Abstract
Burn patients are at high risk of invasive fungal infections, which are a leading cause of morbidity, mortality, and related expense exacerbated by the emergence of drug resistant fungal strains. In this study, we investigated the use of UVC light (254-nm) for the treatment of Candida albicans infection in mouse third degree burns. In-vitro studies demonstrated that UVC could selectively kill the pathogenic yeast C. albicans compared to a normal keratinocyte cell line in a light exposure dependent manner. A mouse model of chronic C. albicans infection in non-lethal 3rd degree burns was developed. The C. albicans strain was stably transformed with a version of the Gaussia princeps luciferase gene that allowed real-time bioluminescence imaging of the progression of C. albicans infection. UVC treatment with a single exposure carried out on day 0 (30 minutes post-infection) gave an average 2.16-log10-unit (99.2%) loss of fungal luminescence when 2.92 J/cm2 UVC had been delivered, while UVC 24-hours post-infection gave 1.94-log10-unit (95.8%) reduction of fungal luminescence after 6.48 J/cm2. Statistical analysis demonstrated that UVC treatment carried out both on both day 0 and day 1 significantly reduced the fungal bioburden of infected burns. UVC was found to be superior to a topical antifungal drug, nystatin cream. UVC was tested on normal mouse skin and no gross damage was observed 24 hours after 6.48 J/cm2. DNA lesions (cyclobutane pyrimidine dimers) were observed by immunofluorescence in normal mouse skin immediately after a 6.48 J/cm2 UVC exposure, but the lesions were extensively repaired at 24-hours after UVC exposure.
Introduction
Despite major advances in burn care and management, infections remain a leading cause of mortality, morbidity, and cost in burn patients 1. While the broad acceptance of topical antibiotics, early excision and grafting, and patient isolation practices have resulted in a significant decline in bacterial wound infections, the incidence of fungal wound infections remains unchanged. Since the most severely burned patients suffer from major suppression of the host immune system, patients with large areas of third degree burns are at the highest risk for fungal infections that are difficult to prevent or eradicate 2, 3. The problem of fungal infections has become even more urgent today due to the increasing emergence of antifungal resistant pathogens 4, 5. Candida albicans is the most common fungal pathogen responsible for fungal infection in burn patients and is now the fourth most common organism found in blood cultures in intensive care unit (ICU) patients 1, 6.
Although it has been known for the last 100 years that ultraviolet (UV) light (particularly UVC with a wavelength range of 240–280 nm) is highly germicidal 7, its use to treat wound or other localized infections remains at an early stage of development. There is a widely held perception that UV light is so damaging to skin and other normal human tissue that any positive benefits of killing microorganisms with UV would be outweighed by known dangers of UV in causing carcinogenesis and other UV photodamage. However most of the studies on the damaging effects of UV on skin have been carried out with UVB light (290–340-nm) and UVA (340–400-nm) because these wavelength ranges are contained in sunlight and humans have been exposed to UVB and UVA throughout human history 8. By contrast there is no natural source of UVC and there has therefore been little impetus to study it 9. It should be noted that mammalian cells have efficient DNA repair systems 10, 11 and since the use of UVC for infections is likely to involve a single treatment or a limited number of treatments, it is possible that any UVC-induced damage to host tissue may be readily repaired. Most carcinogenesis studies of UV have used a large number of chronic exposures over weeks and months 8 and moreover, the extremely limited penetration of UVC through the stratum corneum provides another layer of protection 12.
The mechanism of UVC inactivation of organisms is to damage the genetic material in the cell 13. The UVC-induced damage to the DNA and RNA of an organism often results from the dimerization of adjacent pyrimidine molecules in DNA chains. In particular, thymine (which is found only in DNA) produces cyclobutane dimers. When these molecules are dimerized, it becomes very difficult for the nucleic acids to replicate and if replication does occur it often produces a defect that prevents the organism from being viable. There have been two clinical reports from one group to demonstrate UVC treatment of wound infections 14, 15. It was shown that UVC irradiation had the ability to reduce bacterial burden and improve wound status in patients with chronic ulcers that were infected with methicillin-resistant S. aureus (MRSA) 14, 15. In addition, UVC has been found to be efficient in sterilizing the inner surface of catheters contaminated with bacterial biofilms and subsequently preventing catheter related infections 16–18. We recently showed that UVC irradiation could be used to destroy a pathogenic fungus such as Trichophyton rubrum that causes onychomycosis in toenails 19, and that UVC irradiation could also be used to destroy pathogens in a UVC-transmissive central venous catheter 20. In the present study, we investigated the use of UVC light at 254 nm emitted from a low-pressure mercury lamp for the treatment of C. albicans infection in mouse burns. The C. albicans strain used in the study was stably transformed with a synthetic, codon-optimized version of the Gaussia princeps luciferase gene 21, allowing a real-time monitoring of the extent of C. albicans infection in mouse burns by use of bioluminescence imaging. The efficacy of UVC treatment was compared with that of a topical antifungal drug, nystatin cream. The effects of UVC on mammalian cells and host tissues were also evaluated.
Materials and Methods
Cell lines and culture conditions
The bioluminescent C. albicans strain used in this study was CEC 749 21. The luciferase reporter was constructed by fusing a synthetic, codon-optimized version of the Gaussia princeps luciferase gene to C. albicans PGA59, which encodes a glycosylphosphatidylinositol-linked cell wall protein. Luciferase expressed from this PGA59-gLUC fusion was localized at the C. albicans cell surface 21, allowing the detection of luciferase in intact cells after the addition of the luciferase substrate, coelenterazine, using bioluminescence imaging. C. albicans was routinely grown at 30°C on yeast peptone dextrose (YPD) agar and sub-cultured in YPD medium.
PAM212 cells 22 are an immortalized mouse keratinocyte cell line and were grown in RPMI 1640 supplemented with 10% foetal bovine serum (FBS) and 1% Penicillin-Streptomycin (Sigma Chemical Corporation, Saint Louis, MO).
