Abstract
Female mice are more susceptible to radiation-induced cognitive changes than male mice. Previously, we showed that in female mice, androgens antagonize age-related cognitive decline in aged wild-type mice and androgens and selective androgen receptor modulators (SARMs) antagonize cognitive changes induced by human apolipoprotein E4, a risk factor for developing age-related cognitive decline. In this study, the potential effects of the SARM ACP-105 were assessed in female mice that were either sham-irradiated or irradiated with 137Cesium at a dose of 10 Gy. Behavioral testing started 2 weeks following irradiation. Irradiation impaired sensorimotor function in vehicle-treated mice but not in ACP-105-treated mice. Irradiation impaired cued fear conditioning and ACP-105 enhanced fear conditioning in sham-irradiated and irradiated mice. When immunoreactivity for microtubule-associated protein was assessed in the cortex of sham-irradiated mice, there was a brain area × ACP-105 interaction. While ACP-105 reduced MAP-2 immunoreactivity in the sensorymotor cortex, it increased MAP-2 immunoreactivity in the enthorhinal cortex. No effect on MAP-2 immunoreactivity was seen in the irradiated cortex or sham-irradiated or irradiated hippocampus. Thus, there are relatively early radiation-induced behavioral changes in female mice and reduced MAP-2 levels in the sensorimotor cortex following ACP-105 treatment might contribue to enhanced rotorod performance.
Keywords: androgen receptor, SARM, fear conditioning, rotorod, MAP-2, synaptophysin
1. Introduction
Cranial irradiation is associated with hippocampus-dependent impairments in humans (Roman and Sperduto, 1995) and rodents (Madsen et al., 2003; Monje et al., 2002; Raber et al., 2004; Rola et al., 2004; Shi et al., 2006; Shukitt-Hale et al., 2000; Sienkiewicz et al., 1992; Villasana et al., 2006; Villasana et al., 2008; Villasana et al., 2010b). There may be sex differences in susceptibility to radiation-induced cognitive deficits. When treated for acute lymphoblastic leukemia (ALL), the most common cancer in children and adolescents under age 20 (Butler and Mulhern, 2005), girls are more affected than boys to the effects of radiation exposure on cognitive function (Butler and Mulhern, 2005). Consistent with these human data, female wild-type mice (Villasana et al., 2010b) as well as those expressing human apolipoprotein E3 or E4, a risk factor for developing age-related cognitive decline, (Villasana et al., 2006) are more susceptible to radiation induced-cognitive impairments than genotype-matched male mice. The fear conditioning test, which allows assessment of hippocampus-dependent contextual and hippocampus-independent cued fear conditioning, is particularly sensitive to detect effects of cranial irradiation in female mice (Villasana et al., 2010b).
In addition to cognitive impairments, impairments in motor function have also been reported following cranial irradiation (Ellenberg et al., 2009; Kiehna et al., 2006; Mabbott et al., 2008). For example, five years after the cessation of radiation therapy, motor-evoked potential in the hands and legs elicited by stimulation at the cortex were prolonged and about one third of the patients showed fine or gross motor difficulties and dysdiadochokinesia (Lehtinen et al., 2002).
Selective androgen receptor modulators (SARMs) are tissue specific and do not have side effects associated with the use of conventional androgens. Effects of SARMs outside the brain have been reported ( Gao et al., 2005; Sun et al., 2006). SARMs are also promising for effects in the brain. Androgens were shown to antagonize cognitive impairments in aged 2-year-old wild-type female mice (Benice and Raber, 2009) and androgens and the SARM ACP-105 were shown to antagonize cognitive impairments in adult female mice expressing apoE4 (Raber et al., 2002, Acevedo et al., 2008a). ACP-105 might also be able to antagonize radiation-induced cognitive impairments in female mice.
