes, which are needed to convert OA 2 to cyclopropanone 3. The molecular structure of 1 is characterized by two cyclohexyl groups in chair conformations related by a C2 axis that passes through the center of the four-membered ring (Figure 5). The angle formed by the vectors drawn from the outer cyclohexyl carbons to the center of the cyclubutanedione is 180°, and the corresponding angle to the midpoint of the calculated cyclopropanone C2-C3 bond is 120°, indicating that a ca. 60° angle bending of the two cyclohexane mean planes is needed in going from reactant to product. As shown in Figure 5, the packing structure consists of molecules arranged in layers with the cyclobutanedione planes aligned parallel to the crystal (100) plane, and the carbonyl groups of alternating chains of molecules aligned in orthogonal directions. Each molecule is surrounded by six close neighbors in the plane, which presumably limit the displacement of the cyclohexyl rings. However, calculations of singlet biradical energies and UV-spectra indicate that some torsional relaxation is required to allow for partial conjugation of the CO π bond and the α-carbon radical centers.25
Figure 5.
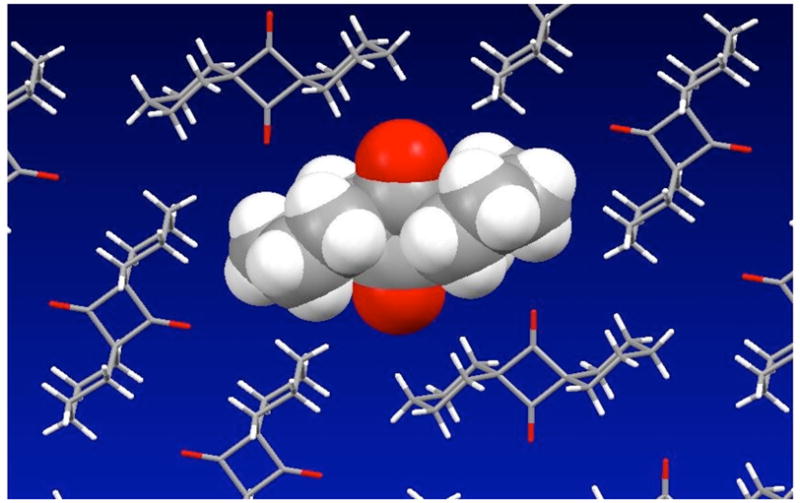
Space-filling model of the molecular structure of 1 and its packing arrangement within crystals in the space group Cmca. The cyclohexane rings of the central molecule must displace close neighbors from their equilibrium positions to form cyclopropanone 3.
In conclusion, we have generated and trapped an OA by the photodecarbonylation of a crystalline spirocyclohexyl-cyclobutanedione and supported its assignment by a combination of solid state UV-Vis absorption, EPR, femtosecond pump-probe spectroscopy, and computational analysis. We have also shown that crystals of cyclobutanedione can extend the lifetime of OA by up to 14 orders of magnitude and, in agreement with recent work by Borden and Lineberger, the electronic structure of 2 is appropriately described as a singlet state biradical.10
Supplementary Material
Acknowledgments
Support by the NSF grants DMR0605688 and CHE0844455, and NIH GM 36700) is acknowledged. G.K. acknowledges NSF: IGERT MCTP grant DGE0114443.
