Abstract
Background and Objectives
Bacterial pneumonia still contributes to morbidity/mortality in HIV-infection despite effective combination antiretroviral therapy (cART). ESPRIT, a trial of intermittent recombinant interleukin-2 (rIL-2) with cART vs.cART alone (control arm) in HIV-infected adults with CD4+≥300 offered the opportunity to explore associations between bacterial pneumonia and rIL-2, a cytokine which increases some bacterial infections.
Methods
Baseline and time-updated factors associated with first-episode pneumonia on study were analysed using multivariate proportional hazards regression models. Smoking/pneumococcal vaccination history was not collected.
Results
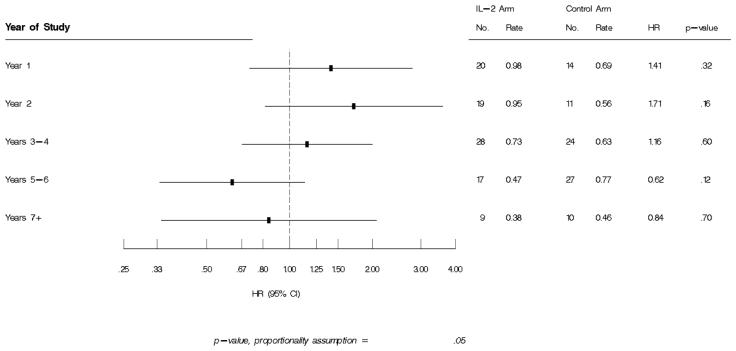
IL-2 cycling was most intense in years 1-2. Over ≈7 years, 93 IL-2 (rate 0.67/100PY) and 86 control (rate 0.63/100PY) patients experienced a pneumonia-event, (HR=1.06,95%CI=0.79,1.42,p=0.68). Median CD4+ prior to pneumonia was 570 (IL-2 arm) and 463cells/uL (control arm). Baseline risks for bacterial pneumonia included older age, IVDU, detectable HIV viral load (VL), previous recurrent pneumonia; Asian ethnicity was associated with decreased risk. Higher proximal VL (HR for 1 log10 higher VL=1.28,95%CI=1.11,1.47,p=<.001) was associated with increased risk; higher CD4+ prior to the event (HR per 100 cells higher=0.94,95%CI0.89,1.0,p=0.04) decreased risk. Compared to controls, the hazard for a pneumonia-event was higher if rIL-2 was received <180 days prior (HR=1.66,95%CI=1.07,2.60,p=0.02) vs.≥180 days (HR=0.98,95%CI=0.70,1.37,p=0.9). Compared to the control group, pneumonia-risk in the IL-2 arm decreased over time with HRs of 1.41, 1.71, 1.16, 0.62 and 0.84 in years 1, 2, 3-4,5-6 and 7, respectively.
Conclusions
Bacterial pneumonia rates in cART-treated adults with moderate immunodeficiency are high. The mechanism of the association between bacterial pneumonia and recent IL-2 receipt and/or detectable HIV-viraemia deserves further exploration.
Keywords: bacterial pneumonia, rIL-2, ESPRIT, combination antiretroviral therapy
Background
Overall, the rates of bacterial pneumonia in HIV-1-infected individuals are 25 fold higher than in their HIV-negative counterparts (1). The risk increases as CD4+ T-cell count declines. Pre-combination antiretroviral therapy (cART) incidence rates of 22.7 episodes per 100 person years (PY) were seen in one large US-based cohort of HIV-infected adults with CD4+ T-cell count <200 cells/uL (2). Rates of pneumonia fell to 9.1 episodes/100 PY in the early cART era (1997) (3,4) and further still in the late cART era (2005-7) to 1.97 episodes/100 PY (5). Other risks identified included, intravenous drug use (IVDU) as the mode of HIV-1 acquistion, low CD4+ T-cell count, lack of protease-inhibitor containing cART, prior Pneumocystis jiroveci pneumonia (PcP), cigarette smoking (3-6) and in one small series smoking illicit substances (7). Other groups have shown that in the absence of cART, cotrimoxazole prophylaxis offers some protection (1). As a consequence of the importance of pneumonia in persons with HIV, ≥two episodes of bacterial pneumonia in a twelve month period was categorised as an AIDS-defining illness (ADI) in 1993 (8).
