Abstract
Osteosarcoma incidence rates in the United States peak in adolescence and in the elderly. Whereas international patterns of osteosarcoma incidence in children have been described, those for young, middle age, or elderly adults have not. Using the Cancer Incidence in Five Continents, International Agency for Cancer Research (IARC) database we compared incidence rates for children and adolescents (age 0–24), the middle age group (25–59) and elderly (≥60) persons by world regions and individual countries. Overall, worldwide osteosarcoma incidence rates were quite similar in the younger age groups. The greatest variation in incidence rates was observed in the elderly.
Keywords: osteosarcoma, bone cancer, epidemiology, incidence
Introduction
Bone tumors make up about 3–5% of childhood cancers and less than 1% of cancers in adults.1–4 Of these, osteosarcoma is the most commonly diagnosed primary malignant bone tumor.1, 5–7 Individuals with localized osteosarcoma have an average 5-year survival of about 80% but those with metastatic disease have much worse outcomes.8 Its incidence is bimodally distributed by age with peaks in adolescence and in the elderly.1, 8–12 Osteosarcoma incidence in childhood and adolescence appears to be relatively consistent throughout the world,5, 13–17 however, international comparisons of incidence for other age groups have not been described.
We recently showed that osteosarcoma in children and adolescents, middle ages, and elderly persons have unique epidemiologic features in the United States using data from the Surveillance, Epidemiology, and End Results (SEER) program.8, 18 Osteosarcoma incidence in the youngest cases (age 0–24 years) was greatest in Asian/Pacific Islanders, while it was greatest in Blacks and Whites in the middle age group (age 25–59 years) and elderly (age 60+ years) patients, respectively.8 This study also found that the first osteosarcoma incidence peak occurred at younger ages in females than in males, and from 1973 to 2004 osteosarcoma incidence increased in the youngest age group, was variable in the middle age group, and decreased in the elderly. The anatomic site distributions were more diverse for the older age groups compared with the 0–24 group; and, survival rates varied by age group, anatomic site and disease stage.8 The disparate features found among these three age groups suggest that they should be studied as separate groups.
Here we expanded the characterization of osteosarcoma incidence to include all age groups: children and adolescents (age 0–24), middle ages (25–59), and elderly (age 60+) based on data from the Cancer Incidence in Five Continents, IARC database, to better understand the international epidemiology of osteosarcoma. Advancing understanding of osteosarcoma epidemiology has the potential to identify new etiologic insights in this complex disease.
Materials and Methods
The world incidence rates and case counts were estimated for osteosarcoma from the Cancer Incidence in Five Continents, IARC CancerBase No. 7, CI5-annual detailed data set (ADDS).19 This database includes information from regional cancer registries in Colombia, Ecuador, Canada, USA, India, Israel, Japan, Kuwait, Philippines, Singapore, Thailand, France, Italy, The Netherlands, Poland, Spain, Switzerland, England, and Australia; and national registries in Costa Rica, Denmark, Estonia, Iceland, Slovakia, and Scotland. We included data from both nationally covered countries (unbiased) and regional cancer registries, which may not be representative of the whole country, to maximize estimates of international coverage. The geographic coverage by country and registry are described in more detail in the referenced database19. Incidence rates were estimated for each regional registry (for the countries with more than one registry), country, world region, and for all of the countries combined (the world rate). For countries with multiple regional registries and for the world estimates, data were pooled from all of the registries for each country, or all of the available countries for the world rate, and the incidence is calculated using the pooled data. All osteosarcoma incidence rates are per 1,000,000, and are age-standardized to a world population to account for differences in the age distribution of patients between countries.
All osteosarcoma cases were classified according to the International Classification of Disease for Oncology (ICD-O-2, codes 9180–9200).20 Data are presented by age group (0–24, 25–59, and 60–85+ years) and by sex. These age groups were chosen to focus on the two incidence peaks in children and adolescents and the elderly, and the plateau in the middle age group. Geographic region and time period are described in the table and figure. Rates that are based on less than 10 cases are considered unstable and should be interpreted with caution (these estimates are indicated by italics).
Results
Osteosarcoma incidence rates among individuals ≤24 years were generally consistent worldwide with peaks around puberty. Most rates ranged from 3–5 per million in males (average of 4.3) and 2–4 per million in females (average of 3.4) (Table 1). Osteosarcoma was more common in males than females in most countries. The overall world male-to-female ratio of osteosarcoma in ages 0–24 was 1.43:1. Incidence peaked in males at age 15–19 and in females at age 10–14 in all regions studied (Figure 1). Incidence rates in females were as high or higher than those observed in males less than 15 years (age 0–14) almost everywhere, but then increased later in puberty for males.
Table 1.
