Abstract
We previously reported that the biological activity of analogues of desmosdumotin B (1) was dramatically changed depending on the B-ring system. A naphthalene B-ring analogue 3 exerted potent in vitro activity against a diverse panel of human tumor cell lines with GI50 values of 0.8–2.1 μM. In contrast, 1-analogues with a phenyl B-ring showed unique selective activity against P-glycoprotein (P-gp) overexpressing multidrug resistance cell line. We have now prepared and evaluated 1-analogues with bicyclic or tricyclic aromatic B-ring systems as in vitro inhibitors of human cancer cell line proliferation. Among all synthesized derivatives, 21 with a benzo[b]thiophenyl B-ring was highly active, with GI50 values of 0.06–0.16 μM, and this activity was not influenced by overexpression of P-gp. Furthermore, 21 inhibited tubulin assembly in vitro with an IC 50 value of 2.0 μM and colchicine binding by 78% as well as cellular microtubule polymerization and spindle formation.
Introduction
We previously reported that desmosdumotin B (1, Figure 1) exerted selective inhibition of a P-glycoprotein (P-gp) overexpressing multidrug resistant (MDR) tumor cell line with significantly lower activity against non-MDR tumor cells.1 The observed selectivity index [collateral sensitivity (CS),2 activity ratio of MDR line versus non-MDR line] was greater than 20. This selective in vitro antitumor activity was further enhanced by replacing the three methyl groups at C-6 and C-8 with ethyl groups (2, Figure 1) and also adding an alkyl group at the C-4′ position. During this study, we also found that analogues in which the phenyl B-ring was replaced with a naphthyl moiety (3, Figure 1) had dramatically different activity profiles, displaying strong cytotoxicity against multiple cancer cells, regardless of MDR expression, with GI 50 values of 0.8–2.1 μM. Thus, placing a larger, more electron-rich aromatic B-ring at C-2 resulted in broader antiproliferative activity and loss of specific activity against the MDR cell line. These compounds also induced rapid cell rounding without immediate detachment, leading us to hypothesize anti-tubulin activity as a mechanism of action, which was tested and confirmed using biochemical assays. Compound 3, therefore, represents a new scaffold for targeting tubulin assembly.
Figure 1. Structures of New Analogues of 1.
Design and synthesis of compounds targeting the microtubule constitutes an attractive strategy for the discovery of new antitumor agents.3 The microtubule network is an essential component of the cytoskeleton, and its timely depolymerization and repolymerization is critical for the cell to construct a functional mitotic spindle. Typically, cells arrested in apparent mitosis eventually undergo apoptosis. Antimitotic agents targeting tubulin are generally classified into two groups, compounds that either stimulate or inhibit microtubule assembly, depending on their effects on the tubulin-microtubule equilibrium. Taxoids and epothilones are well-known enhancers of microtubule polymerization. Colchicine and the vinca alkaloids are the best known inhibitors of microtubule assembly. All of these agents bind to β–tubulin to exert their antimitotic activity. The best characterized drug binding domains are known as the colchicine site, the vinca domain, and the taxoid site.
The vinca alkaloids and the taxoids are among the most useful drugs for cancer therapy, and numerous compounds targeting drug binding sites on tubulin have been developed. Unfortunately, most antitubulin agents that have entered clinical trials have failed due to adverse effects, such as limited therapeutic effects at maximally tolerated doses, perhaps because of drug resistance, high toxicity, or poor physicochemical properties involved in the absorption, distribution, metabolism, and excretion profile. Interestingly, thus far, no colchicine site drug has proved useful in cancer treatment. Despite its lack of utility in cancer therapy, colchicine is used for the treatment of gout, familial Mediterranean fever, secondary amyloidosis, and scleroderma. Moreover, colchicine has long been an important tool for the study of microtubule structure/function4 and a key molecular model for structure-activity relationship studies. Previous research revealed that a two-ring aromatic system with the rings either directly bonded or separated by a one to three atom bridge is a common structural feature for binding to the colchicine site.5 Combretastatin A-4 (CA-4) is an attractive natural product that targets tubulin polymerization via the colchicine site. CA-4 exerts strong cytotoxicity against multiple human tumor cell lines.6 The discovery of CA-4 and its simple molecular structure stimulated research to identify new chemotypes that interact with the colchicine site.
Our interesting discovery of 2-naphthyldesmosdumotin B (3) as an antitubulin agent prompted us to prepare additional 3-analogues with bicyclic and tricyclic aromatic substituents at C-2. In the work presented here, we describe the syntheses and bioactivities of this compound series.
Chemistry
Thirty-four newly synthesized analogues, 4–37, are depicted in Figure 1. They were synthesized from 38 (R = Et for 6–34, R = Me for 4 and 35–37, and R = Pr for 5) 1 through the related chalcones (39) as shown in Scheme 1.1,7 The chalcones (39) were prepared by the Claisen-Schmidt condensation of 38 with various commercially available aromatic aldehydes (ArCHO), except for 55 and 56. In most cases, the condensation was carried out in the presence of 50% aq. KOH in EtOH. However, under these conditions, the reaction with 3-quinoline aldehyde resulted in extremely low yields of product. Finally, the reactions were run with good yields in the presence of piperidine or Ba(OH)2·H2O. The iodine-catalyzed cyclization of 39, followed by demethylation, provided 3–37. The treatment of 3 (R = Et, Ar = naphthyl) with excess Lawesson’s reagent8 resulted in replacement of oxygen with sulfur to yield both the mono-thioketone 41 and the di-thioketone 42. The structure of 41 was determined from the 1H-NMR and MS spectra, which indicated the insertion of a sulfur atom. Compared to the 1H-NMR of the related compound 3, the spectra of 41 showed a 0.79 ppm high field shift of a proton at the C-3 position and no shift for the ethyl protons at the C-6 and C-8 positions. There was, however, a high field shift for the ethyl protons of di-thioketone 42 (Table 1). Bromination of 40a (R = Et, Ar = naphthyl) with nBu4NBr in the presence of PhI(OAc)29 afforded 3-bromo derivative 43, which was converted to 44 by demethylation with BBr3. Meanwhile, flavanone analogue 48 was prepared by the treatment of 39 with HI in HOAc.
Scheme 1. Syntheses of New Analogues of 1.
Reagents and conditions: a) ArCHO, base/solvent [piperidine/EtOH for 3-quinolinecarboxaldehyde, Ba(OH)2·8H2O/MeOH for 4-dimethylamino-1-naphthaldehyde, and KOH/EtOH for others]; b) I2 (cat.), DMSO, H2SO4 (cat.), 90–95 °C, 1 h; c) BBr3, 0° C to rt; d) Lawesson’s reagent, toluene, reflux; e) PhI(OAc)2, nBu4NBr, rt; f) 45%HI, HOAc
Table 1.
1H-NMR Chemical Shifts of 3, 33, and 34
| C-3 | C-6 | C-8 | |||
|---|---|---|---|---|---|
| H | -CH2CH3 | -CH2CH3 | -CH2CH3 | -CH2CH3 | |
| 3 | 6.82 (s, 1H) | 2.49 (q, 2H) | 1.07 (t, 3H) | 2.26–2.12 (m, 2H) 1.98–1.84 (m, 2H) |
0.73 (t, 6H) |
| 41 | 7.62 (s, 1H) | 2.52 (q, 2H) | 1.07 (t, 3H) | 2.31–2.07 (m, 2H) 2.01–1.86 (m, 2H) |
0.74 (t, 6H) |
| 42 | 7.62 (s, 1H) | 2.99 (q, 2H) | 1.10 (t, 3H) | 2.58–2.42 (m, 2H) 2.30–2.16 (m, 2H) |
0.64 (t, 6H) |
Aldehydes 55 and 56 were prepared as shown in Scheme 2. We originally expected that 5- methoxy- and 5-methyl-benzothiophene, 5310 and 54,11 could be obtained from the related 3-carboxyaldehydes through formylation.12 While Rieche formylation of 5-methybenzothiophene (54) using SnCl4 provided the desired 3-carboxaldehyde 56 in good yield, the same treatment of 5-methoxybenzothiophene (53) unexpectively produced 4-formyl product 55. The structure of 55 was identified by 1H-NMR, which showed two doublet signals with a coupling constant of 5.6 Hz at 8.39 and 7.65 ppm for the C-3 and C-2 protons, respectively. Furthermore, nOe crosspeaks were observed between the aldehyde and methoxy protons, the aldehyde and C-3 protons, as well as the methoxy and C-6 protons. The interesting difference in reactivity between the 5-methyl and 5-methoxy groups might be caused by a chelation effect of the Lewis acid with the oxygen atom of the methoxy group.
Scheme 2. Syntheses of 5-Substituted Benzothiophenecarboxaldehyde.
Reagents and conditions: a) 2- chloro-1,1-dimethoxyethane, NaOMe, MeOH, xylene, 110 ºC, b) H3PO4, xylene, 110 ºC, c) Cl2CHOMe, SnCl4, CH2Cl2, rt, 75% for 55, 82% for 56.
Newly synthesized analogues could be classified into the following five groups according to the C-2 substituent: I) naphthalene group (3–12 and 35), II) heterobicyclic aromatic group (13–28, 36 and 37), III) tricyclic aromatic group (29–33), IV) biphenyl group (34) and V) naphthalane group with A- and/or C-ring modifications (40a–44 and 48).
