Abstract
The effects of a mild traumatic brain injury range from white matter disruption to affective disorders. We set out to determine the response to restraint-induced stress after a mild fluid-percussion injury (FPI), an experimental model for brain injury. Hypothalamic-pituitary-adrenal (HPA) axis regulation of corticosterone (CORT) and adrenocorticotropic hormone (ACTH) was determined during the first post-injury weeks, which corresponds to the same time period when rehabilitative exercise has been shown to be ineffective after a mild FPI. Adult male rats underwent either a FPI or sham injury. Additional rats were only exposed to anesthesia. HPA regulation was evaluated by measuring the effects of dexamethasone (DEX) treatment on CORT and ACTH. Tail vein blood was collected following 30-min restraint stress, at post-injury days (PID) 1, 7 and 14, prior to (0 min) and at 30, 60, 90 and 120 min after stress onset. Results from these studies indicate that the stress response was significantly more pronounced after FPI in that CORT and ACTH restraint-induced increases were more pronounced and longer lasting compared to controls. DEX suppression of CORT and ACTH was observed in all groups, suggesting that stress hyper-responsiveness after mild FPI is not attributable to reduced sensitivity of CORT feedback regulation. The increased sensitivity to stressful events in the first two post-injury weeks after a mild FPI may have a negative impact on early rehabilitative therapies.
The cognitive and psychological effects of mild traumatic brain injury (TBI) are becoming more apparent with its high incidence. Approximately 1.7 million Americans sustain a TBI annually (Faul et al., 2010). However, it is likely that this number is higher given that demographics do not generally include mild TBI cases with minimal unconsciousness or no loss of consciousness. The neuropathological consequences stemming from a mild TBI range from white matter damage to long-term cognitive impairments (McAllister et al., 2001, Bazarian et al., 2006, McAllister et al., 2006, Niogi et al., 2008). In addition, mild TBI has affective consequences that notably decrease life quality (Rapoport et al., 2002, Ashman et al., 2006). Here we will address the effects on neuroendocrine regulation within the corticotropic axis. Both human and animal studies suggest that hypothalamic pituitary adrenal (HPA) axis dysregulation is associated with negative mood states (Posener et al., 2000, Sapolsky, 2000, Strohle and Holsboer, 2003, Ising et al., 2005, Vreeburg et al., 2009).
Acute activation of the HPA axis occurs initially as a protective response to injury by promoting intravascular fluid retention and elevating cortisol levels which modulate the immune/inflammatory response and increase availability of substrates for metabolism (Johnson, 2006). However regulation of the hypothalamic-pituitary complex may be impaired due to its vascular and anatomical vulnerability compounded with the diffuse nature of TBI. As a matter of fact, anatomical damage to the pituitary in head injured patients is not uncommon (Benvenga et al., 2000). Clinical studies in patients with different TBI severities, ranging from mild to severe, have described TBI induced HPA disruption resulting from pituitary dysfunction, alterations in adrenocorticotropic hormone (ACTH) and/or adrenal insufficiency (Yuan and Wade, 1991, Cohan et al., 2005, Tanriverdi et al., 2006). Due to the intrinsic characteristics of TBI and the heterogeneity of TBI patients, the type and duration of pituitary deficits is varied. Clinical studies have suggested that neuroendocrine alterations are influenced by injury severity, such that cortisol levels are correlated with the duration of coma and recovery after head trauma (Woolf et al., 1990). Likewise, the post-injury glucocorticoid profile differs depending on the severity/characteristics of the injury. Plasma cortisol levels tend to increase after mild to moderate brain injury in the early post-injury period, whereas after a severe injury, cortisol levels are depressed (Steinbok and Thompson, 1979, Cernak et al., 1999). Indeed patients with a mild TBI are not immune from pituitary dysfunction as indicated in a recent review (Tanriverdi et al., 2010). Similarly animal studies have indicated severity-dependent alterations in HPA function after a controlled cortical impact (CCI) injury, an experimental model of TBI that results in a region of marked cell death (Taylor et al., 2008). .