Incidence of osteosarcoma by age group and sex.
| ASR (n) | |||||||
|---|---|---|---|---|---|---|---|
| Ages 0–24 | Ages 25–59 | Ages 60+ | |||||
| Country (years), regional registry | Male | Female | Male | Female | Male | Female | |
| Europe (1958–1997) | 4.0 (990) | 3.1 (712) | 1.7 (484) | 1.2 (339) | 4.5 (465) | 3.1 (490) | |
| East (1968–1997) | 2.8 (114) | 2.1 (82) | 1.5 (59) | 1.3 (56) | 3.3 (36) | 2.4 (42) | |
| Estonia (1968–1997)* | 2.2 (20) | 2.5 (20) | 1.6 (15) | 1.0 (12) | 3.3 (8) | 2.4 (13) | |
| Poland (1978–1997), Cracow City | 2.0 (7) | 3.2(8) | 0.3 (1) | 0.9 (3) | 1.2 (1) | 2.5 (3) | |
| Slovakia (1973–1997)* | 3.0 (87) | 1.9 (54) | 1.6 (43) | 1.5 (41) | 3.5 (27) | 2.4 (26) | |
| North (1958–1997) | 4.8 (104) | 2.9 (60) | 1.9 (49) | 1.6 (40) | 2.6 (23) | 2.3 (31) | |
| Denmark (1978–1997)* | 4.7 (93) | 2.9 (54) | 1.9 (46) | 1.6 (37) | 2.3 (20) | 2.1 (27) | |
| Iceland (1958–1997)* | 4.9 (11) | 2.9 (6) | 1.7 (3) | 1.7 (3) | 6.2 (3) | 5.8 (4) | |
| West (1970–1997) | 4.5 (127) | 3.2 (84) | 2.3 (76) | 1.7 (54) | 2.6 (29) | 3.1 (48) | |
| The Netherlands (1973–1997), Eindhoven | 4.3 (23) | 3.3 (16) | 2.3 (13) | 1.9 (10) | 3.8 (6) | 3.3 (6) | |
| France (1975–1997) | 4.5 (74) | 3.0 (45) | 2.4 (41) | 1.7 (27) | 2.7 (16) | 2.4 (19) | |
| Bas–Rhin (1975–1997) | 4.3 (20) | 3.6 (15) | 1.7 (9) | 2.0 (10) | 2.3 (4) | 2.3 (4) | |
| Calvados (1978–1997) | 2.9 (8) | 3.0 (7) | 2.7 (7) | 2.7 (7) | 2.1 (2) | 3.0 (3) | |
| Doubs (1978–1997) | 6.9 (15) | 2.6 (5) | 2.3 (5) | 1.4 (3) | 6.2 (4) | 1.2 (2) | |
| Isere (1979–1997) | 5.6 (22) | 2.7 (10) | 2.9 (12) | 1.3 (5) | 2.6 (3) | 1.9 (4) | |
| Somme (1983–1997) | 3.1 (6) | 2.8 (5) | 1.8 (3) | 0.6 (1) | 0.9 (1) | 4.2 (3) | |
| Tarn (1983–1997) | 2.8 (3) | 3.4 (3) | 4.2 (5) | 1.1 (1) | 2.9 (2) | 1.8 (3) | |
| Switzerland (1970–1997) | 4.5 (30) | 3.7 (23) | 2.2 (22) | 1.7 (17) | 2.0 (7) | 4.3 (23) | |
| Basel (1983–1997) | 2.1 (2) | 4.5 (4) | 3.1 (5) | 1.7 (3) | 2.2 (1) | 4.8 (5) | |
| Geneva (1970–1997) | 4.9 (8) | 4.4 (7) | 1.6 (4) | 1.4 (4) | 3.4 (3) | 4.8 (7) | |
| St Gall–Appenzell (1983–1997) | 6.2 (9) | 3.6 (5) | 2.8 (5) | 0.5 (1) | 2.0 (1) | 1.2 (1) | |
| Zurich (1983–1996) | 4.5 (11) | 3.0 (7) | 1.9 (8) | 2.2 (9) | 1.2 (2) | 5.1 (10) | |
| South (1973–1997) | 4.3 (102) | 4.3 (91) | 1.9 (49) | 1.1 (29) | 4.1 (43) | 2.4 (33) | |
| Italy (1978–1997) | 5.3 (51) | 4.5 (38) | 1.6 (20) | 0.8 (11) | 3.0 (16) | 2.6 (20) | |
| Florence (1985–1997) | 6.1 (15) | 4.1 (9) | 1.5 (5) | 0.5 (2) | 1.7 (3) | 1.4 (3) | |
| Parma Province (1978–1997) | 4.4 (6) | 4.7 (5) | 2.0 (3) | 1.6 (3) | 4.3 (4) | 1.4 (2) | |
| Ragusa Province (1983–1997) | 3.1 (3) | 4.1 (3) | 1.1 (1) | 1.0 (1) | 3.0 (1) | 6.9 (3) | |
| Torino (1985–1997) | 5.8 (12) | 3.9 (7) | 1.6 (5) | 0.3 (1) | 4.5 (5) | 2.4 (5) | |
| Lombardy, Varese Province (1978–1997) | 5.4 (15) | 5.0 (14) | 1.6 (6) | 0.9 (4) | 2.2 (3) | 3.8 (7) | |
| Spain (1973–1997) | 3.6 (51) | 4.2 (53) | 2.2 (29) | 1.4 (18) | 5.3 (27) | 2.1 (13) | |
| Granada (1985–1997) | 3.2 (8) | 5.8 (13) | 2.8 (6) | 1.9 (4) | 1.4 (1) | 2.2 (2) | |