Microtubule-associated protein 2 (MAP-2), found mainly in dendrites but also in soma of mature neurons, is necessary for the assembly of microtubules involved in plasticity of the dendritic arbor (Harada et al., 2002). Expression of MAP-2 is increased in the hippocampus and cortex of aged female and male C56BL/6 J wild-type mice (Benice et al., 2006) and Rhesus monkeys (Haley et al., 2010). Three months following cranial irradiation with 137Cs, MAP-2 levels are increased in dentate gyrus, CA1 and CA3 regions of the hippocampus, and enthorhinal and sensorimotor cortex of female targeted replacement mice expressing human apoE3 under control of the mouse apoE promoter (Villasana et al., 2010a). MAP-2 levels might also be altered following cranial irradiation of wild-type female mice. In aged mice (Benice et al., 2006) and monkeys (Haley et al., 2010), there are also changes in the levels of the presynaptic marker synaptophysin. MAP-2 and synaptophysin levels are generally used as markers to assess neuropathology in models of neurodegeneration (Alford et al., 1994; Masliah et al., 1993; Masliah et al., 1996; Masliah et al., 1997; Mucke et al., 1993; Mucke et al., 2000). As radiation effects might mimic some effects of aging, levels of synaptophysin might also be altered following cranial irradiation.
In the current study, the potential effects of ACP-105 (Schienger et al., 2009) on behavioral performance of C57Bl6/J wild-type female mice under baseline conditions and following irradiation were determined. Following behavioral testing, we assessed whether these effects were associated with changes in MAP-2 and synaptophysin immunoreactivity in the hippocampus and cortex.
2. Results
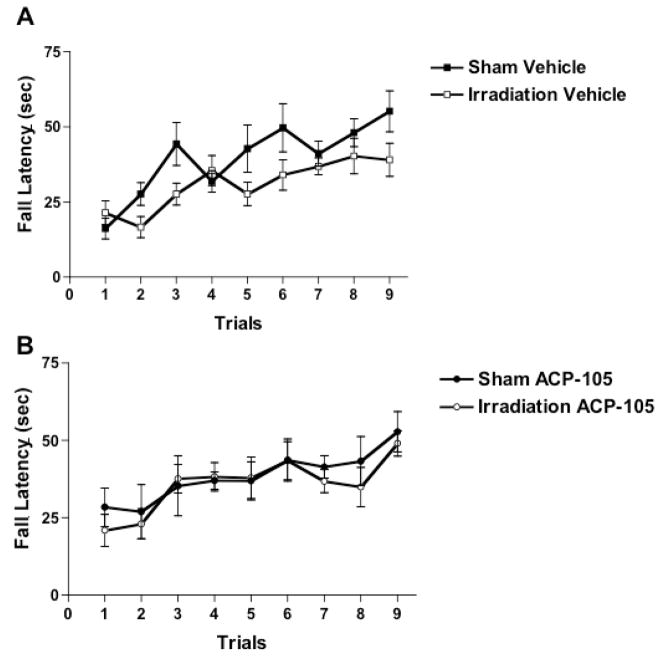
The experimental design is illustrated in Fig. 1. First, sensorimotor function was assessed using the rotorod. Vehicle-treated irradiated mice had significantly lower fall latencies than vehicle-treated sham-irradiated mice (Fig. 2A). There was a day × irradiation interaction (F = 2.381, p = 0.021) and an effect of irradiation (F = 4.996, p = 0.042). In contrast to the vehicle-treated groups, in the presence of ACP-105 there was no day × irradiation interaction (F = 0.294, p = 0.967) or effect of irradiation (F = 0.275, p = 0.609) (Fig. 2B).
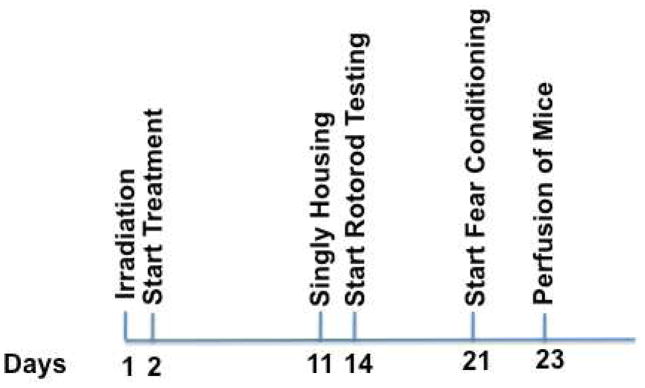
Fig. 1.