Results and Discussion
All new analogues were evaluated against seven human tumor cell lines, specifically, HCT-8 (colon adenocarcinoma), PC-3 (prostate cancer), A549 (lung carcinoma), MCF-7 (breast cancer) or DU145 (prostate cancer), HepG2 (hepatocellular carcinoma), and KB (epidermoid carcinoma of the nasopharynx), and KB-VIN, which is an MDR (P-gp-overexpressing) KB subline selected using increasing concentrations of vincristine. The selected active compounds 3, 6, 8, 11, 15, 21, 22, 26, and 35–37 were also tested for inhibitory effects on tubulin assembly and inhibition of binding of [3H]colchicine to tubulin, together with 2 for comparison. Table 2 lists the antiproliferative data for 1–48 and vincristine, the positive control. The tubulin study data are presented in Table 3.
Table 2.
Antiproliferative Activity of 1 and Analogues of 1
| Group | Cmpd | GI50 (μM)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCT-8 | PC-3 | A549 | DU145 | MCF-7 | HepG2 | KB | KB-VIN | ||
| 1 | NAb | NA | NA | NT | NA | NA | NA | 13.51 | |
| 2 | NA | NA | NA | NT | NA | NA | NA | 1.07 | |
| I | 3 | 1.03 | 1.03 | 1.55 | NT | 1.55 | 2.06 | 1.55 | 0.77 |
| 4 | 1.16 | 1.45 | 1.73 | NT | 1.73 | NT | 0.29 | 0.58 | |
| 5 | 23.26 | 17.44 | 23.26 | NT | NA | NA | 18.60 | 17.44 | |
| 6 | 0.62 | 2.49 | 0.50 | NT | 0.75 | 1.24 | 0.47 | 0.27 | |
| 7 | 17.41 | 12.44 | 17.41 | NT | 13.68 | 16.67 | 19.90 | 19.90 | |
| 8 | 3.35 | 2.39 | 1.48 | NT | 3.59 | 1.48 | 1.67 | 1.56 | |
| 9 | NA | NA | NA | NT | NA | NA | NA | NA | |
| 10 | NA | NT | NA | NT | NT | NT | NA | 35.71 | |
| 11 | 1.29 | 2.58 | 1.16 | NT | 1.42 | 1.55 | 1.03 | 1.16 | |
| 12 | 11.96 | 4.78 | 16.75 | NT | 8.37 | 13.40 | 10.77 | 9.57 | |
| 35 | 2.56 | 13.33 | 1.25 | NT | NT | 1.33 | 0.61 | 0.72 | |
| II | 13 | 12.85 | 12.85 | 12.85 | NT | 19.28 | 14.91 | 15.42 | 14.91 |
| 14 | 13.09 | 5.24 | 26.18 | NT | 11.26 | 11.78 | 18.32 | 17.02 | |
| 15 | 2.27 | 1.52 | 1.52 | NT | 1.89 | 1.52 | 1.26 | 1.14 | |
| 16 | 5.82 | 5.82 | 17.20 | NT | NA | 15.08 | 14.81 | 14.55 | |
| 17 | 19.04 | 3.81 | 13.96 | NT | 15.23 | 15.74 | 11.17 | 9.90 | |
| 18 | >25 | >25 | >25 | >25 | NT | >25 | >25 | >25 | |
| 19 | 15.61 | 28.04 | 9.79 | NT | NT | 17.72 | 4.50 | 2.09 | |
| 20 | 15.86 | 20.46 | 5.63 | NT | 17.39 | 21.74 | 18.67 | 2.81 | |
| 21 | 0.13 | 0.16 | 0.06 | NT | 0.11 | 0.13 | 0.08 | 0.07 | |
| 22 | 2.70 | 90.69 | 1.35 | 3.92 | NT | 2.03 | 0.61 | 0.76 | |
| 23 | 93.14 | >98 | >98 | >98 | NT | >98 | 53.92 >98 | ||
| 24 | 42.19 | 37.97 | 42.19 | 31.65 | NT | 30.59 | 26.37 | 10.55 | |
| 25 | 19.58 | 51.89 | 66.04 | 21.46 | NT | 24.76 | 11.32 | 11.08 | |
| 26 | 3.66 | 21.46 | 3.17 | 1.12 | NT | 1.02 | 0.76 | 0.78 | |
| 27 | 11.17 | 3.81 | 5.84 | 0.74 | NT | 1.90 | 2.49 | 0.94 | |
| 28 | >25 | >25 | >25 | 10.41 | NT | >25 | 11.22 | >25 | |
| 36 | 1.30 | 1.50 | 0.76 | NT | NT | 23.73 | 0.76 | 0.65 | |
| 37 | 1.08 | 0.91 | 0.23 | NT | NT | 0.57 | 0.11 | 0.09 | |
| III | 29 | 2.42 | 6.04 | 6.04 | NT | 3.62 | 4.59 | 2.17 | 1.93 |
| 30 | NA | NA | NA | NT | NA | NA | NA | NA | |
| 31 | NA | NA | NA | NT | NA | NA | NA | NA | |
| 32 | NA | NA | NA | NT | NA | NA | NA | NA | |
| 33 | 12.79 | 13.70 | 18.26 | NT | 9.13 | NT | 11.42 | 9.82 | |
| IV | 34 | NA | 8.45 | 18.12 | NT | 15.70 | 19.32 | 18.12 | 12.08 |
| V | 40a | 11.69 | 14.68 | 16.67 | NT | 20.90 | 20.65 | 11.19 | 9.70 |
| 41 | 8.17 | 16.58 | 1.86 | NT | 3.22 | 8.42 | 1.41 | 2.70 | |
| 42 | 13.33 | 24.05 | 5.95 | NT | 14.05 | 24.05 | 7.38 | 5.95 | |
| 43 | 7.68 | 10.79 | 4.77 | NT | 11.00 | 14.52 | 9.13 | 9.96 | |
| 44 | 13.89 | NA | 25.64 | NT | 24.57 | NA | 6.41 | 11.97 | |
| 48 | 9.28 | 12.63 | 7.99 | NT | NT | 9.02 | 5.93 | 3.87 | |
| VCR | 0.036 | 0.018 | 0.007 | 0.018 | 0.018 | NT | 0.004 | 9.091 | |
Antiproliferative activity as GI50 values for each cell line, the concentration of compound that caused 50% reduction in absorbance at 562 nm relative to untreated cells using the sulforhodamine B assay. Human colon adenocarcinoma (HCT-8), prostate cancer (PC-3 and DU145), lung carcinoma (A549), breast cancer (MCF-7), hepatocellular carcinoma (HepG2), epidermoid carcinoma of the nasopharynx (KB), and MDR line overexpressing P-glycoprotein (KB-VIN).
NA, not active. Test compound (20 μg/mL) did not reach 50% inhibition.
NT, not tested.
Table 3.
| Group | Compound | IC50 (μM) ± SD | Inhibition of colchicine binding (%) ± SD |
|---|---|---|---|
| 2 | NA | - | |
| I | 3 | 2.3 ± 0.08 | 43 ± 10 |
| 6 | 2.7 ± 0.1 | 50 ± 0.4 | |
| 8 | 2.7 ± 0.3 | 31 ± 3 | |
| 11 | Not obtainablec | 29 ± 0.4 | |
| 35 | 2.5 ± 0.2 | 37 ± 5 | |
| II | 15 | 6.5 ± 0.7 | 33 ± 2 |
| 21 | 2.0 ± 0.1 | 78 ± 5 | |
| 22 | 3.4 ± 0.3 | 38 ± 4 | |
| 26 | 3.4 ± 0.2 | 52 ± 0.6 | |
| 36 | 3.7 ± 0.09 | 36 ± 4 | |
| 37 | 2.4 ± 0.007 | 69 ± 3 | |
| CA-4 | 1.1 ± 0.1 | 99 ± 0.7 | |
The tubulin assembly assay measured the extent of assembly of 10 μM tubulin after 20 min at 30 °C.
Tubulin: 1 sμM, [3H]Colchicine: 5 μM, Inhibitor: 5 μM. Incubation was for 10 min at 37 °C.
Partial inhibition observed and was maximal with 4 μM compound. Higher compound concentrations resulted in the same amount of inhibition observed with 4 μM, suggesting poor solubility of 11 in the reaction mixture.
Mono-phenyl analogues 1 and 2 were clearly “non-active” against all non-MDR tumor cells (2 was also inactive in the tubulin assembly assay), while they were “active” against the P-gp overexpressing MDR tumor cell line KB-VIN. In comparison, most analogues with a bicyclic substituent at C-2 (Groups-I and -II) showed in vitro activity against all tested cell lines, with GI50 values ranging from 0.1 to 20 μM, while three of the five analogues (30–32) with a tricyclic substituent at C-2 (Group-III), were inactive.
With respect to the alkyl groups at C-6 and C-8, the 6,8,8-tri-Me (4) and tri-Et (3) analogues showed similar growth inhibitory activity against the tested tumor cell lines, while the tripropyl analogue 5 was much less active.