UVC light source
UVC light was delivered using a low-pressure mercury vapor lamp (American Ultraviolet Co., Lebanon, IN). Emission spectrum measurement of this lamp by a spectroradiometer (SPR-01; Luzchem Research Inc. Ottawa, ON, Canada) showed a peak emission at 254±2 nm. By manipulating the distance between the UVC lamp and the target to be irradiated, the maximum irradiance can reach up to 20 mW/cm2. The irradiance was measured using a model IL-1700 research radiometer/photometer (International Light Inc., Newburyport, MA) over the wavelength range of 250–400 nm.
UVC irradiation of yeast suspensions or monolayer keratinocyte cultures under comparable conditions
Three mL C. albicans suspension at 108 cells/mL in PBS was placed into 35-mm petri dishes without lids that were irradiated with the germicidal lamp at an irradiance of 1.6 mW/cm2. During the irradiation, the C. albicans suspension was stirred by a mini-magnetic bar (Fisher Scientific Co., Norcross, GA,). Aliquots of 40 µL of the suspension were withdrawn at 0, 3, 6, 9, and 12 s, respectively (UVC irradiance ≈ 1.6 mW cm2). Colony forming units (CFU) were then determined by serial dilution on YPD agar plates by the method of Jett et al 23. Colonies were allowed to grow for 24–48 hours at 30C. Experiments were performed in triplicate.
PAM212 cells were seeded into 35 mm petri dishes at a density of approximately 200 cells/dish. The dishes were then incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 hours. Prior to UVC irradiation, the culture medium was replaced with PBS and lids were removed. For each trial, five dishes were illuminated under UVC for 0, 3, 6, 9, and 12 seconds, respectively, at an irradiance of 1.6 mW/cm2. After UVC irradiation, PBS was removed from the 35 mm petri dishes. The cell cultures were then detached using 2 mL trypsin-0.25% EDTA solution (Sigma-Aldrich, St. Louis, MO) per dish and recultured into 150 mm petri dishes inoculated with 50 mL fresh culture medium. The 150 mm culture dishes were then incubated at 37 °C for 10 days for growing keratinocyte colonies to a visible size before counting. Culture medium in the 150 mm dishes was refreshed every three days. After 10 days incubation, the culture medium was removed from the 150 mm petri dishes, and keratinocyte colonies were stained with 0.2% crystal violet solution (ATCC, Manassas, VA). The number of stained keratinocyte colonies was counted manually.
Animals
Adult female BALB/c mice (Charles River Laboratories, Wilmington, MA), 6–8 week old and weighing 17–21 g, were used in all experiments. The animals were housed one per cage with access to food and water ad libitum, and were maintained on a 12-hour light/dark cycle under a room temperature of 21 °C. All animal procedures in this study were approved by the Subcommittee on Research Animal Care (IACUC) of the Massachusetts General Hospital and were in accordance with the guidelines of the National Institutes of Health (NIH).
Production of C. albicans infection in mouse burns
Before the creation of burns, the mice were anesthetized by intraperitoneal (I.P.) injection of a ketamine-xylazine cocktail, shaved on the dorsal surfaces, and then depilated with Nair (Carter-Wallace Inc, New York, NY). Burns were created by applying a single preheated (≈95°C) brass block (Small Parts, Inc., Miami, FL) to the dorsal surface of each mouse for 7 seconds, resulting in a nonlethal, full-thickness, third- degree burn. The brass block area was 10 mm × 10 mm, giving an area of 100 mm2 and corresponding to 2.5% of the total body surface area 24. Immediately after the creation of the burns, the mice were resuscitated with I.P. injections of 0.5 mL sterile saline (Phoenix Scientific Inc., St. Joseph, MO) to prevent dehydration.
Fungal infection took place as described by Ha and Jin 25. Five (5) minutes after the creation of the burns (to allow the burns to cool), a suspension (40 µL) of C. albicans in sterile phosphate buffered saline (PBS) containing 106 cells was inoculated onto the eschar of each burn with a pipette tip and was then smeared onto the eschar with an inoculating loop. The mice were imaged with the luminescence camera immediately after the application of the C. albicans to ensure that the fungal inoculum applied to each burn was consistent.
Bioluminescence imaging
An ICCD photon-counting camera (Model C2400-30H; Hamamatsu Photonics, Bridgewater, NJ) was used for bioluminescence imaging. The camera was mounted in a light-tight specimen chamber, fitted with a light-emitting diode, a set-up that allowed for a background gray-scale image of the entire mouse to be captured. By accumulating many images containing binary photon information (an integration time of 2 minutes was used), a pseudo-color luminescence image was generated. Superimposition of this image onto the grayscale background image yielded information on the location and intensity in terms of photon number. Argus-50 control program (Hamamatsu Photonics) was used to acquire images and to analyze the image data collected.
Prior to imaging, mice were anesthetized by I.P. injections of ketamine/xylazine cocktail. Twenty µL coelenterazine (Gold Biotechnology, Inc, St. Louis, MO; 500 µg/mL in 1:9 methanol-PBS) was topically applied to the eschar of each infected burn. Mice were then placed on an adjustable stage in the specimen chamber, and the infected burns were positioned directly under the camera. A gray-scale background image of each wound was made, and this was followed by a photon count of the same region. This entire burn photon count was quantified as relative luminescence units (RLUs) and was displayed in a false color scale ranging from pink (most intense) to blue (least intense).
UVC treatment of C. albicans infection in mouse burns
UVC light was delivered either at 30 minutes or at 24 hours after infection to investigate the therapeutic efficacies of UVC for both early-stage and established infections. The irradiance used was 2.7 mW/cm2. Mice were given a total UVC exposure of up to 6.48 J/cm2 in aliquots with bioluminescence imaging taking place after each aliquot of light. During the UVC irradiation, the un-burned areas surrounding the burns of the mouse backs were masked using aluminum foil. To record the time course of the extent of fungal infection, fungal luminescence from mouse burns was captured daily after UVC treatment until the infections were cured (characterized by the disappearance of fungal luminescence) or the burns healed.