Experimental schedule. One day following irradiation the treatment started. Two week following irradiation, behavioral testing started. Three days prior to the start of behavioral testing, the mice were singly housed. One week following rotorod testing, the mice were tested for fear conditioning. One day after the second day of fear conditioning, the mice were perfused for immunohistochemistry.
Fig. 2.
Rotorod performance of sham-irradiated and irradiated vehicle- and ACP-105-treated mice. *p < 0.05 versus sham-irradiated vehicle-treated mice. n = 7 sham-irradiated vehicle-treated mice, n = 7 sham-irradiated ACP-105-treated mice, n = 8 irradiated vehicle-treated mice and n = 7 irradiated ACP-105-treated mice.
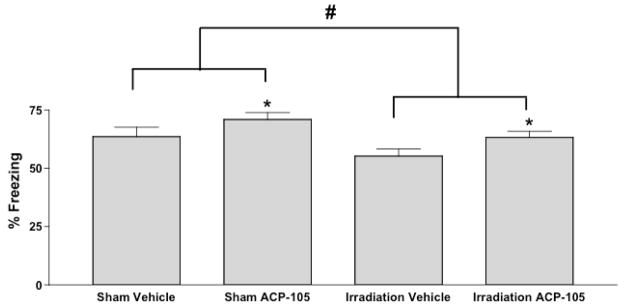
Next the mice were tested for fear conditioning. Irradiation (F = 0.5866, p = 0.8351) or ACP-105 (F = 0.4574, p = 0.5099) did not affect hippocampus-dependent contextual fear conditioning (% freezing; sham-irradiated, vehicle-treated mice: 41.51 ± 2.70, n = 7; sham-irradiated ACP-105-treated mice: 41.15 ± 4.39; irradiated, vehicle-treated mice: 38.81 ± 1.88, n = 8 mice; irradiated ACP-105-treated mice: 43.71 ± 4.40, n = 7 ACP-105-treated mice). However, irradiation did affect hippocampus-independent cued fear conditioning (effect of irradiation: F = 5.965; p = 0.022, Fig. 3). ACP-105 enhanced freezing in both sham-irradiated and irradiated mice (Effect of ACP-105: F = 5.44; p = 0.028).
Fig. 3.
Cued fear conditioning of sham-irradiated and irradiated vehicle- and ACP-105-treated mice. *p < 0.05 versus radiation treatment-matched group; #p < 0.05. n = 7 sham-irradiated vehicle-treated mice, n = 7 sham-irradiated ACP-105-treated mice, n = 8 irradiated vehicle-treated mice and n = 7 irradiated ACP-105-treated mice.
Finally, MAP-2 and synaptophysin immunoreactivity was assessed in the hippocampus and cortex (Table 1). For MAP-2 immunoreactivity in the cortex of sham-irradiated mice, there was a brain area × ACP-105 interaction (F = 6.655; p = 0.0027). While ACP-105 reduced MAP-2 immunoreactivity in the sensorymotor cortex, there was a trend towards increased MAP-2 immunoreactivity in the enthorhinal cortex. In contrast, there was no brain area × ACP-105 interaction or effect of ACP-105 on MAP-2 immunoreactivity in the irradiated cortex (interaction: F = 0.014; p = 0.908; treatment: F = 0.029, p = 0.869) or sham-irradiated (interaction: F = 1.063; p = 0.364; treatment: F= 1.666, p = 0.226) or irradiated (interaction: F = 1.57; p = 0.231; treatment: F = 0.261, p = 0.62) hippocampus. There was no brain area × ACP-105 interaction or effect of ACP-105 on synaptophysin immunoreactivity in the sham-irradiated (interaction: F = 1.30; p = 0.726; treatment: F= 0.370, p = 0.557) or irradiated (interaction: F = 0.216; p = 0.808; treatment: F= 0.110, p = 0.747) cortex or sham-irradiated (interaction: F = 0.082; p = 0.921; treatment: F= 0.08, p = 0.932) or irradiated (interaction: F = 1.282; p = 0.284; treatment: F= 0.418, p = 0.533) hippocampus.
Table 1.