There is a relative paucity of data on the morbidity/mortality associated with bacterial pneumonia in the era of potent cART in those with higher CD4+ T-cells counts, although several cohorts have indicated declining rates associated cART-use and in some studies, pneumococcal polysaccharide vaccine (PPV-23) (9-11). In a Danish study exploring the risks for hospitalisation with pneumonia (including viral pneumonia but excluding PcP), HIV-1-infected patients with nadir CD4+ T cell count >300 cells/uL had a rate of bacterial pneumonia of 1.25 per 100 PY (5). In the SMART study, which enrolled participants with baseline CD4+ T-cells ≥350cells/uL (12), patients on continuous cART, had a rate of bacterial pneumonia of 1.3 per 100 PY.
The clinical benefits of intermittent recombinant interleukin-2 (rIL-2) in HIV-1-infected adults on cART have been explored in two major phase III international studies. In Evaluation of Subcutaneous Interleukin-2 in a Randomized International Trial (ESPRIT), HIV-1-infected adults, on or starting cART, with CD4+ T-cells ≥300 cells/uL, were randomised to intermittent rIL-2 with cART (IL-2 arm) or cART alone (control arm or non-IL-2 arm) (13). The primary endpoints, ADI and death, were reported in both study arms for the duration of follow-up. The main results of ESPRIT have been reported (14). In summary, the receipt of rIL-2 conferred no clinical benefit with respect to ADI and all cause mortality despite a significant CD4+ count difference averaged over follow-up of 159 cells/uL (95%CI145-174, p<0.001) higher than the control arm.
Recombinant IL-2 used in the oncology and/or HIV setting (15) has been associated with an increased risk of some bacterial infections including cellulitis, osteomyelitis, Clostridium difficile (16), bacteraemia and bacterial pneumonia; the mechanism of the association is unclear.
The ESPRIT cohort offered an opportunity to explore both the rate of bacterial pneumonia over several years (≈7 years) in a large cohort of cART treated HIV-1-infected adults with moderate levels of immunodeficiency and the relationship between rIL-2 exposure and bacterial pneumonia.
Methods
The methods (13) and main results of ESPRIT (14) have been published. Key inclusion criteria included CD4+ T-cell count ≥300 cells/uL and on/commencing cART. Category C patients could be enrolled provided there was no active ADI for ≥12 months. Patients randomised to the IL-2 arm, received 3 dosing cycles of rIL-2 (7.5MIU bid SC for five consecutive days every 8 weeks) as induction in year 1. Thereafter dosing cycles were given to achieve/sustain the CD4+ T-cell goal i.e. doubling baseline CD4+ T-cell count in those with baseline counts of 300-499 cells/uL and >1000 cells/uL if baseline was ≥500 cells/uL. Demographics, HIV clinical and treatment history were documented at baseline. Thereafter, patients were seen every 4 months for the study duration, information was captured on standardised case report forms (CRF). Events were reported using specific CRFs with supporting source documentation as soon as sites became aware of them..
Criteria for a confirmed bacterial pneumonia event during follow-up included clinical, radiographic and microbiological evidence; a probable bacterial pneumonia required clinical and radiographic evidence, diagnosis by doctor, physicians' assistant or nurse practitioner without microbiological evidence. For a diagnosis of recurrent bacterial pneumonia, both pneumonia episodes had to occur after enrollment and satisfy the criteria above with the additional requirements i.e. the second pneumonia had onset of symptoms <365 days after the first episode and there was strong evidence that the first episode was cured such as an intervening clear CXR or absence of symptoms after >1 month off antibacterials effective against pathogens commonly producing pneumonia. All endpoints, including the initial episode of bacterial pneumonia, were reviewed by the Endpoint Review Committee (ERC) blinded to treatment group against predetermined criteria as described above and designated as confirmed/probable or did not meet the criteria for an endpoint. CD4+ T-cell count closest to the event and randomisation arm were redacted prior to ERC review. Only bacterial pneumonia events designated by the ERC as confirmed or probable were included in this analysis.
Statistical Analysis
Multivariate proportional hazards regression models were used to compare the treatment groups, IL-2 and control, and to summarize associations between baseline and time-updated factors with bacterial pneumonia – defined as the first episode of confirmed or probable bacterial pneumonia following randomization. The comparison of treatment groups was intention to treat. The proportional hazards assumption was examined by including an interaction term between the treatment indicator and log-transformed failure time. Baseline predictors included age, gender, ethnicity, IVDU, hepatitis B and/or C co-infection, nadir and baseline CD4+, viral load (VL), prior AIDS-defining illness (ADI), prior recurrent bacterial pneumonia as an ADI, and PcP prophylaxis; time-dependent covariates updated during follow-up included, proximal CD4+ T-cell count i.e. the CD4+ closest to the event and VL, incident ADI, and time since rIL2 receipt. Smoking and pneumococcal vaccination histories were not considered in the model as these data were not collected in ESPRIT. Statistical analyses were performed using SAS software, version 9.1. P-values are two-sided.
