Abstract
Risk-reducing salpingo-oophorectomy (RRSO) is the cornerstone of ovarian cancer prevention in BRCA1/2 mutation carriers. Occult fallopian tube and ovarian cancers have been reported in a small percentage of BRCA1/2 mutation carriers undergoing RRSO. Here, we review our single institution experience with RRSO in BRCA1/2 mutation carriers to characterize cases of microscopic cancers in these patients. At the time of RRSO, 7.9% of BRCA1 mutation carriers were diagnosed with microscopic fallopian tube or ovarian cancers and zero cases were diagnosed in BRCA2 mutation carriers. The majority of the microscopic cancers include cases that were confined to the fallopian tubes, although there were also cases involving ovaries only or peritoneal washings only. This suggests that the site of origin may be in the ovary, fallopian tube, or peritoneum for BRCA-associated serous cancers. However, an analysis of early stage (stage I and II) ovarian and fallopian tube cancers diagnosed in BRCA1/2 mutation carriers confirms that the ovary is a preferred site for tumor growth with 11 of 14 early stage cancers having a dominant ovarian mass. Overall, these data suggest that cancer initiation may occur in the ovary, fallopian tube, or peritoneum, but tumor growth and progression is favored in the ovary. We present an updated model for BRCA1/2-associated ovarian and fallopian tube carcinogenesis, which may aid in identifying improved prevention strategies for high-risk women that delay or decline RRSO.
Keywords: BRCA1, BRCA2, ovarian cancer, fallopian tube
Introduction
Women with germline mutations of the tumor suppressor genes BRCA1 or BRCA2 have a high lifetime risk of developing ovarian cancer (approximately 39% and 22%, respectively) (1). Currently, there are no effective screening strategies for ovarian cancer (2, 3); therefore, prevention for this population focuses on prophylactic removal of the fallopian tubes and ovaries. Risk-reducing salpingo-oophorectomy (RRSO) reduces the risk of ovarian, fallopian tube, and peritoneal cancer by 85–90% for BRCA1/2 mutation carriers (4–6). Recent reports have shown that occult cancers in the fallopian tubes and ovary are diagnosed in approximately 3 – 10% of RRSO surgical specimens (5, 7–15). Given this risk, consensus groups have recommended that a complete pathology review, including serial sectioning of the ovaries and fallopian tubes, is necessary for identification of occult cancers (16).
Studying occult microscopic cancers can provide insight into the natural history of BRCA-associated ovarian cancers. Significant debate is ongoing related to the tissue site of origin (ovarian, fallopian tube, or peritoneal surfaces) and also the cell type of origin (ovarian surface epithelial cells, ciliated cells in the fallopian tube, or tubal secretory cells). Furthermore, it is not clear how the ovarian, tubal, or peritoneal microenvironments may differentially impact tumor initiation or progression.
This study reports our single institution experience with microscopic and early-stage cancers diagnosed in BRCA1 and BRCA2 mutation carriers. Observations from these cases are combined with a review of the literature to build an updated model of the early steps of BRCA-associated ovarian and fallopian tube carcinogenesis. By generating a data-driven model of ovarian/fallopian tube carcinogenesis, we can begin to address the many remaining questions that hinder the field of ovarian cancer prevention research.
Materials and Methods
Case Selection and Review
From August 2000 to July 2010, 136 patients with known BRCA mutations underwent RRSO at The University of Texas MD Anderson Cancer Center (MD Anderson). All tissues underwent the recommended complete pathology review, which included full serial sectioning of fallopian tubes and ovaries. Microscopic cancers were undetected prior to RRSO and surgically occult, diagnosed only at subsequent pathology review. Cases were reviewed by a gynecologic pathologist (M.T.D.) to verify histologic diagnosis.