| Murcia (1983–1996) | 4.2 (15) | 2.0 (7) | 1.6 (5) | 0.3 (1) | 5.1 (5) | 0.7 (1) | |
| Navarra (1973–1997) | 2.4 (7) | 4.5 (12) | 1.5 (4) | 0.8 (2) | 2.1 (2) | 3.1 (3) | |
| Tarragona (1983–1997) | 5.2 (9) | 4.6 (7) | 1.8 (3) | 3.5 (6) | 4.8 (3) | 1.0 (1) | |
| Zaragoza (1978–1997) | 3.4 (12) | 5.0 (14) | 2.9 (11) | 1.3 (5) | 10.7 (16) | 2.9 (6) | |
| UK (1960–1997) | 4.0 (543) | 3.1 (395) | 1.6 (251) | 1.0 (160) | 5.4 (334) | 3.4 (336) | |
| England (1960–1997) | 4.1 (450) | 3.2 (333) | 1.7 (217) | 1.0 (132) | 5.3 (282) | 3.3 (273) | |
| Birmingham & West Midlands (1979–1997) | 3.8 (75) | 3.4 (62) | 1.4 (33) | 0.7 (16) | 3.1 (28) | 2.8 (41) | |
| Merseyside & Cheshire (1975–1997) | 3.9 (44) | 2.8 (30) | 1.4 (17) | 1.0 (11) | 6.5 (34) | 2.6 (22) | |
| North Western Region (1979–1997) | 4.2 (64) | 3.1 (44) | 1.8 (31) | 1.2 (20) | 8.1 (57) | 3.7 (45) | |
| Oxford Region (1985–1997) | 3.6 (24) | 3.1 (18) | 2.1 (17) | 1.1 (8) | 8.6 (23) | 2.1 (8) | |
| South Thames Region (1960–1997) | 4.1 (195) | 3.3 (148) | 1.9 (103) | 1.2 (70) | 5.0 (119) | 3.5 (129) | |
| Yorkshire Region (1983–1997) | 4.6 (48) | 3.2 (31) | 1.3 (16) | 0.6 (7) | 4.5 (21) | 3.8 (28) | |
| Scotland (1975–1997)* | 3.8 (93) | 2.8 (62) | 1.3 (34) | 1.0 (28) | 5.4 (52) | 3.9 (63) | |
| Asia (1963–1997) | 4.1 (890) | 2.5 (512) | 1.5 (294) | 1.1 (207) | 3.1 (114) | 2.4 (105) | |
| Singapore (1968–1997) | 3.9 (71) | 3.9 (63) | 1.5 (23) | 1.2 (18) | 1.7 (4) | 1.7 (5) | |
| Chinese (1968–1997) | 3.7 (56) | 4.1 (56) | 1.7 (22) | 1.3 (17) | 2.0 (4) | 1.9(5) | |
| Malay (1968–1997) | 5.0 (15) | 2.6 (7) | 0.8 (1) | 0.4 (1) | 0.0 (0) | 0.0 (0) | |
| Japan (1963–1997) | 3.2 (229) | 2.1 (137) | 1.2 (104) | 0.7 (58) | 2.3 (43) | 1.8 (44) | |
| Miyagi Prefecture (1978–1997) | 4.1 (37) | 2.2 (18) | 1.4 (15) | 0.7 (8) | 3.6 (11) | 2.2 (9) | |
| Nagasaki City (1973–1997) | 6.1 (13) | 3.4 (8) | 1.6 (4) | 1.0 (3) | 5.4 (4) | 4.9 (5) | |
| Osaka Prefecture (1963–1997) | 2.9 (179) | 2.0 (111) | 1.2 (85) | 0.7 (47) | 2.0 (28) | 1.5 (30) | |
| India (1978–1997) | 3.8 (286) | 1.9 (120) | 1.2 (73) | 1.1 (48) | 3.2 (20) | 2.7 (17) | |
| Chennai (1983–1997) | 4.3 (68) | 2.3 (35) | 1.5 (21) | 1.1 (13) | 3.8 (7) | 1.5 (3) | |
| Mumbai (1978–1997) | 3.7 (218) | 1.8 (85) | 1.1 (52) | 1.1 (35) | 2.9 (13) | 3.2 (14) | |
| Israel (1963–1997), Jews | 5.1 (146) | 3.1 (85) | 2.3 (50) | 2.1 (50) | 4.0 (31) | 2.2 (20) | |
| Kuwait (1983–1997), Kuwaitis | 5.4 (14) | 4.3 (11) | 1.7 (2) | 0.0 (0) | 0.0 (0) | 7.9 (1) | |
| Philippines (1983–1997), Manila | 6.7 (125) | 4.4 (90) | 3.1 (34) | 3.0 (32) | 11.8 (14) | 11.1 (18) | |
| Thailand (1983–1997), Chiang Mai | 3.8 (19) | 1.2 (6) | 1.6 (8) | 0.2 (1) | 2.1 (2) | 0.0 (0) | |
| Latin America (1980–1997) | 5.0 (126) | 4.0 (100) | 2.5 (41) | 1.4 (26) | 2.4 (7) | 1.4 (5) | |
| Colombia (1983–1997), Cali | 7.6 (45) | 3.5 (22) | 2.4 (11) | 2.9 (14) | 0.0 (0) | 1.2 (1) | |
| Costa Rica (1980–1997)* | 3.5 (53) | 3.9 (57) | 1.7 (15) | 0.5 (4) | 1.8 (3) | 0.4 (1) | |
