Abstract
The zona pellucida contains three proteins (ZP1, ZP2, ZP3), the precursors of which possess signal peptides, ‘zona’ domains and short (9–15 residue) cytoplasmic tails downstream of a transmembrane domain. The ectodomains of ZP2 and ZP3 are sufficient to form the insoluble zona matrix and yet each protein traffics through oocytes without oligomerization. ZP2 and ZP3 were fluorescently tagged and molecular interactions were assayed by fluorescent complementation in CHO cells and growing oocytes. ZP2 and ZP3 traffic independently, but colocalize at the plasma membrane. However, protein–protein interactions were observed only after release and incorporation of ZP2 and ZP3 into the extracellular matrix surrounding mouse oocytes. In the absence of their hydrophilic cytoplasmic tails, ZP2 and ZP3 interacted within the cell and did not participate in the zona pellucida. A heterologous GPI-anchored ‘zona’ domain protein fused with the cytoplasmic tails was integrated into the zona matrix. We conclude that the cytoplasmic tails are sufficient and necessary to prevent intracellular oligomerization while ensuring incorporation of processed ZP2 and ZP3 into the zona pellucida.
Key words: Cytoplasmic tails, Zona pellucida, Oocytes, Intracellular trafficking, Extracellular matrix, ZP2, ZP3, β-Tectorin
Introduction
Extracellular matrices present in the interstices of multicellular organisms form three-dimensional networks that provide physical support and modulate biological function (Aszodi et al., 2006; Tsang et al., 2010). Their macromolecular components share a common challenge in remaining soluble during biosynthesis and intracellular trafficking while retaining the ability to form an insoluble matrix in the extracellular space. Various strategies have evolved, several of which have been characterized in molecular detail. For example, collagen precursors are synthesized with N- and C-terminal globular domains that are proteolytically removed to allow formation of extracellular intermediates that culminate in formation of mature collagen fibrils (Shoulders and Raines, 2009). Likewise, soluble tropoelastin is secreted and deposited on pre-existing microfibrillar matrices where they undergo conformational changes to enable cross-linking and formation of insoluble elastin polymers (Sato et al., 2007). Another set of extracellular proteins use a ‘zona domain’ (Bork and Sander, 1992) for polymerization of tissues (Jovine et al., 2005), including the tectorin membrane of the inner ear (Richardson et al., 2008) and the zona pellucida formed during folliculogenesis in the mammalian ovary (Wassarman, 2008).
The newborn mouse ovary is composed primarily of primordial follicles in which each oocyte is surrounded by a flattened layer of granulosa cells encased in a basement membrane (Brambell, 1928). With cyclic periodicity, cohorts are selected to enter into a 2 week growth phase in which oocytes grow from ~15 to 80 μm during which time they form the extracellular zona pellucida (Yanagimachi, 1994). The mouse zona is composed of three glycoproteins, ZP1, ZP2 and ZP3 (Bleil and Wassarman, 1980a; Boja et al., 2003) and has an important role in gamete recognition. This is dependent on the cleavage status of ZP2, rendering the zona pellucida either permissive (intact ZP2) or non-permissive (cleaved ZP2) for sperm binding (Gahlay et al., 2010). Although primarily investigated for its role in fertilization and prevention of polyspermy, the zona pellucida also is essential for passage of the early embryo through the oviduct before implantation on the wall of the uterus. Removal of the zona pellucida either biochemically (Modlinski, 1970; Bronson and McLaren, 1970) or genetically (Rankin et al., 1996; Liu et al., 1996; Rankin et al., 2001) results in resorption of eggs and early embryonic loss, with resultant female sterility.
Each zona gene is single copy in the mouse genome (Kinloch et al., 1988; Liang et al., 1990; Lunsford et al., 1990; Epifano et al., 1995a) and zona transcripts accumulate during oogenesis (Epifano et al., 1995b). After translation (Greve et al., 1982; Salzmann et al., 1983), the three mouse zona proteins are secreted to form an extracellular matrix (Bleil and Wassarman, 1980b; Shimizu et al., 1983). Commencing with oocyte growth, the zona matrix is initially observed as discrete patches of amorphous material in the space between the surface of oocytes and the innermost layer of granulosa cells. With time, the patches coalesce to become a continuous, highly porous matrix that reaches a diameter of ~7 μm in fully grown, ovulated mouse eggs (Phillips and Shalgi, 1980).
Genetic ablation of Zp1 indicates that expression of ZP2 and ZP3 is sufficient to form an extracellular zona matrix robust enough for fertilization and early development (Rankin et al., 1999). ZP2 (713 aa) and ZP3 (424 aa) share motifs, including a signal peptide, a ‘zona domain’ (260 aa with eight or ten conserved cysteine residues) and an endoproteinase cleavage site, which is followed by a transmembrane domain and a short, hydrophilic cytoplasmic tail (Ringuette et al., 1988; Liang et al., 1990). The signal peptide directs individual zona proteins into a secretory pathway and the ectodomain is released by cleavage before its incorporation into the insoluble zona pellucida (Boja et al., 2003). These observations present a mechanistic conundrum. How do zona proteins avoid interacting to form polymers during intracellular trafficking and then oligomerize after secretion to form the insoluble, extracellular zona matrix? Here, we explore the role of the cytoplasmic tails of ZP2 and ZP3 in orchestrating these events.
Results
Interactions of ZP2 and ZP3 expressed in heterologous cells
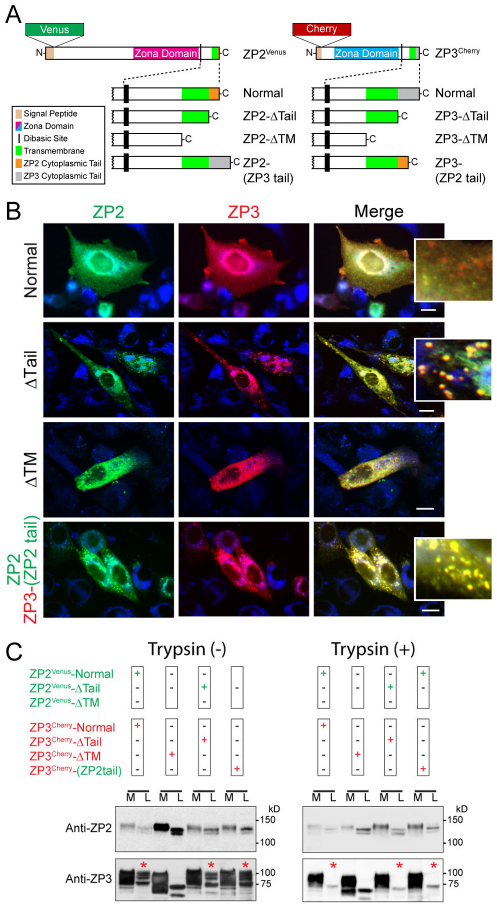
To investigate intracellular trafficking of the zona proteins, expression plasmids encoding ZP2Venus and ZP3Cherry fusion proteins (Fig. 1A) were co-transfected into CHO cells and imaged by fluorescence microscopy. Initially, the two zona proteins colocalized in the endoplasmic reticulum, but appeared to traffic independently through the cell before again colocalizing in the plasma membrane (Fig. 1B). The presence of ZP3 at the plasma membrane was confirmed biochemically by a decrease in the abundance of mature isoforms (larger molecular masses) after digestion of intact cells with trypsin to remove extracellular protein domains before lysis. Similar processing of ZP2 was observed, but the larger molecular mass band was much fainter. Each zona protein was subsequently secreted into the culture medium (Fig. 1C).
Fig. 1.
Cytoplasmic tails direct separate trafficking of ZP2 and ZP3 in CHO cells. (A) ZP2 (713 aa) and ZP3 (424 aa) have a signal peptide, a ‘zona’ domain, a dibasic cleavage site followed by a transmembrane domain and a short cytoplasmic tail. Using cDNA expression vectors, full-length, truncated or modified forms of ZP2 and ZP3 were cloned in-frame with Venus or Cherry fluorescent proteins, respectively. (B) CHO cells were co-transfected with ZP2Venus and ZP3Cherry expression vectors encoding full-length (normal), truncated proteins lacking cytoplasmic tails (ΔTail), transmembrane domains (ΔTM) or ZP3 with a ZP2 cytoplasmic tail, ZP3–(ZP2 tail). Cells were fixed and imaged by fluorescence microscopy using ApoTome technology. Higher-magnification inserts provide images of vesicle-like structures when proteins that lack the cytoplasmic tail or share the same cytoplasmic tail colocalize. Merge includes ER-Tracker, blue. Scale bars: 10 μm. (C) Media and cell lysates treated without (left panel) or with (right panel) trypsin were assayed by immunoblot using monoclonal antibodies against ZP2 or ZP3. The reduction of signal after treatment of cell lysates with trypsin (red asterisks) indicates the presence of expressed protein on the plasma membrane. Note that the upper band is not detected in ΔTM proteins and there is no reduction in signal after treatment with trypsin. Data reflect representative images from experiments that were repeated three times.