Extraction and quantification of C. albican from infected mouse burns
To correlate the fungal luminescence intensity to the viability of C. albicans, we extracted and quantified the colony forming units (CFU) of C. albicans from the infected mouse burns (on day 1 post-infection). Mouse burns exposed to varying doses of UVC were imaged with the luminescence camera immediately after irradiation, and then surgically excised and homogenized in 10 mL PBS with a tissue homogenizer (Omni International, Kennesaw, GA). The suspensions contained a mixture of tissue and C. albicans and were subjected to 5 serial 10-fold dilutions in PBS and then streaked on square YPD agar plates for CFU counting after 24–48 hours incubation at 30 °C.
Nystatin cream treatment of C. albicans infection in mouse burns
Nystatin cream (E. Fougera & Co, Melville, NY) was topically applied on a daily basis by smearing 25 mg/cm2-burn/day onto the burn surface with an inoculating loop from day 1 post-infection to day 6. The formation of the scab prevented effective application of Nystatin cream for longer than day 6. Eight mice were used for this experiment as Nystatin cream was supposed to be a positive control (standard therapy) for the study.
Immunohistochemical analyese of DNA lesions in mouse skin after UVC exposure
Adult female BALB/c mice described above were anesthetized, shaved, and depilated. UVC light was delivered to a square area of 2 cm × 2 cm (defined by a template made of aluminum foil) on anesthetized mice at a radiant exposure of 6.48 J/cm2. Skin biopsies from the irradiated area were taken before, immediately after, and at 24 hours after UVC exposure, respectively. They were fixed in 10% phosphate-buffered formalin at 4°C for 18–24 h, processed, and then embedded in paraffin. For immunohistochemical analyses, serial tissue sections of skin (4 µm) were prepared, deparaffinized, and rehydrated. Skin tissue sections were subjected to antigen retrieval using DAKO Target Retrieval Solution (Cat# S1699) to unmask the formalin cross linked antigens. After blocking in DAKO Universal Protein Blocking Solution (Cat# X0909) for 20 minutes, the tissue sections were incubated with mouse anti-thymine dimer monoclonal antibody (mAb) (Kaniya Biomedical Co., Seattle, WA) 30 min at room temperature. The anti-thymine dimer mAb reacts specifically with thymine cyclobutane dimers produced by UV irradiation in double- or single-stranded DNA. Antibody dilution was 1:50. Skin sections were then incubated with biotinylated anti-mouse IgG antibody (2nd antibody, Vector Labs, Burlingame, CA) for 10 minutes at room temperature. The Vector ® M.O.M biotinylated anti-mouse IgG reagent was used specially to localize mouse primary monoclonal antibodies on mouse tissue according to the kit instructions. Briefly, after specific mouse-on-mouse blocking, the skin tissue sections were incubated in dark for 5 minutes at room temperature with fluorescein avidin DCS (Vector Labs) to attach the fluorescein to the tissue-bound anti-thymine dimer mAb through biotin-avidin interaction. Between the incubations, the skin sections were extensively washed in PBS. The skin sections were then mounted with cover slips using mounting medium with DAPI (diamidino-2-phenylindole, Vector Labs), and observed using an Olympus Fluoview ® FV1000 MPE multiphoton microscope (Olympus Co., Tokyo, Japan). DAPI was used as nuclear counter stain. Masson’ trichrome stained sections were also performed, and appearances of the irradiated skin area at varying time points after UVC exposure were recorded using photography.
Statistical analysis
In a two-dimensional coordinate system, the area-under-the-plot data, which represent the time courses of fungal luminescence of the burns and also the overall fungal bioburden of the mouse burns, were calculated using numerical integration 26. Differences in the areas under the plots between the untreated control and the treatment groups and between different treatments groups were compared for statistical significance using one-way ANOVA. P values of <0.05 were considered significant.
Results
UVC selectively kills C. albicans compared to keratinocytes
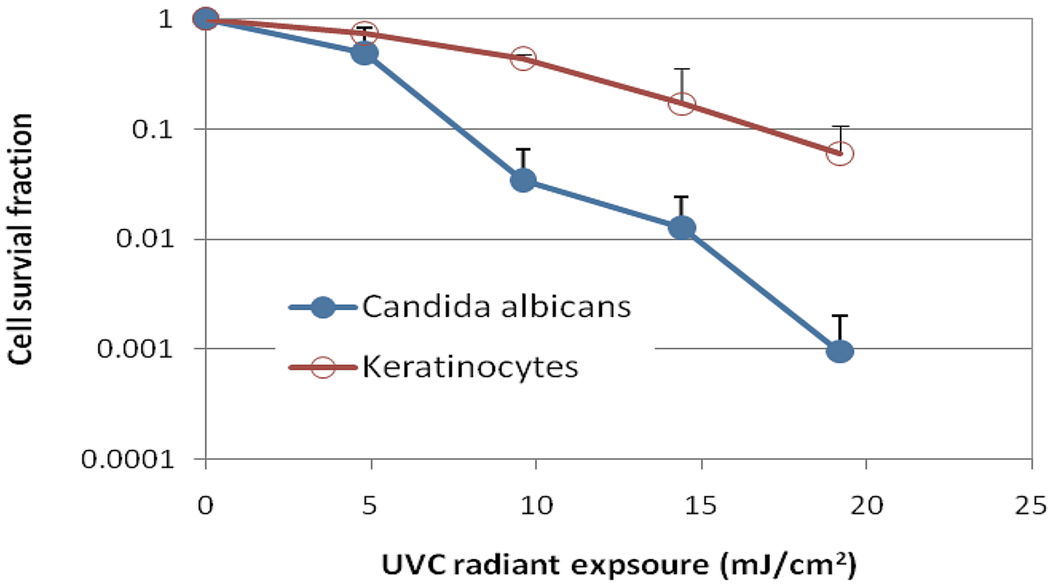
Figure 1 compares the in vitro susceptibilities to UVC irradiation between keratinocytes and C. albicans under strictly comparable conditions and using similar colony forming assays for viability. UVC selectively killed of C. albicans compared to keratinocytes in a UVC dose dependent manner. On average, when 19.2 mJ/cm2 UVC light had been delivered, the viability loss of keratinocytes was approximately 1.22-log10, while a 3.02-log10 inactivation of C. albicans was achieved at the same light dose (p<0.001), resulting in a nearly 2-log10 selective inactivation of C. albicans over keratinocytes. If we consider a 2-log10 inactivation of C. albicans as the therapeutically effective fraction, the viability loss of keratinocytes is approximately 0.77-log10 at the comparable UVC dose.
