Abstract
Endothelial cells express the nucleotide binding oligomerization domain (NOD) receptor 2, which recognizes the bacterial derivate muramyl dipeptide (MDP). MDP stimulation of these cells enhances their IL-6 production and may thus contribute to the immune and inflammatory activities in the skin. However, whether endothelial cells are capable of influencing the development of T cell priming and its polarization remains unknown. We report that in vitro, the murine bEnd.3 endothelial cell line induces, following MDP stimulation, a Th17 polarization at the expense of Th1 and Th2 polarization in the setting of Langerhans cell (LC) antigen presentation to responsive T cells as assessed by IL-17, IL-6, IFN-γ and IL-4 production. Interestingly, IL-22 production, which has been associated with Th17 priming, was not influenced by MDP-treated bEnd.3 cells, illustrating differential regulation of this cytokine from IL-17. Additional analysis confirmed a significantly increased percentage of IL-17+CD4+ T cells by flow cytometry and an increased mRNA level of the specific Th17 transcription factor RORγt in co-cultures of LCs and responsive T cells in the presence of activated bEnd.3 cells. Experiments using the RNA interference technique to knock-down IL-6 in bEnd.3 cells confirmed that IL-6 produced by bEnd.3 cells stimulated by MDP is at least partially involved in Th17 polarization. Our data suggest that activated endothelial cells are capable of influencing LC antigen processing and presentation to T cells and induce a Th17 polarization. These results are important for the understanding of Th17-related disorders of the skin such as psoriasis.
Introduction
Dermal microvascular endothelial cells (DMECs) participate in immune and inflammatory activities in the skin through release of cytokines and chemokines, which modulate immune cell functions, and by expression of adhesion molecules involved in transmigration of leukocytes out of the vasculature. Several studies have demonstrated that endothelial cells express different pattern recognition receptors (PRRs), such as toll like receptors (TLRs) and nucleotide binding oligomerization domain proteins (NODs), and may respond to the correlated pathogen associated molecular patterns (PAMP) (1). The NOD2 receptor is a member of the family of NOD proteins and recognizes the peptidoglycan (PGN) derivate muramyl dipeptide (MDP) (2). Mutations in Nod2 gene have been correlated with the development of Blau syndrome (3) and Crohn’s disease (4). Th17 cells are a recently discovered lineage of effector CD4+ T cells characterized by the production of IL-17, IL-21 and IL-22 and by the expression of the transcription factor retinoid orphan receptor γt (RORγt). In mice, polarization to a Th17 immune response is dependent on the presence of the proinflammatory cytokines IL-6 and TGF-β1 while it is suppressed by IFN-γ, IL-4 and IL-12 (5). Additionally, the Th17 response is amplified by IL-23 (6). In humans, Th17 development is promoted by IL-6, TGF-β1 and IL-1β (7). Recent studies have also proposed the existence in humans of a new lineage of effector CD4+ T cells producing IL-22 but not IL-17 (Th22). These cells appear to develop in an IL-6 and IL-23 rich environment and are inhibited by the presence of TGF-β1 (8, 9). Interestingly, it has been reported that in human endothelial cells IL-6 production is enhanced following MDP stimulation (10). In addition, NOD2 activation in human dendritic cells promotes IL-17 production by memory T cells through induction of IL-23 and IL-1 expression, suggesting a role of this PRR in Th17 development (11). Th17 cells participate in defense against extracellular bacteria and fungi. However, these cells may also play a role in tumor immunity (12), autoimmune disorders (13), graft rejection and host versus graft disease (14, 15). Recent studies have reported the presence in psoriatic lesional skin of enhanced IL-23 and IL-17 expression together with an increased population of Th17 cells (16, 17). Moreover, IL-6, which is necessary for Th17 priming, has previously been found to be over-expressed in lesions of psoriasis (18, 19). In rheumatoid arthritis patients, a Th17 cytokine profile expression is correlated with cartilage and bone destruction (20), while IL-6 and IL-17 expression is significantly increased in multiple sclerosis lesions (21). IL-22 and Th22 cells have also been proposed to be implicated in psoriasis development and to play a major role in atopic dermatitis (22, 23). Langerhans cells (LCs) are professional antigen presenting cells (APCs) resident in the epidermis and characterized by expression of langerin and birbeck granules (24). LCs have classically been thought to be involved in the initiation of cutaneous immune response by processing microbial antigen for presentation to effector T cells (25). However, studies using LC-deficient mice suggest that in the steady-state LCs may induce tolerance (26). Here we hypothesize that DMECs may participate in the skin immune response by modulating LCs antigen processing and presentation. In particular, we show in an in vitro model that the murine bEnd.3 endothelial cells line may respond to microbial stimuli, such as MDP, by increasing IL-6 production and consequently influencing the LCs and T cells microenvironment during antigen presentation. In this scenario, the increased IL-6 concentration biases T cell development to a Th17-type immune response with inhibition of Th1- and Th2-type immunity as assessed by IL-17, IFN-γ and IL-4 production in this study. These data were confirmed by RORγt mRNA expression and flow cytometric analysis of IL-17+CD4+ T cells. These results are important for the understanding of Th17-related pathologies, especially in the skin, where IL-6 and IL-17 production along with Th17 cells have been correlated with the development of autoimmune diseases such as psoriasis.