MAP-2 and synaptophysin immunoreactivity in the hippocampus and cortex of sham-irradiated and irradiated mice treated with or without ACP-105.
| Area Occupied by MAP-2 Immunoreactivity (μm2) |
|||||
|---|---|---|---|---|---|
| DG | Ca1 | Ca3 | SmCtx | EntCtx | |
| Sham-Vehicle | 7030 ± 1536 | 5142 ± 1028 | 7099 ± 1218 | 5471 ± 800 | 4726 ± 716 |
| Sham-ACP-105 | 5670 ± 1464 | 3297 ± 847 | 4016 ± 1280 | 3010 ± 997* | 6012 ± 976 |
| Irradiated-Vehicle | 8748 ± 928 | 4703 ± 891 | 6676 ± 1017 | 4429 ± 1173 | 4757 ± 276 |
| Irradiated-ACP-105 | 6960 ± 1257 | 4928 ± 1649 | 5707 ± 1577 | 4072 ± 1734 | 4572 ± 1314 |
| Area Occupied by Synaptophysin Immunoreactivity (μm2) |
|||||
| DG | Ca1 | Ca3 | SmCtx | EntCtx | |
| Sham-Vehicle | 8904 ± 717 | 3865 ± 1798 | 4730 ± 1711 | 3320 ± 1507 | 3968 ± 895 |
| Sham-ACP-105 | 8742 ± 728 | 4239 ± 1397 | 4964 ± 1122 | 4568 ± 1578 | 4722 ± 895 |
| Irradiated-Vehicle | 8000 ± 1556 | 4518 ± 1162 | 5194 ± 1163 | 5459 ± 1789 | 4058 ± 1085 |
| Irradiated-ACP-105 | 9045 ± 756 | 4774 ± 1346 | 5420 ± 1258 | 3322 ± 1265 | 4360 ± 608 |
DG, dentate gyrus of the hippocampus; Ca1 and Ca3 areas of hippocampus; SmCtx, somatosensory cortex; and EntCtx , entorhinal cortex.
p < 0.05 versus Sham-Vehicle in the SmCtx. N= 6 brains per group
3. Discussion
In this study, we detect for the first time radiation-induced impairments in rotorod function and reduced cued fear conditioning in the absence of detecting hippocampus-dependent cognitive impairments. This pattern of radiation-induced behavioral changes might relate to the fact that behavioral testing started 2 weeks following irradiation. These data show that at relatively early times after irradiation the hippocampus might not be necessarily more susceptible than other brain areas to the effects of radiation on cognitive function. This is consistent with the effects of gamma irradiation on hippocampus-independent novel object recognition (Raber et al., 2009). In contrast to the mitigating effects of ACP-105 against radiation-induced impairments in sensorimotor function on the rotorod, ACP-105 enhanced cued fear conditioning in both sham-irradiated and irradiated mice. ACP-105 might be a cognitive enhancer and not only affect cognition following a challenge such as cranial irradiation. Consistent with such an effect following androgen receptor-mediated signaling, transgenic mice overexpressing androgen receptors showed enhanced spatial memory retention in the water maze under baseline conditions and 3 months following irradiation (Acevedo et al., 2008c).
The mechanisms underlying effects of irradiation on hippocampal function might involve alterations in neurogenesis in the subgranular zone of the hippocampal dentate gyrus and in subsequent migration of newly born cells into the dentate granule cell layer (Kempermann et al., 1997). Significant cell loss occurs in the dentate gyrus within hours after irradiation (Mizumatsu et al., 2003; Villasana et al., 2010a). However, although radiation-induced changes in hippocampal function were seen months following irradiation (Acevedo et al., 2008b; Acevedo et al., 2008c; Monje et al., 2003; Raber et al., 2009; Rabin et al., 2009; Shukitt-Hale et al., 2000; Villasana et al., 2010b), no effects of irradiation on hippocampus-dependent contextual fear conditioning were seen 2 weeks following irradiation in the current study. It is conceivable that 2 weeks is not sufficient for new cells to become functionally integrated into the hippocampal network (Eriksson et al., 1998; Gould et al., 1999; Kempermann et al., 1998; Munoz et al., 2005; Murrell et al., 1996; Prickaerts et al., 2004). Longer periods might be required to detect radiation-induced hippocampus-dependent cognitive impairments. These data highlight the importance of including both hippocampus and non-hippocampus-dependent versions of cognitive tests in the evaluation of irradiation effects on brain function.