| Ecuador (1985–1997), Quito | 7.0 (28) | 4.9 (21) | 5.3 (15) | 2.1 (8) | 8.2 (4) | 5.3 (3) | |
| USA (1973–1997) | 4.4 (708) | 3.6 (552) | 2.0 (353) | 1.7 (300) | 4.9 (245) | 3.8 (261) | |
| California§ (1973–1997) | 4.3 (238) | 3.7 (188) | 2.2 (136) | 2.2 (141) | 5.2 (87) | 4.0 (97) | |
| Connecticut (1973–1997) | 3.8 (61) | 3.2 (50) | 1.9 (33) | 1.0 (19) | 6.3 (39) | 4.2 (37) | |
| Atlanta, Georgia (1975–1997) | 4.3 (41) | 3.2 (29) | 1.5 (18) | 1.8 (21) | 3.3 (7) | 4.1 (13) | |
| New Orleans, Louisiana (1983–1997) | 4.3 (13) | 3.0 (10) | 3.3 (11) | 1.5 (5) | 7.2 (8) | 5.0 (9) | |
| Detroit, Michigan (1973–1997) | 4.2 (90) | 3.4 (70) | 2.2 (49) | 1.2 (27) | 4.9 (30) | 3.1 (29) | |
| Hawaii (1973–1997) | 5.8 (32) | 4.3 (22) | 1.6 (10) | 1.3 (7) | 3.7 (6) | 2.4 (5) | |
| Iowa (1973–1997) | 4.8 (76) | 3.6 (55) | 2.1 (33) | 1.7 (26) | 5.3 (33) | 4.5 (36) | |
| New Mexico (1973–1997) | 4.3 (35) | 4.7 (38) | 1.6 (12) | 1.1 (8) | 3.2 (7) | 2.5 (7) | |
| Utah (1973–1997) | 4.6 (48) | 4.0 (40) | 1.9 (16) | 1.9 (15) | 2.8 (6) | 4.0 (10) | |
| Seattle, Washington (1974–1997) | 4.9 (74) | 3.6 (50) | 2.0 (35) | 1.7 (31) | 4.3 (22) | 3.0 (18) | |
| Australia (1978–1997) | 3.9 (156) | 2.6 (97) | 1.4 (62) | 1.7 (71) | 7.0 (104) | 3.5 (70) | |
| New South Wales (1983–1997) | 3.8 (68) | 2.1 (36) | 1.8 (36) | 1.6 (32) | 7.5 (50) | 3.2 (30) | |
| South (1978–1997) | 3.0 (18) | 2.7 (15) | 1.0 (6) | 1.2 (7) | 7.8 (18) | 2.6 (8) | |
| Tasmania (1978–1997) | 2.4 (5) | 2.6 (5) | 0.9 (2) | 2.3 (5) | 8.4 (7) | 3.9 (3) | |
| Victoria (1983–1997) | 4.6 (65) | 3.3 (41) | 1.2 (18) | 1.8 (27) | 5.8 (29) | 4.3 (29) | |
| Canada (1983–1997)† | 4.4 (360) | 3.7 (281) | 1.8 (178) | 1.6 (159) | 4.9 (148) | 3.6 (139) | |
| Alberta (1973–1997) | 4.5 (59) | 3.7 (47) | 1.8 (23) | 1.2 (14) | 3.8 (13) | 4.9 (18) | |
| British Columbia (1978–1997) | 5.0 (63) | 4.1 (47) | 1.3 (20) | 1.9 (28) | 3.6 (18) | 4.0 (23) | |
| Manitoba (1958–1997) | 5.6 (53) | 3.6 (33) | 2.1 (18) | 2.3 (19) | 5.0 (16) | 4.4 (16) | |
| New Brunswick (1978–1997) | 3.0 (10) | 1.5 (5) | 1.4 (4) | 0.6 (2) | 4.3 (5) | 3.1 (3) | |
| Newfoundland (1978–1997) | 5.6 (17) | 4.4 (12) | 1.5 (4) | 0.9 (2) | 6.5 (5) | 0.9 (1) | |
| Nova Scotia (1978–1997) | 2.8 (11) | 2.1 (7) | 2.3 (10) | 2.0 (8) | 7.8 (10) | 4.5 (7) | |
| Ontario (1978–1997) | 4.7 (188) | 3.4 (125) | 1.8 (86) | 1.4 (66) | 4.1 (56) | 3.1 (56) | |
| Prince Edward Island (1983–1997) | 4.6 (2) | 7.6 (3) | 2.6 (1) | 2.3 (1) | 0.0 (0) | 5.1 (1) | |
| Saskatchewan (1968–1997) | 4.7 (34) | 4.5 (31) | 1.7 (10) | 1.6 (10) | 2.3 (5) | 2.1 (7) | |
| World total | 4.2 (3230) | 3.1 (2254) | 1.7 (1412) | 1.4 (1102) | 4.6 (1083) | 3.3 (1070) | |
ASR, world age–standardized rate per 1,000,000; n, number of cases; Italics indicate potentially unstable rates based on less than 10 cases;
countries with national registries;
includes data from registries in Los Angeles and San Francisco;
Canada has national and regional registries, both data are shown. The world rates include data from all listed countries and registries.