Group-II compound 21, with a tri-Et A-ring and a benzo[b]thiophene B-ring at C-2, had dramatically increased antiproliferative activity, exhibiting GI50 values of 0.06–0.16 μM against the human tumor cell lines. Although the activity of 37, with a tri-Me A-ring and a benzo[b]thiophene B-ring at C-2, was less than that of 21, it still showed potent activity with GI50 values of 0.09–1.08 μM. In comparison, the related analogue 17, with the chromene skeleton attached at the 2′-position of the benzo[b]thiophene system rather than at the 3′-position, showed considerally lower activity against all cell lines (GI50 = 3.8–19.0 μM), with significant activity only against PC-3. The replacement of sulfur (21) with oxygen (19) or N-methyl (20) led to reduced activity. Other relatively active analogues, with mostly submicromolar GI 50 values, were tri-Et compounds 3, 6, 8, 11 and 15, as well as the related tri-Me analogues 4 and 35–37. All of these compounds had a naphthyl B-ring at C-2, except for 15 and 36, which had a 2,3-dihydrobenzo[1,4]dioxine group at C-2. Quinoline 13, benzofurans 16 and 19, as well as indole 20, each bearing a bicyclic heteroaromatic B-ring system, displayed only moderate activity against all tested cell lines. Analogue 14, with a benzo[1,3]dioxolyl B-ring system, also moderately inhibited tumor cell growth, although it was about 10 times less active than 21. These data demonstrated that, although a 10π-electron B-ring system was optimal, it was not essential for activity. Among the Group-III compounds, those with the three rings arranged linearly were inactive, while compound 29 (C-2 dihydroacenaphthyl) showed significant antiproliferative activity. Compounds 33 (C-2 phenanthryl) and 34 (C-2 biphenyl) showed only moderate potency.
When the ketone oxygen at C-7 was replaced with a methoxy group (3 vs 40a), there was a substantial loss of activity (GI50 values of 0.8–2.1 μM for 3 and 9.7–20.9 μM for 40a). The latter compounds would lose the possibility of a hydrogen bond between the C-4 ketone carbonyl and the C-5 hydroxy group, which might account for the difference in potency. Activity also decreased when a bromide group was inserted at the C-3 position (3 vs 44), although the bromine atom had no effect when a C-7 methoxy group was present (cf. 40a and 43). Reduced activity also occurred when both oxygens in the C-4 and C-7 ketones were changed to sulfur (3 vs 42). However, mono-thioketone 41 retained relatively good activity, as compared with 3, against A549 and KB cells, with GI50 values of 1.86 and 1.41 μM, respectively. The saturation of the double bond between C2 and C3 also decreased activity (3 vs 48). Finally, it should be pointed out that MDR KB-VIN cells were as sensitive as the parental KB cells to all active compounds.
Because compound 21, containing a benzo[b]thiophene system, showed potent antiproliferative activity, we investigated additional related derivatives 22–28. While none of these compounds showed greater activity than 21, some interesting facts were revealed. 1) The 2′-Me derivative (22) retained activity against all tested cell lines, except PC-3. 2) 5′-Me (23) and 5′-Br (24) analogues lost activity. 3) Activity varied with the position of attachment of the benzo[b]thiophene system to the chromene skeleton. The rank order of overall activity was 3′ (21) > 7′ (27) > 2′ (17) >5′ (28). 4) Compound 28 displayed selective activity against the DU145 and KB cell lines. 5) Compound 26 (5′-OH) exhibited potent cytotoxicity against all tested cell lines, while 25 (5′-OMe) lost activity against most cell lines. 6) Compounds 24 and 27 possessed “collateral sensitivity” (CS),2 exhibiting over two-fold greater cytotoxicity against the MDR line (KB-VIN) than against the parental line (KB). Figure 2 shows the reversal effects of co-treatment of 24 or 27 together with a nontoxic concentration of verapamil (VERAP), a known P-gp modulator. Co-treatment of 24 and 27 with VERAP partially reversed the cytotoxic activity, showing that the MDR-selectivity was dependent in part on P-gp function and consistent with the effect on P-gp activity measured using the co-incubation treatment protocol. This result is consistent with data obtained with analogues of 1 containing a phenyl B-ring.13
Figure 2.
Figure 2A: Reversal of MDR-selectivity of 24 by verapamil co-treatment
Figure 2B: Reversal of MDR-selectivity of 27 by verapamil co-treatment
We observed that cells treated with cytotoxic concentrations of these agents displayed a characteristic rounded shape, that was morphologically similar to the cells treated with colchicine, when examined microscopically. We explored microtubules and spindles in A549 cells treated with 21. The A549 cells were treated with 21, colchicine, pacritaxel, doxorubicine, or DMSO for 24 hr followed by the immunofluorescence staining of tubulin using monoclonal antibody to α-tubulin. As we expected, microtubule polymerization and spindle formation were significantly inpaired by treatment with 21, that were similar to the effects of colchicine and clearly different from pacritaxel or doxorubicine (Figure 3). The antimicrotubule activity of 21was seen within 24 hr at the concentration of 0.1 nM which was 1000 fold less than colchicine (100 nM).
Figure 3. Effect of compound 21 on microtubule assembly.
A549 cells were cultured and treated with agent for 24 hr as indicated. Cells were labeled immunocytochemically using antibody to α-tubulin (left panels) and DAPI for DNA (right panels). The mitotic spindles were clearly seen in control (DMSO) and pacritaxel (Tax) treated cells (arrow heads), while undetectable in the cells treated with 21, colchicine (Col), or doxorubicine (Dox). The aggregations of tubulin were seen in the prophase cells treated with 21 or colchicine (arrow). In addition, microtubules were undetectable in the cells treated with 21 or colchicine, but detectable in cells treated with DMSO, pacritaxel, or doxorubicine. These observations clearly indicate that 21 inhibits tubulin polymerization in the human tumor cells. Bar, 10 μm.
We therefore evaluated selected active compounds for antitubulin activities, and the results are shown in Table 3. All cytotoxic compounds (GI50 values of less than 1 μg/mL) also exhibited potent inhibition of tubulin assembly. Analogue 21, which showed the most potent cytotoxicity, strongly inhibited tubulin assembly with an IC50 value of 2.0 μM. It was also the most active of these compounds as an inhibitor of colchicine binding to tubulin, inhibiting the binding reaction by 78%. Its trimethyl analogues 35–37 and cytotoxic analogues 3, 6, 8, 21, 22, and 26 also inhibited tubulin assembly, with IC50 values of 2.3–3.7 μM, althoguh these compounds were less active than 21 as inhibitors of colchicine binding. Compounds 11 and 15 were even less active, and 2 had no significant interaction with tubulin at the highest concentration evaluated (40 μM). Thus, we made conclusion that 21 is a potent tubulin polymerization inhibitor.
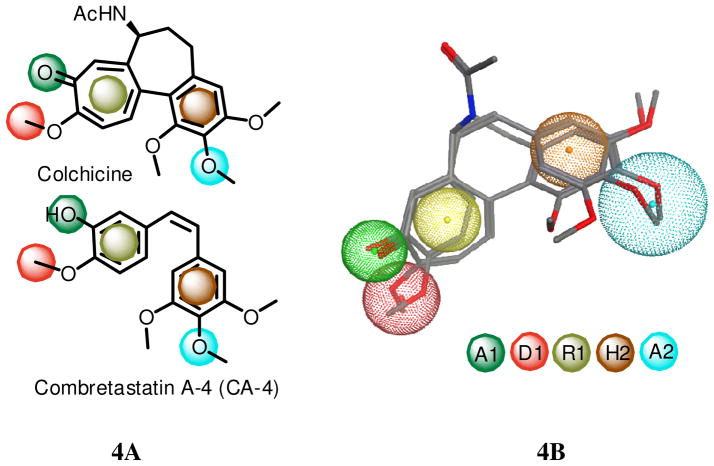
In 2005, Nguyen et al studied fifteen colchicine site inhibitiors (CSIs) and proposed seven pharmacophoric points based on consistent structural features and recurring ligand interactions 14 The seven points were three hydrogen bond acceptors (A1, A2 and A3), one hydrogen bond donor (D1), two hydrophobic centers (H1 and H2) and one planar group (R1). The H2 and R1 points represented the rigid portion of the molecular scaffold, while the other five points were important for binding specificity. Nguyen et al14 also mentioned that A2, H1, H2 and R1 emerged as essential features for inhibitory activity at the colchicines site. Most of the fifteen CSIs contained five to six of the seven pharmacophoric points. For example, Figure 4a shows that the well-known tubulin polymerization inhibitors, colchicine and CA-4, map to five pharmacophoric points, A1, A2, D1, H2, and R1. Figure 4b overlays the structures of colchicine and CA-4 onto a 3D picture of the five pharmacophores. We used Molecular Operating Environment (MOE) software to search for the best-fit 3D conformations of three of our active compounds, 3, 21 and 26, with the five pharmacophore features. The superpositions are shown in Figure 5. The ring systems of 3, 21, and 26 matched well with the planar center (R1) and hydrophobic center (H2), which are critical portions for the molecular scaffold. In addition, the hydrogen acceptor (A1) and hydrogen bond donor (D1) pharmacophores overlapped with the oxygen at the 4-position and hydroxyl group at the 5-position, respectively, of 3, 21, and 26. Compound 21 showed a better fit to the pharmacophore points R1 and H2 than compounds 3 and 26, which could explain the greater inhibitory activity of 21 against the colchicine binding site. Another possible reason of its great potency is that the sulfur atom in 21 might interact positively with the binding site. Further structural modifications to enhance expression of one or more of the other three pharmacophore features, A2, A3, and H1, could increase the potency of these tubulin-destabilizing agents targeting the colchicine site.
Figure 4. Common pharmacophores of colchicine and CA-4.
4A: Five point pharmacophores, H2 (hydrophobic center), R1 (planar group), A1, A2 (hydrogen bond acceptors), and D1 (hydrogen bond donor) for colchicine and CA-4 by Nguyen.14 4B: The structures of colchicine and CA-4 onto a 3D picture of the five pharmacophores by MOE. R1 is shown as hydrophobic/aromatic center in this model.
Figure 5. The alignment of pharmacophore and compounds 3, 21, and 26.