To further characterize the interactions of ZP2Venus and ZP3Cherry after secretion from heterologous cells, medium from stably transfected cells was evaluated by size exclusion chromatography. Individual column fractions were analyzed by immunoblots using antibodies against ZP2 and ZP3. A peak with a molecular mass of ~240 kDa was observed and contained each zona protein (supplementary material Fig. S2A). Using an antibody against the fluorescent tag on ZP2, it was possible to co-immunoprecipitate ZP3 from medium of the transfected cells (supplementary material Fig. S2B). This assay was extended for evaluation of individual column fractions and co-immunoprecipitation of ZP2 and ZP3 in the peak fractions (31–28) confirmed interactions between the two secreted zona proteins (supplementary material Fig. S2C).
Cytoplasmic tails direct ZP2 and ZP3 separately to the plasma membrane
In contrast to results observed with intact zona proteins, ZP2 and ZP3 truncated before their cytoplasmic tails (ΔTail, Fig. 1A) colocalized during intracellular trafficking and at the plasma membrane before secretion into the medium (Fig. 1B). With the additional removal of their transmembrane domains (ΔTM), ZP2 and ZP3 continued to co-traffic, albeit in smaller vesicles that were diffusely present throughout the cells (Fig. 1B). Unlike the two zona proteins that lacked just their cytoplasmic tails, those also lacking their transmembrane domains (ZP2Venus-ΔTM; ZP3Cherry-ΔTM) were not detected at the plasma membrane before secretion (Fig. 1B) and therefore ZP3 was not subject to digestion with trypsin (Fig. 1C).
These results suggested that the cytoplasmic tails of ZP2 and ZP3 were sufficient to prevent co-trafficking of the two zona proteins. To confirm these observations, the ZP3 expression plasmid was modified to replace the normal tail with that of ZP2 [ZP3–(ZP2 tail)] (Fig. 1A). The two different zona proteins, but with identical tails, co-trafficked to the plasma membrane, where they colocalized before secretion into the medium (Fig. 1B,C). Taken together, these data indicate that the transmembrane domain is essential for the presence of zona proteins on the plasma membrane and that distinct cytoplasmic tails ensure that ZP2 and ZP3 traffic independently through the cell.
Cytoplasmic tails prevent ZP2–ZP3 polymerization within the cell
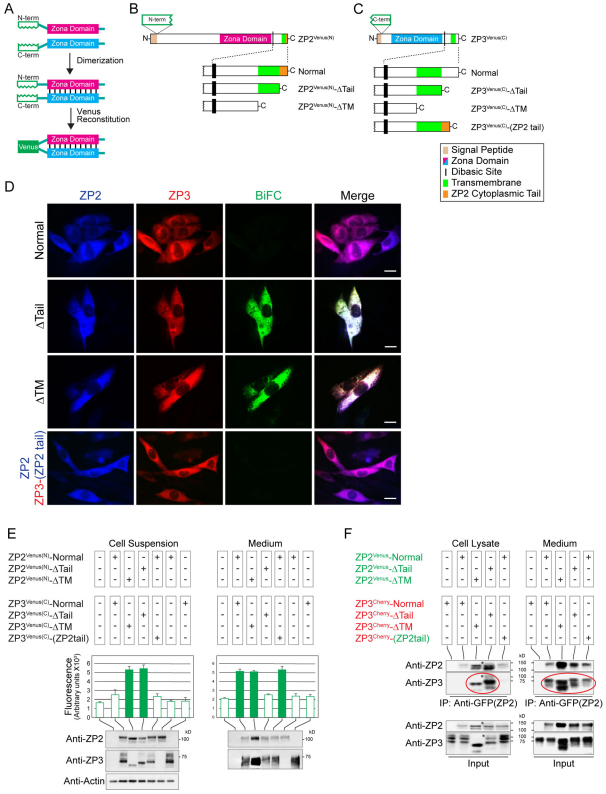
To investigate ZP2 and ZP3 interactions before incorporation into the extracellular zona matrix, we established a fluorescent-based protein fragment complementation (BiFC) assay (Fig. 2A). BiFC is based on the formation of a fluorescent complex from two separate non-fluorescent fragments, which are brought together by the association of two interacting partner proteins fused to the fragments (Hu et al., 2002; Magliery et al., 2005; Kerppola, 2006). The N-terminal fragment (aa 1–156) of Venus was fused to ZP2 just after the signal peptide, ZP2NTVenus(N), whereas the C-terminal fragment of Venus (aa 157–239) was fused just after the N-terminal signal peptide of ZP3 [ZP3Venus(C)] (Fig. 2B,C). No fluorescence was observed in CHO cell lysates or media after expression of ZP2NTVenus(N) alone, ZP3Venus(C) alone or if the N-terminus of Venus was inserted at the end of the ZP2 zona domain [ZP2CTVenus(N), supplementary material Fig. S3B]. However, fluorescence was readily detected in the medium, but not in CHO cells, after co-expression of ZP2NTVenus(N) and ZP3Venus(C). Intact zona proteins did not interact within the cell (Fig. 2D–F), but did fluoresce (positive BiFC) after secretion into the medium (Fig. 2E). Only ZP2NTVenus(N) was co-expressed with ZP3Venus(C) in subsequent experiments and was abbreviated to ZP2Venus(N).
Fig. 2.
ZP2 and ZP3 cytoplasmic tails prevent premature interactions within cells. (A) Schematic representation of BiFC assay in which the N- and C-termini of Venus fluorescent protein were inserted in-frame just downstream of the signal peptide of ZP2 and ZP3, respectively. Correct zona protein dimerization resulted in Venus complementation and production of a fluorescent signal. (B) Expression vectors encoding ZP2Venus(N) that were full-length (Normal), truncated before the cytoplasmic tail (ΔTail) or truncated before the transmembrane domain (ΔTM). (C) ZP3Venus(C) with an additional construct encoding ZP3 with a ZP2 cytoplasmic tail, ZP3Venus(C)–(ZP2 tail). (D) After transfection, fixed cells were imaged by fluorescence microscopy using monoclonal antibodies against ZP2 and an antibody that binds to the C-terminal fragment of Venus in ZP3. The fluorescent signal of ZP2 (blue), ZP3 (red) and Venus complementation (green) were recorded separately and as a merged image. Scale bars: 10 μm. (E) Venus complementation (BiFC) was quantified by fluorometric analysis of cell suspensions (left) and medium (right). Normal, full-length ZP2 and ZP3 did not complement within cells (open bars), but did after secretion into the medium (green bars). Zona proteins lacking their transmembrane domains and cytoplasmic tails complemented prematurely in the cell and complementation was observed in the medium. ZP2 and ZP3, lacking only their cytoplasmic tails, also complemented within the cell, but complementation was not detected in the medium. Data are the mean ± s.e.m. of three independent experiments. ZP2 and ZP3 were detected by immunoblots of cell suspensions and media. Actin levels documented protein equivalence among samples. (F) Cell lysates and media were immunoprecipitated (IP) with mouse anti-GFP monoclonal antibody that recognizes ZP2Venus, but not ZP3Cherry. Resultant protein samples were separated by SDS-PAGE and analyzed by immunoblot using monoclonal antibodies against ZP2 and ZP3. Normal, full-length ZP2 and ZP3 did not interact within the cells but co-immunoprecipitated from the medium. When truncated to remove their cytoplasmic tails alone (ΔTail) or in conjunction with their transmembrane domains (ΔTM), ZP2 and ZP3 interactions were detected in both cell lysate and the medium. The upper band (asterisk), identified as the TM isoforms based on their absence in the ΔTM samples, were not well precipitated in cell lysates expressing proteins lacking their cytoplasmic tails suggesting premature release from the transmembrane domain. After secretion into the medium, ZP2 and ZP3 interactions were detected in the medium for all constructions. No signal was detected in control cells, transfected with only ZP3Cherry. Photomicrographs and immunoblots are representative images from experiments that were repeated three times.
The BiFC assay was extended to zona proteins lacking their cytoplasmic tails. When ZP2Venus(N)-ΔTail and ZP3Venus(C)-ΔTail were co-expressed, fluorescence was observed within cells indicating protein–protein interactions of the two zona proteins in the absence of their cytoplasmic tails (Fig. 2D). The fluorescence pattern was discretely localized in the cytoplasm, which is consistent with complementation within secretory vesicles. Fluorescence was not observed in the medium (Fig. 2E), indicating that complementation of ZP2 and ZP3 within the cell precluded secretion. However, interactions with ZP2Venus-ΔTail and ZP3Cherry-ΔTail proteins were detected in the medium by immunoprecipitation (Fig. 2F), which indicated that non-complementing zona proteins (negative BiFC signal) can be secreted. Thus, we propose that tail-less (ΔTail) zona proteins traffic through heterologous cells via two pathways. In the first, premature release of ZP2 and ZP3 from the membrane (perhaps by convertases during passage through the Golgi) results in BiFC complementation, which precludes secretion. In the second, ZP2 and ZP3 remain colocalized and tethered to the membrane as they progress to the plasma membrane. Although ZP2 and ZP3 are released from their transmembrane domains in the absence of their cytoplasmic tails, there are subtle structural differences that prevent complementation and formation of a secreted protein complex with a positive BiFC signal.