Here we set out to determine the stress response, during the first two post-injury weeks, following a mild TBI. We were particularly interested in this time-window since we have previously observed that an exercise-based therapeutic intervention was counter-productive during the first post-injury week (Griesbach et al., 2004a). Because we are interested in the effects of mild TBI and because TBI produces and affects cells that in spite of surviving do not function normally (Lyeth et al., 1990, Harris et al., 2001, Singleton and Povlishock, 2004), we utilized the fluid percussion injury (FPI) model, an experimental injury model that, in our hands, results in cognitive impairments with no substantial cortical and hippocampal cell loss (Osteen et al., 2001, Griesbach et al., 2004b, Gurkoff et al., 2006). Similarly, behavioral deficits have been observed after experimental TBI with little histological degeneration (Lyeth et al., 1990). Because HPA dysregulation may not be apparent unless provocative testing is performed (Agha et al., 2004a, Agha et al., 2004b), we studied the effects of dexamethasone (DEX), a synthetic glucocorticoid, on corticosterone (CORT) and ACTH during restraint-induced stress.
Experimental Procedures
Subjects
A total of 216 male Sprague-Dawley rats (mean weight: 302g ± 2.46 SEM) from Charles River Breeding Labs (Hollister, CA) were utilized in these experiments. Rats underwent surgery to induce either sham injury (n=81) or FPI (n=81). Additional control rats were only exposed to anesthesia (n=54). Rats were handled daily and habituated to a reversed lighting schedule (lights off: 09:00 to 21:00 hrs) commencing the week prior to experimental procedures. During the experiments, rats were single-housed in opaque plastic bins (50.8 × 25.4 × 25.4 cm), which were lined with bedding material. Rats had ad lib access to water and rat chow. All procedures were performed in accordance with the United States National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Chancellor’s Animal Research Committee.
Lateral Fluid Percussion Injury
As previously done (Griesbach et al., 2004a, 2007), rats were anesthetized with isofluorane (4% for induction and 2.0% for maintenance in 100% O2) via a nose mask. The level of anesthesia was monitored by level of respiration, muscular relaxation and the corneal and pedal reflexes. After loss of corneal and pedal reflexes, the scalp and scapular regions were shaved, the animal was secured in a stereotaxic head frame, and the scalp was cleansed with ethanol and betadine. Rectal temperature was monitored and maintained between 36.5–38.0°C with a thermostatically controlled heating pad (Braintree Scientific Inc., Braintree, MA, USA). The scalp and temporal muscle were reflected and a 3-mm diameter circular craniotomy was made over the left parietal cortex, centered at 3 mm posterior to bregma and 6 mm lateral to the midline. The bone flap was removed and the dura left intact in all animals to receive FPI. The dura was inspected with the aid of a microscope (Wild, Heerburg, Switzerland) in order to assure that it was intact and thus allow for animal inclusion. A plastic injury cap was placed over the craniotomy with silicone adhesive, cyanoacrylate, and dental cement. When the dental cement hardened, the cap was filled with 0.9% NaCl solution. Anesthesia was discontinued and the animal was removed from the stereotaxic device. The injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a mild fluid percussion pulse (1.5 atm) was administered. Apnea times were determined as the time from injury to the return of spontaneous breathing. Time of unconsciousness was determined from the time of injury until the return of the hind-limb withdrawal reflex. Immediately upon responding to a paw pinch, anesthesia was restored, the injury cap removed, and the scalp was sutured. Sham animals underwent an identical preparation with the exception of the FPI. Animals used as controls were placed under anesthesia for a period of time similar to that of the rats that underwent FPI or sham surgery. A skin incision was also made, as described above, to assure that there would be experimenter blindness for the following procedures. After suturing, Bupivacaine (0.25 mg) was injected into the margins of the scalp incision and triple antibiotic ointment was applied over the incision. The rat was placed in a recovery chamber for approximately one hour before being returned to its cage.
Dexamethasone Sensitivity Test, Restraint Stress
At post-injury days (PID) 1 (20–24 hrs after surgery), 7 and 14 rats were subjected to restraint stress and DEX experiments were performed. Prior to restraint, basal blood collections were obtained by tail venepuncture. Immediately thereafter, rats were tightly restrained in plexiglas flat-bottom tubes (13×6×8 cm, Harvard Apparatus, Co) for 30 min. After 30 min, the restraining gate was released allowing mobility while the rats remained in the plexiglas tubes for an additional 90 min. Blood was collected by tail venepuncture at 30, 60, 90 and 120 min after onset of restraint. All blood samples were obtained between 14:00 and 16:00 hours during the active dark cycle.