Hydrophobic center (H2), planer group (R1), hydrogen bond acceptors (A1 and A2), hydrogen bond donor (D1)
In summary, we discovered that analogues of 1 with bicyclic aryl substituents at C-2 possessed promising antitumor activity, with significant antiproliferative effects against HCT-8, PC-3, A549, MCF-7, HepG2, KB, and KB-VIN tumor cells. Benzo[b]thiophene analogue 21 displayed the most potent inhibitory effects on tumor cell growth, with GI50 values of 0.06–0.16 μM. The activity of the compounds, including 21, was not influenced by overexpression of P-gp (GI50 = 0.07 μM against KB-VIN vs 0.08 μM for the parental KB line). Furthermore, cytotoxicic analogues 3, 6, 8, 11, 15, 21, 22, 26, and 35–37, which all possess a bicyclic B-ring at C-2, displayed significant antitubulin activities with 30–80% inhibition of binding to the colchicine site. Compounds 3, 21, and 26 overlapped with four out of seven pharmacophoric points that were derived from the binding models of fifteen selected CSIs. Thus, they are a new class of CSIs, and further modifications would be useful to develop more potent antitubulin agents. However, analogues of 1 with a phenyl B-ring, including the natural product 1 itself, showed selective cytotoxicity against KB-VIN cells relative to KB cells. Thus, the results presented here indicate that the B-ring structure is critically important in the interaction of this compound class with P-gp. This distinction may well prove valuable in designing more effective chemotherapeutic agents.
Experimental
All chemicals and solvents were used as purchased. All melting points were measured on a Fisher-Johns melting point apparatus without correction. 1H and 13C NMR spectra were recorded on a Varian Gemini 2000 (300 MHz) or a Varian Inova (400 MHz) NMR spectrometer with TMS as the internal standard. All chemical shifts are reported in ppm. NMR spectra were referenced to the residual solvent peak, chemical shifts d in ppm, apparent scalar coupling constants J in Hz. Mass spectroscopic data were obtained on a Shimazu LC-MS2010 instrument. Analytical thin-layer chromatography (TLC) was carried out on Merck precoated aluminum silica gel sheets (Kieselgel 60 F-254). Biotage Flash or Isco Companion systems were used for flash chromatography. All target compounds were characterized and determined as at least >95% pure by 1H-NMR, MS, and elemental analyses or analytical HPLC.
General synthetic procedures for 31
A solution of 30 in EtOH–50% aq. KOH (1:1, v/v) and an appropriate aromatic aldehyde (excess) was stirred at room temperature. After the reaction was complete by TLC analysis, the mixture was poured into ice-cold 1 N HCl, and then extracted with CH2Cl2. The extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was chromatographed on silica gel with CH2Cl2–hexane as eluent to afford the target compound, which was crystallized from CH2Cl2–hexane.
General synthetic procedures for 3–37
Compound 39 was dissolved separately in 1% H 2SO4 in DMSO, then I2 (0.1 eq. mol) was added and the reaction mixture heated at 90 °C for 1 h. The reaction mixture was treated in the same manner as described above to afford compound 40, which was dissolved in anhydrous CH2Cl2. BBr3 (3 eq. mol, 1.0 M solution in CH2Cl2) was added to the solution at 0 ºC, which was warmed to rt spontaneously and stirred overnight. After addition of water, the reaction mixture was extracted three times with CH2Cl2. The extracts were combined, washed with brine, dried over Na2SO4 and concentrated in vacuo. The residues were chromatographed on silica gel eluting with EtOAc-hexane (1:4) to obtain analogs 5–37.
2-(Naphthalen-1′-yl)-6,8,8-tripropyldesmosdumotin B (5)
1H NMR (300 MHz, CDCl3): δ̣ 13.07 (s, 1H, chelated-OH), 8.09 (d, 1H, J = 7.9 Hz, 5′-H), 8.01–7.97 (m, 1H, 4′ or 8′-H), 7.92–7.86 (m, 1H, 4′ or 8′-H), 7.70–7.58 (m, 4H, J = 7.3 Hz, 2′, 3′ 6′ and 7′-H), 6.80 (s, 1H, 3-H), 2.43 (t, 2H, J = 7.5 Hz, 6-CH2CH2CH3), 2.12 (dt, 2H, J = 12.5 and 4.3 Hz, 8-CH2CH2CH3), 1.83 (dt, 2H, J = 12.5 and 4.3 Hz, 8-CH2CH2CH3), 1.49 (q, 2H, J = 7.7 Hz, 6-CH2CH2CH3), 1.24–1.11 (m, 2H, 8- CH2CH2CH3), 1.10–0.50 (m, 2H, 8- CH2CH2CH3), 0.96 (t, 3H, J = 7.7 Hz, 6-CH2CH2CH3), 0.82 (t, 6H, J = 7.5 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 431 (M++1). Anal. (C28H30O4) C, H, O.
2-(4′-Methylnaphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (6)
1H NMR (300 MHz, CDCl3):δ 13.11 (s, 1H, chelated-OH), 8.17–8.12 (m, 1H, 5′-H), 7.97–7.92 (m, 1H, 8′-H), 7.70–7.56 (m, 2H, 6′- and 7′-H), 7.58 (d, 1H, J = 7.3 Hz, 2′-H), 7.46 (d, 1H, J = 7.3 Hz, 3′-H), 6.79 (s, 1H, 3-H), 2.80 (s, 3H, 4′-CH3), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.26–2.12 (m, 2H, 8-CH2CH3), 1.96–1.84 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). Anal. (C26H26O4·1/4H2O) C, H, O.
2-(2′-Methylnaphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (7)
1H NMR (300 MHz, CDCl 3): δ̣ 13.05 (s, 1H, chelated-O 3 H), 7.96 (d, 1H, J = 8.7 Hz, 4′-H), 7.94–7.88 (m, 1H, 5′-H), 7.58–7.48 (m, 3H, 6′, 7′and 8′-H), 7.46 (d, 1H, J = 8.7 Hz, 3′-H), 6.64 (s, 1H, 3-H), 2.49 (q, 2H, J = 7.5 Hz, 6-CH2CH3), 2.46 (s, 3H, 2′-CH3), 2.20–2.06 (m, 2H, 8-CH2CH3), 1.88–1.74 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.69 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 403 (M++1). Anal. (C26H26O4·1/4H2 O) C, H, O.
2-(4′-Methoxynaphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (8)
1H NMR (300 MHz, CDCl3):δ 13.19 (s, 1H, chelated-OH), 8.40 (dd, 1H, J = 7.3 and 2.2 Hz, 5′-H), 7.94 (dd, 1H, J = 7.3 and 2.2 Hz, 8′-H), 7.65 (d, 1H, J = 8.0 Hz, 2′-H), 7.65–7.56 (m, 2H, 6′ and 7′-H), 6.93 (d, 1H, J = 8.0 Hz, 3′-H), 6.78 (s, 1H, 3-H), 4.10 (s, 3H, -OCH3), 2.48 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.24–2.12 (m, 2H, 8-CH2CH3), 1.99–1.84 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 419 (M++1). Anal. (C26H26O5) C, H, O.
2-(4′,7′-Dimethoxynaphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (9)
1H NMR (300 MHz, CDCl3): δ̣ 13.22 (s, 1H, chelated-OH), 8.31 (d, 1H, J = 10.0 Hz, 5′-H), 7.59 (d, 1H, J = 8.2 Hz, 2′-H), 7.24 (d, 1H, J = 10.0 Hz, 6′-H), 7.23 (s, 1H, 2′-H), 6.80 (d, 1H, J = 8.2 Hz, 3′-H), 6.77 (s, 1H, 3- H), 4.07 (s, 3H, -OCH3), 3.89 (s, 3H, -OCH3), 2.48 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.27–2.13 (m, 2H, 8-CH2CH3), 2.01–1.87 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 449 (M++1). Anal. (C27H28O6·1/4H2O) C, H, O.
2-(2′,3′-Dimethoxynaphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (10)
1H NMR (300 MHz, CDCl3): δ̣ 13.09 (s, 1H, chelated-OH), 7.81 (d, 1H, J = 8.2 Hz, 5′-H), 7.54–7.38 (m, 3H, 6′,7′ and 8′-H), 7.49 (s, 1H, 4′-H), 6.67 (s, 1H, 3-H), 4.05 (s, 3H, -OCH3), 3.89 (s, 3H, -OCH3), 2.49 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.20–2.06 (m, 2H, 8-CH2CH3), 1.92–1.78 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.69 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 449 (M++1). Anal. (C27H28O6·H2O) C, H, O.
2-(Naphthalen-2′-yl)-6,8,8-triethyldesmosdumotin B (11)
1H NMR (300 MHz, CDCl3):δ̣ 13.10 (s, 1H, chelated-OH), 8.33 (d, 1H, J = 1.8 Hz, 1′-H), 8.04–7.96 (m, 2H, 5′- and 8′-H), 7.96–7.90 (m, 1H, 4′-H), 7.80 (dd, 1H, J = 1.8 and 8.5 Hz, 3′-H), 7.69–7.60 (m, 2H, 6′ and 7′-H), 7.04 (s, 1H, 3-H), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.38–2.24 (m, 2H, 8-CH2CH3), 2.15–2.01 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 389 (M++1). Anal. (C25H24O4) C, H, O.
2-(6′-Methoxynaphthalen-2′-yl)-6,8,8-triethyldesmosdumotin B (12)
1H NMR (300 MHz, CDCl3): δ̣ 13.18 (s, 1H, chelated-OH), 8.24 (d, 1H, J = 1.8 Hz, 1′-H), 7.88 (d, 2H, J = 8.7 Hz, 4′ and 8′-H), 7.76 (dd, 1H, J = 8.7 and 1.8 Hz, 3′-H), 7.27 (dd, 1H, J = 8.7 and 1.8 Hz, 7′-H), 7.19 (d, 1H, J = 1.8 Hz, 5′-H), 6.99 (s, 1H, 3-H), 3.98 (s, 3H, -OCH3), 2.47 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.38–2.23 (m, 2H, 8-CH2CH3), 2.14–2.00 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). Anal. (C26H26O5) C, H, O.