We have chosen to adopt terminology that is different than what is frequently used to define microscopic cancers of the fallopian tube. Recent studies that include extensive sampling of the fallopian tube for histologic analysis have identified small lesions confined to the tubal mucosa, often labeled serous tubal intraepithelial carcinoma (STIC) or carcinoma in situ. We have chosen to use descriptive definitions instead (for example, high grade serous carcinoma involving the fimbria, confined to the mucosa). By using a descriptive label that is entirely case-specific, we hope to avoid the connotations often associated with the labels “in situ” or “STIC”. In our experience, these labels are sometimes interpreted to indicate disease of minimal severity that does not require chemotherapy. As some fallopian tube lesions identified as carcinoma in situ have been shown to recur (17), it is essential that we do not inadvertently diminish the significance of disease.
Early stage ovarian or fallopian tube cancer cases (stage I – stage II) were identified by reviewing records from patients with known germline BRCA1 or BRCA2 mutations who sought genetic counseling, treatment, or an opinion at MD Anderson from January 1999 to March 2010. This group includes patients with early stage cancers who were initially diagnosed at outside institutions. These early stage cancers were not surgically occult. This study was approved by the MD Anderson Institutional Review Board.
Literature Review
Literature search terms included- BRCA1, BRCA2, prophylactic, ovarian, fallopian tube, cancer, occult, and unexpected. For inclusion in our analysis, literature reports were required to meet the following criteria: microscopic cancers occurred in known BRCA1/2 mutation carriers (germline testing required) without suspicion of cancer prior to surgery (risk-reducing salpingo-oophorectomy), cancers were surgically occult, and diagnosed only upon further pathology review. In addition, a description of the fallopian tube review methodology was required for inclusion. Individual case reports were not included in our analysis.
Results
As of July 2010, 136 patients with known BRCA mutations have undergone risk-reducing salpingo-oophorectomy at MD Anderson (76 women with BRCA1 mutations and 60 women with BRCA2 mutations). Age at RRSO ranged from 28 – 73 years for BRCA1 mutation carriers (mean age 47 years) and 30 – 67 years for BRCA2 mutation carriers (mean age 48 years).
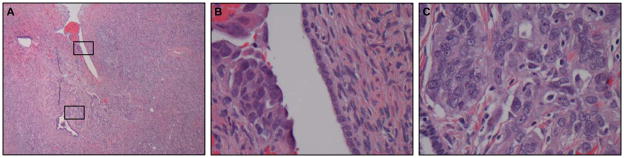
Six of 76 patients with BRCA1 mutations (7.9%) and 0 of 60 patients with BRCA2 mutations were diagnosed with microscopic cancers at the time of RRSO at MD Anderson (Table 1). These 6 microscopic cancers were all high grade serous carcinoma (HGSCa). Of the 6 HGSCa, four cases involved the fallopian tubes only and one case (case # 2) was confined to the ovary (Fig. 1). The other case (case # 1) involved HGSCa in only the peritoneal washings and no carcinoma was identified in the tubes or ovaries. Three of the 4 fallopian tube cancer cases were confined to the mucosa, and one case involved the mucosa and submucosa. All patients diagnosed with microscopic cancer at the time of RRSO were without symptoms and CA-125 levels were within normal limits. In addition, two BRCA1 mutation carriers were diagnosed with ovarian serous tumors of low malignant potential at the time of RRSO at MD Anderson (not shown).
Table 1.
Microscopic cancers identified at the time of RRSO for BRCA mutation carriers
| Case # | Age at RRSO | BRCA1/2 Mutation | Primary Site | Histology | Washings |
|---|---|---|---|---|---|
| 1 | 51 | BRCA1 | primary peritoneal (washings only) | Washings- HGSCa. No carcinoma identified in ovaries or fallopian tubes | HGSCa |
| 2 | 53 | BRCA1 | ovary | HGSCa near surface of ovary (1 mm) | NE |
| 3 | 60 | BRCA1 | fallopian tube | 2 foci of HGSCa involving the fimbria of RFT, confined to mucosa (1.7 mm and 0.4 mm) | negative |
| 4 | 52 | BRCA1 | fallopian tube | 2 foci of HGSCa confined to mucosa in RFT, first focus is in tube proper (1 mm), second focus in fimbria (0.4 mm) | negative |
| 5 | 70 | BRCA1 | fallopian tube | HGSCa involving the fimbria, confined to mucosa (2 × 3 mm) | negative |
| 6 | 55 | BRCA1 | fallopian tube | HGSCa involving fimbria involving mucosa and submucosa (2 mm) | negative |
HGSCa, high grade serous carcinoma; NE, not evaluated; RFT, right fallopian tube; RRSO, risk-reducing salpingo-ophorectomy.