Results
ESPRIT enrolled 4111 patients from 257 sites in 25 countries; 2071 and 2040 were randomised to the IL-2 and control arms respectively and followed for a median of 7 years. Overall, there were 179 patients who experienced a bacterial pneumonia event following randomization, of these, 93 were rIL-2 patients (rate 0.67/100 patient years) and 86 control patients (rate 0.63/100 patient years). Of these pneumonia events, 9% met the ERC criteria for a confirmed bacterial pneumonia, 81% were classified as probable. A total of 8 patients experienced recurrent bacterial pneumonia on study (4 in each arm).
CD4+ T-cell count and bacterial pneumonia
The median CD4+ T-cell count prior to pneumonia diagnosis was 570 cells/uL and 463 cells/uL in the IL-2 and control arms respectively. The baseline characteristics of the participants in the IL-2 and Control arms experiencing a pneumonia event compared to those who did not experience a pneumonia event are shown in Table 1. There was an interaction of borderline significance (p=.052 for trend) between treatment group and baseline CD4+ cell count. For the 300-499 cell stratum, the hazard ratio was 1.16 (95% CI = 0.81 - 1.68) while for the stratum with baseline CD4+ ≥500, the hazard ratio was 0.94 (95% CI = 0.57 – 1.54). For the 3269 patients who were virologically suppressed at baseline differences between treatment group effects for the two CD4+ strata are more pronounced. Hazard ratios were 1.11 (95% CI=0.72-1.72) and 0.76 (94% CI+0.42-1.36) for the lower (300-499) and high CD4+ (≥500) strata respectively, leading to a CD4+ by treatment group interaction of .025.
Table 1.
Baseline Characteristics of Study Participants with and without Bacterial Pneumonia Events
| IL-2 with event |
Control with event |
All patients without event |
|
|---|---|---|---|
| Demographics | |||
| Age in Years (Median,IQR) | 43 (37, 50) | 41 (37, 49) | 40 (34, 46) |
| Female Gender (%) | 16.1 | 12.8 | 18.8 |
| Race (%) | |||
| Asian | 1.1 | 2.3 | 11.2 |
| Black | 10.8 | 5.8 | 9.2 |
| White | 83.9 | 86.0 | 74.9 |
| Other/unknown | 4.3 | 5.8 | 4.8 |
| Injection drug use (%) | 16.1 | 19.8 | 10.2 |
| CD4+ count (cells/μL)(median,IQR) | 420 (356, 556) | 425 (364, 634) | 458 (373, 584) |
| CD4+ Nadir (cells/ μL)(median,IQR) | 175 (55, 270) | 169 (93, 273) | 198 (91, 310) |
| HIV RNA ≤ 500 copies (%) | 66.7 | 74.4 | 80.1 |
| Medical History | |||
| AIDS diagnosis (%) | 34.4 | 33.7 | 25.5 |
| History of recurrent BP (%) | 15.1 | 3.5 | 5.9 |
| Hepatitis B (% HBsAg pos.) | 2.4 | 6.3 | 6.5 |
| Hepatitis C (% antibody pos.) | 22.9 | 23.1 | 14.9 |
| ART History | |||
| Years since 1st ART (median, IQR) | 5.1 (2.1, 7.9) | 4.9 (2.9, 7.8) | 4.2 (2.1, 6.4) |
| PcP prophylaxis (%) | 7.5 | 4.7 | 6.2 |
More than one can be checked.
Table 2 summarises the rate of bacterial pneumonia event by closest CD4+ to the event and by randomisation arm; the hazards for bacterial pneumonia are higher at the lowest CD4+ T-cell count in particular those with an absolute count <100 cells/μL in both arms. In the multivariate analysis (Table 3b) lower CD4+ T-cell closest to the event is associated with increased risk of bacterial pneumonia event.
Table 2.