Similar complementation of BiFC was detected within cells expressing ZP2Venus(N)-ΔTM and ZP3Venus(C)-ΔTM, but these proteins, which also lack their transmembrane domains, remained complemented after secretion into the medium (Fig. 2D,E). There was no evidence of either zona protein on the plasma membrane (Fig. 1B,C). Thus, ZP2 and ZP3 truncated before their transmembrane domains, appear to follow a constitutive secretory pathway, with no obligatory presence at the plasma membrane. To further investigate the role of cytoplasmic tails, ZP2Venus(N)-Normal and ZP3Venus(C)-(ZP2tail) (Fig. 2B,C) were co-expressed in CHO cells. The two proteins colocalized in the cell, but did not interact, as determined by the BiFC assay. However, fluorescence complementation was observed in secreted proteins (Fig. 2E). These observations suggest that release from the plasma membrane represents an important step in the physiological interactions of ZP2 and ZP3. Thus, the presence of either cytoplasmic tail is sufficient for physiological interaction of the two proteins after release from the cell surface. These data are consistent with a model in which the cytoplasmic tail ensures that ZP2 and ZP3 traffic independently in the cell before they colocalize at the plasma membrane, where their release from the transmembrane domain provokes structural changes reflected in fluorescent complementation.
Cytoplasmic tails are required for incorporation of ZP2 and ZP3 into the zona pellucida
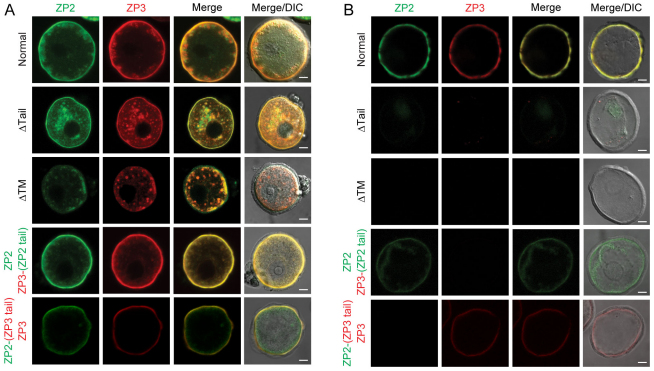
To investigate the role of ZP2 and ZP3 cytoplasmic tails in determining cellular localization under more physiological conditions, expression plasmids were microinjected into the nucleus of isolated mouse oocytes and imaged by confocal microscopy. To distinguish localization of peripheral signals between the plasma membrane and the closely opposed extracellular zona pellucida matrix, 50% of the injected oocytes were lysed with non-ionic detergent and high salt to obtain zona ‘ghosts’ which were then imaged by confocal microscopy (Shimizu et al., 1983; Zhao et al., 2003).
The presence of fluorescence in >85% of injected oocytes indicated that the plasmid construct encoding ZP2Venus and ZP3Cherry were transcribed and translated into protein. Each protein appeared to traffic through the cell independently of the other before detection at the periphery (Fig. 3A) and incorporation into the zona pellucida (Fig. 3B). However, when truncated before their cytoplasmic tails, ZP2Venus-ΔTail and ZP3Cherry-ΔTail colocalized within the oocyte and, although detected in the plasma membrane (Fig. 3A), they were not incorporated into the zona pellucida (Fig. 3B). After the additional removal of the transmembrane domain of ZP2Venus-ΔTM and ZP3Cherry-ΔTM, the two proteins continued to colocalize in the oocytes, but were not detected in the plasma membrane and were not incorporated into the zona pellucida (Fig. 3A,B). Following replacement of the ZP3 tail with that of ZP2, ZP3Cherry-(ZP2 tail) colocalized with ZP2Venus in the oocyte and trafficked to the periphery (Fig. 3A), but was not incorporated into the extracellular zona pellucida (Fig. 3B). Similar results were obtained by replacing the cytoplasmic tail of ZP2 with that of ZP3 (Fig. 3A,B). These data suggest that not only are cytoplasmic tails necessary for incorporation of ZP2 and ZP3 into the zona matrix, but they need to differ from one another (i.e. they cannot both be ZP2 or ZP3).
Fig. 3.
Distinct cytoplasmic tails are essential for incorporation of ZP2 and ZP3 into the zona pellucida. (A) Oocytes were co-microinjected with ZP2Venus and ZP3Cherry expression vectors encoding full-length (normal), truncated proteins lacking cytoplasmic tails (ΔTail), transmembrane domains (ΔTM), ZP2 with a ZP3 cytoplasmic tail, ZP2–(ZP3 tail) or ZP3 with a ZP2 cytoplasmic tail, ZP3–(ZP2 tail). After 40 hours in culture, the oocytes were fixed and imaged by confocal microscopy. Fluorescent signal of ZP2Venus (green) and ZP3Cherry (red) were imaged individually and merged with and without differential interference contrast (DIC) microscopy. Images are representative of three independent experiments, each with 20–30 oocytes. (B) Zona ‘ghosts’ were obtained by freeze-thawing in presence of 0.5 M NaCl and 1% NP-40 before imaging. Photomicrographs are representative images from experiments that were repeated three times with 10–20 oocytes. Scale bars: 20 μm.
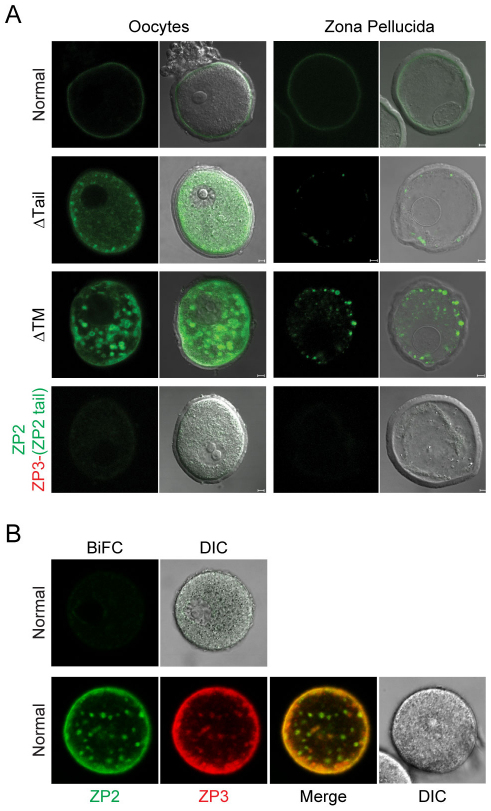
These studies were extended using the BiFC assay. Expression of full-length Venus inserted at the end of the ZP2 zona domain was observed in CHO cells and in oocytes (supplementary material Fig. S3A,C,E). When the N-terminal fragment of Venus was similarly positioned [ZP2CTVenus(N)], it was expressed (supplementary material Fig. S3B,D), but did not complement ZP3Venus(C) in oocytes (supplementary material Fig. S3F). Thus, complementation was only observed when both Venus fragments were positioned near the N-termini of ZP2 and ZP3 and only these constructs were used in the experiments described below.
After microinjection into oocytes, complementation between ZP2Venus(N) and ZP3Venus(C) was not observed within the cytoplasm, indicating that the two zona proteins do not interact within the endomembrane system. However, complementation and fluorescence was observed in the periphery of the cells (Fig. 4A). To determine whether the peripheral signal reflected ZP2–ZP3 interactions in the plasma membrane, the zona pellucida was removed biochemically after microinjection. No complementation fluorescence (BiFC) of ZP2Venus(N) and ZP3Venus(C) was detected (Fig. 4B), although the two proteins trafficked to the plasma membrane as ZP2Venus and ZP3Cherry (Fig. 4B) and the presence of the complementation signal in isolated zona ‘ghosts’ indicated incorporation into the extracellular zona pellucida (Fig. 4A).
Fig. 4.
Cytoplasmic tails prevent ZP2 and ZP3 interaction within growing oocytes. (A) Oocytes were co-microinjected with ZP2Venus(N) and ZP3Venus(C) expression vectors encoding full-length (normal), truncated proteins lacking cytoplasmic tails (ΔTail), transmembrane domains (ΔTM) or ZP3 with a ZP2 cytoplasmic tail, ZP3–(ZP2 tail). After 40 hours in culture, oocytes or zona ‘ghosts’ were imaged by confocal microscopy alone (left) or merged with DIC images (right). Fluorescence (green) in the BiFC assay indicated protein-protein of ZP2 and ZP3, rather than mere colocalization. (B) Oocytes were co-microinjected with full-length ZP2Venus(N) and ZP3Venus(C) expression vectors and the zona pellucida was removed by brief exposure to Tyrode's acidified media, before incubation and imaging by confocal for BiFC and DIC microscopy (upper panels). As controls, oocytes were injected with ZP2Venus and ZP3Cherry before removal of the zona pellucida, incubation and imaging (lower panels). Photomicrographs are representative images from experiments that were repeated three times with 10–20 oocytes.
By contrast, ZP2Venus(N) and ZP3Venus(C), lacking their cytoplasmic tails alone or in conjunction with their transmembrane domains, interacted in oocytes as assayed by fluorescent complementation, but no complementation was observed at the periphery or in isolated zona ‘ghosts’ (Fig. 4A). After replacement of the ZP3 cytoplasmic tail with that of ZP2, the two proteins did not interact to produce a BiFC signal (Fig. 4A) either in the oocyte or in the extracellular zona pellucida, although both were able to traffic to the plasma membrane (Fig. 3A). Thus, ZP2 and ZP3 traffic independently through the cell and, although they colocalize in the plasma membrane, they only interact to provide a BiFC signal after release from the membrane and incorporation into the zona pellucida.