6,8,8-Triethyl-2-(quinolin-3′-yl)desmosdumotin B (13)
1H NMR (300 MHz, CDCl3): δ̣ 12.88 (s, 1H, chelated-OH), 9.29 (d, 1H, J = 2.2 Hz, 2′-H), 8.56 (d, 1H, J = 2.2 Hz, 4′-H), 8.21 (d, 1H, J = 8.5 Hz, 5′-H), 8.00 (d, 1H, J = 7.4 Hz, 8′-H), 7.94–7.86 (m, 1H, 7′-H), 7.72 (dd, 1H, J = 8.5 and 7.4 Hz, 6′-H), 7.09 (s, 1H, 3-H), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.39–2.25 (m, 2H, 8-CH2CH3), 2.12–1.99 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 390 (M++1). Anal. (C24H23O4N·1/8H2O) C, H, O.
2-(Benzo[d][1′,3′]dioxol-5′-yl)-6,8,8-triethyldesmosdumotin B (14)
1H NMR (300 MHz, CDCl3): δ̣ 13.13 (s, 1H, chelated-OH), 7.37 (dd, 1H, J = 8.2 and 1.8 Hz, 6′-H), 7.20 (d, 1H, J = 1.8 Hz, 4′-H), 6.97 (d, 1H, J = 8.2 Hz, 7′-H), 6.76 (s, 1H, 3-H), 6.12 (s, 2H, 2′-CH2-), 2.45 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.32–2.17 (m, 2H, 8-CH2CH3), 2.03–1.90 (m, 2H, 8-CH2CH3), 1.04 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.67 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 383 (M++1). Anal. (C22H22O6·1/4H2O) C, H, O.
2-(2′,3′-Dihydrobenzo[d][1′,4′]dioxin-6′-yl)-6,8,8-triethyldesmosdumotin B (15)
1H NMR (300 MHz, CDCl3): δ̣ 13.17 (s, 1H, chelated-OH), 7.34–7.28 (m, 2H, 5′ and 7′-H), 7.02 (d, 1H, J = 9.2 Hz, 8′-H), 6.77 (s, 1H, 3-H), 4.40–4.30 (m, 4H, -OCH2CH2O-), 2.45 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.31–2.16 (m, 2H, 8-CH2CH3), 2.05–1.91 (m, 2H, 8-CH2CH3), 1.04 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.66 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 397 (M++1). Anal. (C23H24O6·1/4H2O) C, H, O.
2-(Benzofuran-2′-yl)-6,8,8-triethyldesmosdumotin B (16)
1H NMR (300 MHz, CDCl3): δ̣ 12.98 (1H, chelated-OH), 7.71 (d, 1H, J = 7.7 Hz, 4′-H), 7.60 (d, 1H, J = 7.9 Hz, 7′-H), 7.49 (dd, 1H, J = 7.9 and 7.2 Hz, 6′-H), 7.43 (s, 1H, 3′-H), 7.36 (dd, 1H, J = 7.7 and 7.4 Hz, 5′-H), 7.01 (s, 1H, 3-H), 2.46 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.34–2.18 (m, 2H, 8-CH2CH 3), 2.06–1.92 (m, 2H, 8-CH2CH3), 1.04 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.69 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS m/z 351 (M+-1). MS (ESI+) m/z: 379 (M++1). Anal. (C23H22O5) C, H, O.
2-(Benzo[b]thiophen-2′-yl)-6,8,8-triethyldesmosdumotin B (17)
1H NMR (300 MHz, CDCl3): δ̣ 12.96 (s, 1H, chelated-OH), 7.94–7.88 (m, 2H, 4′ and 7′-H), 7.91 (s, 1H, 3′-H), 7.52–7.46 (m, 2H, 5′ and 6′-H), 6.83 (s, 1H, 3-H), 2.46 (q, 2H, J = 7.4, Hz, 6-CH2CH3), 2.34–2.21 (m, 2H, 8-CH2CH3), 2.10–1.96 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 395 (M++1). Anal. (C23H22O4S) C, H, O.
2-(3′-Methylbenzo[b]thiophen-2′-yl)-6,8,8-triethyldesmosdumotin B (18)
1H NMR (400 MHz, CDCl3): δ 13.00 (1H, chelated-OH), 7.91–7.82 (m, 2H, Ar-H), 7.55–7.45 (m, 2H, Ar-H), 6.82 (s, 1H, 3-H), 2.72 (s, 3H, 3′-CH3), 2.46 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.32–2.20 (m, 2H, 8-CH2CH3), 2.07–1.95 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.3 Hz, 6-CH 2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 409 (M++1). Anal. (C24H24O4S) C, H, O.
2-(Benzofuran-3′-yl)-6,8,8-triethyldesmosdumotin B (19)
1H NMR (400 MHz, CDCl3): δ̣ 13.03 (s, 1H, chelated-OH), 8.23 (s, 1H, 2′-H), 7.90–7.84 (m, 1H, 4′ or 7′-H), 7.68–7.63 (m, 1H, 4′ or 7′-H), 7.53–7.42 (m, 2H, 5′ and 6′-H), 6.89 (s, 1H, 3-H), 2.5–2.42 (m, 2H, 6-CH2CH3), 2.38–2.24 (m, 2H, 8-CH2CH3), 2.06–1.94 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 379 (M++1). Anal. Not available due to limited quantity.
2-(1′-Methyl-1H-indol-3′-yl)-6,8,8-triethyldesmosdumotin B (20)
1H NMR (300 MHz, CDCl3): δ̣13.61 (1H, chelated-OH), 8.00–7.93 (m, 1H, 4′-H), 7.70 (s, 1H, 2′-H), 7.48–7.34 (m, 3H, 5′, 6′ and 7′-H), 6.79 (s, 1H, 3-H), 3.94 (s, 3H, N-CH3), 2.46 (q, 2H, J = 7.4 Hz, 6- CH2CH3), 2.36–2.21 (m, 2H, 8-CH2CH3), 2.10–1.96 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 392 (M++1). Anal. (C24H25O4N) C, H, O.
2-(Benzo[b]thiophen -3′-yl)-6,8,8-triethyldesmosdumotin B (21)
1H NMR (300 MHz, CDCl3): δ̣ 13.08 (s, 1H, chelated-OH), 8.12–8.06 (m, 1H, 4′-H), 8.07 (s, 1H, 2′-H), 8.02–7.96 (m, 1H, 7′-H), 7.61–7.48 (m, 2H, 5′ and 6′-H), 6.94 (s, 1H, 3-H), 2.47 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.36–2.08 (m, 2H, 8-CH2CH3), 2.07–1.93 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 395 (M++1). Anal. (C23H22O4S·1/8H2O) C, H, O.
2-(2′-Methylbenzo[b]thiophen-3′-yl)-6,8,8-triethyldesmosdumotin B (22)
1H NMR (400 MHz, CDCl3): δ̣ 13.08 (1H, chelated-OH), 7.86–7.82 (m, 1H, 4′-H), 7.72–7.68 (m, 1H, 7′-H), 7.48–7.38 (m, 2H, 5′ and 6′-H), 6.67 (s, 1H, 3-H), 2.97 (s, 3H, 2′- CH3), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.28–2.16 (m, 2H, 8-CH2CH3), 1.98–1.86 (m, 2H, 8-CH2 CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 409 (M++1). HPLC.
2-(5′-Methylbenzo[B]thiophen-3′-yl)-6,8,8-triethyldesmosdumotin B (23)
1H NMR (400 MHz, CDCl3): δ̣ 13.09 (1H, chelated-OH), 8.03 (s, 1H, 2′-H), 7.89 (br s, 1H, 4′-H), 7.84 (d, 1H, J = 8.4 Hz, 7′-H), 7.34 (dd, 1H, J = 0.98 and 8.4 Hz, 6′-H), 6.93 (s, 1H, 3-H), 2.54 (s, 3H, 5′-CH3), 2.48 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.34–2.22 (m, 2H, 8-CH2CH3), 2.06–1.95 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 409 (M++1). Anal. (C24H24O4S) C, H, O.
2-(5′-Bromobenzo[b]thiophen-3′-yl)-6,8,8-triethyldesmosdumotin B (24)
1H NMR (400 MHz, CDCl3): δ̣ 12.97 (1H, chelated-OH), 8.25 (d, 1H, J = 1.9 Hz, 4′-H), 8.07 (s, 1H, 2′-H), 7.84 (d, 1H, J = 8.8 Hz, 7′-H), 7.61 (dd, 1H, J = 1.9 and 8.8 Hz, 6′-H), 6.88 (s, 1H, 3-H), 2.47 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.37–2.24 (m, 2H, 8-CH2CH3), 2.05–1.94 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.71 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 473 and 475 (M++1). Anal. (C23H21BrO4S) C, H, O.
2-(5′-Methoxybenzo[b]thiophen-4′-yl)-6,8,8-triethyldesmosdumotin B (25)
1H NMR (400 MHz, CDCl3): δ̣ 13.19 (1H, chelated-OH), 8.00 (d, 1H, J = 8.8 Hz, Ar-H), 7.63 (d, 1H, J = 5.6 Hz, Ar-H), 7.25 (d, 1H, J = 5.6 Hz, Ar-H), 7.16 (d, 1H, J = 8.8 Hz, Ar-H), 6.75 (s, 1H, 3-H), 3.92 (s, 3H, 5′-OCH3), 2.47 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.20–2.10 (m, 2H, 8-CH2CH3), 1.94–1.85 (m, 2H, 8-CH2CH 3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.69 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 425 (M++1). Anal. (C24H24O5S) C, H, O.