Materials and Methods
Mice
Balb/c (H-2d) inbred mice and transgenic DO11.10 (C.Cg-Tg(DO11.10)10Dlo/J, H-2d) mice (Balb/c background) were purchased from The Jackson Laboratory, Bar Harbor, Maine. DO11.10 mice express T cellα andβ receptor transgenes, which recognize a fragment of chicken ovalbumin (cOVA 323–339) peptide. All mice were maintained in the Weill Cornell Medical College animal facility under specific pathogen-free conditions, food and water ad libitum and under a standard 12 hours photoperiod at a constant temperature of 21°C. Seven to ten weeks old female mice were used for all experiments. All experiments were approved by the Institutional Animal Care and Use Committee of the WeillCornell Medical College.
Media and cell lines
The bEnd.3 cell line (27) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). This cell line is an endothelial cell line established from the cerebral cortex of Balb/c mice and has many characteristics of freshly isolated endothelial cells including expression of von Willebrand factor (28), intracellular adhesion molecule 1 (ICAM-1) (29) and vascular cell adhesion molecule 1 (VCAM-1). The PAM212 cell line is a transformed Balb/c murine keratinocyte cell line provided by Stuart Yuspa (National Cancer Institute, Bethesda, MD).
bEnd.3 and PAM212 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Mediatech, Manassas, VA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Gemini Bio-Products, Sacramento, CA), 100 U/ml penicillin (Mediatech), 100 μg/ml streptomycin (Mediatech) and 2 mM L-glutamine (Mediatech). LCs and T cells were cultured in complete medium (CM): RPMI 1640 (Mediatech) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM non essential amino acids (Mediatech), 0.1 mM essential amino acids (Mediatech), 2 mM L- glutamine, 1 mM sodium pyruvate (Mediatech) and 10 mM HEPES buffer (Mediatech).
Primary cells
Primary mouse dermal endothelial cells were obtained from Celprogen (San Pedro, CA) and maintained in dermal endothelial cell complete growth medium (Celprogen) in precoated flasks (Celprogen).
Primary mouse keratinocytes (Kera) were isolated from the negative fraction of LC purification on the basis of a previously described protocol (30). Briefly, epidermal cells depleted of LC were treated with dead cell removal kit (Miltenyi Biotec, Auburn, CA) following the manufacturer instruction to eliminate dead cells. The keratinocytes obtained were plated in DMEM medium supplemented with 10% FBS, 100 U/ml penicillin (Mediatech), 100 μg/ml streptomycin (Mediatech) and 2 mM L-glutamine (Mediatech) and used for the experiments the next day.
Cell stimulation
In order to investigate the effect of NOD2 and TLR-4 activation on the bEnd.3 and PAM212 cell lines and on primary dermal endothelial cells and on primary keratinocytes, cells were plated at a concentration of 0.2 × 106 cells per well in a 12-well plate in the presence or absence of 2 μg/ml of MDP (InvivoGen, San Diego, CA) or 0.1 μg/ml of Lipopolysaccharide (LPS, Invitrogen, Carlsbad, CA). The supernatants were collected 3, 6, 9 and 24 hours later and analyzed for cytokine content by ELISA.
LC purification
LCs were obtained following a modified standard protocol (31, 32). Briefly, the truncal skin of mice was shaved and chemically depilated. The subcutaneous fat and panniculum carnosum were removed by blunt dissection. The skin was then floated dermis side down in PBS (Mediatech) containing 0.5 U/ml of dispase (Mediatech) and 0.25% trypsin (Mediatech). Epidermal sheets were collected by gentle scraping, washed, and dissociated by continuous mild agitation in Hank’s Buffered Salt Solution (HBSS, Mediatech) supplemented with 2% FBS. Epidermal cells were then filtered through a 40 μm cell strainer (BD Biosciences, San Jose, CA). LCs were enriched by incubation with mouse anti-mouse I-Ad antibody (AMS 32.1) (BD Biosciences) followed by incubation with goat anti-mouse IgG antibody conjugated to magnetic microspheres (Dynabeads, Invitrogen). LCs were isolated by placing the tube in a magnet (Invitrogen), discarding the supernatant and washing the bead-bound cells three times with HBSS containing 2% FBS. This procedure yields to a cell population of ~95% LCs as shown by flow cytometry.
RNA interference
Twenty-thousand bEnd.3 or PAM212 cells per well were plated in 96-well round-bottom plates (BD Falcon, San Jose, CA) in antibiotic free medium and incubated at 37°C until adherence. The appropriate amount of ON-TARGET plus SMARTpool Mouse IL-6 siRNA (Dharmacon, Lafayette, CO, target sequences: CCUAGUGCGUUAUGCCUAA; U U A C A C A U G U U C U C U G G G A ; G G A C C A A G A C C A U C C A A U U ; CUACCAAACUGGAUAUAAU) or the corresponding ON-TARGET plus non-targeting pool were diluted in OptiMEM medium (Invitrogen) to obtain a final concentration of 100 nM. Lipofectamine 2000 (Invitrogen) was also diluted following the manufacturer instruction in OptiMEM. The diluted siRNA and Lipofectamine 2000 were mixed and incubated for 20 minutes at RT for complex formation. Twenty-five μl of complexed siRNA was added to each well. After overnight incubation at 37°C, the medium was replaced with complete medium and cells used for the in vitro antigen presentation experiments.
In vitro antigen presentation to DO11.10 T cells
In order to evaluate possible involvement of endothelial cells in the skin immune response, 0.5 × 105 bEnd.3 or 0.25 × 105 PAM212 cells per well were plated in 96 well, round-bottom plates and incubated overnight at 37°C. The following day, cells were treated with 2 μg/ml of MDP or medium alone for 3 hours at 37°C. Cells were then washed extensively and 1 × 104 purified LCs in complete medium were added to each well. T cells were isolated from the spleens of DO11.10 transgenic mice using the previously described nylon-wool protocol (33). Briefly, spleens were collected and mechanically disrupted to obtain a single cells suspension. After erythrocyte lysis by exposure to hypotonic medium, spleen cells were filtered through a 70 μm cell strainer (BD Falcon) and extensively washed before application to a nylon-wool column. After 1 hour incubation at 37°C, T cells were eluted from the column and 2 × 105 T cells in complete medium were added to each well along with 10 μM cOVA323–339 (Peptides International, Louisville, KY). Supernatants were harvested 48 hours later and analyzed by sandwich ELISA for cytokine content.