β-tectorin with a ZP3 cytoplasmic tail is incorporated into the zona pellucida
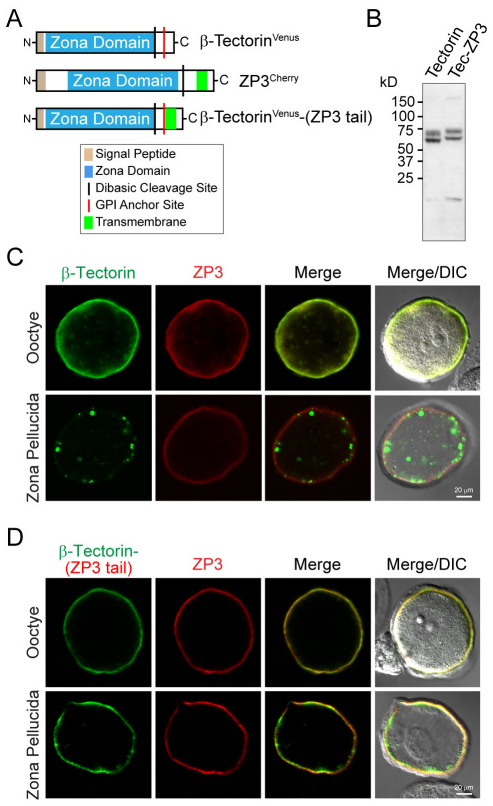
The tectorin membrane of the inner ear is composed of α-tectorin (2155 aa) and β-tectorin (329 aa), the latter composed primarily of a ‘zona’ domain that is analogous to ZP3. Mouse β-tectorin has a signal peptide to direct it into a secretory pathway and a zona domain by which it interacts with α-tectorin. Unlike ZP3, β-tectorin lacks a hydrophilic cytoplasmic tail and forms a glycosylphosphatidylinositol-linked membrane-bound precursor that is released into the extracellular space following cleavage at a tetrabasic site (Legan et al., 1997). Venus was inserted downstream of the β-tectorin signal peptide and cloned as cDNA into an expression vector either with its native sequence or after the replacement of its C-terminus with that of ZP3 (Fig. 5A). When expressed in CHO cells, two isoforms were observed by immunoblot with those of β-tectorin–ZP3 tail having greater molecular mass than native β-tectorin (Fig. 5B). When β-tectorinVenus was co-injected with ZP3Cherry into oocytes, both proteins were observed in the periphery, but only ZP3Cherry was incorporated into the extracellular zona pellucida (Fig. 5C). However, after replacement of the β-tectorinVenus C-terminus with that of ZP3, β-tectorin–ZP3-tail was incorporated into the extracellular zona pellucida (Fig. 5D). Thus, processing by the ZP3 tail and transmembrane domain was sufficient for integration of the zona domain of β-tectorin into the mouse zona pellucida.
Fig. 5.
β-Tectorin with the ZP3 cytoplasmic tail is incorporated into the zona pellucida. (A) β-Tectorin (329 aa) has a signal peptide, a ‘zona’ domain, a potential dibasic cleavage site followed by a GPI-anchor site and a hydrophobic tail that is removed in the mature protein. β-TectorinVenus cDNA was modified by replacing its C-terminus with that of ZP3 and cloned into the expression vector β-tectorin–(ZP3 tail). (B) Lysates from CHO cells transfected with normal (Tectorin) and modified (Tec-ZP3) β-tectorinVenus were assayed by immunoblot using monoclonal antibody against GFP that recognizes Venus. (C) Oocytes were co-injected with β-tectorinVenus and ZP3Cherry before imaging oocytes and zona ‘ghosts’ by confocal microscopy. Scale bar: 20 μm. (D) C-terminus of β-tectorinVenus was replaced by that of ZP3 containing its cytoplasmic tail. Photomicrographs are representative images from experiments that were repeated three times with 10–20 oocytes.
Discussion
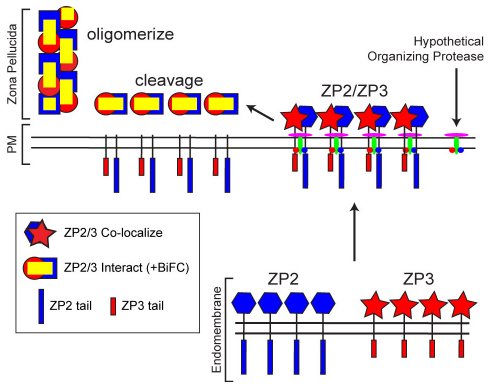
Little is known about the intracellular trafficking of the zona pellucida proteins or of the processing that ensures release of N-terminal ectodomains for assembly into the insoluble, extracellular matrix. By tagging zona proteins with different fluorescent markers and assaying molecular interactions using bimolecular fluorescent complementation (BiFC), we have investigated the molecular processing of ZP2 and ZP3 in growing oocytes. Our results suggest a model in which signal peptides direct ZP2 and ZP3 into a secretory pathway where their ectodomains remain tethered to a transmembrane domain. While traversing the endomembrane, cytoplasmic tails prevent interactions between ZP2 and ZP3 and ensure passage through the Golgi complex without cleavage by resident convertases. At the plasma membrane, a hypothetical transmembrane protease (single protein or part of a complex) recognizes the intracellular cytoplasmic tails of ZP2 and ZP3 and releases extracellular domains of each zona protein. This cleavage alters the conformation of ZP2 and ZP3 ectodomains, permitting oligomerization and formation of the insoluble zona pellucida (Fig. 6).
Fig. 6.
Model for ZP2 and ZP3 incorporation into the zona pellucida. The signal peptides of intact ZP2 and ZP3 direct each into a secretory pathway. While traversing the endomembrane system, the cytoplasmic tails prevent interactions of the two zona proteins (either directly or indirectly via interactions with binding proteins) and ensure passage through the Golgi without cleavage by resident convertases (e.g. furin). At the plasma membrane, a hypothetical transmembrane protease (single protein or part of a complex) recognizes the intracellular cytoplasmic tails of ZP2 and ZP3 and releases the extracellular ectodomain of each. This cleavage alters the conformation of the two proteins permitting their oligomerization and incorporation into the zona pellucida
Intracellular trafficking
During protein synthesis and before incorporation into the extracellular zona pellucida, ZP2 (713 amino acids) and ZP3 (424 aa) undergo disulfide bond formation, proteolytic processing and glycosylation. The signal peptide cleavage sites, as well as the C-termini of the released ectodomains, have been defined by microscale mass spectrometry for native ZP2 (Val35-Ser633) and ZP3 (Gln23-Asn351) (Boja et al., 2003). As the two proteins transit the endoplasmic reticulum and Golgi, they are heavily and heterogeneously glycosylated by complex N-glycans and, to a much less extent, O-glycans. Together these post-translational modifications account for roughly one-half of the mass of ZP2 and ZP3 on SDS-PAGE gels (Nagdas et al., 1994; Easton et al., 2000; Boja et al., 2003). The two proteins are observed in peripherally located multivesicular aggregates (MVAs) consisting of 1–5 μm vesicles embedded in an amorphous matrix before transit to the plasma membrane and ultimate incorporation into the extracellular zona matrix (Merchant and Chang, 1971; El Mestrah et al., 2002; Hoodbhoy et al., 2006).
ZP2 and ZP3 with intact transmembrane domains co-expressed in heterologous cells and oocytes are localized in vesicle-like structures, but seem more diffuse when transmembrane domains are absent (ΔTM). These data are consistent with tethering of ZP2 and ZP3 to the lipid bilayer of distinct transport vesicles while they are trafficking through the cell, and implies the action of a cytoplasmic regulator(s) that distinguishes between the two proteins. We now implicate the relatively short (9–15 aa) cytoplasmic tails as crucial for the independent trafficking of the zona proteins. Upon their removal (ΔTail) or when genetically engineered to be the same, ZP2 and ZP3 colocalize in the endomembrane system of growing oocytes, including the MVA. Each cytoplasmic tail contains 3–5 basic residues that might have a role in ensuring that the ectodomains of the two proteins do not interact precociously and form insoluble zona matrices within the oocyte. Similar basic residues in the cytoplasmic tails of other transmembrane proteins have been implicated (1) in coat assembly, which is essential for vesicular transport (Dominguez et al., 1998; Bremser et al., 1999); (2) in providing signals for localization in the endomembrane system (Schoberer et al., 2009); and (3) in apical sorting in polarized cells in which Tctex, the dynein light-chain, binds to the cytoplasmic tails of a variety of proteins (Chuang and Sung, 1998; Marzolo et al., 2003; Hodson et al., 2006; Braiterman et al., 2009; Carmosino et al., 2010).
