Abstract
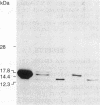
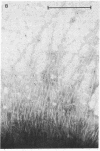
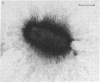
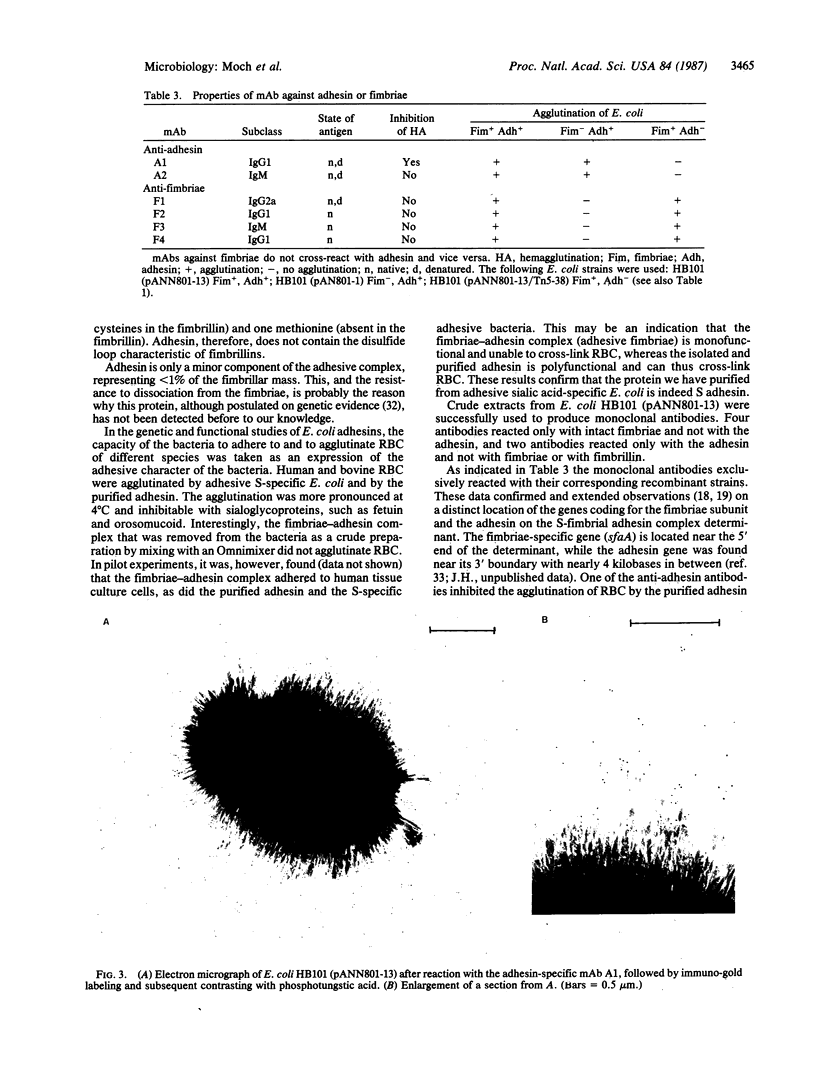
The alpha-sialyl-beta-2,3-galactosyl-specific adhesin (S adhesin) was isolated from cells of a recombinant Escherichia coli K-12 strain expressing the S-fimbrial adhesin complex. A crude cell extract was partially dissociated into fimbriae and an adhesin-enriched fraction by heating to 70 degrees C. From the latter, adhesin was purified to apparent homogeneity (by fast protein liquid chromatography, immunoblot, and NaDodSO4/PAGE) by differential ammonium sulfate precipitation, dissociation in 8 M guanidine hydrochloride, and high-resolution anion-exchange chromatography in 8 M urea. The purified adhesin formed an aggregate of Mr approximately 10(6) that was made up of one type of 12-kDa polypeptide (fimbrillin is 16.5 kDa). It had pI value of 4.7 (fimbriae has a pI value of 6). Adhesin and fimbrillin had different amino acid compositions. The purified adhesins agglutinated human and bovine erythrocytes with the same specificity as the whole bacteria; purified fimbriae were not adhesive. Monoclonal anti-adhesin and anti-fimbriae antibodies were obtained. Monoclonal anti-adhesin, but none of the anti-fimbriae, antibodies inhibited the agglutination of erythrocytes. The anti-adhesive antibodies were used in immuno-gold electron microscopy to localize adhesin exclusively on the fimbriae, with a possible preference to their tips.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr Non-flagellar appendages of bacteria. Nature. 1959 Mar 21;183(4664):782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Burchiel S. W. Purification and analysis of monoclonal antibodies by high-performance liquid chromatography. Methods Enzymol. 1986;121:596–615. doi: 10.1016/0076-6879(86)21059-9. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Eshdat Y., Speth V., Jann K. Participation of pili and cell wall adhesion in the yeast agglutination activity of Escherichia coli. Infect Immun. 1981 Dec;34(3):980–986. doi: 10.1128/iai.34.3.980-986.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F. P., Båga M., Normark S. Globoside-specific adhesins of uropathogenic Escherichia coli are encoded by similar trans-complementable gene clusters. J Bacteriol. 1985 Jun;162(3):1293–1301. doi: 10.1128/jb.162.3.1293-1301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Kaplan J., Hammond M. E., Larson J. K., Buchanan T. M., Schoolnik G. K. Ultrastructural localization of specific gonococcal macromolecules with antibody-gold sphere immunological probes. Infect Immun. 1984 Nov;46(2):361–366. doi: 10.1128/iai.46.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- van Die I., Zuidweg E., Hoekstra W., Bergmans H. The role of fimbriae of uropathogenic Escherichia coli as carriers of the adhesin involved in mannose-resistant hemagglutination. Microb Pathog. 1986 Feb;1(1):51–56. doi: 10.1016/0882-4010(86)90031-8. [DOI] [PubMed] [Google Scholar]