Figure 1.
In vitro susceptibility to UVC of keratinocytes and C. albicans. Bars: Standard deviation.
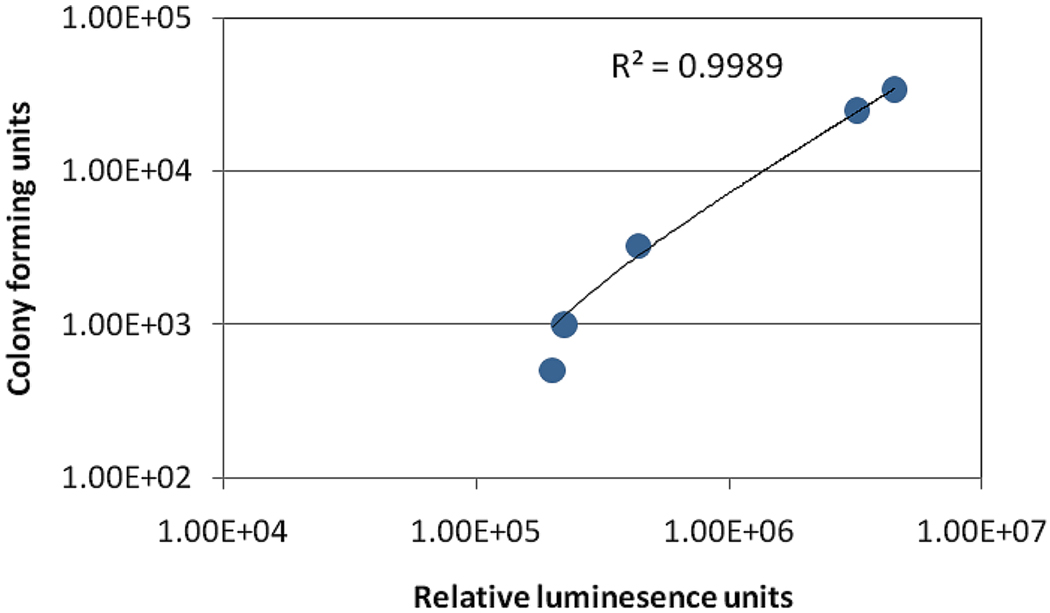
Quantification of CFU of C. albicans extracted from infected mouse burns correlated well with the fungal bioluminescence
Mice were imaged for bioluminescence and then sacrificed for removal of the infected tissue, followed by homogenization and CFU determination. Some of the mouse burns were exposed to UVC irradiation on day 1 post-infection and some were untreated. Figure 2 correlates the C. albicans CFU extracted from the excised mouse burns to the fungal bioluminescence signal measured in relative light units (RLU) from the same mouse burn. An excellent linear correlation (R2=0.9989, P< 0.001) between the CFU and RLU can be observed in Figure 2.
Figure 2.
In vivo linear correlation of C. albicans CFU extracted from tissue at necropsy to fungal bioluminescence from infected mouse burns.
Chronic C. albicans infection was developed in mouse burns as characterized by bioluminescence imaging and histological evaluation
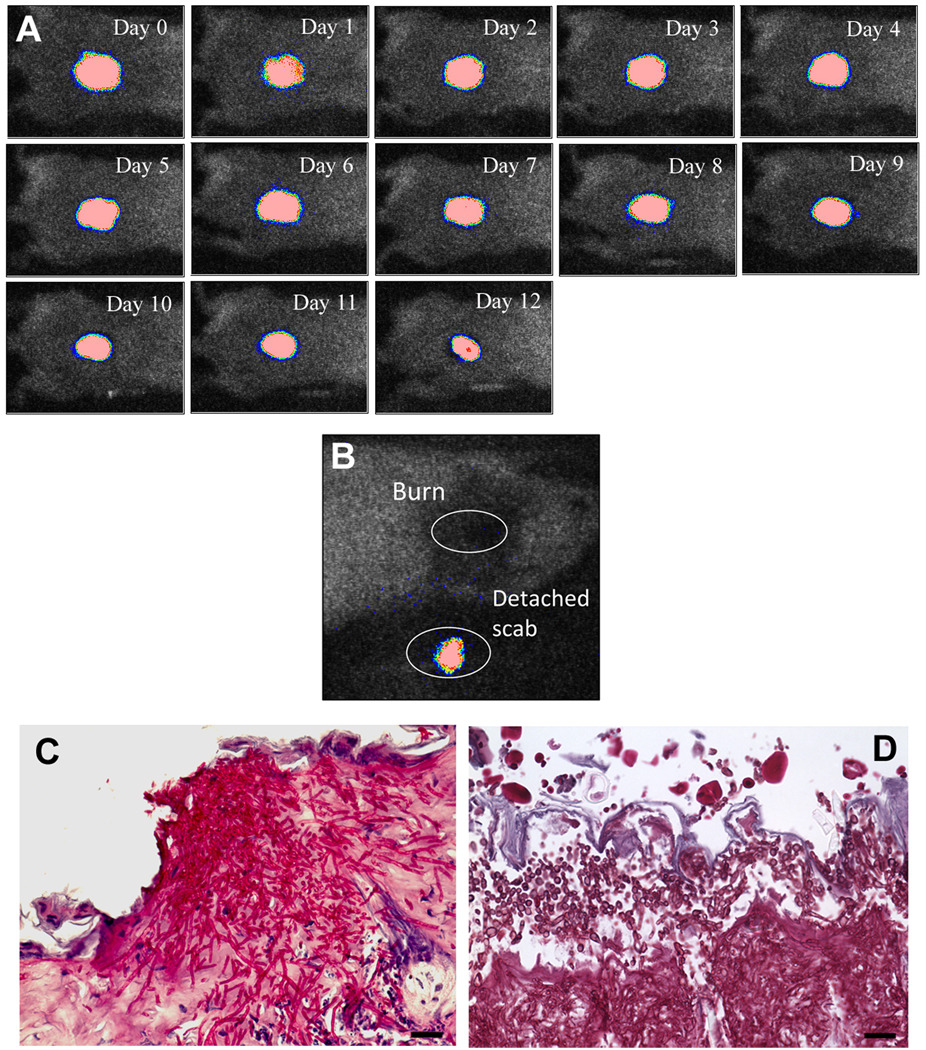
By applying 106 CFU of C. albicans in PBS topically onto the 7-second burns made on the shaved backs of adult female BALB/c mice, a stable and chronic infection was developed as characterized by bioluminescence imaging. Figure 3A shows the successive bioluminescence images of a representative mouse burn infected with C. albicans. After infection, the bioluminescence remained strong and stable until day 12 before the scab peeled off from the mouse back. In all the mice examined in the study, almost no remaining fungal bioluminescence was detected from the burns after the scabs peeled-off, but the detached scabs were all detected as bioluminescent immediately after they came off (Figure 3B). Figures 3C and 3D show representative periodic acid-Schiff (PAS)-stained histological sections of C. albicans infected mouse burns biopsied on day 1 and day 4 post-infection, respectively. Massive C. albicans invasion was demonstrated on both days by numerous conidia and hyphae branching out in every direction in the burned tissue.