Prevention of intracellular polymerization
Two short hydrophobic patches have been identified in ZP3, one of which is C-terminal to the cleavage site that releases the zona ectodomain (Zhao et al., 2003; Jovine et al., 2004). It has been proposed that the two hydrophobic motifs interact to ensure an ‘inactive’ conformation of individual zona proteins, which prevents intracellular polymerization. Once cleaved from the transmembrane domain, the zona ectodomain retains only one of the hydrophobic patches, which could interact with other zona ectodomains to promote polymerization and formation of the extracellular zona pellucida (Jovine et al., 2004). Consistent with this model, we find that molecular complementation (BiFC) of ZP2 and ZP3 only occurs after release from the plasma membrane and incorporation into the inner aspect of the zona pellucida. However, we also observe that in the absence of their cytoplasmic tails, ZP2 and ZP3 interact within growing oocytes, suggesting either adventitious release from their transmembrane domains, which promotes protein–protein interaction according to the above model, or an ability of the two different cytoplasmic tails to physically separate ZP2 and ZP3 while trafficking within cells.
To distinguish between these two possibilities, we genetically switched the tails and co-expressed proteins sharing the same cytoplasmic tails (either both ZP2 or both ZP3). The absence of intracellular molecular interaction assayed by BiFC and the inability to co-immunoprecipitate the two proteins from cell lysates indicates that the cytoplasmic tails do not inhibit polymerization but rather maintain the zona proteins in an ‘inactive’ conformation. In support of this, proteins lacking cytoplasmic tails exhibit fluorescent (BiFC) complementation within the cells, but not at the cell surface membrane. Together, these data indicate that: (1) the cytoplasmic tails prevent cleavage of the ectodomain and premature interactions between ZP2 and ZP3 within the endomembrane during intracellular trafficking, and (2) the C-terminus of zona proteins (including the external hydrophobic patch, the transmembrane domain and the cytoplasmic tails) modulate zona protein assembly required for incorporation into the zona pellucida.
Processing zona proteins at the plasma membrane
Upon reaching the plasma membrane, ZP2 and ZP3 colocalize, but do not interact molecularly until released from the plasma membrane before incorporation into the zona pellucida. A well-conserved convertase cleavage site (RX[R/K]R↓) has been implicated in the release of the zona ectodomains (Yurewicz et al., 1993), but microscale mass spectrometry of native zonae pellucidae defined the C-terminus upstream of a dibasic motif within the same site (RX↓[R/K]R) (Boja et al., 2003).
Once secreted from heterologous cells, ZP2 and ZP3 interact even when lacking their transmembrane domain and/or cytoplasmic tails. The cleavage site is heterogeneous in heterologous cells (Zhao et al., 2004) and differs from that observed in the native proteins as documented by changes in gel mobility (current manuscript) and definition of the C-terminus by mass spectrometry (Boja et al., 2003). The precision in the cleavage site in the native ZP2 and ZP3, suggests involvement of oocyte-specific mechanisms in processing zona proteins. The proteins lacking their transmembrane domains and/or cytoplasmic tails are not incorporated into the zona pellucida of growing oocytes (Jovine et al., 2004) (current manuscript). More recently, it has been reported that mutations of the EHP and IHP of uromodulin resulted in the release of monomers unable to assemble into filaments (Schaeffer et al., 2009). Together these data suggest that the absence of tails may affect correct cleavage of zona proteins and precise cleavage may determine the ability of zona proteins to assembly and participate in the zona pellucida.
Sheddases or secretases release ectodomains from membrane-spanning domains in a variety of proteins (Hooper et al., 1997). Particularly well studied are the selectins, three members of the CAM (cell adhesion molecules) family identified in endothelial cells (E-selectin), platelets (P-selectin) and leukocytes (L-selectin) (Gonzalez-Amaro and Sanchez-Madrid, 1999). The extracellular domains of the three proteins have similar structural features, but the divergence of their cytoplasmic tails suggests differences in regulation. The 17 residue cytoplasmic tail of L-selectin regulates proteolytic cleavage, microvillar positioning and the tethering/rolling behavior of leucocytes. The tail interacts with at least three proteins, including CaM (calmodulin), α-actinin, and the members of the ERM (erzin–radixin–moesin) family of cytoskeletal proteins (Ivetic and Ridley, 2004; Killock et al., 2009). CaM negatively regulates shedding by interacting with the L-selectin tail and inducing a conformational changes in the extracellular domain that renders the cleavage site resistant to proteolysis (Kahn et al., 1998; Diaz-Rodriguez et al., 2000). Conversely, when CaM dissociates from the tail, the conformational change in the extracellular domain promoted proteolytic cleavage by a sheddase.
To further assay the requirement for a cytoplasmic tail in formation of the zona pellucida, we expressed β-tectorin, a GPI-anchored ‘zona’ domain containing protein that normally complexes with α-tectorin to form the extracellular tectorin membrane in the vertebrate inner ear (Legan et al., 1997; Petit et al., 2001). When expressed in oocytes, β-tectorin trafficked correctly to the plasma membrane, but was not incorporated into the zona pellucida. However, after genetically replacing its C-terminus with the transmembrane and cytoplasmic tail of ZP3, β-tectorin was incorporated into the zona pellucida. This suggests that the cytoplasmic tail must be specifically recognized at the plasma membrane for the ectodomain to be released and incorporated into the zona matrix. The importance of cytoplasmic tails has been documented in other experimental systems including Herpes simplex Virus 1 glycoproteins H and D which are virion envelope proteins required for fusion with infected cells. Each has a transmembrane domain with a short cytoplasmic tail. Insertional mutation in the cytoplasmic tail of glycoprotein H completely abrogate cell fusion and viral infectivity (Jackson et al., 2010) as does tethering glycoprotein D to the viral envelop with a GPI-anchor sequence (Browne et al., 2003).
Conclusion
We propose a model in which the cytoplasmic tails of ZP2 and ZP3 control the release of zona ectodomains to form the extracellular zona pellucida. Before arrival at the plasma membrane, zona tails prevent premature cleavage of the proteins and their absence renders ZP2 and ZP3 susceptible to cleavage (perhaps by a convertase in the Golgi) and precocious intracellular polymerization. We speculate that upon arrival at the plasma membrane, the ZP2 and ZP3 cytoplasmic tails bind (directly or indirectly) to a putative membrane-associated protease. The cleavage of each protein would alter the conformation of its ectodomains, permitting oligomerization and incorporation into the zona matrix. Identification of a molecular basis of these hypothetical interactions and determining whether specificity is based on specific protein–protein or protein–phospholipid (Deford-Watts et al., 2009) interactions will be of considerable interest.
ZP2 and ZP3 have different cytoplasmic tails, albeit with similar basic charges. When their tails are genetically switched to be the same, the ZP2 and ZP3 ectodomains were not incorporated into the extracellular matrix surrounding growing oocytes. This is consistent with ZP2 and ZP3 tails being recognized at the plasma membrane to ensure correct stoichiometry of the two zona proteins for incorporation into the zona pellucida. We note that although the zona matrix is generally thought to be formed from heterodimers of ZP2 and ZP3, a role for homodimers of either protein has not been excluded. To validate these models it will be important to biochemically identify and genetically confirm the molecular basis of zona protein processing at the plasma membrane. Understanding of these molecular details might provide insight into formation of other matrices formed by proteins containing ‘zona’ domains (Jovine et al., 2005), including the tectorin membrane of the inner ear (Cosgrove and Grotton, 2001), as well as other extracellular polymers (Aszodi et al., 2006; Larsen et al., 2006).
Materials and Methods
Construction of expression plasmids
The C-terminal domains of ZP2 and ZP3 are well conserved among mammals (supplementary material Fig. S1). To insert Venus at the N-terminus of ZP2, the 5′ region (nucleotides 21–150) of ZP2, including the signal sequence (aa 1–34), was amplified by PCR with two primers (supplementary material Table S1), each containing either an NheI or an AgeI site (ZP2IF, ZP2IR). cDNA encoding full-length Venus fluorescent protein (aa 1–239) (gift from Eneko Urizar, Center for Molecular Recognition, Columbia University College of Physicians and Surgeons, New York, NY) was amplified by PCR with two primers, each containing an AgeI or an EcoRI recognition site (VenusF, VenusR). The resultant PCR products were assembled and subcloned into pcDNA3.1(+) (Invitrogen, Carlsbad, CA), previously digested with NheI and EcoRI. To construct ZP2Venus(N), the N-terminal fragment 1–156 of Venus, Venus(N), was amplified by PCR with a 5′ primer (VenusF) containing an AgeI recognition site and a 3′ primer (NVenIR) containing a sequence coding for a five amino acid linker (GGGGS) and an EcoRI site. The Venus(N) fragment was subcloned in-frame with the 5′ region of ZP2 in the pcDNA vector. The remainder of ZP2 (bp 151–2201) was amplified by PCR product containing EcoRI and PciI recognition sites (ZP2IIF, ZP2IIR) and the rest of ZP2 (bp 413–2169) was isolated from ZP2 cDNA after digestion with PciI and EcoRI. These two products were assembled and cloned into the EcoRI recognition site of pcDNA. The resultant EcoRI fragments were isolated and subcloned into the EcoRI site downstream and in-frame with full length ZP2 to complete the ZP2Venus and ZP2Venus(N) plasmids.