ESPRIT - First Bacterial Pneumonia Event by Proximal CD4
| IL-2 |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4 | Pt yrs | Events | Ratea) | Pt yrs | Events | Ratea) | HRb) | p-val |
| < 100 | 64 | 2 | 3.13 | 60 | 1 | 1.66 | 1.93 | .59 |
| 100 - 199 | 194 | 2 | 1.03 | 212 | 3 | 1.41 | 0.77 | .77 |
| 200 - 349 | 1117 | 12 | 1.07 | 1879 | 21 | 1.12 | 1.01 | .97 |
| 350 - 499 | 2540 | 23 | 0.91 | 4011 | 24 | 0.60 | 1.55 | .13 |
| 500 - 699 | 3639 | 22 | 0.60 | 4253 | 23 | 0.54 | 1.11 | .73 |
| ≥ 700 | 6281 | 32 | 0.51 | 3131 | 14 | 0.45 | 1.10 | .77 |
| Total | 13836 | 93 | 0.67 | 13547 | 86 | 0.63 | ||
|
| ||||||||
| HR (p) for 100 cells c) | 0.89 (.001) | 0.90 (.05) | Int.d) | |||||
| CD4 Coeff (SE) | −0.0012 (0.0004) | −0.0010 (0.0005) | .71 | |||||
| Log10 CD4 Coeff (SE) | −1.537 (0.375) | −1.194 (0.461) | .62 | |||||
Rate per 100 person yrs of follow-up in that category. Events are attributed to last CD4 recorded prior to the event. Assumes patient stays in that category until a CD4 is measured in an alternate category, or until the censoring date. Uses interim and visit-associated lab values.
Hazard ratio (IL-2 vs. control) and p-value from Cox model with treatment group and time-updated CD4 strata.
HR and p-value for time-updated CD4 (100 cells) from Cox model, run separately by treatment group.
Interaction p-value is for treatment group by time-updated CD4 in a model containing time-updated CD4, treatment group, and an interaction term.
Table 3b.
Time-Varying Predictors of 1st Bacterial Pneumonia Event During ESPRIT
| Covariate | Coeff.* | HR | 95% CI | p-value |
|---|---|---|---|---|
| CD4 (per 100 cells) | −0.0611 | 0.94 | 0.89, 1.00 | .04 |
| HIV-RNA (per one log10) | 0.2439 | 1.28 | 1.11, 1.47 | < .001 |
| Cycled within previous 180 days | 0.5451 | 1.72 | 1.12, 2.65 | .01 |
| Incident POD Progression of Disease (POD)** event |
0.3108 | 1.36 | 0.50, 3.73 | .54 |
| Patients included in analysis | 4097 | |||
| Number of events | 179 |
Results are from a proportional hazards regression model with all covariates listed, plus indicators for race, age, IV drug use, and history of bacterial pneumonia.
the definitions of POD are described in the methods manuscript (13).
IL-2 dosing and bacterial pneumonia
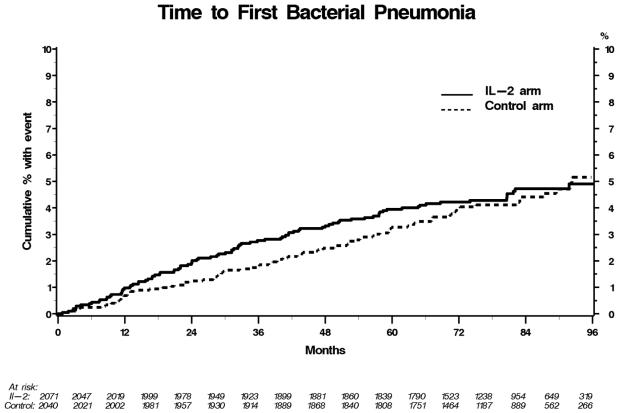
The IL-2 arm received a median of 4 dosing cycles during follow-up (IQR3,6). In years 1, 2, 3-4, 5-6, 7-8 and 9-10, the percentage of IL-2 patients cycling with rIL-2 was 96, 38, 39, 25, 16 and 19 respectively. Patients in the IL-2 arm with CD4+ cells between 300-499 at study entry compared to those with CD4+ ≥500 cells/uL received a median of 5 vs. 4 dosing cycles of IL-2. The overall hazard ratio (HR) for bacterial pneumonia in the IL-2 compared to the control arm was 1.06 (95% CI=0.79,1.42, p=0.68), however, the HR for pneumonia in the IL-2 groups compared to controls varied by year of follow-up as shown in Figure 1 with the risk highest in years 1 and 2 i.e. HR for a bacterial pneumonia event was 1.41 (p=.32) and 1.71 (trend towards significance, p=0.16) in year 1 and 2 respectively. In contrast, in years 5-6, when only 25% of IL-2 patients cycled with rIL-2, the HR for bacterial pneumonia in the IL-2 arm compared to the control group was 0.62 (p=0.12). Only forty-five percent of the IL-2 patients who experienced bacterial pneumonia received further dosing cycles of rIL-2 subsequently. The Kaplan-Meier table showing the time to bacterial pneumonia in the IL-2 and control arms is shown in Figure 2.
Figure 1.
Hazard ratio for first bacterial pneumonia event by year of follow-up in ESPRIT
Figure 2.