Figure 3.
A) Successive bioluminescence images captured daily for 12 days of a representative mouse burn infected with bioluminescent C. albicans. B) A representative bioluminescence image of a mouse burn immediately after the peel-off of its scab as well as the detached scab from the same mouse. C) –D) Representative periodic acid–Schiff (PAS)-stained histological sections of C. albicans infected mouse burns biopsied on day 1 and day 4 post-infection, respectively. Scale bars: 20 µm.
UVC irradiation significantly reduced the fungal bioburden in mouse burns with either early-stage or established C. albicans infections
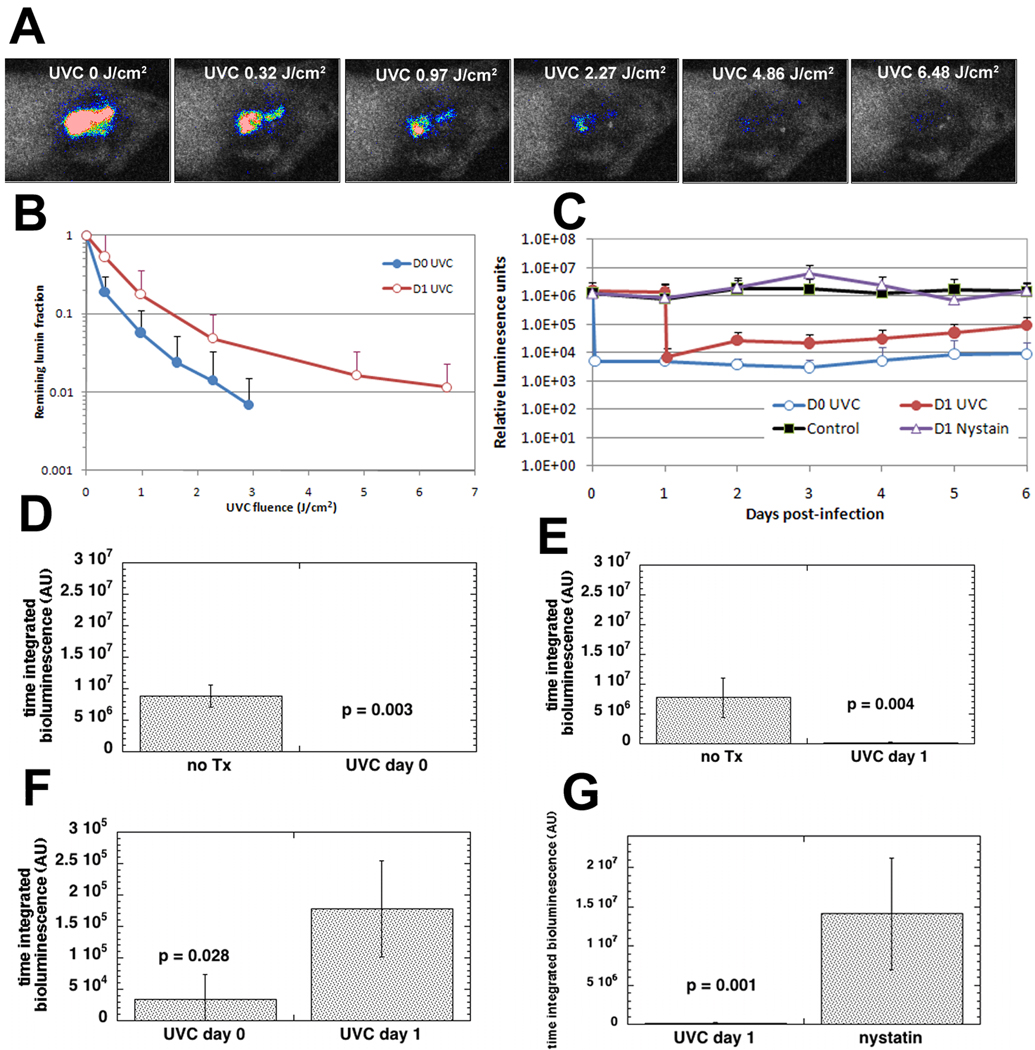
Figure 4A shows a set of bioluminescence images after increasing doses of UVC delivered to a representative mouse burn infected with C. albicans and treated with UVC on day 1 (24 hours) after infection. As quantified by fungal bioluminescence intensity, approximately 2.03-log10-unit (99.1%, data not shown) reduction in fungal bioluminescence from this particular mouse burn was achieved in a light exposure dependent manner when 6.48 J/cm2 UVC had been delivered.