Figure 1.
A, microscopic high grade serous carcinoma near the surface of the ovary (case #2), boxes indicate area shown at higher magnification in panels B and C.
We have performed a comprehensive review of reports of microscopic cancer cases diagnosed in BRCA1/2 mutation carriers at the time of RRSO to determine if our observation holds true in a larger sample set (9–15, 18–22). In total, 38 cases of microscopic cancer were reported in the literature (Table 2). Of these, 23 cases (60.5%) were confined to the fallopian tube only. In addition, 8 cases (21.1%) involved the ovary only. An additional 6 cases (15.8%) involved both the fallopian tube and ovary. One case (2.6%) of primary peritoneal cancer was also identified. Although a thorough description is not present for all cases, reported ovarian tumors include those confined to the surface of the ovary and an intracystic tumor. (Supplementary Table S1). Furthermore, 7 cases were diagnosed with bilateral disease.
Table 2.
Literature review of microscopic ovarian, fallopian tube, and peritoneal tumors in BRCA1/2 mutation carriers
| ID | BRCA1/2 Mutation | Primary Site | Washings | Reference |
|---|---|---|---|---|
| L1 | BRCA1 | fallopian tube | positive | (18) |
| L2 | BRCA1 | fallopian tube | negative | (19) |
| L3 | BRCA1 | fallopian tube | negative | (19) |
| L4 | BRCA1 | fallopian tube | negative | (19) |
| L5 | BRCA1 | fallopian tube | negative | (9) |
| L6 | BRCA1 | fallopian tube | negative | (9) |
| L7 | BRCA1 | fallopian tube | negative | (9) |
| L8 | BRCA1 | fallopian tube | negative | (9) |
| L9 | BRCA1 | fallopian tube | negative | (10, 20) |
| L10 | BRCA1 | fallopian tube | negative | (11) |
| L11 | BRCA1 | fallopian tube | negative | (11) |
| L12 | BRCA1 | fallopian tube | negative | (21) |
| L13 | BRCA1 | fallopian tube | negative | (21) |
| L14 | BRCA1 | fallopian tube | positive | (12, 21) |
| L15 | BRCA1 | fallopian tube | positive | (12, 21) |
| L16 | BRCA1 | fallopian tube | NR | (14) |
| L17 | BRCA1 | fallopian tube | NR | (14) |
| L18 | BRCA1 | fallopian tube | positive | (15) |
| L19 | BRCA2 | fallopian tube | positive | (19) |
| L20 | BRCA2 | fallopian tube | negative | (19) |
| L21 | BRCA2 | fallopian tube | negative | (12, 21) |
| L22 | BRCA2 | fallopian tube | negative | (15) |
| L23 | BRCA2 | fallopian tube | negative | (15) |
| L24 | BRCA1 | ovary | positive | (10, 20) |
| L25 | BRCA1 | ovary | NR | (13) |
| L26 | BRCA1 | ovary | NR | (14) |
| L27 | BRCA1 | ovary | NR | (14) |
| L28 | BRCA1 | ovary | negative | (15) |
| L29 | BRCA2 | ovary | NR | (13) |
| L30 | BRCA1 | both ovaries | negative | (9) |
| L31 | BRCA1 | both ovaries | negative | (11) |
| L32 | BRCA1 | both ovaries, one fallopian tube | negative | (10) |
| L33 | BRCA1 | both ovaries, one fallopian tube | positive | (10, 20) |
| L34 | BRCA1 | both ovaries, one fallopian tube | NR | (13) |
| L35 | BRCA1 | ovary and fallopian tube | negative | (19) |
| L36 | BRCA1 | ovary and both fallopian tubes | NR | (14) |
| L37 | BRCA2 | both ovaries and one fallopian tube | negative | (11) |
| L38 | BRCA2 | primary peritoneal | negative | (10) |
NR; not reported
To expand our study to include the process of tumor progression, we have also reviewed all cases of early stage ovarian and fallopian tube cancers in BRCA1/2 mutation carriers. MD Anderson has identified 131 ovarian and fallopian tube cancer cases in BRCA1/2 mutation carriers (84 BRCA1, 46 BRCA2, and 1 patient with both BRCA1 and BRCA2 mutations). From these cases, 14 cases of early stage ovarian and fallopian tube cancers were identified (Table 3). We define early stage to include stage IA – stage IIC.