In sham-irradiated mice, enhanced rotorod performance in ACP-105 treated mice was associated with reduced MAP-2 levels in the sensorimotor cortex. A similar trend was seen in the somotosensoricortex of irradiated mice. Interestingly, with age (Benice et al., 2006; Haley et al., 2010)) and 3 months following cranial 137Cs irradiation with (Villasana et al., 2010a), enhanced MAP-2 levels are associated with reduced performance. Therefore, it is possible that reduced MAP-2 levels might contribute to enhanced cognitive performance. Future studies are warranted to further explore this hypothesis. However, as SARMs are shown to improve muscle strength, body composition, and bone function, and reduce body fat (Allan et al., 2007; Gao et al., 2005; Kearbey et al., 2007), we cannot exclude that peripheral effects of SARMs contributed to the enhanced rotorod performance.
In summary, irradiation impaired sensorimotor function in vehicle-treated mice but not in ACP-105-treated mice. In contrast, irradiation reduced fear conditioning and ACP-105 enhanced fear conditioning in sham-irradiated and irradiated mice. ACP-105 enhanced rotorod performance. In sham-irradiated ACP-105-treated mice, enhanced rotororod performance was associated with reduced MAP-2 immunoreactivity. These data support SARMs as a potential therapeutic to enhance brain function under baseline conditions and following radiation.
4. Experimental Procedure
Mice
Two-month-old C56Bl/6J female mice were kept on a 12:12 hr light-dark schedule (lights on at 6AM) with lab chow (PicoLab Rodent Diet 20, #5053; PMI Nutrition International, St. Louis, MO) and water given ad libitum. All procedures were according to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the Oregon Health and Science University (OHSU). OHSU has an Association for the Assessment and Accreditation of Laboratory Animal Care approved animal facility. The estrous cycle was not assessed in the mice.
Irradiation and SARM treatment
Following i.p. anesthesia (ketamine (Sigma), 80 mg/kg and xylazine (Sigma), 20 mg/kg), mice were sham-irradiated (n = 7 sham vehicle-treated mice and n = 7 ACP-105-treated mice) or irradiated (n = 8 vehicle-treated mice and n = 7 ACP-105-treated mice) using a dose of 10 Gy in a Mark 1 Cesium Irradiator (Shepherd and Associates, San Fernando, CA). The cerebellum, eyes, and body were shielded with lead.
Twenty-four hours following irradiation, the mice were implanted with Alzet minipumps filled with ACP-105 (provided by Acadia Pharmaceuticals) at 1 mg/kg/day or 1.09 mg/200 μl in 10% Tween in saline or vehicle. Behavioral testing started two weeks after irradiation. For the structure of ACP-105, see (Acevedo et al., 2008a). Note that ACP-105 was referred to as AC-264184 in earlier publications.
Behavioral testing
Mice were housed singly starting 3 days prior to testing sensorimotor function on the rotorod (Raber et al., 2009) in week 1. Mice were singly housed, as placing a behaviorally tested mouse back in a cage of other mice that have not been tested yet might affect their performance. Mice were not singly housed directly after irradiation as living alone for a short period (7 days) did not affect any physiological indexes of stress in female mice and had marginal effects on emotional behavior (Bartolomucci et al., 2009). The person testing the mice was blinded to the treatments of the mice. To assess sensorimotor ability, mice were tested using a rotorod apparatus (Kinder Scientific, Poway, CA). Mice were placed on an elevated rod (7.0 mm in diameter) initially rotating at 4 rpm. Every 15 sec, the rod was accelerated by 15 rpm. Fall latency was recorded by timers, which stopped when the mouse broke the photobeams at the bottom of the chamber. Mice received three trials per day for three subsequent days.