Figure 4.
A) Dose-response of fungal luminescence from a representative mouse burn infected with C. albicans and treated with UVC exposure on day 1 (24 hour) post-infection. B) Dose-responses of mean fungal luminescence of the mouse burns infected with C. albicans and treated by use of a single UVC exposure on day 0 (30 min, n=11) and day 1 (24 hour, n=12) post-infection, respectively. C) Time courses of mean fungal luminescence from infected mouse burns treated with a single UVC exposure on day 0 (n=11), day 1 (n=12), daily application of nystatin cream from day 1 to day 6 (n=8), and no treatment (n=12), respectively. D) –G) Mean areas under the bioluminescence-time plots (in the two-dimensional coordinate system in panel C) representing the overall fungal bioburden of the mouse burns in different groups (day 0 UVC treatment vs. no treatment, p=0.003; day 1 UVC treatment vs. no treatment, p=0.004; day 0 treatment vs. day 1 treatment, p=0.006; day 1 UVC treatment vs. daily treatment with nystatin cream, p=0.028).
When UVC was delivered on day 0 (30 minutes after infection) the Candida was even more susceptible to inactivation by UVC. Figure 4B shows the average values of the fungal bioluminescence from the infected mouse burns treated with an exposure to UVC on day 0 (30 minutes post-infection; n =11) or receiving UVC on day 1 (24 hours post-infection; n=12). UVC treatment carried out on day 0 gave an average 2.16-log10-unit (99.2%) loss of fungal luminescence when 2.92 J/cm2 UVC had been delivered, while UVC treatment carried out on day 1 resulted in an approximately 1.94-log10-unit (95.8%) reduction when more than twice as much UVC (6.48 J/cm2) had been delivered.
Figure 4C shows the time courses of the mean fungal luminescence values from day 0 to day 6 of the infected burns in mice treated with a single exposure of UVC on day 0 (2.92 J/cm2), a single exposure of UVC on day 1 (6.48 J/cm2), daily application of nystatin cream from day 1 to day 6, and no treatment (the scabs of the burns peeled off as early as on day 8 post-infection, and almost no remaining fungal luminescence was detected from the wounds after the peel-off of scabs). Although some fungal re-growth in the UVC treated burns was observed, especially in the burns treated on day 1, the averaged fungal luminescence intensities of the burns treated on day 0 and day 1 were at least 2-log10 and 1-log10-unit respectively lower than that of the untreated burns at all time points sampled. Statistical comparison of the areas under the plots of bioluminescence time course in the two-dimensional coordinate system in Figure 4C demonstrated that UVC treatment significantly decreased fungal bio-burden of the infected burns (Figures 4D and 4E). In addition, the fungal bioburden of the mouse burns treated with UVC on day 0 was significant lower than that of the mouse burns treated with UVC on day 1 (Figure 4F).
UVC was superior to nystatin cream in reducing the fungal burden of mouse burns with established infections
Daily application of nystatin cream (25 mg per cm2 burned skin per day) from day 1 to day 6 hardly had any effect on controlling the infection. The formation of the scab prevented effective application of Nystatin cream for longer than day 6. The fungal bioburden in the mouse burns treated with a single UVC exposure on day 1 was significantly lower than that in mouse burns treated with daily application of nystatin cream (Figure 4G).
Mouse skin could tolerate UVC irradiation at the effective antifungal dose
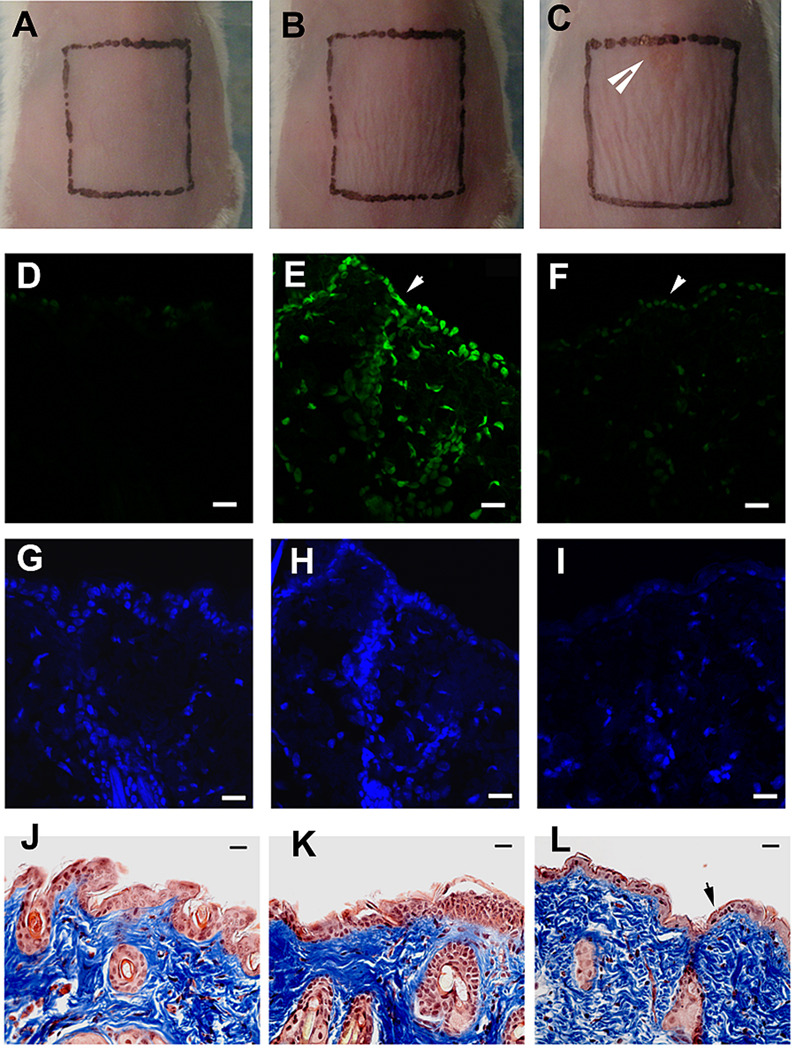
Figures 5A–5C show representative morphologies of mouse skin before, immediately after, and 24 hours after being exposed to UVC at a radiant exposure of 6.48 J/cm2, which is the effective antifungal dose for treating infections on day 1 post-infection. It can be observed that there is mild wrinkling of the skin evident immediately after UVC and this is slightly more pronounced at 24 hours after UVC exposure, but the wrinkling disappeared in succeeding days. A small lesion (top of square in Fig 5C) was also observed occurred at 24 hours after UVC exposure, but the lesion eventually healed without problem. Cyclobutane pyrimidine dimer (CPD) positive nuclei were observed in the immunofluorescence micrograph of the biopsy taken immediately after UVC exposure (Figure 5E). However, the damage was extensively repaired at 24 hours after UVC exposure where only traces of fluorescence remained (Figure 5F). Mild epidermal shrinkage was seen at 24 hours after UVC exposure (Figure 5L).