Table 3.
Early stage ovarian or fallopian tube cancers identified in BRCA mutation carriers
| Case # | Age at dx | BRCA1/2 Mutation | Grade | Dominant Mass | Histology | Bilateral or Unilateral? | Primary size | Stage |
|---|---|---|---|---|---|---|---|---|
| 7 | 32 | BRCA1 | high | ovary | serous carcinoma | bilateral | 12 × 6 × 6 cm | IB |
| 8 | 43 | BRCA1 | high | ovary | serous carcinoma | unknown | unknown | IIA |
| 9 | 56 | BRCA1 | high | ovary | serous carcinoma | bilateral | 11 cm | IC |
| 10 | 55 | BRCA1 | high | ovary | serous carcinoma | unilateral | 8 cm | IIC |
| 11 | 54 | BRCA1 | high | ovary | undifferentiated carcinoma | unilateral | unknown | IA |
| 12 | 50 | BRCA1 | high | fallopian tube | poorly differentiated carcinoma | unilateral | 2 cm | IA |
| 13 | 40 | BRCA1 | high | fallopian tube | serous carcinoma | unilateral | 8 × 5 × 3 cm | II |
| 14 | 50 | BRCA1 | high | fallopian tube | serous carcinoma | unilateral | 2 × 1 × 1 cm | IA |
| 15 | 54 | BRCA2 | high | ovary | poorly differentiated carcinoma | unilateral | 7 × 7 × 5 cm | IIB |
| 16 | 59 | BRCA2 | high | ovary | serous carcinoma | bilateral | 7 cm | IIC |
| 17 | 55 | BRCA2 | high | ovary | serous carcinoma | bilateral | 14 × 7 × 6 cm | IB |
| 18 | 50 | BRCA2 | high | ovary | serous carcinoma | bilateral | 7 × 5 × 3 cm | IIA |
| 19 | 61 | BRCA2 | high | ovary | serous carcinoma | unilateral | 15 cm | IIA |
| 20 | 41 | BRCA1 and BRCA2 | high | ovary | serous carcinoma involving left ovary and detached fragments in RFT lumen | unilateral | 6 cm | IIB |
RFT, right fallopian tube.
Early stage cancers were identified in 8 BRCA1 mutation carriers (5 stage I and 3 stage II). Many of these cases were high grade serous carcinoma, though undifferentiated and poorly differentiated cases were also observed. Of these 8 early stage BRCA1 cases, 5 cases had dominant ovarian masses and 3 cases had dominant masses in the fallopian tube. Two early stage cases with BRCA1 mutations were diagnosed with bilateral ovarian disease.
Five early stage ovarian cancers were identified in BRCA2 mutation carriers (1 stage I and 4 stage II). These cases were predominantly serous. Three of these five patients were diagnosed with bilateral ovarian disease. There were no women diagnosed with fallopian tube primaries. In addition, one case (case #20) of early stage ovarian cancer was diagnosed in a patient with germline mutations in both BRCA1 and BRCA2. This woman was diagnosed with a stage IIB high grade serous ovarian carcinoma.
Overall, 11 of 14 stage I and II cancers in BRCA1/2 mutation carriers occurred with a dominant ovarian mass. The other 3 cases were fallopian tube primaries. This is in contrast to the majority of our microscopic cancers, which occurred more frequently in the fallopian tubes.