Mice were tested for fear conditioning in week 2 (Villasana et al., 2010). During contextual fear conditioning, mice learn to associate the environmental context (conditioned stimulus, CS) with a mild foot shock (unconditioned stimulus, US). On day 1, each mouse was placed in a sound attenuated fear conditioning chamber (Med Associates, St. Albans, VT) and allowed to explore for 2 minutes before delivery of a 30 second tone (3.8kHz, 80 db) which was immediately followed by a 2 sec foot shock (0.60 mA). Two min later, a second CS-US pair was delivered. On day 2, each mouse was first placed in the fear conditioning chamber containing the exact same context with the exception of the tone or foot shock. Hippocampus-dependent contextual freezing was assessed for 3 min. One hour later, each mouse was placed in a new context containing a different odor, cleaning solution, floor texture, walls and shape. Three minutes later each mouse was re-exposed to the fear conditioning tone for 3 min and hippocampus-independent cued fear conditioning was assessed. Freezing behavior was acquired and analyzed using the Ethovision XT video tracking system (Noldus Information Technology, Sterling, VA) which tracks changes in pixels to measure immobility (freezing). Contextual conditioned fear was assessed during the first 3 minutes of the contextual test trial when freezing behavior is most robust. Cued conditioned fear was assessed during the presentation of the tone (the last 3 minutes of the trial).
MAP-2 and synaptophysin immunoreactivity
Following behavioral testing, all mice were anesthetized (100 mg/kg ketamine, 10mg/kg xylazine, 2 mg/kg acepromazine) and transcardially perfused with saline followed by 4% paraformaldehyde. The brains were removed, allowed to equilibrate in 30% sucrose overnight at 4°C, then stored at −80°C and later processed for MAP-2 and synaptophysin immunohistochemistry as summarized below.
Coronal sections (30μm) containing relevant brain regions, guided by a brain atlas (Franklin and Paxinos, 1997), were generated from the fixed frozen brains of female mice using a cryostat (Microm). Sections were serially mounted on Superfrost microscope slides (Fisher Scientific) and stored at −80°C. Upon use, slides were allowed to air dry for 10 minutes at room temperature and then placed on a slide warmer for 45 minutes to promote adhesion. Slides were rehydrated for 10 minutes at room temperature (RT).
All slides were washed twice with phosphate buffered saline (PBS) for 10 minutes at RT and once with PBS containing 0.2% BSA + 0.2% Triton X-100 (PBT) for 15 minutes at RT. A 5% normal donkey serum (Jackson Immunoresearch, West Grove, PA) plus 1% fish skin gelatin (Sigma-Aldrich, St. Louis, MO) was used to block nonspecific binding (120 min RT). Subsequently, slides were incubated with mouse anti-synaptophysin or anti-MAP-2 antibody (1:500, Millipore, Billerica, MA) overnight at 4°C in a humidifying chamber. Sections were washed three times (every 15 minutes) with PBT and incubated with Texas Red conjugated donkey-anti-mouse IgG F(ab′)2 (1:100, Jackson Immunoresearch) in a dark humidifying chamber (240 min RT). Sections were washed, in the dark, once with PBT for 10 minutes and then three times with PBS for 10 minutes at RT. Slides were then treated with a 10 mM CuSO4 in a 50 mM NH4OAc buffer (pH 5.0) for 7 minutes in the dark. The slides were again washed three times with PBS for 10 minutes at RT in the dark. Anti-fade mounting media containing DAPI (Vectashield) was added to the slides, before cover slipping, and sealed with enamel. For each mouse, 8 sections serially sectioned through the hippocampus were analyzed using an unbiased stereological approach. Every 7th section was analyzed. Immunoreactivity was analyzed using an Olympus spinning disk confocal microscope, a 60X oil objective, and Slidebook software (Intelligent Imaging Innovations, Denver, CO) for the following areas: dentate gyrus, CA1 and CA3 regions of the hippocampus, sensorimotor (SMCX) and enthorhinal cortex (ENTCX). Each image was generated using 12, 1 micron layers of a Z-stack. Each Z series stack was collapsed into a single projection. The images were analyzed for area occupied by MAP-2 and synaptophysin immunoreactivity using the Slidebook software. Thresholds were calculated and set for each region of interest, based on averaging the low intensity range value across all test groups.