Footnotes
Supporting Information Available: Computational, synthetic, and photochemical procedures. Raman, IR, 1H and 13C NMR, and X- ray diffraction spectra and complete references 24 and 30. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Matlin AR, Lahti PM, Appella D, Straumanic A, Lin S, Patel H, Schrieber KP, Pauls J, Raulerson P. J Am Chem Soc. 1999;121:2164. [Google Scholar]; (b) Liang G, Trauner D. J Am Chem Soc. 2004;126:9544. doi: 10.1021/ja0476664. [DOI] [PubMed] [Google Scholar]; (c) He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. J Am Chem Soc. 2008;130:1003. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lee E, Yoon CH. J Chem Soc, Chem Commun. 1994:479. [Google Scholar]; (b) Noriko T, Yamazaki S, Shinichi Y. Org Biomol Chem. 2008;6:3109. doi: 10.1039/b806577b. [DOI] [PubMed] [Google Scholar]; (c) Hamblin GD, Jimenez RPl, Sorensen TS. J Org Chem. 2007;72:9439. doi: 10.1021/jo701351p. [DOI] [PubMed] [Google Scholar]
- 3.(a) Foehlisch B, Herrscher I. Chem Ber. 1986;119:524. [Google Scholar]; (b) Bhargava S, Hou J, Parvez M, Sorensen TS. J Am Chem Soc. 2005;127:3704. doi: 10.1021/ja043646q. [DOI] [PubMed] [Google Scholar]; (c) Moiseev AG, Manabu A, Danilov EO, Neckers DC. J Org Chem. 2007;72:2777. doi: 10.1021/jo062259r. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zimmerman HE, Suryanarayan V. Eur J Org Chem. 2007;24:4091. [Google Scholar]; (b) Chung WK, Lam SK, Lo B, Lui LL, Wong WT, Chiu P. J Am Chem Soc. 2009;131:4556. doi: 10.1021/ja807566t. [DOI] [PubMed] [Google Scholar]
- 5.Hess BA, Jr, Smentek L, Brash AR, Cha JK. J Am Chem Soc. 1999;121:5603. [Google Scholar]; (b) Kolijak Rl, Boutaud O, Shieh B-H, Samel N, Brash AR. Science. 1997;277:1994. doi: 10.1126/science.277.5334.1994. [DOI] [PubMed] [Google Scholar]
- 6.(a) Malona JA, Cariou K, Frontier AJ. J Am Chem Soc. 2009;131:7560. doi: 10.1021/ja9029736. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang J, Hsung RP. J Am Chem Soc. 2005;127:50. doi: 10.1021/ja044760b. [DOI] [PubMed] [Google Scholar]; (c) Wang Y, Schill BD, Arif AM, West FG. Org Lett. 2003;5:2747. doi: 10.1021/ol034985b. [DOI] [PubMed] [Google Scholar]
- 7.Ichimura AS, Lahti PM, Matlin AR. J Am Chem Soc. 1990;112:2868. [Google Scholar]
- 8.Osamura Y, Borden WT, Morokuma K. J Am Chem Soc. 1984;106:5112. [Google Scholar]
- 9.Lim D, Hrovat DA, Borden WT, Jorgensen WL. J Am Chem Soc. 1994;116:3494. [Google Scholar]
- 10.Ichino T, Villano SM, Gianola AJ, Goebbert DJ, Velarde L, Sanov A, Blanksby SJ, Zhou X, Hrovat DA, Thatcher Borden WT, Lineberger WC. Angew Chem Int Ed. 2009;48:8509. doi: 10.1002/anie.200904417. [DOI] [PubMed] [Google Scholar]
- 11.(a) Cordes MHJ, Berson JA. J Am Chem Soc. 1996;118:6241. [Google Scholar]; (b) Sorensen TS, Sun F. J Am Chem Soc. 1995;117:5592. [Google Scholar]
- 12.Campos LM, Garcia-Garibay MA. Reactive Intermediates in Crystals: Form and Function. In: Platz MS, Jones M, Moss R, editors. Reactive Intermediates. Hoboken, NJ: 2007. [Google Scholar]
- 13.Mortko CJ, Garcia-Garibay MA. Topics in Stereochem. 2006;25:205. [Google Scholar]
- 14.Turro NJ, Leermakers PA, Wilson HR, Neckers DC, Byers GW, Vesley GF. J Am Chem Soc. 1965;87:2613. [Google Scholar]
- 15.Krapcho AP, Abegaz B. J Org Chem. 1974;39:2251. [Google Scholar]
- 16.Veerman M, Resendiz MJE, Garcia-Garibay MA. Org Lett. 2006;8:2615. doi: 10.1021/ol060978m. [DOI] [PubMed] [Google Scholar]
- 17.Irradiation of lumisantonin at 77 K results in a similar transient that could be assigned to oxyallyl: Fisch MH, Richards JH. J Am Chem Soc. 1968;90:1547.