To insert Venus and Venus(N) at the C-terminal position of ZP2, the 3′ region (bp 1921–2201), including the transmembrane domain (aa 684–703) and cytoplasmic tail (aa 704–713), was amplified by PCR with two primer (ZP2IVF, ZP2IVR) containing AgeI and XhoI sites. cDNA encoding full-length Venus and Venus(N) fragments were amplified by PCR with 5′ primers (VenusF, NVenIIF) containing EcoRI, and 3′ primers (VenusR, NVenIIR) containing the AgeI site. The resultant PCR products were assembled and subcloned into EcoRI and XhoI digested pcDNA to generate a fusion protein with the C-terminus of ZP2 in-frame and downstream of Venus and Venus(N) fragments. The remainder of ZP2 (bp 21–1920) was amplified by PCR using primers with NheI and EcoRI recognition sites (ZP2IF, ZP2IIIR). The PCR product was isolated after digestion with NheI-EcoRI and subcloned upstream and in-frame with Venus and Venus(N) fragments.
For ZP3Cherry, pmCherry was cloned by PCR from pmCherry-N1 vector (Clontech, Mountain View, CA) with two primers (CherryF, CherryR) containing AgeI and BglII sites, respectively. The PCR product was subcloned into pSEGFP-MoZP3 (Zhao et al., 2002), replacing EGFP, but remaining in-frame and downstream of the signal sequence of ZP3 and upstream of the remainder of ZP3. For ZP3Venus(C), the Venus(C), C-terminus of the Venus fragment (157–239) was amplified by PCR with a 5′ primer (CVenF) containing an AgeI recognition site and 3′ primer (CVenR) containing a sequence encoding a five amino acid linker (GGGGS) and a BglII site. The Venus(C) fragment was subcloned into pSEGFP-MoZP3. cDNA encoding ZP3 fused in-frame to pmCherry and the Venus(C) fragments was isolated from the original vector after digestion with NheI and XhoI and subcloned into pcDNA3.1/Zeo(+) vector (Invitrogen).
For ZP3Cherry(ZP2-tail) and ZP3Venus(C) (ZP2-tail), the 5′ regions of ZP3Cherry and ZP3Venus(C) (1938 and 1497 bp, respectively) were amplified by PCR with two primers (ZP3ctZP2F, ZP3ctZP2R) each containing either an NheI or an EcoRI recognition site. The C-terminus of ZP2 (bp 2017–2169) was amplified by PCR using two primers (ZP2ctF, ZP2ctR), containing EcoRI and XhoI sites. The PCR products were subcloned into pcDNA after digestion with NheI and XhoI sites to establish cDNA encoding a protein fusion in which the C-terminus of ZP2 was in-frame and downstream of ZP3.
For ZP2Venus(ZP3 tail), the 5′ region of ZP2Venus (2776 bp) was amplified from the above cDNA by PCR using the primers ZP2ctZP3F and ZP2ctZP3R containing NheI and HindIII sites. The C-terminus of ZP3 (bp 1167–1317) was amplified by PCR using two primers (ZP3ctF, ZP3ctR) containing HindIII and XhoI sites. The PCR products were subcloned in pcDNA vector digested with NheI and XhoI generating a cDNA encoding a fusion protein with the C-terminus of ZP3 in-frame and downstream of the ZP2Venus protein.
Truncated proteins were generated by inserting a stop codon using specific primers (supplementary material Table S2) and QuikChange site-directed mutagenesis (Stratagene, Garden Grove, CA) following the manufacturer's instructions. Briefly, for ZP2-ΔTM, Asp (673) from ZP2Venus or ZP2Venus(N), was replaced with a stop codon using ZP2ΔTMF and ZP2ΔTMR primers. For the ZP2-ΔTail, Tyr (704) from ZP2Venus or ZP2Venus(N), was replaced with a stop codon using ZP2ΔCTF and ZP2ΔCTR primers. For ZP3-ΔTM, Trp (380) from ZP3Cherry and ZP3Venus(C) was replaced with a stop codon using ZP3ΔTMF and ZP3ΔTMF primers. For ZP3-ΔTail, Val (409) was replaced with a stop codon using ZP3ΔCTF and ZP3ΔCTR primers.
For the β-tectorinVenus expression plasmid, the 5′ region (bp 11–181) of β-tectorin, including the signal sequence (aa 1–17), was amplified by PCR with two primers (BtecIF, BTecIR), each containing either an NheI or an AgeI recognition site. cDNA encoding full-length Venus fluorescent protein (aa 1–239) was cloned as described above. The resultant PCR products were assembled and subcloned into pcDNA3.1(+) digested with NheI and EcoRI. The remainder of β-tectorin (bp 182–1120) was amplified by PCR product (151–413) with primers (BTecIIF, BTecIIR) containing EcoRI and XhoI recognition sites. pcDNA was digested with EcoRI and XhoI and the products were cloned in-frame and downstream of the Venus protein. For β-tectorinVenus-(ZP3 tail) the 5′ region of β-tectorinVenus (encoding 1815 aa) was amplified from the above cDNA by PCR using the primers BTecIIF and BTecIIR containing NheI and HindIII sites. The C-terminus of ZP3 (bp 1167–1317) was amplified by PCR using two primers containing HindIII and XhoI sites (ZP3ctF, ZP3ctR). The PCR products were subcloned in pcDNA digested with NheI and XhoI to generate a cDNA encoding fusion protein in which the C-terminus of ZP3 was in-frame and downstream of β-tectorinVenus.
Junction fragments and PCR products of all plasmids were verified by DNA sequencing and the presence of recombinant protein was confirmed by immunoblot after expression in heterologous cells. Expression plasmids were purified with the GenEluted Plasmid Kit (Sigma).
Expression in heterologous cells
CHO-K1 cells (American Type Culture Collection, Manassas, VA) were grown (37°C, 5% CO2) for 24 hours to 50–70% confluence in F-12 medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin (Gibco-Invitrogen). Transient transfections were performed with FuGene HD (Roche Applied Science, Indianapolis, IN) in accordance with the manufacturer's protocol. For each transfection, 6 μl FuGene HD transfection reagent was added to 100 μl Opti-MEM reduced-serum medium (Gibco-Invitrogen) predissolved with 2 μg template plasmid and incubated for 15 minutes at room temperature. The complex was diluted with 2 ml Opti-MEM and overlaid on the growing cells. Transiently transfected cells were harvested at 24 hours and stable clones were selected after culture for an additional 1–2 weeks in the presence of Geneticin (200 μg/ml) and Zeocin (350 μg/ml).
Photomicroscopy
Transfected cells expressing fluorescent-tagged proteins were grown on coverslips for 48 hours after which pre-warmed (37°C) medium (Opti-MEM) containing 20 μM ER-Tracker-Blue-White DPX (Molecular Probes–Invitrogen) was added. The cells were incubated for an additional 30 minutes, fixed with 3% paraformaldehyde and imaged at room temperature with an Axioplan 2 fluorescence microscope (Carl Zeiss, Thornwood, NY) equipped with C-Apochromat 63×/1.2W Corr objective, ApoTome technology, CCD camera and AxioVision 2 software. Venus fluorochrome was excited with BP450–490 nm filter and emission detected with BP515–565 nm filter; Cherry and Alexa Fluor 568 fluorochromes were excited with BP534–558 nm filter and was detected through a LP590 nm filter; Alexa Fluor 633 fluorochrome was excited with HQ590–650 nm filter and emission was detected through a HQ667–738 nm filter; and Dapoxyl (DPX) fluorochrome was excited with a G365 and emission detected through a BP420–470 nm filter.
For BiFC, cells were incubated an additional 2 hours at 30°C to allow maturation of the Venus fluorophore (Hu et al., 2002). Cells were then fixed (3% paraformaldehyde, 15 minutes), washed in PBS (3×), permeabilized (PBS and Triton X-100, 5 minutes), blocked (PBS with BSA, 1 hour) and incubated with rat monoclonal antibodies to ZP2 (1:200) and mouse antibodies against (1:250)(Roche Applied Science), which binds to the C-terminus of Venus (Nyfeler et al., 2005) contained in ZP3. Antibody binding was detected with either Alexa-Fluor-633-conjugated goat anti-rat antibody (1:100) or Alexa-Fluor-568-conjugated goat anti-mouse (1:100) secondary antibodies (Invitrogen).
Confocal images from growing oocytes were obtained at room temperature with an LSM confocal microscope (Carl Zeiss) equipped with differential contrast optics using a C-Apochromat 63×/1.2W Corr objective and scan zoom of 1.7. The Venus fluorochrome was excited with a 488 nm argon laser and emission detected through a BP500–550 nm filter and Cherry fluorochrome was excited with 561 nm DPSS laser and detected through a BP575–615 nm filter.
Immunoblots
Supernatants and cells were recovered 48 hours after transfection, after which cells were homogenized with M-PER Mammalian Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) supplemented with Complete Protease Inhibitor Cocktail (Roche Applied Science) according to the manufacturer's instructions. Following centrifugation (16,000 g, 4°C), cell supernatants and lysates were separated by SDS-PAGE, transferred to PVDF membranes which were probed with monoclonal antibodies to ZP2 (East and Dean, 1984), ZP3 (East et al., 1985) or actin (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by chemiluminescence (Rankin et al., 2003).