ELISA
To quantitate protein production in culture supernatants IL-1β, IL-4, IL-6, IL-10, IL-17 DuoSet ELISA kits (R&D, Minneapolis, MN), IL-22 kits (InvivoGen), IL-12, IL-23p19 and TGF-β1 kits (eBioscience, San Diego, CA) and IFN-γ kits (BD Biosciences) were used following the manufacturer instructions. Optical density was determined at 450 nm in a Versamax microplate reader (Molecular devices, Sunnyvale, CA) and data analyzed with the Softmax software.
Real-time RT PCR
For gene expression analysis, T cells and LCs were gently collected from mixed culture wells after 24 hours incubation and total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from 1 μg of total RNA using random hexamer primer and Superscript III (Invitrogen). RORγt expression was analyzed by real-time PCR using Power SYBR Green Master Mix (Applied Biosystems, Foster City CA) and the ABI 7900HT instrument (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as internal control and melting curve analysis were performed to assess the specificity of the amplification. Data were analyzed using the REST 2008 v2.0.7 software from Corbett life Science (Valencia, CA) (34).
Staining and FACS
Cells were cocultured for 48 hours as described above and then restimulated with 10 ng/ml PMA and 1 μg/ml ionomycin for 3 hours prior to enrichment of IL-17 secreting cells using the mouse IL-17 secretion assay-cell enrichment and detection kit (Miltenyi Biotec) following the manufacturer’s instruction. This kit allows for enrichment and staining of IL-17 secreting cells with a phycoerythrin (PE) dye. Cells were then also stained with FITC-conjugated rat anti-mouse CD4 (H129.19) antibody (BD Biosciences). The corresponding isotype, FITC rat IgG2a,κ (BD Biosciences), was used as a negative control. Lymphocytes were gated on the basis of forward and side scatter properties (FSC/SSC) prior analysis. Samples were analyzed using the BD FACScan cytometer and BD CellQuest software (BD Biosciences).
Statistical Analyses
Differences between groups were analyzed using the Statview software and the repeated measure ANOVA test or the student t-test. Real-time PCR data were analyzed using the REST 2008 v2.0.7 software from Corbett life Science.
Results
NOD2 and TLR-4 activation in bEnd.3 cells increases IL-6 production
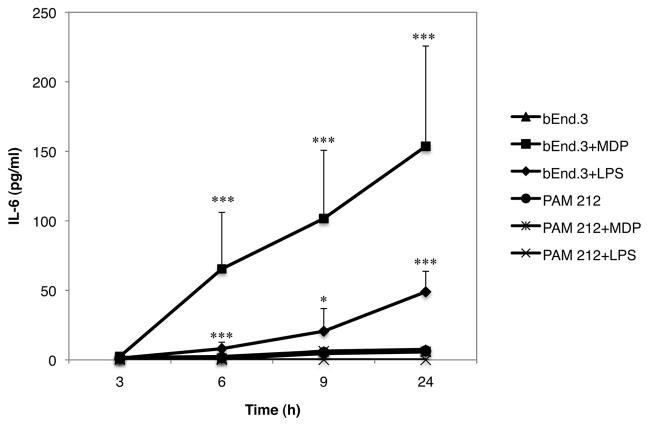
It has been previously reported that human endothelial cells express the intracellular NOD2 receptor and increase IL-6 secretion following MDP stimulation (10). To examine the effect of the NOD2 agonist MDP on cytokine production in the murine transformed endothelial cell line bEnd.3 and in the keratinocyte cell line PAM212, 0.2 × 106 cells were incubated in the presence or absence of 2 μg/ml of MDP. In some wells, cells were stimulated with the TLR-4 agonist LPS as a control for the specificity of MDP. The supernatants were collected 3, 6, 9 and 24 hours later and analyzed for cytokine content by ELISA. IL-6 was significantly higher in supernatants of bEnd.3 cells treated with MDP compared to untreated cells while PAM212 cells did not produce IL-6 with or without MDP stimulation (Fig. 1). Interestingly, LPS was also able to induced IL-6 production in bEnd.3 cells but only after 24 hours incubation and in a much lower amount than MDP. None of the others cytokines tested (IL-1β, IL-10, IL-12, IL-17, IL-23) were detected in supernatants conditioned by stimulated bEnd.3 or PAM212 cells. TGF-β1 was found in the supernatants of both cell lines but was not modulated by MDP (data not shown).
Fig. 1. NOD2 and TLR-4 activation in bEnd.3 endothelial cells promotes IL-6 production.
bEnd.3 or PAM212 cells were incubated in the presence or absence of 2 μg/ml of the NOD2 ligand MDP or 0.1 μg/ml of LPS. Supernatants were collected 3, 6, 9 and 24 hours later and analyzed for cytokine content by ELISA. The graph represents the mean of 3 independent experiment ± SD (***p<0.0001; *p<0.05).