2-(5′-Hydroxybenzo[B]thiophen-4′-yl)-6,8,8-triethyldesmosdumotin B (26)
1H NMR (400 MHz, CDCl3): δ̣ 313.11 (1H, chelated-OH), 7.90 (d, 1H, J = 8.8 Hz, Ar-H), 7.82 (br s, 1H, 5′-OH), 7.63 (d, 1H, J = 5.6 Hz, Ar-H), 7.32 (d, 1H, J = 5.6 Hz, Ar-H), 7.14 (d, 1H, J = 8.8 Hz, Ar-H), 7.12 (s, 1H, 3-H), 2.49 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.25–2.14 (m, 2H, 8-CH2CH3), 2.02–1.91 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 411 (M++1). Anal. (C23H22O5S) C, H, O.
2-(Benzo[b]thiophen-7′-yl)-6,8,8-triethyldesmosdumotin B (27)
1H NMR (400 MHz, CDCl3): δ 13.10 (1H, chelated-OH), 8.06 (dd, 1H, J = 7.8 and 0.8 Hz, 4′-H), 7.79 (d, 1H, J = 7.6 Hz 6′-H), 7.62 (d, 1H, J = 5.6 Hz, 2′-H), 7.57 (dd, 1H, J = 7.8 and 7.6 Hz, 5′-H), 7.50 (d, 1H, J = 5.6 Hz, 3′-H), 7.12 (s, 1H, 3-H), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.34–2.18 (m, 4H, 8-CH2CH3×2), 1.06 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 395 (M++1). HPLC
2-(Benzo[b]thiophen-5′-yl)-6,8,8-triethyldesmosdumotin B (28)
1H NMR (400 MHz, CDCl3): δ 13.10 (1H, chelated-OH), 8.26 (d, 1H, J = 1.9 Hz, 4′-H), 8.05 (d, 1H, J = 8.5 Hz, 7′-H), 7.72 (dd, 1H, J = 1.9 and 8.5 Hz, 6′-H), 7.62 (d, 1H, J = 5.4 Hz, 2′-H), 7.49 (d, 1H, J = 5.4 Hz, 3′-H), 6.98 (s, 1H, 3-H), 2.46 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.36–2.24 (m, 2H, 8-CH2CH3), 2.10–2.00 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.70 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z 395 (M++1). HPLC
2-(1′,2′-Dihydroacenaphthylen-5′-yl)-6,8,8-triethyldesmosdumotin B (29)
1H NMR (300 MHz, CDCl3): δ̣ 13.21 (1H, chelated-OH), 7.81 (d, 1H, J = 8.2 Hz, 8′-H), 7.72 (d, 1H, J = 7.5 Hz, 4′-H), 7.61 (dd, 1H, J = 8.2 and 7.5 Hz, 7′-H), 7.46 (d, 1H, J = 7.5 Hz, 3′ or 6′-H), 7.42 (d, 1H, J = 7.5 Hz, 3′ or 6′-H), 6.88 (s, 1H, 3-H), 3.49 (s, 4H, -CH2-×2), 2.48 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.30–2.15 (m, 2H, 8-CH2CH3), 2.04–1.90 (m, 2H, 8-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 415 (M++1). Anal. (C27H26O4) C, H, O.
2-(9′-Ethyl-9′H-carbazol-2′-yl)-6,8,8-triethyldesmosdumotin B (30)
1H NMR (300 MHz, CDCl3): δ̣ 13.31 (1H, chelated-OH), 8.47 (d, 1H, J = 1.8 Hz), 8.29 (d, 1H, J = 1.8 Hz), 7.91 (dd, 1H, J = 1.8 and 8.7 Hz), 7.65 (dd, 1H, J = 1.8 and 8.7 Hz), 7.55 (d, 1H, J = 8.7 Hz), 7.37 (d, 1H, J = 8.7 Hz), 6.97 (s, 1H, 3-H), 4.42 (q, 2H, J = 7.3 Hz, N-CH2CH3), 2.46 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.40–2.27 (m, 2H, 8-CH2CH3), 2.16–2.03 (m, 2H, 8-CH2CH3), 1.48 (t, 3H, J = 7.3 Hz, N-CH2CH3), 1.06 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 455 (M++1). Anal. Not available due to limited quantity.
2-(Dibenzo[b,d]furan-4′-yl)-6,8,8-triethyldesmosdumotin B (31)
1H NMR (300 MHz, CDCl3): δ̣ 13.14 (s, 1H, chelated-OH), 8.18 (dd, 1H, J = 7.8 and 1.4 Hz, 9′-H), 8.03 (dd, 1H, J = 6.9 and 1.4 Hz, 1′-H), 7.94 (dd, 1H, J = 7.8 and 1.4 Hz, 6′-H), 7.73 (s, 1H, 3-H), 7.70 (d, 1H, J = 8.2 Hz, 3′-H), 7.62–7.42 (m, 3H, 2′, 7′ and 8′-H), 2.48 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.38–2.24 (m, 2H, 8-CH2CH3), 2.18–2.04 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.72 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 429 (M++1). Anal. (C27H24O5·1/2H2O) C, H, O.
2-(Anthracen-9-yl)- 6,8,8-triethyldesmosdumotin B (32)
1H NMR (300 MHz, CDCl3): δ̣ 13.06 (1H, chelated-OH), 8.70 (s, 1H, 10′-H), 8.17–8.09 (m, 2H, 5′- and 8′-H, or 1′- and 3′-H), 7.83–7.75 (m, 2H, 5′- and 8′-H, or 1′- and 3′-H), 7.61–7.54 (m, 4H, 2′-, 3′-, 6′- and 7′-H), 6.83 (s, 1H, 3- H), 2.51 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.18–2.04 (m, 2H, 8-CH2CH3), 1.84–1.70 (m, 2H, 8-CH2 CH3), 0.88 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.74 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 439 (M++1). Anal. (C29H26O4·1/2H2O) C, H, O.
2-(Phenanthren-9′-yl)- 6,8,8-triethyldesmosdumotin B (33)
1H NMR (300 MHz, CDCl3): δ̣ 13.06 (1H, chelated-OH), 8.86–8.72 (m, 2H, 4′ and 5′-H), 8.04–7.94 (m, 2H, 1′- and 8′-H), 7.92–7.62 (m, 5H, 2′, 3′, 6′, 7′ and 10′-H), 6.89 (s, 1H, 3- H), 2.49 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.24–2.11 (m, 2H, 8-CH2CH3), 1.98–1.83 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.75 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 439 (M++1). Anal. (C29H26O4·1/2H2O) C, H, O.
2-(Biphen-4′-yl)- 6,8,8-triethyldesmosdumotin B (34)
1H NMR (300 MHz, CDCl3): δ̣ 13.08 (1H, chelated-OH), 7.88 (d, 2H, J = 7.8 Hz, 2′- and 6′-H), 7.78 (d, 2H, J = 7.8 Hz), 7.64 (d, 2H, J = 8.2 Hz), 7.55–7.43 (m, 3H, 8′-, 9′- and 10′-H), 6.95 (s, 1H, 3-H), 2.46 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.36–2.21 (m, 2H, 8-CH2CH3), 2.10–1.96 (m, 2H, 8-CH2CH3), 1.05 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.69 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). Anal. (C27H26O4) C, H, O.
2-(4′-Methylnaphthalen-1′-yl)-6,8,8-trimethyldesmosdumotin B (35)
1H NMR (400 MHz, CDCl3): δ 13.12 (s, 1H, chelated-OH), 8.16–8.12 (m, 1H, 5′-H), 8.00–7.96 (m, 1H, naphtyl-H), 7.68–7.57 (m, 3H, naphtyl-H), 7.45 (d, 1H, J = 7.3 Hz, naphtyl-H), 6.79 (s, 1H, 3-H), 2.80 (s, 3H, 4′-CH3), 1.90 (s, 3H, 6-CH3), 1.53 (s, 6H, 8-CH3×2). Anal. (C23H20O4) C, H, O.
2-(2′,3′-Dihydrobenzo[d][1′,4′]dioxin-6′-yl)-6,8,8-trimethyldesmosdumotin B (36)
1H NMR (400 MHz, CDCl3): δ̣ 13.22 (s, 1H, chelated-OH), 7.34–7.30 (m, 2H, Ar-H), 7.03–7.00 (m, 1H, Ar-H), 6.77 (s, 1H, 3-H), 4.40–4.30 (m, 4H, -OCH2CH2O-), 1.86 (s, 3H, 6-CH3), 1.56 (s, 6H, 8-CH3×2). MS (ESI+) m/z: 355 (M++1). Anal. (C20H18O6·1/2H2O) C, H, O.
2-(Benzo[b]thiophen-3′-yl)-6,8,8-trimethyldesmosdumotin B (37)
1H NMR (400 MHz, CDCl3): δ̣13.08 (s, 1H, chelated-OH), 8.11 (d, 1H, J = 8.1 Hz, 4′ or 7′-H), 8.07 (s, 1H, 2′-H), 7.98 (d, 1H, J = 8.1 Hz, 4′ or 7′-H), 7.60–7.48 (m, 2H, 5′ and 6′-H), 6.92 (s, 1H, 3-H), 1.90 (s, 3H, 6-CH3), 1.60 (s, 6H, 8-CH3×2). MS (ESI+) m/z: 351 (M+-1). Anal. (C20H16O4S·1/4H2O) C, H, O.