Figure 1.
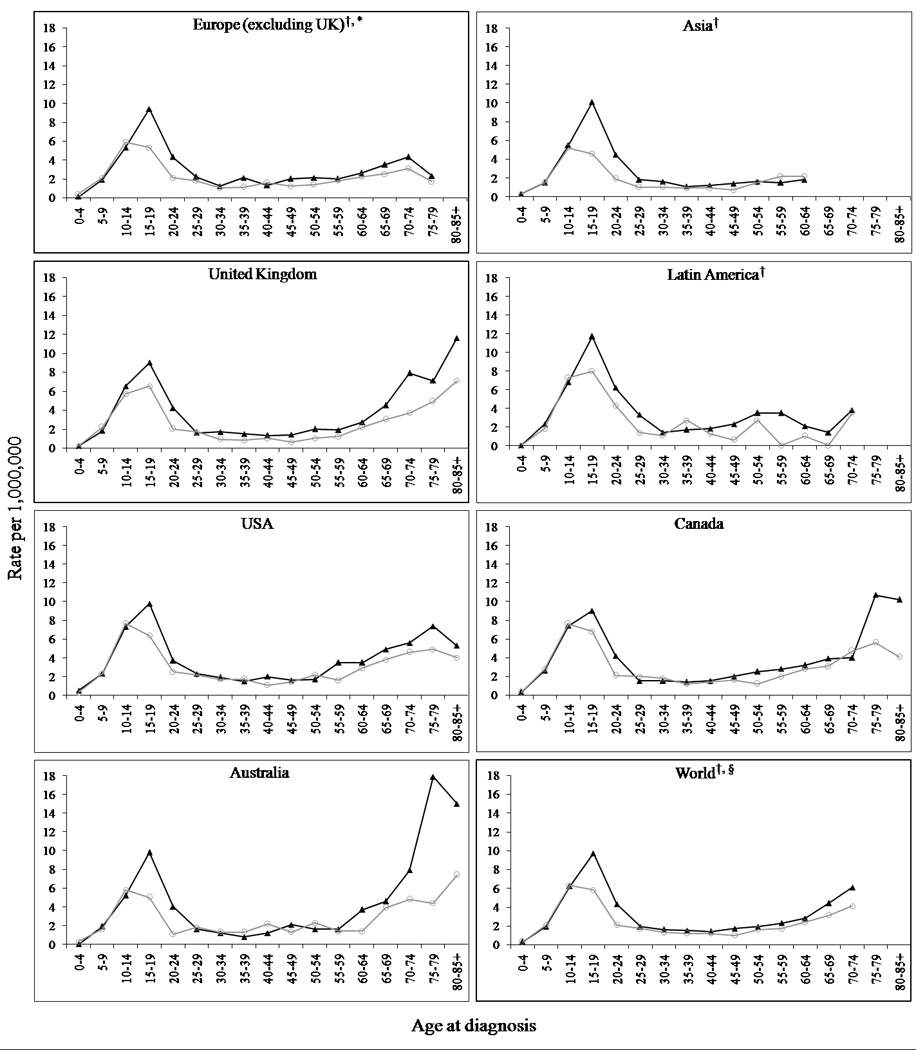
Osteosarcoma incidence by country or region. Calendar period and the countries with national or regional registries are shown in Table 1. The world rates include data from all countries and registries listed in Table 1. Black triangles are male rates, grey circles are female rates; †, number of osteosarcoma cases were available for all ages in this region but rate data was not; *, 75–79 age group includes data from only East, West, and South European countries; §, 65–69 and 70–74 age groups do not include data from Asia, Latin America or North European countries.
The majority of osteosarcoma incidence rates among persons age 25–59 were in the range of 1.5–2.5 per million in males for each country (average of 1.9) and 0.5–2 in females (average 1.36) (Table 1). There was a plateau of incidence observed in all regions in this age group, although more variation was observed in Latin America (Figure 1). Osteosarcoma was more common in males than females in most countries, with a male-to-female ratio of 1.28:1.