NOD2 but not TLR-4 activation in primary dermal endothelial cells increases IL-6 production
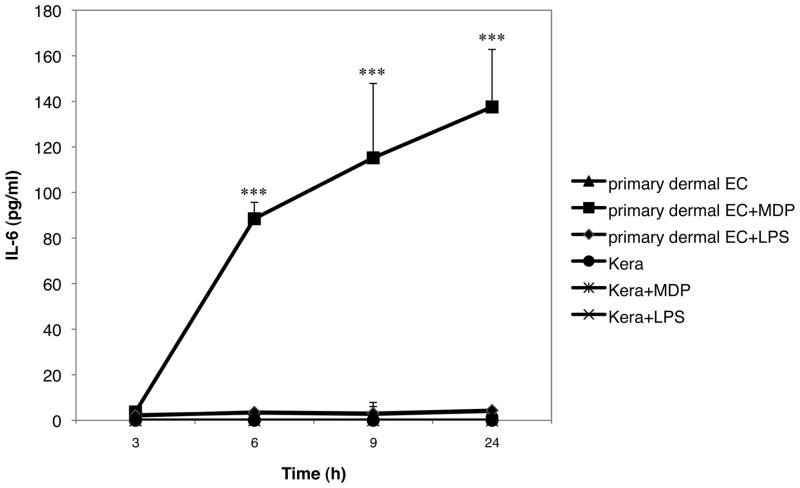
To verify that the endothelial cell line bEnd.3 behave in a similar way as primary dermal endothelial cells, primary cells were plated and treated at the same conditions as described above and supernatants were analyzed for IL-6 content. Freshly isolated keratinocytes were used as a control. MDP induced IL-6 production in primary endothelial cells in the same manner than in the bEnd.3 cell line (Fig. 2). Accordingly, freshly isolated keratinocytes did not produce IL-6 when stimulated with either MDP or LPS. Interestingly, LPS failed to induce IL-6 production in primary dermal endothelial cells in contrast to the results obtained with the cell line.
Fig. 2. NOD2 but not TLR-4 activation in primary endothelial cells promotes IL-6 production.
Primary dermal endothelial cells or primary keratinocytes were incubated in the presence or absence of 2 μg/ml of MDP or 0.1 μg/ml of LPS. Supernatants were collected 3, 6, 9 and 24 hours later and analyzed for cytokine content by ELISA. The graph represents the mean of 3 independent experiment ± SD (***p<0.0001).
NOD2 activation of bEnd.3 cells polarizes LC antigen presentation to a IL-17 immune response
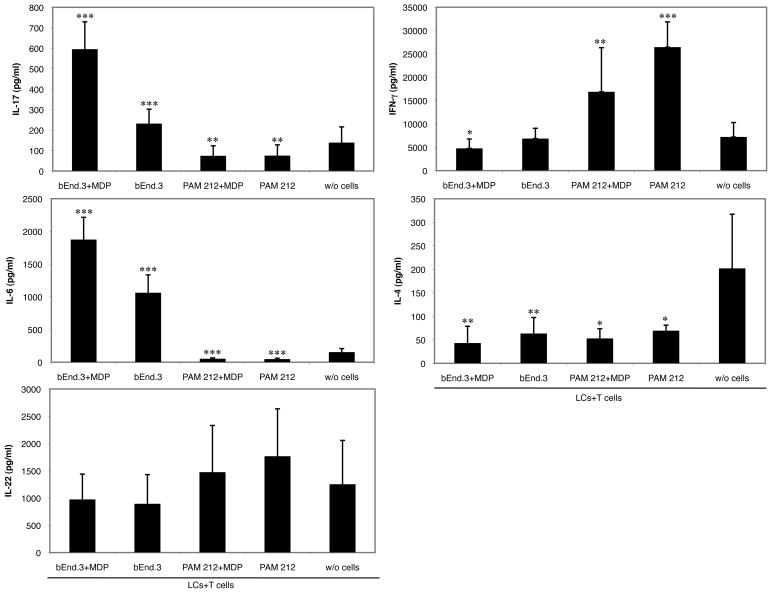
To determine whether IL-6 production by endothelial cells activated by MDP may influence the skin immune response to an immunogenic antigen, we developed an in vitro model where MDP-treated or untreated bEnd.3 cells were cultured together with a mixed population of LCs and T cells from DO11.10 transgenic mice in the presence of cOVA323–339. The bEnd.3 cell line, which we established respond to MDP in the same way as primary endothelial cells, was used as a surrogate for dermal endothelial cells. After 48 hours incubation, supernatants were harvested and analyzed for cytokine content by ELISA. We evaluated IL-6 along with IL-17, IL-22, IL-4, and IFN-γ production. Figure 3 shows that in the presence of activated bEnd.3 cells, IL-6 and IL-17 production was significantly higher compared to the wells where LCs and T cells were cultured alone while IL-4 and IFN-γ were inhibited. The resulting cytokine pattern in these wells suggests Th17 polarization with inhibition of Th1- and Th2-type immunity. Interestingly, IL-22 production, which is often correlated to Th17 priming, was not influenced by the presence of activated bEnd.3 cells. This suggests that in our in vitro model IL-17 and IL-22 production are differentially regulated.
Fig. 3. NOD2 activation in bEnd.3 endothelial cells promotes Th17-related cytokine release in a LCs and T cells antigen presentation culture.
bEnd.3 or PAM212 cells were preincubated in the presence or absence of the NOD2 ligand MDP for 3 hours. After extensive washing, LCs and T cells from transgenic mice were added along with cOVA323–339. Supernatants were harvested 48 hours later for cytokine content analysis by ELISA. The graphs represent the mean of 4 independent experiments ± SD (***p<0.0001; **p<0.002; *p<0.02).