Kaplan-Meier curves showing the time to first bacterial pneumonia event, the IL-2 arm is indicated by the solid line, and control arm by the dotted line
Risk Factors for bacterial pneumonia (both treatment groups combined)
Overall, as shown in Table 3a in the multivariate model, baseline risk factors for bacterial pneumonia were older age (HR per 10 years increase in age of 1.34, 95% CI: 1.14-1.59,p=<.001), IVDU (HR 1.78 (95% CI: 1.09,2.90, p=0.02), HIV RNA ≥500 copies/mL (HR 2.02, 95% CI: 1.46,2.81, p=<.001), and history of recurrent bacterial pneumonia as an ADI (HR 5.38, 95% CI: 2.86,10.11, p=<.001). Asian ethnicity was associated with decreased risk of bacterial pneumonia, HR 0.17 (95%CI0.05,0.56, p=0.003).
Table 3a.
Baseline Predictors of First Bacterial Pneumonia Event
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Pts | Events* | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Treatment group | ||||||
| IL-2 | 2071 | 93 | 1.06 (0.79, 1.42) | .68 | 1.07 (0.80, 1.44) | .65 |
| Control | 2040 | 86 | ref | ref | ||
| Age (10 yrs) | 4111 | 179 | 1.36 (1.17, 1.58) | < .001 | 1.34 (1.14, 1.59) | < .001 |
| Gender | ||||||
| Male | 3345 | 153 | ref | ref | ||
| Female | 766 | 26 | 0.72 (0.48, 1.10) | .13 | 0.97 (0.63, 1.49) | .89 |
| Race | ||||||
| Asian | 442 | 3 | 0.13 (0.04, 0.40) | < .001 | 0.17 (0.05, 0.56) | .003 |
| Black | 376 | 15 | 0.79 (0.47, 1.35) | .39 | 0.73 (0.42, 1.25) | .25 |
| White/other | 3279 | 161 | ref | ref | ||
| IV drug use | ||||||
| Yes | 433 | 32 | 1.94 (1.32, 2.84) | < .001 | 1.78 (1.09, 2.90) | .02 |
| No/unknown | 3678 | 147 | ref | ref | ||
| Hep B/C status | ||||||
| Positive to either | 730 | 44 | 1.44 (1.01, 2.04) | .04 | 1.09 (0.70, 1.69) | .71 |
| Unknown status | 776 | 23 | 0.67 (0.43, 1.05) | .08 | 0.66 (0.42, 1.04) | .08 |
| Negative to both | 2605 | 112 | ref | ref | ||
| Nadir CD4 (100 cells) | 4110 | 179 | 0.88 (0.79, 0.97) | .01 | 0.93 (0.82, 1.05) | .24 |
| BL CD4 (100 cells) | 4111 | 179 | 0.94 (0.86, 1.03) | .17 | 0.98 (0.88, 1.09) | .69 |
| HIV-RNA | ||||||
| ≥ 500 copies | 832 | 53 | 1.66 (1.21, 2.29) | .002 | 2.02 (1.46, 2.81) | < .001 |
| < 500 copies | 3269 | 126 | ref | ref | ||
| Prior disease | ||||||
| Bact pneumonia as ADI | 51 | 11 | 6.43 (3.46, 11.93) | < .001 | 5.38 (2.86, 10.11) | < .001 |
| Other ADI | 1012 | 50 | 1.38 (0.99, 1.93) | .06 | 1.16 (0.80, 1.67) | .43 |
| No ADI | 3048 | 118 | ref | ref | ||
| PJP prophylaxis | ||||||
| yes | 253 | 11 | 0.98 (0.53, 1.80) | .95 | 0.68 (0.36, 1.26) | .22 |
| no | 3858 | 168 | ref | ref | ||
Patients with a first (after randomization) bacterial pneumonia event.
IL-2 arm
In the multivariate analysis of bacterial pneumonia events in the IL-2 arm the baseline associations were similar to the overall findings, Asian ethnicity was protective (HR 0.10,95%CI0.01.0.74, p=0.02); being older (HR1.46, 95%CI1.15, 1.85, p=0.002), having detectable plasma VL (HR2.27, 95%CI1.45,3.55, p=<.001) and prior history of recurrent bacterial pneumonia (HR4.46, 95%CI1.72,11.54, p=0.002) were associated with increased pneumonia risk. However, IVDU was not associated with an increased pneumonia risk (HR1.46,95%CI0.72,2.96,p=0.30).