Gel filtration chromatography
Stably transfected cells co-expressing ZP2Venus and ZP3Cherry were grown in 75 cm2 tissue culture flasks (Corning Life Sciences, Lowell, MA) to 80% confluence and serum starved for 48 hours. Cell supernatants were dialyzed and concentrated against 50 mM Tris-HCl, 0.1 M NaCl, 10% glycerol, pH 7.5 before FPLC chromatography on a Superose 6 10/300 GL column (GE Healthcare Life Sciences) in the same buffer. Using a flow rate of 0.5 ml/minute, the protein elution pattern was monitored (A280) and collected fractions (0.5 ml) were precipitated with 20% trichloroacetic acid. Following cold acetone washes (1.0 ml, 1×), the precipitated samples were analyzed by immunoblot using monoclonal antibodies against ZP2 and ZP3. The data (average of three experiments) were quantified by on a LAS-3000 (Fujifilm Medical Systems, Stamford, CN). Protein standards from Gel Filtration Calibration Kits (GE Healthcare) run in the same buffer were used to calibrate the column.
BiFC assay in CHO cells
48 hours after transfection, medium was recovered and centrifuged to remove detritus. Cells were washed with PBS, harvested by scraping and resuspended in 1 ml PBS. 20 μl aliquots were counted on a Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA) and all samples were normalized to 5×105 cells. After centrifugation and resuspension in 300 μl PBS, the cells and media were transferred to 96-well, clear-bottom, black microtiter plates (Nunc-Thermo Fischer Scientific). Fluorescence was determined on a Spectramax GEMINI EM (Molecular Devices, Sunnyvale, CA) using excitation and emission wavelengths of 470 and 535 nm, respectively. The C-terminal fragment of Venus was cloned downstream of the ZP3 zona domain (see above). The N-terminal fragment of Venus was cloned downstream of the ZP2 signal peptide and of the ZP2 zona domain; fluorescence was observed in the BiFC assay with the former, but not the latter. Mock-transfected cells and medium were used as negative controls. Data from three independent experiments were averaged and s.e.m. calculated.
Immunoprecipitation
For immunoprecipitation of ZP2Venus and ZP3Cherry normal and mutated proteins, monoclonal antibody specific to GFP (Roche Applied Science) was incubated with magnetic Dynabeads® Protein G (Invitrogen) according to the manufacturer's instructions. The complex was incubated with supernatant and lysate from transfected cells and analyzed by immunoblot.
Microinjection of isolated oocytes
Oocytes isolated from 11- to 13-day-old mouse ovaries (Millar et al., 1991) were incubated in M2 medium (Chemicon-Millipore, Billerica, MA) supplemented with IBMX (3-isobutyl-1-methylxanthine) at 37°C before microinjection. 10 pl plasmid DNA (50 ng/ml) was injected into the nucleus and surviving oocytes were cultured (37°C, 5% CO2) for 48 hours in KSOM medium (Millipore) containing IBMX. The oocytes were then separated into two groups. One group was fixed with 2% paraformaldehyde for 1 hour at room temperature. The other group was transferred into 20 mM Tris-HCl, pH 7.4, containing 1% NP-40 and 0.5 M NaCl and freeze-thawed ten times on ethanol in dry ice to isolate zona ‘ghosts’ (Shimizu et al., 1983; Zhao et al., 2003). To obtain zona-free oocytes after nuclear microinjection, the zona matrix was removed from growing oocytes by brief exposure to acidified Tyrode's medium (Hogan et al., 1994) and fixed as above after 48 hours of incubation.
Oocytes co-injected with BiFC fragments were incubated for 48 hours, then placed at 30°C for 2 hours and fixed. Individual plasmids carrying the Venus fragments were also microinjected as a negative control of the complementation. The fixed oocytes and treated zona ‘ghosts’ were washed three times with PBS and placed on a slide chambered (20 μl cavity) with Gene Frame (Advanced Biotechnologies, Leatherhead, UK). Images were obtained by confocal microscopy.
Supplementary Material
Acknowledgments
For their help and advice, we thank Lyn Gauthier (microinjection of oocytes) and Boris Baibakov (confocal microscopy). This research was supported by the Intramural Research Program of the National Institutes of Health, NIDDK. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/6/940/DC1
References
- Aszodi A., Legate K. R., Nakchbandi I., Fassler R. (2006). What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 22, 591-621 [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. (1980a). Structure and function of the zona pellucida: Identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev. Biol. 76, 185-202 [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. (1980b). Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc. Natl. Acad. Sci. USA 77, 1029-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja E. S., Hoodbhoy T., Fales H. M., Dean J. (2003). Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 278, 34189-34202 [DOI] [PubMed] [Google Scholar]
- Bork P., Sander C. (1992). A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett. 300, 237-240 [DOI] [PubMed] [Google Scholar]
- Braiterman L., Nyasae L., Guo Y., Bustos R., Lutsenko S., Hubbard A. (2009). Apical targeting and Golgi retention signals reside within a 9-amino acid sequence in the copper-ATPase, ATP7B. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G433-G444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambell F. W. R. (1928). The development and morphology of the gonads of the mouse. Part III. The growth of the follicles. Proc. R. Soc. Lond. B Biol. Sci. 103, 258-272 [Google Scholar]
- Bremser M., Nickel W., Schweikert M., Ravazzola M., Amherdt M., Hughes C. A., Sollner T. H., Rothman J. E., Wieland F. T. (1999). Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, 495-506 [DOI] [PubMed] [Google Scholar]
- Bronson R. A., McLaren A. (1970). Transfer to the mouse oviduct of eggs with and without the zona pellucida. J. Reprod. Fertil. 22, 129-137 [DOI] [PubMed] [Google Scholar]
- Browne H., Bruun B., Whiteley A., Minson T. (2003). Analysis of the role of the membrane-spanning and cytoplasmic tail domains of herpes simplex virus type 1 glycoprotein D in membrane fusion. J. Gen. Virol. 84, 1085-1089 [DOI] [PubMed] [Google Scholar]
- Carmosino M., Valenti G., Caplan M., Svelto M. (2010). Polarized traffic towards the cell surface: how to find the route. Biol. Cell 102, 75-91 [DOI] [PubMed] [Google Scholar]
- Chuang J. Z., Sung C. H. (1998). The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J. Cell Biol. 142, 1245-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Grotton M. A. (2001). Extracellular matrix and inner ear development and function. Adv. Dev. Biol. 15, 169-201 [Google Scholar]
- Deford-Watts L. M., Tassin T. C., Becker A. M., Medeiros J. J., Albanesi J. P., Love P. E., Wulfing C., van Oers N. S. (2009). The cytoplasmic tail of the T cell receptor CD3 epsilon subunit contains a phospholipid-binding motif that regulates T cell functions. J. Immunol. 183, 1055-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Rodriguez E., Esparis-Ogando A., Montero J. C., Yuste L., Pandiella A. (2000). Stimulation of cleavage of membrane proteins by calmodulin inhibitors. Biochem. J. 346, 359-367 [PMC free article] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard K., Fullekrug J., Dahan S., Fazel A., Paccaud J. P., Thomas D. Y., Bergeron J. J., Nilsson T. (1998). gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol. 140, 751-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East I. J., Dean J. (1984). Monoclonal antibodies as probes of the distribution of ZP-2, the major sulfated glycoprotein of the murine zona pellucida. J. Cell Biol. 98, 795-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East I. J., Gulyas B. J., Dean J. (1985). Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: Effects on fertilization and early development. Dev. Biol. 109, 268-273 [DOI] [PubMed] [Google Scholar]
- Easton R. L., Patankar M. S., Lattanzio F. A., Leaven T. H., Morris H. R., Clark G. F., Dell A. (2000). Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J. Biol. Chem. 275, 7731-7742 [DOI] [PubMed] [Google Scholar]
- El Mestrah M., Castle P. E., Borossa G., Kan F. W. (2002). Subcellular distribution of ZP1, ZP2, and ZP3 glycoproteins during folliculogenesis and demonstration of their topographical disposition within the zona matrix of mouse ovarian oocytes. Biol. Reprod. 66, 866-876 [DOI] [PubMed] [Google Scholar]
- Epifano O., Liang L.-F., Dean J. (1995a). Mouse Zp1 encodes a zona pellucida protein homologous to egg envelope proteins in mammals and fish. J. Biol. Chem. 270, 27254-27258 [DOI] [PubMed] [Google Scholar]
- Epifano O., Liang L.-F., Familari M., Moos M. C., Jr, Dean J. (1995b). Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 121, 1947-1956 [DOI] [PubMed] [Google Scholar]
- Gahlay G., Gauthier L., Baibakov B., Epifano O., Dean J. (2010). Gamete recognition in mice depends on the cleavage status of an egg's zona pellucida protein. Science 329, 216-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Amaro R., Sanchez-Madrid F. (1999). Cell adhesion molecules: selectins and integrins. Crit. Rev. Immunol. 19, 389-429 [PubMed] [Google Scholar]
- Greve J. M., Salzmann G. S., Roller R. J., Wassarman P. M. (1982). Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell 31, 749-759 [DOI] [PubMed] [Google Scholar]