Similar results were obtained when non-activated bEnd.3 cells were used. However, the amount of IL-17 produced was significantly lower compared to wells with MDP-treated bEnd.3 cells and IFN-γ was not inhibited, signifying that NOD2 activation in endothelial cells is necessary for a marked Th17 polarization. Although we previously reported that non-activated bEnd3 cells produce low amount of IL-6, we observed an increased concentration of this cytokine in wells where LC and T cells were cultivated in presence of bEnd.3 cells. This likely resulted from the cross-talk between the three cell types in the wells. When PAM212 cells with or without MDP stimulation were used instead of bEnd.3 cells, the resulting cytokine pattern was suggestive of a Th1 polarization at the expense of Th17 and Th2. Indeed, IL-17 and IL-4 were both inhibited, while IFN-γ production was strongly induced in supernatants of wells containing PAM212 compared to those of wells without bEnd.3 or PAM212 cells, regardless of whether PAM212 cells were stimulated with or without MDP. These data confirm that MDP activation in bEnd.3 cells is required for inducing Th17 development.
We attempted to repeat this experiment with primary murine dermal microvascular endothelial cells. However, primary endothelial cells are difficult to use in coculture experiments due to stringent media requirements, such as the necessity for growth factors, which may affect the other cells in the coculture and the experiment final outcome. Indeed, when the coculture experiment was carried out in the primary endothelial cell line medium, no effective antigen presentation was observed even in the absence of endothelial cells (data not shown). On the other hand, when the antigen presentation assay was performed in complete medium, we observed a significant increase in cell death (as assessed by release of lactate dehydrogenase) in wells where endothelial cells were present, which also resulted in unsuccessful antigen presentation (data not shown).
NOD2 activation in bEnd.3 cells induces RORγt expression and drives Th cells polarization to a Th17 type
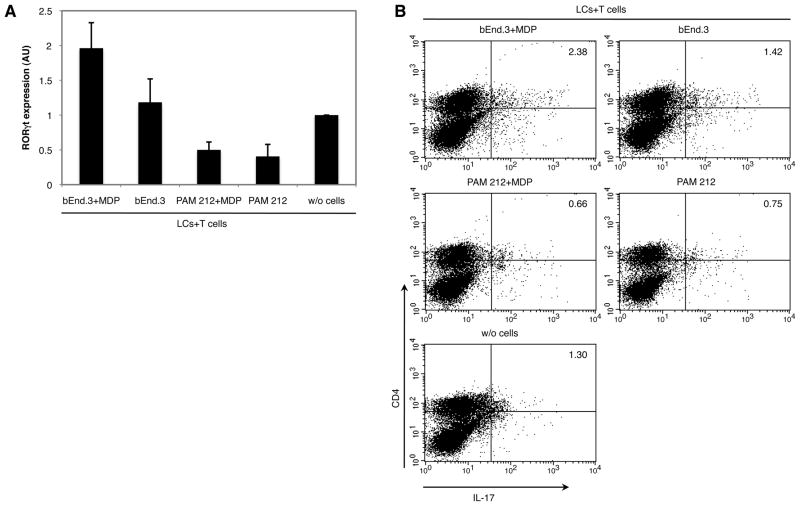
In order to confirm the ability of activated bEnd.3 cells to drive Th17 polarization, we analyzed the expression of the Th17-specific transcription factor RORγt in LCs and T cells cultivated in presence or absence of bEnd.3 or PAM212 cells cultured with or without MDP. We found that RORγt expression was induced in the MDP-stimulated bEnd.3 cells group while lower mRNA expression was detected in cells cultivated with untreated bEnd.3 cells (Fig. 4A). On the other hand, no significant difference in RORγt mRNA expression was found in wells containing MDP-stimulated bEnd.3 cells compared to the control group. These results were confirmed by flow cytometric analysis of IL- 17+CD4+ T cells (Fig. 4B). In wells where LCs and T cells were cultivated in the presence of activated bEnd.3 cells, the percentage of IL-17+CD4+ T cells was higher than in wells where LC and T cells were cultured alone. Addition of untreated bEnd.3 cells to LCs and T cells culture was also able to induce the differentiation of IL-17+CD4+ T cells, though this increase was much lower than in the bEnd.3+MDP group. With regard to cells cultivated in the presence of MDP-stimulated or not PAM212 cells, the percentage of IL-17+CD4+ T cells was even lower than the control group where LC and T cells were cultured alone. These results correlate with cytokine production in supernatants and confirm the ability and specificity of activated bEnd.3 cells to induce Th17 differentiation.
Fig. 4. NOD2 activation in bEnd.3 endothelial cells promotes Th17 cells differentiation.
bEnd.3 or PAM212 cells were preincubated in the presence or absence of the NOD2 ligand MDP for 3 hours. After extensive washing, LCs and T cells from transgenic mice were added along with cOVA323–339. Cells were collected 24 hours later for RNA extraction or 48 hours later for CD4 and IL-17 staining. (A) Expression of RORγt was quantitated by real-time RT-PCR using GAPDH as an internal control. In every experiment RORγt was analyzed using the REST 2008 software. The bars represent the mean of 3 independent experiments ± SD. In all three experiments RORγt was found to be significantly increased in cells cultivated in the presence of activated bEnd.3 cells compared to cultures of LCs and T cells without bEnd.3 cells. (B) Cells were restimulated after 48 hours incubation with 10 ng/ml PMA and 1 μg/ml ionomycin for 3 hours prior to enrichment with the mouse IL-17 secretion assay-cell enrichment and detection kit, which simultaneously enrich and stain IL-17 cells with a PE dye. Cells were then also stained with FITC-conjugated rat anti-mouse CD4 (H129.19) antibody and analyzed by flow cytometry. IL-17+CD4+ cells were significantly increased in cells cultivated in presence of activated bEnd.3 cells. The figure shows one representative experiment out of 3 independent significant experiments.