6,8,8-Triethyl-7-methoxy-2-(naphthalen-1′-yl)-4H-chromene-4,5(8H)-dione (40a)
40a was prepared according to the previously reported procedure.1b
1H NMR (300 MHz, CDCl3): δ̣ 8.04 (d, 1H, J = 8.2 Hz, Ar-H), 8.00–7.92 (m, 2H, Ar-H), 7.67–7.54 (m, 4H, Ar-H), 6.72 (s, 1H, 3-H), 4.00 (s, 3H, OCH3), 2.60 (q, 2H, J = 7.3 Hz, 6-CH2CH3), 2.16–2.00 (m, 2H, 8-CH2CH3), 2.24–1.89 (m, 2H, 8-CH2CH3), 1.18 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.76 (t, 6H, J = 7.3 Hz, 8-CH2CH3×2). MS (ESI+) m/z: 403 (M++1). Anal. (C26H26O4) C, H, O.
2-(Naphthalen-1-yl)-4-thioxo-6,8,8-triethyldesmosdumotin B (41)
Lawesson’s reagent (48 mg, 0.12 mmol) was added to a solution of 3 (40 mg, 0.10 mmol) in toluene (1.5 mL). After refluxing for 6.5 h, the volatile solvent was removed in vacuo. The residue was purified by SiO2 column chromatography (EtOAc/hexane, gradient) to obtain 41 (20 mg, 50%) and 42 (3 mg, 7%) along with starting material (8 mg, 20%). 1H NMR (300 MHz, CDCl3): δ̣ 13.50 (1H, chelated-OH), 8.10 (d, 1H, J = 7.9 Hz, 5′-H), 8.02–7.95 (m, 2H, 3′- and 8′-H), 7.73 (dd, 1H, J = 7.2 and 1.1 Hz, 2′-H), 7.66–7.60 (m, 3H, 4′-, 6′- and 7′-H), 7.62 (s, 1H, 3-H), 2.52 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.31–2.07 (m, 2H, 8-CH2CH3), 2.01–1.86 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.74 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z 405 (M++1). Anal. (C25H24O3S) C, H, O.
4,7-Dithioxo-2-(naphthalen-1-yl)-6,8,8-triethyldesmosdumotin B (42)
1H NMR (300 MHz, CDCl3): δ̣ 14.30 (1H, chelated-OH), 8.11 (d, 1H, J = 8.2 Hz, 5′-H), 8.03–7.94 (m, 2H, 3′- and 8′-H), 7.76 (dd, 1H, J = 7.4 and 1.3 Hz, 2′-H), 7.67–7.57 (m, 3H, 4′-, 6′- and 7′-H), 7.62 (s, 1H, 3-H), 2.99 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.58–2.42 (m, 2H, 8-CH2CH3), 2.30–2.16 (m, 2H, 8-CH2CH3), 1.10 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.64 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z 421 (M++1). Anal. (C25H24O2S2·H2O) C, H, O.
3-Bromo-6,8,8-triethyl-7-methoxy-2-(naphthalen-1′-yl)-4H-chromene-4,5(8H)-dione (43)
PhI(OAc)2 (280 mg, 0.87 mmol) was suspended in anhydrous CH2Cl2 (1.5 mL) under argon at room temperature. Bu4NBr (281 mg, 0.87 mmol) was added, and the mixture was stirred for 30 min. 40a (64 mg, 0.16 mmol) in anhydrous CH2Cl2 (1.0 mL) was added, and the mixture was stirred at room temperature for 4 days. The reaction was quenched with saturated NH4Cl aq. and extracted with CH2Cl2. The organic layer was dried over Na2SO4, and the solvent evaporated in vacuo. The residue was purified by SiO2 column chromatography (EtOAc/hexane, gradient) to obtain 43 (22 mg, 29%).1H NMR (300 MHz, CDCl3): δ̣ 8.07 (dd, 1H,3J = 2.1 and 7.4 Hz, 5′-H), 8.01–7.96 (m, 1H, Ar- H), 7.68–7.52 (m, 5H, Ar-H), 3.99 (s, 3H, OCH3), 2.61 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.10–1.95 (m, 2H, 8-C H2CH3), 1.90–1.78 (m, 2H, 8-CH2CH3), 1.17 (t, 3H, J = 7.3 Hz, 6-CH2CH3), 0.73 (br s, 6H, 8-CH2CH3×2). MS (ESI+) m/z 481 and 483 (M++1). Anal. (C26H25 BrO4) C, H, O.
3-Bromo-2-(naphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (44)
To a solution of 43 (18 mg, 0.038 mmol) in anhydrous CH2Cl2 (1.0 ml), BBr3 (0.1 ml, 0.1 mmol, 1.0 M solution in CH2Cl2) was added at −78 °C under N2. The mixture was stirred overnight at −78 °C to 0 °C. The reaction mixture was quenched with water and extracted with CH2Cl2. The organic phase was dried over Na2SO4, and concentrated. The residue was purified by SiO2 column chromatography (EtOAc/hexane, gradient) to obtain 44 (13 mg, 73%).
1H NMR (300 MHz, CDCl3): δ̣ 12.63 (1H, chelated-OH), 8.14–8.08 (m, 1H, 5′-H), 8.03–7.98 (m, 1H, 8′-H), 7.71–7.52 (m, 5H, 2′-, 3′-, 4′-, 6′-, and 7′-H), 2.49 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.20–2.07 (m, 2H, 8-CH2CH3), 1.87–1.72 (m, 2H, 8-CH2CH3), 1.07 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.74 (t, 6H, J = 7.4 Hz, 8-CH2CH3×2). MS (ESI+) m/z 467 and 469 (M++1). Anal. (C22H23BrO4) C, H, O.
2,3-Dihydro-2-(naphthalen-1′-yl)-6,8,8-triethyldesmosdumotin B (48)
Compound 39 (R = Et, Ar = naphthyl,1b 58 mg, 0.14 mmol) was dissolved in HOAc (2.0 mL). 45% HI (1.6 mL) was added to the mixture, which was refluxed for 20 h. The reaction mixture was cooled to rt, and the solvent was removed in vacuo. Water was added to the residue. The whole mixture was neutralized to pH 7 with sat. aq. NaHCO3 and extracted with CH2Cl2. The organic phase was dried over Na2SO4, and concentrated. The residue was purified by SiO2 column chromatography (EtOAc/hexane, gradient) to obtain 48 (20 mg, 37%). 1H NMR (400 MHz, CDCl3): δ̣ 11.64 (1H, chelated-OH), 8.01–7.92 (m, 3H, naphtyl-H), 7.64–7.52 (m, 4H, naphthyl- H), 6.37 (dd, 1H, J = 12.3 and 4.1 Hz, 2-H), 3.19 (dd, 1H, J = 12.3 and 17.3 Hz, 3-Hax), 3.14 (dd, 1H, J = 4.1 and 17.3 Hz, 3-Heq), 2.40 (q, 2H, J = 7.4 Hz, 6-CH2CH3), 2.12–1.98 (m, 2H, 8-CH2CH3), 1.90–1.66 (m, 2H, 8-CH2CH3), 1.00 (t, 3H, J = 7.4 Hz, 6-CH2CH3), 0.77 (t, 3H, J = 7.4 Hz, 8-CH2CH3), 0.50 (t, 3H, J = 7.4 Hz, 8-CH2CH3). MS (ESI+) m/z 389 (M+–1). Anal. (C25H26O4) C, H, O
5-Methoxybenzothiophene-3-carboxaldehyde (55)
To a solution of 53 (122 mg, 0.74 mmol) in anhydrous CH2Cl2 (1.5 mL), SnCl4 (1.5 mL, 1.5 mmol, 1.0 M solution in CH2Cl2) was added dropwise at 0 °C under inert gas. Subsquently, dichloromethyl methyl ether (0.1 mL, 1.11 mmol) was added dropwise at 0 °C. The mixture was allowed to warm to room temperature and stirred overnight. The mixture was quenched with sat. NaHCO3 at 0 °C, and stirred for 2 h at room temperature. After extraction with CH2Cl2, the organic layer was washed with brine, dried over Na2SO4, and concentrated. The residue was chromatographed on SiO2 with EtOAc-hexane gradient to give 55 (107 mg, 0.56 mmol, 75%). 1H NMR (400 MHz, CDCl3): δ̣ 10.71 (1H, s, CHO), 8.39 (d, 1H, J = 5.6 Hz, 3 -H), 7.97 (d, 1H, J = 9.0 Hz, 7-H), 7.65 (d, 1H, J = 5.6 Hz, 2-H), 7.04 (d, 1H, J = 9.0 Hz, 6-H) 3.96 (s, 3H, OCH3). MS (ESI+) m/z 193 (M++1).
5-Methylbenzothiophene-3-carboxaldehyde (56)
The title compound was prepared in 82% yield following the same procedure as 55 starting from 54 (119.5 g, 0.81 mmol), SnCl4 (2.4 mL, 2.4 mmol, 1.0 M solution in CH2Cl2) and dichloromethyl methyl ether (0.11 mL, 1.21 mmol). 1H NMR (400 MHz, CDCl3): δ̣ 10.13 (1H, s,3 CHO), 8.51–8.48 (m, 1H, 4-H), 8.32 (s, 1H, 2-H), 7.86 (d, 1H, J = 8.2 Hz, 7-H), 7.30 (dd, 1H, J = 8.2 and 1.6 Hz, 6-H) 2.5 (s, 3H, CH3). MS (ESI+) m/z 177 (M++1).