Control arm
Consistent with the overall findings in control patients, IVDU (HR2.11, 95% CI: 1.06, 4.20,p=0.03), recurrent bacterial pneumonia and detectable plasma VL were associated with a significantly increased hazard for pneumonia (HR5.61,95%CI2.38,13.24,p<=0.001 and HR1.85,95%CI1.13,3.03,p=0.01 respectively). In contrast to the overall findings, there was only a trend towards decreased risk with Asian ethnicity (HR 0.27, 95% CI: 0.06,1.11,p=0.07) and a trend toward increased risk with older age (HR1.26,95%CI0.99.1.61,p=0.06).
Time-updated predictors of pneumonia event (both treatment groups combined)
As shown in Table 3b, Higher proximal VL on study (HR for one log10 higher VL=1.28, 95% CI1.11,1.47, p=<.001) and receipt of rIL-2 within the last 180 days (HR=1.72, 95% CI1.12,2.65, p=0.01) were predictors of increased risk for a bacterial pneumonia event; higher proximal CD4+ T-cell count was associated with decreased risk (HR=0.94; 95%CI0.89,1.00, p=0.04).
Proximity of IL-2 cycling and risk of bacterial pneumonia
When adjusted for baseline predictors (age, IVDU, ethnicity, history of recurrent bacterial pneumonia) and time-updated CD4+ T-cell count and HIV-RNA, the hazards for IL-2 patients cycling within 180 days and ≥180 days of a bacterial pneumonia event were 1.66 (95%CI 1.07,2.60, p=0.02) and 0.98 (95%CI 0.70,1.37, p=0.90) respectively compared to the control arm. In years 1 and 2 in the IL-2 group, the hazard for bacterial pneumonia when rIL-2 cycling was within <30 days, 30-119 days, 120-179 days compared to receipt ≥180 days previously, was 2.59 (95%CI0.88,7.62, p=0.08), 1.74 (95%CI0.70,4.30, p=0.23) and 1.21(95%CI0.36,4.04, p=0.75), respectively. During the first 2 years of the study when rIL-2 cycling was most intense, there was a trend towards an increased risk of bacterial pneumonia associated with receipt of more rIL-2 cycles (HR 1.28,95%CI0.96,1.69, p=0.09 for each additional cycle received), which was independent of proximal CD4+ T-cell count.
Discussion
During a median follow-up of 7 years, 4.4% of ESPRIT participants experienced bacterial pneumonia. Single episode bacterial pneumonia was the most commonly reported infection in ESPRIT. These data indicate that bacterial pneumonia still contributes substantially to morbidity in the era of potent cART and in a group of patients with relatively high CD4+ T-cells. As expected, the greatest risk for bacterial pneumonia occurred in those with very low CD4+ T-cell counts, with lower risks in those with CD4+ T-cells above 350 cells/μL compared to those below. Recurrent bacterial pneumonia (two or more episodes in a 12-month period) during follow-up was rare. As bacterial pneumonia events seem to be related in part to more recent IL-2 it is possible that the lack of further receipt of rIL-2 in just under half of the IL-2 arm experiencing a pneumonia event is part of the explanation for our not seeing higher rates of recurrent bacterial pneumonia.
It is likely these figures are an underestimate of the risk of bacterial pneumonia as we only included events meeting the criteria for a probable or confirmed pneumonia-event. Traditional risk factors for bacterial pneumonia in HIV-1-infected patients were identified in the ESPRIT cohort including older age, IVDU, prior recurrent bacterial pneumonia as an ADI, lower CD4+ T-cell count and detectable HIV viraemia (defined as ≥500 copies/mL). These data are consistent with the findings of the SMART study on bacterial pneumonia (12), where detectable viraemia (>400 cp/mL vs. <400 cp/mL) in the patients on continuous cART even when CD4+ T-cell count was >500 cells/uL was associated with an increased hazard for bacterial pneumonia (overall HR2.65,95% CI1.49,4.72,p=0.001) and treatment interruption (associated with viral rebound and CD4+ T-cell decline) compared to continuous cART was also associated with an increased hazard (HR1.55 95% CI1.07-2.25 p=0.02) for bacterial pneumonia. However, in the SMART study the strongest predictors of bacterial pneumonia in both study arms, were prior history of recurrent bacterial pneumonia and current cigarette smoking. For patients on continuous cART the risk of bacterial pneumonia was 3 fold higher in current smokers than life-long non-smokers. A limitation of this analysis is the lack of smoking data.
It is noteworthy that the majority of pneumonia events did not have a microbiological diagnosis and this is in keeping with other studies (12) and indeed in clinical practice, where the microbiological yield is low, either because the appropriate cultures were not taken or cultures taken were negative. As a consequence we were not able to use these data as a surrogate for pneumococcal vaccination, data that was not collected in ESPRIT.