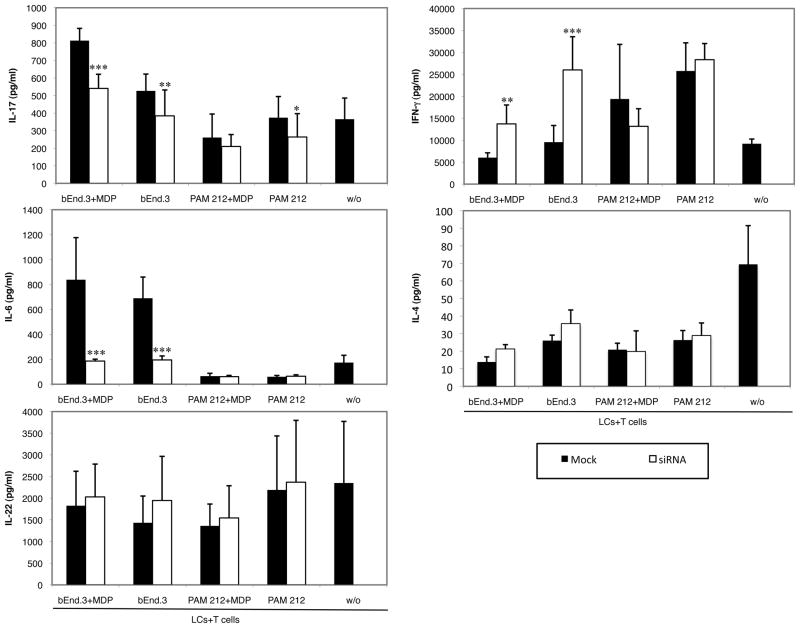
IL-6 knock-down in bEnd.3 cells inhibits Th17 polarization
To investigate the mechanism by which activated endothelial cells induce Th17 polarization, we set-up an experiment where bEnd.3 cells were pretreated with IL-6 siRNA to knock down the production of this cytokine prior to treatment with MDP and cultivation with LCs and T cells. IL-6, along with TGF-β1, is known to promote Th17 differentiation in mice (5) and, as shown above, NOD2 activation in bEnd.3 cells but not in PAM212 cells stimulates IL-6 production.
Pretreatment of bEnd.3 cells with IL-6 siRNA significantly inhibited IL-6 release into supernatants of LCs and T cells cultured in the presence of bEnd.3 cells treated with or without MDP (Fig. 5). The portion of IL-6 resulting from bEnd.3 production following MDP treatment and cross-talk between the cells in the wells was totally inhibited and the final amount of IL-6 in these wells was comparable to the amount found in wells of LCs and T cells cultured alone. The IL-6 inhibition in these groups was also reflected in the IL-17 production, which was significantly lower when bEnd.3 cells were treated with IL- 6 siRNA. On the other hand, siRNA treatment significantly boosted IFN-γ production and had no effect on IL-4 in these wells. Interestingly, IL-22 production was not affected by siRNA treatment, confirming that IL-17 and IL-22 production are differentially regulated. These results suggest that IL-6 produced by bEnd.3 cells is responsible for the Th17 polarization and may at the same time inhibit the Th1 immune response. siRNA treatment of PAM212 cells had no influence on the final amount of IL-6, IL-22, IL-4 and IFN-γ produced. In wells where LCs and T cells were cultured along with PAM212 cells there was a small, although significant, inhibition of IL-17 production. However, this inhibition did not correlate with increased IFN-γ induction as in wells containing MDP-stimulated bEnd.3 cells.
Fig. 5. IL-6 downregulation in bEnd.3 endothelial cells inhibits Th17 polarization.
bEnd.3 or PAM212 cells were cultured overnight with 100 nM mouse IL-6 siRNA or the corresponding non-targeting control siRNA. Cells were then stimulated with or without the NOD2 ligand MDP for 3 hours. After extensive washing, LCs and T cells from transgenic mice were added along with cOVA323–339. Supernatants were harvested 48 hours later for cytokine content analysis. The graphs represent the mean of 3 independent experiments ± SD (***p<0.0001; **p<0.01; *p<0.05).
Discussion
Although it is generally believed that the first responder to pathogens are often infected non-immune cells rather than innate immune cells, very little is known about their active contribution to the innate immune response and their subsequent influence on the adaptive immune response. Endothelial cells are amongst the first non-immune cells in the body to directly contact antigens and PAMPs, such as TLR and NOD agonists, during skin infection and they may contribute to the innate and adaptive immune response by producing inflammatory cytokines and chemokines. In this study we demonstrated that endothelial cells have likely an important role in the modulation of the development of the adaptive immune response.
We found that NOD2 activation of the bEnd.3 endothelial cell line, but not of PAM212 keratinocyte cell line, resulted in an increased production of IL-6. These data are consistent with the previous report that activation of human ocular endothelial cells by MDP resulted in increased IL-6 secretion (10). TLR-4 activation in bEnd.3 cells was also able to induce IL-6 production but in a much lower amount than NOD2. In addition, TGF-β1 was also present in the supernatant of bEnd.3 cells but, in contrast to IL-6, its concentration was not influenced by MDP. The data concerning NOD2 activation were further confirmed by substituting the cell lines with primary dermal endothelial cells and primary keratinocytes. This experiment established that the bEnd.3 and PAM212 cell lines behave as the corresponding primary cells when stimulated with MDP. Interestingly, when primary endothelial cells were stimulated with LPS there was no increase of IL-6 production suggesting that the cell line response to TLR-4 stimulation diverges from that of primary endothelial cells. However, it should be noted that the amount of IL-6 produced by LPS treated bEnd.3 cells was minimal. IL-6 and TGF-β1 are known to be implicated in the polarization of the adaptive immune response toward a Th17 type (5). Therefore we further questioned whether NOD2 activated endothelial cells might be implicated in the Th17 development.