Statistical Analysis
Differences among means were evaluated by ANOVA, followed by Tukey-Kramer post hoc test if indicated. Data are expressed as mean ± SEM. To compare rotorod performance curves, repeated measures ANOVA was used. For immunoreactivity analysis, irradiation treatment was used as between-group subject factors and brain region was used as the within-group subject factor. The null hypothesis was rejected at the 0.05 level for all analyses.
Acknowledgments
We would like to thank Krista McFarland from Acadia Pharmaceuticals for generously providing ACP-105. This work was supported by a NIAID Cooperative Agreement U19 AI067734.
Footnotes
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. 2. References
- Acevedo S, Gardell L, Bradley SR, Piu F, Raber J. Selective androgens receptor modulators antagonize apolipoprotein E4-induced cognitive impairments. Lett Drug Design Discovery. 2008a;5:271–276. [Google Scholar]
- Acevedo S, McGinnis G, Raber J. Effects of 137Cesium irradiation on cognitive performance and measures of anxiety of Apoe-/- and wild-type female mice. Radiat Res. 2008b;170:168–184. doi: 10.1667/rr1494.1. [DOI] [PubMed] [Google Scholar]
- Acevedo S, Tittle S, Raber J. Transgenic expression of androgen receptors improves spatial memory retention of sham-irradiated and 137Cesium irradiated female mice. Radiat Res. 2008c;170:161–167. doi: 10.1667/RR1435.1. [DOI] [PubMed] [Google Scholar]
- Alford MF, Masliah E, Hansen LA, Terry RD. A simple dot-immunobinding assay for quantification of synaptophysin-like immunoreactivity in human brain. J Histochem Cytochem. 1994;42:283–287. doi: 10.1177/42.2.8288869. [DOI] [PubMed] [Google Scholar]
- Allan G, Lai M, Sbriscia T, Linton O, Haynes-Johnson D, Bhattacharjee S, Dodds R, Fiordeliso J, Lanter J, Sui Z, Lundeen S. A selective androgen receptor modulator that reduces prostate tumor size and prevents orchidectomy-induced bone loss in rats. J Steroid Biochem Mol Biol. 2007;103:76–83. doi: 10.1016/j.jsbmb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Parmigiani S, Gioiosa L, Ceresini G, Palanza P. Effects of housing social context on emotional behavior and physiological responses in female mice. Scan J Lab Anim Sci. 2009;36:87–95. [Google Scholar]
- Benice T, Rizk A, Pfankuch T, Kohama S, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Benice T, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn Mem. 2009;16:479–485. doi: 10.1101/lm.1428209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R, Mulhern R. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer R, Mertens A, Donaldson S, Stovall M, Kadan-Lottick N, Armstrong G, Robison L, Zeltzer L. Neurocognitive Status in Long-Term Survivors of Childhood CNS Malignancies: A Report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Gao W, Reiser P, Coss C, Phelps M, Kearbey J, Miller D, Dalton J. Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–4897. doi: 10.1210/en.2005-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Haley G, Kohama S, Urbanski H, Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus nacaque prefrontal cortex and hippocampus. AGE. 2010;32:283–296. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP-2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearbey J, Gao W, Narayanan R, Fisher S, Wu D, Miller D, Dalton J. Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res. 2007;24:328–335. doi: 10.1007/s11095-006-9152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehna E, Mulhern R, Li C, Xiong X, Merchant T. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. Clin Oncol. 2006;24:5283–5290. doi: 10.1200/JCO.2005.03.8547. [DOI] [PubMed] [Google Scholar]
- Lehtinen S, Huuskonen U, harila-Saari A, Tolonen U, Vainionpaa L, Lanning B. Motor nervous impairment persists in long-term survivors of childhood lymphoblast leukemia. Cancer. 2002;94:2466–2473. doi: 10.1002/cncr.10503. [DOI] [PubMed] [Google Scholar]
- Mabbott D, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:158–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Masliah E, Abraham CR, Johnson W, Forss-Petter S, Mallory M, Terry R, Mucke L. Synaptic alterations in the cortex of APP transgenic mice. J Neuropathol Exp Neurol. 1993;52:307. [Google Scholar]
- Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F b-amyloid precursor protein and Alzheimer’s disease. J Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Westland CE, Rockenstein EM, Abraham CR, Mallory M, Veinberg I, Sheldon E, Mucke L. Amyloid precursor proteins protect neurons of transgenic mice against acute and chronic excitotoxic injuries in vivo. Neuroscience. 1997;78:135–146. doi: 10.1016/s0306-4522(96)00553-2. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Monje M, Toda H, Palmer T. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monje MJ, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nature Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Rockenstein E, Toggas S. Neuropathologic alterations induced in brains of transgenic mice by expression of the HIV-1 envelope protein gp120. J Neuropathol Exp Neurol. 1993;52:314. [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Ab1–42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Stoutenger B, Robinson A, Spees J, Prockop D. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W, Bushell GR, Livesey J, McGrath J, MacDonald KPA, Bates PR, Mackay-Sim A. Neurogenesis in adult human. NeuroReport. 1996;7:1189–1194. doi: 10.1097/00001756-199604260-00019. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: Survival with or without proliferation? Neurobiol Learn Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Fan Y, Matsumori Y, Weistein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis in the dentate gyrus and exacerbates ischemia-induced functional deficits. Ann Neurol. 2004;55:381–390. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- Raber J, Villasana L, Rosenberg J, Zou Y, Huang T-T, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2009 doi: 10.1002/hipo.20724. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin B, Carrihill-Knoll K, Hinchman M, Shukitt-Hale B, Joseph J, Foster B. Effects of heavy particle irradiation and diet on object recognition memory in rats. Adv Space Res. 2009;43:1193–1199. [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg S, Morhardt D, Fike J. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- Schienger N, Lund B, Pawlas J, Badalassi F, Bertozzi F, Lewinsky R, Fejzic A, Thygesen M, Tabatabaei A, Bradley SR, Gardell L, Plu F, Olsson R. Synthesis, structure-activity relationships, and characterization of novel nonsteroidal and selective androgen receptor modulators. J Med Chem. 2009;52:7186–7191. doi: 10.1021/jm901149c. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams M, Long A, Carter C, Bennett C, Sonntag W, Nicolle M, Robbins M, D’Agostino R, Brunso-Bechtold J. patial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Rad Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Casadesus G, McEwen J, Rabin B, Joseph J. Spatial learning and memory deficits induced by exposure to iron-56- particle radiation. Rad Res. 2000;154:28–33. doi: 10.1667/0033-7587(2000)154[0028:slamdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz ZJ, Saunders RD, Butland BK. Prenatal irradiation and spatial memory in mice: investigation of critical period. Int J Radiat Biol. 1992;62:211–219. doi: 10.1080/09553009214552031. [DOI] [PubMed] [Google Scholar]
- Sun C, Robl J, Wang T, Huang Y, Kuhns J, Lupisella J, Beehler B, Golla R, Sleph P, Seethala R, Fura A, Krystek SJ, An Y, Malley M, Sack J, Salvati M, Grover G, Ostrowski J, Hamann L. Discovery of potent, orally-active, and muscle-selective androgen receptor modulators based on an N-aryl-hydroxybicyclohydantoin scaffold. J Med Chem. 2006;49:7596–7599. doi: 10.1021/jm061101w. [DOI] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and ApoE Isoform-dependent effects of radiation on cognitive function. Rad Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Villasana L, Poage C, Van Meer P, Raber J. Passive avoidance learning and memory of 56Fe sham-irradiated and irradiated human apoE transgenic mice. Radiat Biol Radioecol. 2008;48:191–194. [PubMed] [Google Scholar]
- Villasana L, Pfankuch T, Raber J. Isoform-Dependent Effects of apoE on Doublecortin-Positive Cells and Microtubule-Associated Protein 2 Immunoreactivity following 137Cs Irradiation. Radiat Environ Biophys. 2010a doi: 10.1007/s00411-010-0290-4. in press. [DOI] [PubMed] [Google Scholar]
- Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe Irradiation on contextual fear conditioning in C56BL/6J mice. Hippocampus. 2010b;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible B, Hawryluk P, Ficker E, Kuryshev Y, Kirsch G, Brown A. HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. J Pharmacol Toxicol Methods. 2005;52:136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]