Discussion
The data reported here provide a view of the early natural history of ovarian and fallopian tube cancers in women with BRCA1/2 mutations, including differences between BRCA1 and BRCA2 mutation carriers. Although 76 women with BRCA1 mutations and 60 women with BRCA2 mutations underwent RRSO at MD Anderson, 6 cases of microscopic cancer were diagnosed in BRCA1 patients, but no microscopic cancer cases were observed in BRCA2 patients. This is consistent with previous estimates of higher risk of ovarian cancer and earlier age of onset of ovarian cancer in BRCA1 mutation carriers compared to BRCA2 mutation carriers (1, 23). Furthermore, while microscopic cancers were identified primarily in the fallopian tubes, they also occurred in the ovaries and peritoneal washings of BRCA1/2 mutation carriers. A review of microscopic cancers reported in the literature confirms our observation in BRCA1/2 mutation carriers and adds a case of an intracystic ovarian tumor. Our data also suggest that while many ovarian cancers actually originate from the fallopian tube, the ovary may be the preferred site for tumors to progress beyond the microscopic stage in BRCA1/2 mutation carriers.
With the data described here, we propose an updated model of the early stages of BRCA-associated ovarian cancer (Fig. 2), which expands the number of potential sites for ovarian cancer initiation. The fallopian tube epithelium, ovarian surface epithelium, ovarian cystic structures, and peritoneum may give rise to the early steps of carcinogenesis. Our single-institution experience and cases reported in the literature show that the majority of “ovarian” cancers begin in the distal fallopian tube and fimbria. Some of these microscopic fallopian tube cancers will progress and grow larger, but remain confined to the tube, creating a primary tubal cancer (Fig. 2A). However, because of the close association between the fimbria and ovarian surface, fallopian tube tumor cells could also spread to the ovary. This tumor may appear confined to the ovarian surface (Fig. 2B), or due to the cyclic rupture, repair, and invaginations that occurs with ovulation, the tumor may be incorporated into the ovarian stroma, where it grows and expands (Fig. 2C). Alternatively, early carcinogenic changes may occur in the ovary (either on the surface or within cystic structures) where it quickly grows (Fig. 2D). In many cases, the ovary seems to provide a particularly rich environment to support tumor growth and the majority of tumor mass is associated with the ovary. As the tumor grows larger, involving both the fallopian tube and the ovaries, it becomes difficult to identify whether the site of origin was ovary or fallopian tube. The observation of carcinoma in isolated peritoneal washings suggests that, less commonly, tumorigenesis might also begin in the peritoneum (not shown). However, additional cases with isolated positive peritoneal washings or cases of early peritoneal disease alone would be required to better address this issue. Furthermore, this study does not have sufficient cases to comment on other possible sequences of initiation or progression involving the peritoneum.
Figure 2.
Possible pathways of BRCA-associated ovarian/fallopian tube cancer initiation and progression. A, Some tumors begin in the fallopian tube and continue to grow larger while remaining confined to the tube, creating a primary tubal cancer. However, because of the close association between the fimbria and ovarian surface, fallopian tube tumor cells could also spread to the ovary (B and C). B, Cancer initiation occurs in the fallopian tube fimbria and a small number of cancer cells are deposited on to the ovarian surface, which provides a supportive environment for tumor growth. C, Due to the cyclic rupture, repair, and invaginations that occurs with ovulation, cancer cells from the fimbria can be transferred into the ovarian stroma where it grows and expands. D, Alternatively, early carcinogenic changes may occur within ovarian cysts where the tumor grows, again producing a dominant ovarian mass. As the tumor grows larger, involving both the fallopian tube and the ovaries, it becomes difficult to identify whether the site of origin was ovary or fallopian tube. OSE, ovarian surface epithelium.
The dominant size of ovarian masses observed in stage I and stage II ovarian/fallopian tube cancers in BRCA1/2 mutation carriers indicates that the ovary may be the preferred site for tumor growth. While early stage cancers are rarely reported, our observation is also confirmed in a screening study by Lewin et al. (24). Three early stage cancers were identified in BRCA1 mutation carriers (1 case at stage IA and 2 cases at stage IC). All 3 of these cancers were ovarian primaries. These observations imply that the ovarian microenvironment provides a permissive environment for tumor progression, regardless of site of tumor initiation. We can hypothesize factors that may play a role (inflammation, hormonal changes, etc), but detailed molecular studies will be required to better understand the contributions of the ovarian microenvironment during these early steps in tumorigenesis.