In our experiments we used the bEnd.3 cell line, as a surrogate for primary microvascular endothelial cells. We determined that when LCs and the responsive T cells were cultured in the presence of MDP-activated bEnd.3 cells with cOVA323–339, the resulting supernatant cytokine profile was suggestive of Th17 polarization. Indeed, IL-17 and IL-6 were both significantly induced compared to wells where LCs and T cells were cultured alone or in presence of untreated bEnd.3 cells. In addition, IFN-γ and IL-4, a Th1- and a Th2- type cytokine, respectively, were both inhibited in accordance with the paradigm that Th17 cell expansion inhibits Th1- and Th2-type immunity (35).
To better characterize the cells responsible for the increased IL-17 concentration seen in the in vitro model, we analyzed by FACS the IL-17+CD4+ T cells content in the wells after the period of antigen presentation. We found that in wells where LCs and T cells were cultured in presence of activated bEnd.3 cells, the percentage of IL-17+CD4+ T cells was almost doubled compared to control wells. In addition the expression of IL-17 was much more intense in bEnd.3+MDP wells as shown by the shift of IL-17+cells toward the right of the FACS dot plot. These data further confirmed the idea that activated bEnd.3 cells induce Th17 polarization as did increased expression of mRNA for the Th17-associated transcription factor RORγt. In contrast, when untreated bEnd.3 cells were used, the percentage of IL-17+CD4+ T cells and the expression of RORγt mRNA were much lower than wells containing MDP-stimulated bEnd.3 cells, correlating well with the ELISA results and suggesting that activation of endothelial cells by MDP and the consequent IL-6 production are crucial for Th17 priming. On the other hand when PAM212 cells were used as a control, the cytokine profile in the supernatants was suggestive of a Th1 polarization, a decrease of IL-17+CD4+ T cells was observed in the FACS experiment and there was an inhibition of RORγt mRNA expression. These results demonstrate the specificity of endothelial cells in stimulating a Th17 immune response and suggest that keratinocytes might have a similar ability to favor a Th1 immune response. To further investigate the mechanism by which endothelial cells induce Th17 polarization we blocked IL-6 production in bEnd.3 and PAM212 cells using the siRNA technique prior to stimulation with MDP and coculture with LCs and T cells. IL-6 blockade of bEnd.3 cells partially, although significantly, impaired IL-17 secretion in wells containing MDP-stimulated bEnd.3 cells while allowing for significantly induced IFN-γ production. These results further validate the ability of MDP-treated bEnd.3 cells to promote Th17 cells expansion while inhibiting Th1 immunity and confirmed the importance of IL-6 in the Th17 development as previously described in others studies (5, 36, 37). However, the inhibition of IL-17 found in our experiments was only partial, suggesting the presence of others bEnd.3-derived factors involved in the generation of Th17 cells. Interestingly, IL-22 production was not affected by the MDP treatment of bEnd.3 cells or by the IL-6 blockade. This demonstrates differential regulation of IL-17 and IL-22 production in our in vitro model. Given the recent reports of the existence of a distinct subset of CD4+T cells, different from Th17 cells, producing IL-22 but not IL-17 (Th22) (8, 9, 38), one could speculate that MDP-activated bEnd.3 cells favor the development of T cells that produce predominantly IL-17 but not those that produce only IL-22 (Th22 cells).
We also attempted to reproduce these findings with primary murine dermal microvascular endothelial cells. Unfortunately, we faced a number of technical difficulties. First, when we performed the assay in complete medium as in the original experiment, effective antigen presentation did not occur in wells where endothelial cells were present. As discussed above in Results, we discovered that the percentage of dead cells in these wells was significantly higher compared to wells where LC and T cells were cultured alone or in presence of the bEnd.3 cell line, as assessed by release of lactate dehydrogenase. These results suggested that carrying out the antigen presentation assay in complete medium did not provide a suitable environment for endothelial cell survival with cell death and a consequent toxic environment for antigen presentation. Next, we replaced the complete medium with the primary endothelial cell medium in an attempt to provide better conditions for primary endothelial cell survival. However, under these conditions antigen presentation was also inhibited, possibly due to the presence of hydrocortisone in the endothelial cell medium. Thus, we have been unable to perform the experiment using primary endothelial cells. We realize that the use of primary cells in these experiments would further support our hypothesis. Nonetheless, we believe our results using bEnd.3 cells as a surrogate for primary microvascular endothelial cells is highly suggestive that activated endothelial cells in vivo are capable of biasing local immune responses towards the Th17 pole.
Although primary endothelial cells do not produce IL-6 following LPS stimulation, we repeated the antigen presentation experiment using bEnd.3 cells stimulated with LPS and we obtained similar results to the previous experiment where MDP was used (Data not shown). These results suggest that Th17 polarization does not specifically require NOD-2 activation in endothelial cells but necessitate the production of IL-6 from these cells. IL-6 production may be induced by different type of PAMPs, such as TLR agonists, depending on the expression of the corresponding PRRs on endothelial cells. Our experiments are most likely going to be reproducible using others PAMPs able to induce IL-6 production in endothelial cells.