Figure 5.
A)–C) Morphologies of a representative mouse skin before, immediately after, and 24 hour after being exposed to UVC at a dose of 6.48 J/cm2, respectively. D) –F) Representative immuno-fluorescence micrographs of cyclobutane pyrimidine dimers (CPDs) in skin cell nuclei. G) –I) Micrographs of DAPI counterstaining of cell nuclei. J)–L) Micrographs of Masson’s trichrome stained sections. Biopsies were taken before (D, G, J), immediately after (E, H, K), and 24 hours after (F, I, L) being exposed to UVC at a dose of 6.48 J/cm2, respectively, from the same mouse. Arrow in panel C: UVC-induced lesion on mouse skin. Arrows in panels D-F: mouse skin surface. Arrow in panel 5L shows epidermal shrinkage. Scale bars: 20 µm.
Discussion
In this study, we developed a mouse model of non-lethal 3rd degree burn infected with a bioluminescent strain of C. albicans, and investigated the use of UVC light for treatment of C. albicans infection in mouse burns. Extraction of C. albicans from the infected mouse burns confirmed the fungal luminescence to be accurate at predicting the viability of C. albicans in the infected burns. It was found in this study that UVC significantly reduced the fungal bioburden in mouse burns with both early-stage and established infections. To the best of our knowledge, this study is the first report on both the development of the animal model and the use of UVC to treat C. albicans burn infection.
Fungal cells prefer relatively low temperatures for growth compared to bacteria. While most pathogenic bacteria grow well at body temperature (37°C), this property is rare amongst fungi and yeasts 27. The requirement for lower temperatures means that fungal infections on skin, mucosa, toenails and burns tend to be superficial in nature and therefore more susceptible to UVC treatment. UVC does not penetrate to any significant extent in living tissue (less than 1 mm) so its use as an antimicrobial therapy is limited to superficial infections. We previously reported 19 that UVC could be used for treatment of onychomycosis, a fungal infection of nails caused by dermatophytes such as Trichophyton rubrum. A clinical trial was conducted of UVC for toenail onychomycosis with careful masking of the periungual skin 28.
It is well known that UV radiation is damaging to human tissue and particularly damaging to skin. UVB irradiation of skin has been particularly well studied, and is accepted as the main cause of skin cancer and photoaging. Using appropriate risk-benefit analysis, UVB treatments are still delivered to various areas of the bodies of millions of patients in the United States per year for the treatment of psoriasis, dermatitis, vitiligo, etc 29–31. Although the dangers of UVC exposure to host cells and tissues are well-known, we would argue that the selectivity for UVC inactivation of C. albicans over keratinocytes, combined with the ability of host cells to repair DNA lesions, the likelihood that antimicrobial UVC will not be repeated in a chronic manner, and the ability to mask healthy skin around the burns with UVC-opaque material will combine together to mitigate these dangers. For burn infections, the use of UVC light is especially compelling in that third degree burns are usually composed of dead tissue. As a result, there is minimal concern about the side effects of UVC on host tissues when the healthy skin surrounding the burns is carefully protected. We believe there may be situations where the risk benefit ratio is favorable for the use of UVC for treating wound infections particularly when the microbes responsible are antibiotic resistant. While permissible UVC dose exposure limits for human tissue do not exist, it is expected that there would be an acceptable maximum number of repetition times of UVC irradiation and possibly a total lifetime cumulative exposure in J/cm2.
The infections on day 1 were shown to be more resistant to UVC treatment than they were on day 0. According to the results from the present study, to achieve an approximately 2-log10-unit inactivation of C. albicans in vivo, over a double dose of UVC light was required for day 1 treatment in comparison to day 0 treatment. On day 1 post-infection, the infections were fully established as demonstrated by the histological sections (see Figure 3C). In fully established infections, C. albicans exists predominantly as biofilms 32, which are structured microbial communities attached to the wound surface. It was found in previous mechanistic studies that the biofilm matrix can block UV light 33 and make fungal cells less susceptible to UV light 32. In addition, when the infections were fully established, the fungal cells may have proliferated into the deeper layer of the tissue, where the UVC light was attenuated.
Regrowth of C. albicans was sometimes observed especially in the mouse burns treated with a single UVC exposure on day 1 post-infection, although the overall fungal bioburden of the treated mouse burns was still significantly lower than the untreated mouse burns. This problem, if proved to occur predictably, might be overcome by multiple UVC exposures during the following days after the initial treatment. Future studies in this regard are warranted.
The scabs of the mouse burns in this study formed as early as on day 5 post-infection and peeled off from the mouse backs as early as on day 8. When the scabs peeled off, almost no remaining fungal luminescence could be detected from the wounds but the peeled-off scabs were bioluminescent, indicating that C. albicans cells were mostly located within the scabs and isolated from the host when approaching the peel-off of the scabs.