This updated model of ovarian cancer initiation and progression has implications for current clinical management strategies for BRCA mutation carriers. In recent years, a great deal of momentum has gathered behind the idea that the fallopian tube is the singular site of origin for ovarian cancer. However, our data indicate caution should be exercised in allowing this hypothesis to impact clinical management. For example, due to the emphasis on the fallopian tube as the primary site of initiation, there has been discussion about the possibility of risk-reducing surgery to remove the fallopian tubes only. By leaving the ovaries, patients would avoid estrogen deprivation symptoms and perhaps be more likely to undergo surgery at a younger age. However, based on this updated model of BRCA-associated ovarian carcinogenesis, salpingectomy may not be an effective preventive strategy. Initiated cells may have spread to the ovary at a very early point, resulting in a risk of carcinogenesis even if the tubes were removed. In addition, some BRCA-associated cancers may initiate in the ovaries or peritoneum, and this cancer risk would be unaffected by salpingectomy alone. In both of these scenarios, the patient might have a false sense of protection against ovarian cancer. We propose that future, large-scale collaborative studies should evaluate the relationship between age at diagnosis of microscopic cancers versus early stage invasive cancers to determine if there might be an age at which salpingectomy alone could be used as a temporizing risk-reducing strategy.
Several fundamental issues regarding ovarian cancer initiation and progression remain to be addressed. Future studies must address preneoplastic lesions that could be part of the continuum of BRCA-associated ovarian/fallopian tube carcinogenesis. For example, Crum et al. has proposed the “p53 signature” as an early lesion in the fallopian tube that precedes microscopic tumor development. The “p53 signature” is defined as 12 or more consecutive cells with strongly positive p53 nuclear staining within benign-appearing epithelium (25, 26). Further analysis of p53 signatures and continued efforts to identify additional precancerous lesions will be advanced through studying microscopic ovarian/fallopian tube cancers identified in BRCA mutation carriers. Studies are also necessary to evaluate the molecular implications of an updated model of ovarian cancer initiation and progression. For example, are the same early molecular events responsible for carcinogenesis in cancers that initiate in the ovary, fallopian tube, or peritoneum? Furthermore, are there common microenvironment contributions to carcinogenesis at these different sites? Bilateral microscopic cancers were diagnosed in several cases described here. This raises the question of whether metastasis occurs early in tumor development or if this is an indication of multifocal initiation. Future studies must also determine whether all ovarian cancers proceed through a linear progression from early stage to late stage disease or perhaps some microscopic tubal carcinomas might bypass early stage ovarian disease and progress directly to advanced-stage disease Addressing these basic issues in BRCA-associated ovarian tumorigenesis will be essential to creating improved in vitro and in vivo models to support development of prevention strategies for these high-risk patients.
It is important to note that this model is specific to BRCA-associated ovarian carcinogenesis; the relevance to sporadic ovarian cancer is unknown. Developing this type of model for sporadic ovarian cancer is problematic because microscopic cancers are rarely diagnosed in average-risk women and the intensive pathology review applied to BRCA1/2 mutation carriers is rarely performed in average-risk women. Due to the rarity of these cancers, large-scale collaborative studies will be invaluable to successfully creating a model of early events in sporadic ovarian cancer. While late stage ovarian tumor tissues are readily-available, it is difficult to extrapolate late-stage molecular findings to understand early molecular events.
In conclusion, our data suggest that the fallopian tube, ovary, and peritoneal surfaces may give rise to cancer initiation. Interestingly, the majority of BRCA-associated early stage cancers have a dominant ovarian mass, suggesting that the ovarian microenvironment should be further explored to better understand factors that support tumor progression. This updated model is important for an improved understanding of early events in BRCA-associated ovarian and fallopian tube carcinogenesis and may aid in identifying improved prevention strategies for women with BRCA1/2 mutations that delay or decline RRSO. By highlighting the many gaps in our basic knowledge of ovarian cancer, this study also emphasizes the need for a broad cooperative effort to continue to refine this data-driven model of early events in BRCA-associated ovarian carcinogenesis.