There was a second peak of osteosarcoma incidence in the elderly observed in the majority of countries and regions, although it was comparatively lower in Europe (excluding U.K.; Figure 1). Osteosarcoma incidence rates were more varied among the elderly, however the majority were in the range of 2.5–5 per million in males (average of 4.0) and 1.5–4 in females (average 3.1) (Table 1). Males age 75+ had strikingly higher incidence rates in Australia (15–18) and Canada (10–11), and for male patients age 80+ in the U.K. (11.6), as compared to other countries (rates in the range of 1–7 for 75+ males) (Figure 1). Osteosarcoma was more common in males age 60+ than females of the same age group in most countries, although rates were higher in females in Western Europe (Table 1). The worldwide male-to-female ratio was 1.01:1 for this age group.
Discussion
This is the first report of international osteosarcoma incidence in subjects of all ages to our knowledge. The recent study of incidence and survival patterns in the U.S. found that osteosarcoma incidence, time-trends, survival, pathologic subtype, and anatomic site were varying among children and adolescents, middle ages, and elderly persons.8 This suggested that there may be important osteosarcoma incidence differences in these age groups throughout the world.
In individuals ≤24 years, the incidence rates were minimally variable between countries. Previously, a higher incidence of childhood osteosarcoma was reported in Southern Europe,15 particularly in Italy.5 We also found a slightly higher incidence in Italy (males: 5.3, females: 4.5) for ages 0–24, particularly for males in Florence and Torino. However, the highest rates were in Latin America (males: 7.0–7.6, females: 3.5–4.9). Rates were particularly high in all age groups in the Philippines and Ecuador. A high incidence of osteosarcoma has been observed in an earlier report in two African countries, Sudan and Uganda, compared to the relative frequency in populations of European origin.14 This is consistent with the finding that young and middle aged individuals of African American descent in the US had higher rates of osteosarcoma than whites.8 It is important to note that other differences between ethnic groups may be observed, but data are less complete in those populations
The earlier world-wide incidence peak observed in adolescent girls compared to boys links osteosarcoma to bone growth and an earlier occurrence of the adolescent growth spurt in girls, supporting the idea that bone growth and/or hormonal changes during puberty may have a role in osteosarcoma pathogenesis.21–23 The observation that the vast majority of adolescent tumors develop in the long bone epiphyses of the lower limbs1, 8, 24, 25 lends further support to this hypothesis.
An interesting difference between the age groups is the overall 1:1 male-to-female ratio of osteosarcoma cases in the elderly compared to the younger age groups male predominance (ages ≤24, 1.43:1; ages 25–59, 1.28:1). In the U.S. using SEER incidence data, osteosarcoma in the elderly was found to occur slightly less commonly in males than females (0.9:1).8 The rates were strikingly high for elderly male patients in the U.K. (particularly in the North Western and Oxford Regions), Australia, and Canada, to our knowledge the first time this has been reported. The interpretation of data from some Asian and Latin American countries was limited because some of these rates are based on less than 10 cases and may be unstable. Rates were not available for each elderly age stratum in these two regions, so it was difficult to determine an incidence peak in the elderly; interestingly, numbers of cases reported and the overall rates were comparatively lower in these regions.
The greater geographic variation observed in elderly patients could be due to several reasons: (1) diagnosis and/or classification differences among countries; (2) differences in completeness of cancer registration; (3) differences in environmental exposures that could alter risk of primary osteosarcoma; (4) exposures, such as irradiation, that could increase the chance of secondary osteosarcoma; and/or (5) different genetic components to osteosarcoma in the elderly, such as Paget’s disease.26 In elderly patients, osteosarcoma is often considered a secondary neoplasm attributed to the sarcomatous transformation of Paget’s disease of bone.27, 28 We were unable to separate osteosarcomas occurring with Paget’s disease from those that did not with this database. There is marked geographic variation observed in the prevalence of Paget’s disease, with a high prevalence noted in the UK, Australia, and North America, and lower levels in Asia and the Middle East.29, 30 which is similar to the high and low regions of elderly osteosarcoma incidence.
Competing risks due to different environmental and/or genetic risk factors could also be involved in the geographic variation observed in the elderly patients. For example, osteosarcoma in this age group may occur in body sites that have been previously irradiated for a different cancer,27, 28, 31 a variable that we were unable to assess. Differences based on geographic location in access to radiotherapy and other cancer treatment modalities could also exist which would affect the rates of secondary osteosarcoma..
It is theoretically possible that vitamin D deficiency could contribute to risk of osteosarcoma since vitamin D is required for bone development and vitamin D deficiency has been associated with risk of developing colon, prostate, breast, and several other cancers.32, 33 Vitamin D deficiency is recognized as pandemic34–38 and the elderly may be particularly affected.37, 39 One could also speculate that the increased rates in subjects of African descent are related to differences in vitamin D absorption. This hypothesis in osteosarcoma susceptibility has yet to be tested.