Some of the findings identified in this study are more difficult to explain, in particular the reduced rate of bacterial pneumonia in those of Asian ethnicity. One bacterial pneumonia event among 365 patients was reported from Thailand which recruited the majority of patients of Asian ethnicity. Rates of PcP prophylaxis were lower in Thailand (0.8%) compared to other countries (6.7%) in which the study was enrolled, and this lower use of PcP prophylaxis, if anything could potentially favour an increased risk of bacterial pneumonia; geographic and other country characteristics, are potential confounders.
In ESPRIT, more recent receipt of rIL-2 was associated with a greater risk of bacterial pneumonia, although the confidence intervals were very wide. The reasons why more recent receipt of rIL-2 is associated with increased risk of pneumonia are uncertain, but there are a number of potential mechanisms.
Polymorphonuclear neutrophils (PMN) are a major effector cell against pathogenic bacteria including those causing pneumonia; the T-cell response (17) is also thought to be important in the normal immune response to pneumococci. Interleukin-2 may activate PMN by inducing the secretion of tumour necrosis-alpha (15) thus contributing to protective immunity, but at higher doses (600,000 IU/Kg) IL-2 causes a chemotaxis defect which impairs neutrophil function. Recent data in mice show that exogenous IL-2 can impair sequestration of neutrophil into the peritoneal cavity although the same effect was not seen in the lung in response to LPS-induced inflammation (18). In ESPRIT as in the SMART study, detectable HIV-viraemia was associated with an increase risk of bacterial pneumonia event. Gordin and colleagues (12) suggested that increased inflammatory markers (IL-6 and D-dimer) in patients with detectable HIV virus replication might be associated with higher rates of bacterial pneumonia, although there was no direct evidence in support of this. Porter and colleagues (19) have recently demonstrated that in a group of patients exposed to rIL-2 with cART, there were significant increases in hsCRP and D-dimer occurring by the end of the initial rIL-2 cycle and these increases were independent of changes in HIV-RNA, CD4+ T-cell count and T-cell proliferation.
These findings suggest the following hypotheses that might in part, explain the increased hazard of bacterial pneumonia associated with very recent receipt of rIL-2. First, the inflammatory surge associated with recent interleukin-2 receipt (19-24), second, the transient burst of HIV-viraemia known to occur around rIL-2 dosing cycles (20) and last, impairment of neutrophil function associated with rIL-2 exposure. Overall, however, it is harder to reconcile why the increased risk associated with rIL-2 receipt should continue for several months after the dosing cycle and long after the die back of secondary cytokines and the reduction in immune activation that occur following rIL-2 exposure (20,25).
Despite the limitations of this subanalysis of the ESPRIT, including lack of data on smoking status and pneumococcal vaccination, these data are informative. In summary, rates of bacterial pneumonia are high in a large cohort of cART treated HIV-infected adults with moderate levels of immunodeficiency followed for an average of more than 7 years. In the absence of smoking and pneumococcal vaccination history, the strongest recommendation arising from these data, is to try and reduce the pneumonia-risk associated with detectable HIV-viraemia by utilising cART that is fully virologically suppressive, at least to levels below 500 copies/mL. Why detectable HIV-viraemia and recent rIL-2 are associated with increased risk of bacterial pneumonia is unclear; we need further studies to understand the pathogenesis of bacterial pneumonia and its relationship with inflammatory biomarkers.
Acknowledgements
The writing group acknowledges the efforts of the many ESPRIT and SILCAAT investigators who collected these data, the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) Executive Committee (JD Neaton, D Abrams, A Babiker, J Baxter, DA Cooper, CJ Cohen, D Cohn, JH Darbyshire, W El-Sadr, S Emery, F Gordin, HC Lane, G Larson, MH Losso, JD Lundgren, J Nadler, AN Phillips) for their oversight of the ESPRIT study and valuable editorial assistance.
ESPRIT was supported by grants U01 AI46957 and U01 AI068641 from the National Institute of Allergy and Infectious Diseases (NIAID). rIL-2 was provided by Chiron and Novartis.
Footnotes
For a full listing of the Writing Group, conflicts of interest, the INSIGHT ESPRIT Study Group, and investigators please refer to the acknowledgements section.
ClinicalTrials.gov numbers, NCT00004978
Conflicts of Interest disclosures
The US government has been issued a patent for the use of IL-2 in HIV infection naming H.C. Lane as a co-inventor.