Antiproliferative Activity Assay
All stock cultures were grown in T-25 flasks. Freshly trypsinized cell suspensions were seeded in 96-well microtiter plates at densities of 1500–7500 cells per well, with compounds added from DMSO stock solutions and then successively diluted into medium. The highest concentration of DMSO in the cultures (0.5% v/v) was without effect on cell replication under the culture conditions used. After three days in culture, attached cells were fixed with cold 50% trichloroacetic acid and then stained with 0.4% sulforhodamine B. The absorbency at 562 nm was measured using a microplate reader after solubilizing the bound dye. The mean GI50 is the concentration of agent that reduced cell growth by 50% under the experimental conditions and is the average from at least three independent and similar determinations. All values presented in Table 2 are statistically significant and standard deviations are shown in the support information. For the verapamil reversal experiments, cells were co-treated with verapamil (1 μg/mL). Control experiments showed this concentration had no effect on the replication of KB-VIN cells. The following human tumor cell lines were used in the assay: A549 (lung carcinoma), MCF-7 (breast cancer), HCT-8 (colon adenocarcinoma), PC-3 (prostate cancer), KB (nasopharyngeal carcinoma), and KB-VIN (vincristine-resistant KB subline). All cell lines were obtained from the Lineberger Cancer Center (UNC-CH) or from ATCC (Manassas, VA), except KB-VIN, which was a generous gift of Professor Y.-C. Cheng, Yale University. Cells were cultured in RPMI-1640 medium supplemented with 25 mM HEPES, 0.25% sodium bicarbonate, 10% fetal bovine serum, and 100 μg/mL kanamycin.
Tubulin assays
Tubulin assembly was measured by turbidimetry at 350 nm as described previously.15 Assay mixtures contained 1.0 mg/mL (10 μM) tubulin and varying compound concentrations and were preincubated 15 min at 30 °C without guanosine 5′-triphosphate (GTP). The samples were placed on ice, and 0.4 mM GTP was added. Reaction mixtures were transferred to 0 °C cuvettes, and turbidity development was followed for 20 min at 30 °C following a rapid temperature jump. Compound concentrations that inhibited increase in turbidity by 50% relative to a control sample were determined.
Inhibition of the binding of [3H]colchicine to tubulin was measured as described previously.16 Incubation of 1.0 μM tubulin with 5.0 μM [3H]colchicine and 5.0 μM inhibitor was for 10 min at 37°C, when about 40–60% of maximum colchicine binding occurs in control samples.
Immunofluorescence staining of tubulin
A549 tumor cells were maintained in 4-well chamber slides (Lab-Tech) for 12 h prior to treatment with DMSO, 0.1 nM compound 21, 100 nM colchicine, 1.5 nM pacritaxel, or 500 nM doxorubicin for 24 h at 37 °C. Cells were fixed with 4% paraformaldehyde in phospate buffered saline (PBS), permeabilized with 0.5% Triton X-100 in PBS, and then tubulin was immunostained with monoclonal antibody to α-tubulin (B5-1-2, Sigma) followed by fluorescein 5-isothiocyanate (FITC)-conjugated secondary antibody. Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI).17 Fluorescence labeled tubulin and nuclei were observed using a Zeiss Axioplan fluorescence microscope and images were captured by a XL16 Excel cooled digital camera controled by the Dage Exponent Software (Dage-MTI). Final images were prepared using Adobe Photoshop.
Pharmacophore analysis
Pharmacophore analysis was conducted using Molecular Operating Environment (MOE) software (version 2009.10, Chemical Computing Group, Inc.). First, low-energy 3D conformations were calculated for compounds 3, 21 and 26, as well as two known antitubulin compounds, colchicine and combretastatin-A4 (CA-4). The pharmacophore was determined by aligning the 3D structures of colchicine and CA-4. This resulting pharmacophore was later used to search the best-fit 3D confirmations of compounds 3, 21 and 26.
Supplementary Material
Acknowledgments
This study was supported by grant CA-17625 from the National Cancer Institute, NIH, awarded to K. H. L, and by a grant from the University Research Council, awarded to K.N.G. We thank Dr. Mitch Eddy (NIEHS/NIH) for providing a fluorescence microscope.
Abbreviations
- CS
collateral sensitivity
- CA-4
Combretastatin A-4
- VERAP
verapamil
- CSI
colchicine site inhibitior
- MOE
Molecular Operating Environment
Footnotes
Supporting Information Available: Elemental analysis data for compounds 5–36 and HPLC analysis for 46–53. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.a) Nakagawa-Goto K, Bastow KF, Wu JH, Tokuda H, Lee KH. Total synthesis and bioactivity of unique flavone desmosdumotin B and its analogs. Bioorg Med Chem Lett. 2005;15:3016–3019. doi: 10.1016/j.bmcl.2005.04.070. [DOI] [PubMed] [Google Scholar]; b) Nakagawa-Goto K, Bastow KF, Chen TH, Morris-Natschke SL, Lee KH. Antitumor agents 260. New desmosdumotin B analogues with improved in vitro anticancer activity. J Med Chem. 2008;51:3297–3303. doi: 10.1021/jm701208v. [DOI] [PubMed] [Google Scholar]; c) Nakagawa-Goto K, Chang PC, Lai CY, Hung HY, Chen TH, Wu PC, Zhu H, Sedykh A, Bastow KF, Lee KH. Antitumor agents 280. Multidrug resistance-selective desmosdumotin B analogues. J Med Chem. 2010;53:6699–6705. doi: 10.1021/jm100846r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol Sci. 2009;30:546–556. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For selected recent reviews: Kingston DG. Tubulin-interactive natural products as anticancer agents. J Nat Prod. 2009;72:507–515. doi: 10.1021/np800568j.Carlson RO. New tubulin targeting agents currently in clinical development. Expert Opin Investig Drugs. 2008;17:707–722. doi: 10.1517/13543784.17.5.707.Eddy P, Maria K. Microtubules: a dynamic target in cancer therapy. IUBMB Life. 2008;60:165–170. doi: 10.1002/iub.25.Kiselyov A, Balakin KV, Tkachenko SE, Savchuk N, Ivachtchenko AV. Recent progress in discovery and development of antimitotic agents. Anticancer Agents Med Chem. 2007;7:189–208. doi: 10.2174/187152007780058650.
- 4.a) Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchicine-3H to cellular protein. J Cell Biol. 1967;34:525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Borisy GG, Taylor EW. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967;34:535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Mol Pharmacol. 1988;34:200–208. [PubMed] [Google Scholar]; b) Medarde M, Maya ABS, Rérez-Melero C. Naphthalene combretastatin analogues: synthesis, cytotoxicity and antitubulin activity. J Enz Inhibit Med Chem. 2004;19:521–540. doi: 10.1080/14756360412331280473. [DOI] [PubMed] [Google Scholar]
- 6.Recent review: Chaudhary A, Pandeya SN, Kumar P, Sharma PP, Gupta S, Soni N, Verma KK, Bhardwaj G. Combretastatin A–4 analogs as anticancer agents. Mini Rev Med Chem. 2007;7:1186–1205. doi: 10.2174/138955707782795647.
- 7.a) Nakagawa-Goto K, Chen TH, Peng CY, Bastow KF, Wu JH, Lee KH. Antitumour Agents 259. Design, syntheses, and structure-activity relationship study of desmosdumotin C analogs. J Med Chem. 2007;50:3354–3358. doi: 10.1021/jm0702534. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nakagawa-Goto K, Wu JH, Lee KH. First total synthesis of desmosdumotin C. Synth Commun. 2005;35:1–5. [Google Scholar]
- 8.Ozturk T, Ertas E, Mert O. Use of Lawesson ‘s reagent in organic syntheses. Chem Rev. 2007;107:5210–5278. doi: 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- 9.Rho HS, Ko BS, Kim HK, Ju YS. Synthesis of 3-bromo derivatives of flavones. Synth Commun. 2002;32:1303–1310. [Google Scholar]
- 10.Martin-Smith M, Reid ST. Thianaphthene derivatives. III. Characterization of some 5-substituted derivatives. J Chem Soc. 1960:938–944. [Google Scholar]
- 11.Tarbell DS, Fukushima DK, Dam H. Synthesis and antihemorrhagic activity of 5-methyl-4,7-thionaphthenequinone. J Am Chem Soc. 1945;67:1643–1644. [Google Scholar]
- 12.Jackson PM, Moody CJ. Preparation and Diels-Alder reactivity of thieno[2,3-c]- and thieno[3,2-c]pyran-3-ones, stable 2,3-dimethylenethiophene derivatives; synthesis of benzothiophenes. J Chem Soc Perkin Trans. 1990;1:681–687. [Google Scholar]
- 13.Nakagawa-Goto K, Chang PC, Lai CY, Hung HY, Chen TH, Wu PC, Zhu H, Sedykh A, Bastow KF, Lee KH. Antitumor Agents 280. Multidrug resistance-selective desmosdumotin B analogues. J Med Chem. 2010;53:6699–6705. doi: 10.1021/jm100846r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen TL, McGrath C, Hermone AR, Burnett JC, Zaharevitz DW, Day BW, Wipf P, Hamel E, Gussio R. A common pharmacophores for a diverse set of colchicines site inhibitors using a structure-based approach. J Med Chem. 2005;48:6107–6116. doi: 10.1021/jm050502t. [DOI] [PubMed] [Google Scholar]
- 15.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–22. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 16.Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Márquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Structure-activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol Pharmacol. 1998;53:62–76. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Goto M, Eddy EM. Speriolin is a novel spermatogenic cell-specific centrosomal protein associated with the seventh WD motif of Cdc20. J Biol Chem. 2004;279:42128–4238. doi: 10.1074/jbc.M403190200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.