It is difficult to compare international survival rates for osteosarcoma because there are few estimates in the literature, few available data sources for older persons, and time frames have been reported differently. However, data from the Automated Childhood Cancer Information System (ACCIS; for ages 0–19 from 1993–1997)40 and the Eurocare-3 Study: Survival of Cancer Patients in Europe (for ages 0–14 from 1990–1994)41, 42 show that the 5-year survival rates for children and adolescents are similar in most European countries (55–75%), except for lower rates observed in Slovakia (19–20%), Estonia (26%), and Denmark (39%). Similar 5-year survival rates have also been observed in the U.S. (62%, ages 0–24; 68%, ages 0–14)8, 43 and Japan (51%, ages 0–14).44 The lower survival rates observed in Estonia, Slovakia, and Denmark could be a consequence of limited access to early diagnosis and appropriate treatment.43 The 5-year osteosarcoma survival rates in the U.S.8 and Japan44 increased significantly from the 1970s to the 1980s (U.S.: 51 to 63%; Japan: 18 to 51%), but have not improved further since the 1980s to the 1990s in the U.S.8 and Europe.42, 45 This lack of improvement during the last decade indicates an international need for more efficient management and new treatment strategies.
In summary, worldwide osteosarcoma incidence rates varied most in the elderly, with little geographic variation among the younger age groups. These data illustrate the importance of separating osteosarcoma incidence data by these age groups and inclusion of this information in more studies and databases.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. We thank anonymous reviewers for very helpful comments.
References
- 1.Dorfman HA, Czerniak B. Bone Cancers. Cancer supplement. 1995;75:203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Cancer facts and figures 2008. Atlanta: American Cancer Society Inc.; 2008. [Google Scholar]
- 3.Gurney JG, Swensen AR, Bulterys M. Malignant bone tumors. In: Reis LAG Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program, 1975–1995. Bethesda, MD: National Cancer Institute; 1999. pp. 99–110. NIH Pub. No. 99-4649. [Google Scholar]
- 4.Mascarenhas L, Siegel S, Spector L, Arndt C, Femino D, Malogolowkin M. Malignant bone tumors: cancer in 15- to 29-year-olds in the United States. In: Bleyer A, O'Leary M, Barr R, Ries LAG, editors. Cancer Epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Cancer Institute; 2006. pp. 98–109. NIH Pub. No. 06-5767. [Google Scholar]
- 5.Parkin DM, Kramárová E, Draper GJ, Masuyer E, Michaelis J, Neglia J, Qureshi S, Stiller CA. IARC Scientific Publications No. 144. vol. 2. Lyon: 1998. International incidence of childhood cancer. [Google Scholar]
- 6.Damron TA, Ward WG, Stewart A. Osteosarcoma, Chrondrosarcoma, and Ewing's Sarcoma. National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin DC, Unni KK. Bone Tumors: general aspects and data on 8,542 cases. 4th ed. Springfield, IL: Thomas; 1986. [Google Scholar]
- 8.Mirabello L, Troisi RJ, Savage S. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2008 doi: 10.1002/cncr.24121. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unni KK. Dahlin's bone tumors: general aspects and data on 11,087 cases. 5th ed. Philadelphia: Lippincott-Raven; 1996. pp. 143–183. [Google Scholar]
- 10.Larsson SE, Lorentzon R. The incidence of malignant primary bone tumors in relation to age, sex, and site: a study of osteogenic sarcoma, chondrosarcoma, and Ewing's sarcoma diagnosed in Sweden from 1958 to 1968. J Bone Joint Surg. 1974;56B:534–540. [PubMed] [Google Scholar]
- 11.Polednak AP. Primary bone cancer incidence in black and white residents of New York state. Cancer. 1985;55:2883–2888. doi: 10.1002/1097-0142(19850615)55:12<2883::aid-cncr2820551231>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Hemminki K. Familial risk for histology-specific bone cancers: An updated study in Sweden. Eur J Cancer. 2006;42:2343–2349. doi: 10.1016/j.ejca.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Stiller CA. International patterns of cancer incidence in adolescents. Cancer Treatment Reviews. 2007;33:631–645. doi: 10.1016/j.ctrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Stiller CA, Draper GJ, Bieber CA. The international incidence of childhood cancer. Int J Cancer. 1988;42:511–520. doi: 10.1002/ijc.2910420408. [DOI] [PubMed] [Google Scholar]
- 15.Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–2135. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. Br Med Bull. 1996;52:682–703. doi: 10.1093/oxfordjournals.bmb.a011577. [DOI] [PubMed] [Google Scholar]
- 17.Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW. ACCIS Scientific Committee. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet. 2004;364:2097–2105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973–2004 varying) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch released April 2007, based on the November 2006 submission