Supplementary Material
Acknowledgments
This research was supported, in part, by a cancer prevention fellowship for Melinda S. Yates (National Cancer Institute grant R25T CA57730, Shine Chang, Ph.D., Principal Investigator). This research was also supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24:863–71. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velde NM, Mourits MJ, Arts HJ, et al. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int J Cancer. 2009;124:919–23. doi: 10.1002/ijc.24038. [DOI] [PubMed] [Google Scholar]
- 3.Woodward ER, Sleightholme HV, Considine AM, Williamson S, McHugo JM, Cruger DG. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population risk women is ineffective. BJOG. 2007;114:1500–9. doi: 10.1111/j.1471-0528.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 4.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–92. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 7.Domchek SM, Friebel TM, Garber JE, et al. Occult ovarian cancers identified at risk-reducing salpingo-oophorectomy in a prospective cohort of BRCA1/2 mutation carriers. Breast Cancer Res Treat. doi: 10.1007/s10549-010-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirst JE, Gard GB, McIllroy K, Nevell D, Field M. High rates of occult fallopian tube cancer diagnosed at prophylactic bilateral salpingo-oophorectomy. Int J Gynecol Cancer. 2009;19:826–9. doi: 10.1111/IGC.0b013e3181a1b5dc. [DOI] [PubMed] [Google Scholar]
- 9.Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol. 2006;30:1222–30. doi: 10.1097/01.pas.0000202161.80739.ac. [DOI] [PubMed] [Google Scholar]
- 10.Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan TJ. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 11.Laki F, Kirova YM, This P, et al. Prophylactic salpingo-oophorectomy in a series of 89 women carrying a BRCA1 or a BRCA2 mutation. Cancer. 2007;109:1784–90. doi: 10.1002/cncr.22603. [DOI] [PubMed] [Google Scholar]
- 12.Leeper K, Garcia R, Swisher E, Goff B, Greer B, Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol. 2002;87:52–6. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 13.Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728–32. doi: 10.1200/JCO.2000.18.14.2728. [DOI] [PubMed] [Google Scholar]
- 14.Olivier RI, van Beurden M, Lubsen MA, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer. 2004;90:1492–7. doi: 10.1038/sj.bjc.6601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell CB, Kenley E, Chen LM, et al. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J Clin Oncol. 2005;23:127–32. doi: 10.1200/JCO.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 16.ACOG. ACOG Practice Bulletin No. 103: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–66. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 17.Agoff SN, Garcia RL, Goff B, Swisher E. Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol. 2004;28:1112–4. doi: 10.1097/01.pas.0000131554.05732.cd. [DOI] [PubMed] [Google Scholar]
- 18.Agoff SN, Mendelin JE, Grieco VS, Garcia RL. Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or -2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol. 2002;26:171–8. doi: 10.1097/00000478-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 20.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–9. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lamb JD, Garcia RL, Goff BA, Paley PJ, Swisher EM. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol. 2006;194:1702–9. doi: 10.1016/j.ajog.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 23.Iversen ES, Chen S. Population-Calibrated Gene Characterization: Estimating Age at Onset Distributions Associated With Cancer Genes. J Am Stat Assoc. 2005;100:399–409. doi: 10.1198/016214505000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewin SN, Kemel Y, Kosarin J, et al. Utility of ovarian cancer screening in women with BRCA mutations. J Clin Oncol. 2008 May 20;26(suppl) abstr #5531. [Google Scholar]
- 25.Jarboe EA, Pizer ES, Miron A, Monte N, Mutter GL, Crum CP. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol. 2009;22:345–50. doi: 10.1038/modpathol.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleemuddin A, Folkins AK, Garrett L, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol Oncol. 2008;111:226–32. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.