Conclusions
UVC offers a selective inactivation of C. albicans over keratinocytes in a light dose dependent manner. UVC appears to be an effective treatment strategy for mouse burns with both early-stage and established C. albicans infections and is worth further investigation for potential clinical utility. UVC is superior to nystatin cream in reducing the fungal bioburden of mouse burns with established infections. Normal mouse skin could tolerate UVC irradiation at the effective antifungal doses.
Acknowledgements
This work was supported by the NIH (grant RO1 AI050875 to MRH) and US Air Force MFEL Program (FA9550-04-1-0079). TD was partially supported by a Bullock-Wellman Fellowship Award and an Airlift Research Foundation grant (grant #109421).
References
- 1.Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, Wibbenmeyer L, Voight D, Palmieri T, Greenhalgh D, Kemalyan N, Caruso D. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 2.Horvath EE, Murray CK, Vaughan GM, Chung KK, Hospenthal DR, Wade CE, Holcomb JB, Wolf SE, Mason AD, Jr., Cancio LC. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg. 2007;245:978–985. doi: 10.1097/01.sla.0000256914.16754.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker WK, Cioffi WG, Jr., McManus AT, Kim SH, McManus WF, Mason AD, Pruitt BA., Jr Fungal burn wound infection. A 10-year experience. Arch Surg. 1991;126:44–48. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 4.Struck MF. Infection control in burn patients: are fungal infections underestimated? Scand J Trauma Resusc Emerg Med. 2009;17:51. doi: 10.1186/1757-7241-17-51. author reply 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker KS, Rogers PD. Recent insights into the mechanisms of antifungal resistance. Curr Infect Dis Rep. 2006;8:449–456. doi: 10.1007/s11908-006-0019-3. [DOI] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 7.Hockberger PE. A history of ultraviolet photobiology for humans, animals and microorganisms. Photochem Photobiol. 2002;76:561–579. doi: 10.1562/0031-8655(2002)0760561AHOUPF2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 8.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 9.Radonovich LJ, Martinello RA, Hodgson M, Milton DK, Nardell EA. Influenza and ultraviolet germicidal irradiation. Virol J. 2008;5:149. doi: 10.1186/1743-422X-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eker AP, Quayle C, Chaves I, van der Horst GT. DNA repair in mammalian cells: Direct DNA damage reversal: elegant solutions for nasty problems. Cell Mol Life Sci. 2009;66:968–980. doi: 10.1007/s00018-009-8735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouspikel T. DNA repair in mammalian cells : Nucleotide excision repair: variations on versatility. Cell Mol Life Sci. 2009;66:994–1009. doi: 10.1007/s00018-009-8737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruls WA, Slaper H, van der Leun JC, Berrens L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984;40:485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49:1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thai TP, Houghton PE, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48:52–60. [PubMed] [Google Scholar]
- 15.Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manage. 2005;51:32–45. [PubMed] [Google Scholar]
- 16.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25:289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- 17.Bak J, Ladefoged SD, Begovic T, Winding A. UVC fluencies for preventative treatment of Pseudomonas aeruginosa contaminated polymer tubes. Biofouling. 2010;26:821–828. doi: 10.1080/08927014.2010.520314. [DOI] [PubMed] [Google Scholar]
- 18.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Disinfection of Pseudomonas aeruginosa biofilm contaminated tube lumens with ultraviolet C light emitting diodes. Biofouling. 2010;26:31–38. doi: 10.1080/08927010903191353. [DOI] [PubMed] [Google Scholar]
- 19.Dai T, Tegos GP, Rolz-Cruz G, Cumbie WE, Hamblin MR. Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis. Br J Dermatol. 158:1239–1246. doi: 10.1111/j.1365-2133.2008.08549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai T, Tegos GP, St. Denis TG, Anderson D, Sinofsky E, Hamblin MR. Ultraviolet-C irradiation for prevention of central venous catheter related infections: an in-vitro study. Photochem Photobiol. 2010 doi: 10.1111/j.1751-1097.2010.00819.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, Brown AJ, d'Enfert C. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun. 2009;77:4847–4858. doi: 10.1128/IAI.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marthinuss J, Lawrence L, Seiberg M. Apoptosis in Pam212, an epidermal keratinocyte cell line: a possible role for bcl-2 in epidermal differentiation. Cell Growth Differ. 1995;6:239–250. [PubMed] [Google Scholar]
- 23.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 24.Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 1996;22:607–611. doi: 10.1016/s0305-4179(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 25.Ha U, Jin S. Expression of the soxR gene of Pseudomonas aeruginosa is inducible during infection of burn wounds in mice and is required to cause efficient bacteremia. Infect Immun. 1999;67:5324–5331. doi: 10.1128/iai.67.10.5324-5331.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis PJ, Rabinowitz P. Methods of numerical integration. Dover Publications; 2007. [Google Scholar]
- 27.Robert VA, Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis. 2009;200:1623–1626. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]
- 28.Boker A, Cumbie B, A. Kimball B. A single-center, prospective, open-label, pilot study of the safety, local tolerability, and efficacy of ultraviolet-C (UVC) phototherapy for the treatment of great toenail onychomycosis. 2008. [Google Scholar]
- 29.Nolan BV, Yentzer BA, Feldman SR. A review of home phototherapy for psoriasis. Dermatol Online J. 2010;16:1. [PubMed] [Google Scholar]
- 30.Gambichler T. Management of atopic dermatitis using photo(chemo)therapy. Arch Dermatol Res. 2009;301:197–203. doi: 10.1007/s00403-008-0923-5. [DOI] [PubMed] [Google Scholar]
- 31.Abdulla SJ, Desgroseilliers JP. Treatment of vitiligo with narrow-band ultraviolet B: advantages and disadvantages. J Cutan Med Surg. 2008;12:174–179. doi: 10.2310/7750.2008.07054. [DOI] [PubMed] [Google Scholar]
- 32.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 33.Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73:4592–4601. doi: 10.1128/AEM.02506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]