- 19.Parkin DM, Whelan S, Ferlay J, Storm H, editors. CancerBase No. 7. Vol. I to VIII. Lyon: IARC; 2005. Cancer Incidence in Five Continents. [Google Scholar]
- 20.World Health Organization. Van Holten V, Muir C, editors. Geneva: World Health Organization; International classification of diseases for oncology: Morphology. 1990;vol. 2nd
- 21.Glass AG, Fraumeni JF. Epidemiology of bone cancer in children. J Natl Cancer Inst. 1970;44:187–199. [PubMed] [Google Scholar]
- 22.Miller RW. Contrasting epidemiology of childhood osteosarcoma, Ewing's sarcoma, and rhabdomyosarcoma. Nat Cancer Inst Monogr. 1981;56:9–15. [PubMed] [Google Scholar]
- 23.dos Santos Silva I, Swerdlow AJ. Sex differences in the risks of hormonedependent cancers. Am J Epidemiol. 1993;138:10–28. doi: 10.1093/oxfordjournals.aje.a116773. [DOI] [PubMed] [Google Scholar]
- 24.Fraumeni JF., Jr Stature and malignant tumors of the bone in childhood and adolescence. Cancer. 1967;20:967–973. doi: 10.1002/1097-0142(196706)20:6<967::aid-cncr2820200606>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher CDM, Unni KK, editors. Pathology and genetics of soft tissue and bone. Lyon, France: IARC Press; 2002. World Health Organization classification of tumours. [Google Scholar]
- 26.Daroszewska A, Ralston SH. Genetics of Paget's disease of bone. Clin Sci (Lond) 2005;109:257–263. doi: 10.1042/CS20050053. [DOI] [PubMed] [Google Scholar]
- 27.Price CHG. Osteogenic sarcoma: an analysis of the age and sex incidence. Br J Cancer. 1955;9:558–574. doi: 10.1038/bjc.1955.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray RO. Tumor and tumorlike lesions of bone. In: Sutton D, Grainger RG, editors. A textbook of radiology. 2nd edition. New York: Churchill Livingstone; 1975. pp. 107–137. [Google Scholar]
- 29.Colina M, La Corte R, De Leonardis F, Trotta F. Paget’s disease of bone: a review. Rheumatol Int. 2008 doi: 10.1007/s00296-008-0640-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Cooper C, Harvey NC, Dennison EM, van Staa TP. Update on the Epidemiology of Paget’s Disease of Bone. J Bone Miner Res. 2007;21:P3–P8. doi: 10.1359/jbmr.06s201. [DOI] [PubMed] [Google Scholar]
- 31.Sabanas AO, Dahin DC, Childs DS, Ivings JC. Postirradiation sarcoma of bone. Cancer. 1956;9:528–542. doi: 10.1002/1097-0142(195605/06)9:3<528::aid-cncr2820090316>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol. 2006;92:65–79. doi: 10.1016/j.pbiomolbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Hull S. Vitamin D deficiency. Br J Gen Pract. 2007;57:836–837. [PMC free article] [PubMed] [Google Scholar]
- 35.Mark S, Gray-Donald K, Delvin EE, O’Loughlin J, Paradis G, Levy E, Lambert M. Low Vitamin D Status in a Representative Sample of Youth From Quebec, Canada. Clin Chem. 2008;54:1283–1289. doi: 10.1373/clinchem.2008.104158. [DOI] [PubMed] [Google Scholar]
- 36.van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW, Blizzard L, Dwyer T, Lucas R, Jones G. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115:1132–1139. doi: 10.1289/ehp.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 38.Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust. 2002;177:149–152. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 39.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 40.Automated Childhood Cancer Information System. Cancer incidence and survival by registry and tumour. last updated 11/03/2003. [Google Scholar]
- 41.Berrino F, Capocaccia R, Coleman MP, Esteve J, Gatta G, Hakulinen T, Micheli A, Sant M, Verdecchia A. Survival of Cancer Patients in Europe: The Eurocare-3 Study. Annals of Oncolology. 2003;vol. 14 Suppl 5 [Google Scholar]
- 42.the EUROCARE Working Group. Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M. Childhood cancer survival trends in Europe: a EUROCARE Working Group studyroup study. J Clin Oncol. 2005;23:3742–3751. doi: 10.1200/JCO.2005.00.554. [DOI] [PubMed] [Google Scholar]
- 43.Gatta G, Capocaccia R, Coleman MP, Ries LAG, Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95:1767–1772. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- 44.Ajiki W, Hanai A, Tsukuma H, Hiyama T, Fujimoto I. Survival rates of childhood cancer patients in Osaka, Japan, 1975–1984. Jpn J Cancer Res. 1995;86:13–20. doi: 10.1111/j.1349-7006.1995.tb02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arndt V, Lacour B, Steliarova-Foucher E, Spix C, Znaor A, Pastore G, Stiller C, Brenner H. Up-to-date monitoring of childhood cancer long-term survival in Europe: tumours of the sympathetic nervous system, retinoblastoma, renal and bone tumours, and soft tissue sarcomas. Ann Oncol. 2007;18:1722–1733. doi: 10.1093/annonc/mdm189. [DOI] [PubMed] [Google Scholar]