The skin is the largest organ of the body and plays a central role in the host defense. The resident APCs of the skin are LCs, which reside in the epidermis, and dermal DCs. Our data suggests that in vivo MDP-activated endothelial cells may influence the LC microenvironment during antigen processing and presentation by increasing IL-6 production and subsequently modulate the development of the adaptive immune response by enhancing the Th17 polarization. Possibly, the use of other PAMPs to activate endothelial cells and induce IL-6 production from these cells would result in a similar effect. In this regard, as LCs traffic through lymphatics to regional lymph nodes, they would be ideally situated to be exposed to factors produced by microvascular endothelial cells. Preliminary results using bone marrow-derived DCs as an alternative to LCs, showed that MDP activated bEnd.3 cells failed to induce an IL-17-type immune response in our in vitro model. These results agree with previous studies which showed a discrete ability of skin resident APCs in inducing Th17 priming (39) and suggest that LCs possess a distinctive ability in promoting Th17 differentiation.
Interestingly, it has been reported that in vivo MDP treatment failed to induce resistance to Mycobacterium leprae and Mycobacterium marinum in mice (40). In addition, in vitro treatment of T cells with murabutide, an MDP analog, reversed T cell anergy to mycobacteria leprae antigens (41) suggesting that in vivo the inability to mount an effective response to muramyl dipeptide may play a role in the exacerbation of the infection. Our findings, along with the fact that multibacillary leprosy is highly angiogenic (42), suggest that activation of the MDP pathway in vivo might be useful in the treatment of this disease.
In conclusion our results may be of great significance for a more complete understanding of the mechanisms underlying Th17-related disorders of the skin, such as psoriasis and other autoimmune diseases, in particular multiple sclerosis, which have been correlated with high IL-6 and IL-17 production along with the presence of Th17 cells. In the future, study of signaling inhibitors which block or modulate the IL-6/ RORγt/ IL-17 pathway may be of great interest in the development of potential treatments of such diseases.
Acknowledgments
The study was supported by NIH grant 5R01 AR042429, a gift from the Jacob L. and Lillian Holtzmann Foundation (RDG), a grant from the Edith C. Blum Foundation (RDG), contributions from the Carl and Fay Simons Family Trust (RDG), a contribution from the Seth Sprague Educational and Charitable Foundation and a grant from the Lewis B. and Dorothy Cullman Foundation (RDG).
Abbreviations
- DC
dendritic cell
- DMEC
dermal microvascular endothelial cells
- EC
endothelial cell
- Kera
keratinocytes
- LC
Langerhans cell
- MDP
muramyl dipeptide
- NOD2
nucleotide binding oligomerization domain receptor 2
- PAMP
pathogen associated molecular pattern
- PRR
pattern recognition receptor
References
- 1.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 2.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Kambe N, Nishikomori R, Kanazawa N. The cytosolic pattern-recognition receptor Nod2 and inflammatory granulomatous disorders. J Dermatol Sci. 2005;39:71–80. doi: 10.1016/j.jdermsci.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn’s disease. Mucosal Immunol. 2008;1(Suppl 1):S5–9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Garra A, Stockinger B, Veldhoen M. Differentiation of human T(H)-17 cells does require TGF-beta! Nat Immunol. 2008;9:588–590. doi: 10.1038/ni0608-588. [DOI] [PubMed] [Google Scholar]
- 8.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 9.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 10.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res. 2006;71:103–107. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 13.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14:326–331. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 15.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 17.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 18.Neuner P, Urbanski A, Trautinger F, Moller A, Kirnbauer R, Kapp A, Schopf E, Schwarz T, Luger TA. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J Invest Dermatol. 1991;97:27–33. doi: 10.1111/1523-1747.ep12477880. [DOI] [PubMed] [Google Scholar]
- 19.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB, Gottlieb AB. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 22.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, Lecron JC, Morel F. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. e1242. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells -dendritic cells of the epidermis. APMIS. 2003;111:725–740. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 27.Montesano R, Pepper MS, Mohle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 28.Azuma M, Tamatani T, Fukui K, Bando T, Sato M. Enhanced proteolytic activity is responsible for the aberrant morphogenetic development of SV40-immortalized normal human salivary gland cells grown on basement membrane components. Lab Invest. 1994;70:217–227. [PubMed] [Google Scholar]
- 29.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 30.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granstein RD, Askari M, Whitaker D, Murphy GF. Epidermal cells in activation of suppressor lymphocytes: further characterization. J Immunol. 1987;138:4055–4062. [PubMed] [Google Scholar]
- 32.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 36.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, Morelli AE, Larregina AT. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol. 2009;182:921–933. doi: 10.4049/jimmunol.182.2.921. [DOI] [PubMed] [Google Scholar]
- 40.Krahenbuhl JL, Humphres RC. Effects of treatment with muramyl dipeptide on resistance to Mycobacterium leprae and Mycobacterium marinum infection in mice. Immunopharmacology. 1983;5:329–339. doi: 10.1016/0162-3109(83)90048-6. [DOI] [PubMed] [Google Scholar]
- 41.Sridevi K, Khanna N, Chattree V, Pal PC, Haq W, Rao DN. Reversal of T cell anergy in leprosy patients: in vitro presentation with Mycobacterium leprae antigens using murabutide and Trat peptide in liposomal delivery. Int Immunopharmacol. 2003;3:1589–1600. doi: 10.1016/S1567-5769(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 42.Bhandarkar SS, Cohen C, Kuruvila M, Rea TH, Mackelfresh JB, Lee DJ, Modlin RL, Arbiser JL. Angiogenesis in cutaneous lesions of leprosy: implications for treatment. Arch Dermatol. 2007;143:1527–1529. doi: 10.1001/archderm.143.12.1527. [DOI] [PubMed] [Google Scholar]