- Hodson C. A., Ambrogi I. G., Scott R. O., Mohler P. J., Milgram S. L. (2006). Polarized apical sorting of guanylyl cyclase C is specified by a cytosolic signal. Traffic 7, 456-464 [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington R., Costantini F., Lacy E. (1994). Manipulating the Mouse Embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Hoodbhoy T., Aviles M., Baibakov B., Epifano O., Jimenez-Movilla M., Gauthier L., Dean J. (2006). ZP2 and ZP3 traffic independently within oocytes prior to assembly into the extracellular zona pellucida. Mol. Cell. Biol. 26, 7991-7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Karran E. H., Turner A. J. (1997). Membrane protein secretases. Biochem. J. 321, 265-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. D., Chinenov Y., Kerppola T. K. (2002). Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9, 789-798 [DOI] [PubMed] [Google Scholar]
- Ivetic A., Ridley A. J. (2004). The telling tail of L-selectin. Biochem. Soc. Trans. 32, 1118-1121 [DOI] [PubMed] [Google Scholar]
- Jackson J. O., Lin E., Spear P. G., Longnecker R. (2010). Insertion mutations in herpes simplex virus 1 glycoprotein H reduce cell surface expression, slow the rate of cell fusion, or abrogate functions in cell fusion and viral entry. J. Virol. 84, 2038-2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L., Qi H., Williams Z., Litscher E. S., Wassarman P. M. (2004). A duplicated motif controls assembly of zona pellucida domain proteins. Proc. Natl. Acad. Sci. USA 101, 5922-5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovine L., Darie C. C., Litscher E. S., Wassarman P. M. (2005). Zona pellucida domain proteins. Annu. Rev. Biochem. 74, 83-114 [DOI] [PubMed] [Google Scholar]
- Kahn J., Walcheck B., Migaki G. I., Jutila M. A., Kishimoto T. K. (1998). Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell 92, 809-818 [DOI] [PubMed] [Google Scholar]
- Kerppola T. K. (2006). Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell Biol. 7, 449-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killock D. J., Parsons M., Zarrouk M., Ameer-Beg S. M., Ridley A. J., Haskard D. O., Zvelebil M., Ivetic A. (2009). In vitro and in vivo characterization of molecular interactions between calmodulin, ezrin/radixin/moesin, and L-selectin. J. Biol. Chem. 284, 8833-8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Roller R. J., Fimiani C. M., Wassarman D. A., Wassarman P. M. (1988). Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc. Natl. Acad. Sci. USA 85, 6409-6413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M., Artym V. V., Green J. A., Yamada K. M. (2006). The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463-471 [DOI] [PubMed] [Google Scholar]
- Legan P. K., Rau A., Keen J. N., Richardson G. P. (1997). The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 272, 8791-8801 [DOI] [PubMed] [Google Scholar]
- Liang L.-F., Chamow S. M., Dean J. (1990). Oocyte-specific expression of mouse Zp-2: Developmental regulation of the zona pellucida genes. Mol. Cell. Biol. 10, 1507-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Litscher E. S., Mortillo S., Sakai Y., Kinloch R. A., Stewart C. L., Wassarman P. M. (1996). Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl. Acad. Sci. USA 93, 5431-5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford R. D., Jenkins N. A., Kozak C. A., Liang L.-F., Silan C. M., Copeland N. G., Dean J. (1990). Genomic mapping of murine Zp-2 and Zp-3, two oocyte-specific loci encoding zona pellucida proteins. Genomics 6, 184-187 [DOI] [PubMed] [Google Scholar]
- Magliery T. J., Wilson C. G., Pan W., Mishler D., Ghosh I., Hamilton A. D., Regan L. (2005). Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J. Am. Chem. Soc. 127, 146-157 [DOI] [PubMed] [Google Scholar]
- Marzolo M. P., Yuseff M. I., Retamal C., Donoso M., Ezquer F., Farfan P., Li Y., Bu G. (2003). Differential distribution of low-density lipoprotein-receptor-related protein (LRP) and megalin in polarized epithelial cells is determined by their cytoplasmic domains. Traffic 4, 273-288 [DOI] [PubMed] [Google Scholar]
- Merchant H., Chang M. C. (1971). An electron microscopic study of mouse eggs matured in vivo and in vitro. Anat. Rec. 171, 21-37 [DOI] [PubMed] [Google Scholar]
- Millar S. E., Lader E., Liang L.-F., Dean J. (1991). Oocyte-specific factors bind a conserved upstream sequence required for mouse zona pellucida promoter activity. Mol. Cell. Biol. 12, 6197-6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinski J. A. (1970). The role of the zona pellucida in the development of mouse eggs in vivo. J. Embryol. Exp. Morphol. 23, 539-547 [PubMed] [Google Scholar]
- Nagdas S. K., Araki Y., Chayko C. A., Orgebin-Crist M.-C., Tulsiani D. R. P. (1994). O-linked trisaccharide and N-Linked poly-N-acetyllactosaminyl glycans are present on mouse ZP2 and ZP3. Biol. Reprod. 51, 262-272 [DOI] [PubMed] [Google Scholar]
- Nyfeler B., Michnick S. W., Hauri H. P. (2005). Capturing protein interactions in the secretory pathway of living cells. Proc. Natl. Acad. Sci. USA 102, 6350-6355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Hardelin J. P. (2001). Molecular genetics of hearing loss. Annu. Rev. Genet. 35, 589-646 [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Shalgi R. (1980). Surface architecture of the mouse and hamster zona pellucida and oocyte. J. Ultrastruct. Res. 72, 1-12 [DOI] [PubMed] [Google Scholar]
- Rankin T., Familari M., Lee E., Ginsberg A. M., Dwyer N., Blanchette-Mackie J., Drago J., Westphal H., Dean J. (1996). Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 122, 2903-2910 [DOI] [PubMed] [Google Scholar]
- Rankin T., Talbot P., Lee E., Dean J. (1999). Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 126, 3847-3855 [DOI] [PubMed] [Google Scholar]
- Rankin T. L., O'Brien M., Lee E., Wigglesworth K. E. J. J., Dean J. (2001). Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development 128, 1119-1126 [DOI] [PubMed] [Google Scholar]
- Rankin T. L., Coleman J. S., Epifano O., Hoodbhoy T., Turner S. G., Castle P. E., Lee E., Gore-Langton R., Dean J. (2003). Fertility and taxon-specific sperm binding persist after replacement of mouse ‘sperm receptors’ with human homologues. Dev. Cell 5, 33-43 [DOI] [PubMed] [Google Scholar]
- Richardson G. P., Lukashkin A. N., Russell I. J. (2008). The tectorial membrane: one slice of a complex cochlear sandwich. Curr. Opin. Otolaryngol. Head Neck Surg. 16, 458-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. (1988). Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev. Biol. 127, 287-295 [DOI] [PubMed] [Google Scholar]
- Salzmann G. S., Greve J. M., Roller R. J., Wassarman P. M. (1983). Biosynthesis of the sperm receptor during oogenesis in the mouse. EMBO J. 2, 1451-1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F., Wachi H., Ishida M., Nonaka R., Onoue S., Urban Z., Starcher B. C., Seyama Y. (2007). Distinct steps of cross-linking, self-association, and maturation of tropoelastin are necessary for elastic fiber formation. J. Mol. Biol. 369, 841-851 [DOI] [PubMed] [Google Scholar]
- Schaeffer C., Santambrogio S., Perucca S., Casari G., Rampoldi L. (2009). Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol. Biol. Cell 20, 589-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J., Vavra U., Stadlmann J., Hawes C., Mach L., Steinkellner H., Strasser R. (2009). Arginine/lysine residues in the cytoplasmic tail promote ER export of plant glycosylation enzymes. Traffic 10, 101-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Tsuji M., Dean J. (1983). In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J. Biol. Chem. 258, 5858-5863 [PubMed] [Google Scholar]
- Shoulders M. D., Raines R. T. (2009). Collagen structure and stability. Annu. Rev. Biochem. 78, 929-958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K. Y., Cheung M. C., Chan D., Cheah K. S. (2010). The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res. 339, 93-110 [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. (2008). Zona pellucida glycoproteins. J. Biol. Chem. 283, 24285-24289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. (1994). Mammalian fertilization. In The Physiology of Reproduction (ed. Knobil E., Neil J.), pp. 189-317 New York: Raven Press; [Google Scholar]
- Yurewicz E. C., Hibler D., Fontenot G. K., Sacco A. G., Harris J. (1993). Nucleotide sequence of cDNA encoding ZP3 alpha, a sperm-binding glycoprotein from zona pellucida of pig oocyte. Biochim. Biophys. Acta 1174, 211-214 [DOI] [PubMed] [Google Scholar]
- Zhao M., Gold L., Ginsberg A. M., Liang L.-F., Dean J. (2002). Conserved furin cleavage site not essential for secretion and integration of ZP3 into the extracellular egg coat of transgenic mice. Mol. Cell. Biol. 22, 3111-3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Gold L., Dorward H., Liang L.-F., Hoodbhoy T., Boja E., Fales H., Dean J. (2003). Mutation of a conserved hydrophobic patch prevents incorporation of ZP3 into the zona pellucida surrounding mouse eggs. Mol. Cell. Biol. 23, 8982-8991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Boja E., Hoodbhoy T., Nawrocki J., Kaufman J. B., Kresge N., Ghirlando R., Shiloach J., Pannell L., Levine R., et al. (2004). Mass spectrometry analysis of recombinant human ZP3 expressed in glycosylation deficient CHO cells. Biochemistry 43, 12090-12104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.