- 18.Generation of 2 by irradiation of dione 1 in neat furan gave both alkene 5 (95%) and 6 (5%). Examples involving sterically hindered oxyally show that it can be untrappable: Greene FD, Sclove DB, Pazos JF, Camp RL. J Am Chem Soc. 1970;92:7488.and ref 11a
- 19.Previous EPR measurements on proposed oxyallyl at 77 K were silent: Hirano T, Kummagai T, Miyashi T. J Org Chem. 1991;56:1907.
- 20.Zang H, Neckers DC. J Org Chem. 1999;64:2103. doi: 10.1021/jo9806862. [DOI] [PubMed] [Google Scholar]
- 21.Steiner G, Huisgen R. J Am Chem Soc. 1973;95:5056. [Google Scholar]
- 22.(a) West FG. CRC Handbook of Organic Photochemistry and Photobiology. 2. CRC Press LLC; Boca Raton: 2004. pp. 22–1. [Google Scholar]; (b) Matlin AR. CRC Handbook of Organic Photochemistry and Photobiology. 2. CRC Press LLC; Boca Raton: 2004. p. 81. [Google Scholar]
- 23.Roos BO, Taylor PR, Siegbahn PEM. Chem Phys. 1980;48:157. [Google Scholar]
- 24.All CASSCF structures were optimized using GAMESS 2009 (R1).
- 25.For analysis of the singlet biradical in crystals please see the SI section.
- 26.(a) Nakano H. J Chem Phys. 1993;99:7983. [Google Scholar]; (b) Nakano H. Chem Phys Lett. 1993;207:372. [Google Scholar]
- 27.(a) Koch H, Jørgensen P. J Chem Phys. 1990;93:3333. [Google Scholar]; (b) Stanton JF, Bartlett RJ. J Chem Phys. 1993;98:7029. [Google Scholar]; (c) Koch H, Kobayashi R, Sánchez de Merás A, Jørgensen P. J Chem Phys. 1994;100:4393. [Google Scholar]; (d) Kállay M, Gauss J. J Chem Phys. 2004;121:9257. doi: 10.1063/1.1805494. [DOI] [PubMed] [Google Scholar]
- 28.Black C, Lario P, Masters AP, Sorensen TS, Sun F. Can J Chem. 1993;71:1910. [Google Scholar]
- 29.Holroyd RA, Blacet FE. J Am Chem Soc. 1957;79:4830. [Google Scholar]
- 30.These calculations were carried out using UB3LYP/6-31+G(d) as implemented in Gaussian 03. ( Frisch MJ, et al. Gaussian 03, revision C.02. Gaussian, Inc; Wallingford, CT: 2004. ).
- 31.An applied scaling factor of 0.971 was obtained from calculations with 2,4 dimethyl 3-pentanone as a model. See SI for details.
- 32.Mozhayskiy V, Goebbert DJ, Velarde L, Sanov A, Krylov AI. J Phys Chem A. 2010;114:6935. doi: 10.1021/jp102183z. [DOI] [PubMed] [Google Scholar]
- 33.Resonance Raman spectra acquired with a laser probe centered within the absorption band of the blue chromophore at λex.= 530 nm were hampered by concomitant photobleaching.
- 34.Haller I, Srinivasan R. Can J Chem. 1965;43:3165. [Google Scholar]
- 35.Diffraction data from crystal of diketone 1 were solved in the space group Cmca (a=9.922, b=6.689, c=18.285, α=β=γ=90°)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.