References
- 1.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4(7):445–55. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- 2.Wallace JM, Rao AV, Glassroth J, Hansen NI, Rosen MJ, Arakaki C, Kvale PA, Reichman LB, Hopewell PC. Respiratory illness in persons with human immunodeficiency virus infection. The Pulmonary Complications of HIV Infection Study Group. Am Rev Respir Dis. 1993;148(6 Pt 1):1523–9. doi: 10.1164/ajrccm/148.6_Pt_1.1523. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan JH, Moore RD, Keruly JC, Chaisson RE. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med. 2000;162(1):64–7. doi: 10.1164/ajrccm.162.1.9904101. [DOI] [PubMed] [Google Scholar]
- 4.Nagappan V, Kazanjian P. Bacterial infections in adult HIV-infected patients. HIV Clin Trials. 2005;6(4):213–28. doi: 10.1310/a3q4-uqqn-x9en-y4he. Review. [DOI] [PubMed] [Google Scholar]
- 5.Sogaard OS, Lohse N, Gerstoft J, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995-2007: a Danish population-based, nationwide cohort study. Clin Infect Dis. 2008;47(10):1345–53. doi: 10.1086/592692. [DOI] [PubMed] [Google Scholar]
- 6.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–51. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 7.Caiaffa WT, Vlahov D, Graham NM, et al. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1493–8. doi: 10.1164/ajrccm.150.6.7952605. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41 [PubMed] [Google Scholar]
- 9.Rodriguez-Barradas MC, Goulet J, Brown S, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008;46(7):1093–100. doi: 10.1086/529201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teshale EH, Hanson D, Flannery B, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, 1998—2003. Vaccine. 2008;26(46):5830–4. doi: 10.1016/j.vaccine.2008.08.032. Epub 2008 Sep 9. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2009 April 10; www.aidsinfo.nih.gov.
- 12.Gordin FM, Roediger MP, Girard PM, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008;178(6):630–6. doi: 10.1164/rccm.200804-617OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery S, Abrams DI, Cooper DA, et al. The Evaluation of Subcutaneous Proleukin (R) (interleukin-2) in a Randomized International Trial: Rationale, design, and methods of ESPRIT. Control Clin Trials. 2002;23:198–220. doi: 10.1016/s0197-2456(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 14.INSIGHT-ESPRIT Study Group. SILCAAT Scientific Committee. Abrams D, Lévy Y, Losso MH, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361(16):1548–59. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Investigator's Brochure Clinical Development Proleukin® / Aldesleukin Recombinant Human Interleukin-2 / PRL002A for use in Oncologic disease. Novartis Edition number 1- Edition date: April 30, 2007. [Google Scholar]
- 16.Gifford AH, Kirkland KB. Risk factors for Clostridium difficile-associated diarrhea on an adult hematology-oncology ward. Eur J Clin Microbial Infect Dis. 2006;25:751–755. doi: 10.1007/s10096-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 17.Kemp K, Bruunsgaard H, Skinhøj P, Klarlund Pedersen B. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect Immun. 2002;70(9):5019–25. doi: 10.1128/IAI.70.9.5019-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno SE, Alves-Filho JC, Bertozi G, et al. Systemic administration of interleukin-2 inhibits inflammatory neutrophil migration: role of nitric oxide. Br J Pharmacol. 2006;148(8):1060–6. doi: 10.1038/sj.bjp.0706835. Epub 2006 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter BO, Shen J, Kovacs JA, et al. Interleukin-2 cycling causes transient increases in high-sensitivity C-reactive protein and D-dimer that are not associated with plasma HIV-RNA levels. AIDS. 2009;23(15):2015–9. doi: 10.1097/QAD.0b013e32832d72c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs JA, Imamichi H, Vogel S, et al. Effects of intermittent interleukin-2 therapy on plasma and tissue human immunodeficiency virus levels and quasi-species expression. J Infect Dis. 2000;182(4):1063–9. doi: 10.1086/315821. Epub 2000 Sep 5. [DOI] [PubMed] [Google Scholar]
- 21.Fortis C, Soldini L, Ghezzi S, et al. Tumor necrosis factor alpha, interleukin 2, and soluble interleukin 2 receptor levels in human immunodeficiency virus type 1-infected individuals receiving intermittent cycles of interleukin 2. AIDS Res Hum Retroviruses. 2002;18:491–499. doi: 10.1089/088922202317406637. [DOI] [PubMed] [Google Scholar]
- 22.Mier JW, Vachino G, van der Meer JW, et al. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988;8:426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- 23.Heaton KM, Ju G, Grimm EA. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 1993;53:2597–2602. [PubMed] [Google Scholar]
- 24.Sereti I, Herpin B, Metcalf JA, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. AIDS. 2001;15:1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 25.Sereti I, Anthony KB, Martinez-Wilson H, et al. IL-2-induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104(3):775–80. doi: 10.1182/blood-2003-12-4355. Epub 2004 Apr 13. [DOI] [PubMed] [Google Scholar]