In the DEX experiments, immediately following basal blood collection, DEX was administered by s.c. injection of DEX (Gensia Pharmaceuticals; Irvine CA) [0.01 (low) or 0.1 (high) mg/kg diluted in physiological saline] or saline vehicle (SAL). The 0.1 mg/kg dose has been found to significantly suppress stress-induced increases in CORT and ACTH in control rats (Houshyar et al., 2001, Taylor et al., 2010). Two hours later, rats were restrained for 30 min as described above. Rats were sacrificed after the last blood collection at 120 min after restraint onset and brains were saved for further analysis.
Corticosterone and ACTH Radioimmunoassays
Blood samples (250ul/sample) were collected in an EDTA-micro collection tube with added trasyol (Aprotinin, Sigma; 200 kIU/ml). Blood samples were centrifuged at 2,000 rpm for 20 min at 4°C, plasma was separated, aliquoted and stored at −80°C until assayed for CORT and ACTH. Plasma CORT was assayed with a commercial rat CORT 125I-RIA kit (MP Biomedicals, Inc, Orangeburg, NY) according to the vendor’s instructions, as done previously (Taylor et al, 2008). The reported detection limit for the CORT assay is 8 ng/ml and intra- and inter-assay coefficients of variation are lower than 10.3% and 7.2%, respectively. The results are expressed as nanograms per milliliter of plasma.
Plasma ACTH levels were assessed with a human ACTH 125I-RIA kit (DiaSorin Corp., Stillwater, MN), according to the vendor’s instructions, as done previously (Taylor et al 2008). The reported detection limit of this assay is 15 g/ml and intra- and inter-assay coefficients of variation are lower than 10.7% and 5.7%, respectively. The results are expressed as picograms per milliliter of plasma.
Statistical Analysis
Basal concentration values of CORT and ACTH were analyzed by comparing different treatment groups [FPI, Sham and Anesthesia Controls (Control)] with a one-way analysis of variance (ANOVA). Responses to restraint stress were analyzed with a between groups and within post-restraint time (30min, 60min, 90min and 120min) repeated measures ANOVA. Differences in individual group means were then detected with Bonferroni corrected comparisons. Responses to DEX suppression were analyzed with a between groups and within post-restraint time (30min, 60min, 90min and 120min) and PID (1, 7 and 14) ANOVA. In order to facilitate group comparisons, CORT and ACTH values are also shown as Area Under the Curve (AUC). AUC was calculated by using the trapezoidal rule. Normalized AUC values are shown given that they are indicative of the responses compared after baseline hormone levels.
Results
Subjects
Injured rats had a mean (± SEM) unconsciousness time of 108 ± 5.1 s and a mean apnea time of 15 ± 2.5 s. Four animals were deleted from the study after FPI because the severity of injury was determined to be too high (over 300 s unconsciousness) and it was the purpose of these experiments to study mild TBI. Additionally, severely injured animals have an increased risk of cardiac arrest. Although we did not measure cardiac output in our animals, by eliminating severely injured subjects we eliminated cardiac arrest-induced increases in CORT (Neigh et al., 2009). No significant differences in weight gain were observed between the groups. No gross motor impairments of ambulatory ability were observed in any of the injured rats.
Injury-Induced Effects on Corticosterone and ACTH
Basal levels of plasma CORT, prior to restraint-induced stress were analyzed. CORT values for the anesthesia controls were pooled given that no significant differences were observed across post injury days. At PID 1, a significant group effect was observed (F2,97 = 6.15, p < 0.0005). This effect was due to significantly lower CORT levels in the FPI group compared to the Control group (p < 0.005). A significant group effect was also observed at PID 7 (F2,96 = 3.67, p < 0.05); which was again attributed to a significant CORT decrease in the FPI rats compared to the Control group (p < 0.05). At PID 14, a CORT decrease in FPI rats compared to the Control group approached significance (p = 0.09) (Table 1).
Table 1.
Basal levels of plasma CORT and ACTH on PID 1, 7 and 14
| Group | CORT, ng/ml | ACTH, pg/ml | ||||
|---|---|---|---|---|---|---|
| PID 1 | PID7 | PID 14 | PID 1 | PID 7 | PID 14 | |
| Control (54) | 251±19 | 251±19 | 251±19 | 130±16 | 75±3 | 75±3 |
| Sham (81) | 216±18 | 207±23 | 238±24 | 132 ±8# | 92±5 | 76±3 |
| FPI (81) | 159±15** | 176±18* | 188±17 | 188 ±16* | 101±7** | 75±4 |
Results are means + SEM; parentheses indicate number per group. Control values were pooled across days as indicated above.
p<0.05 FPI vs Control;
p<0.005 FPI vs Control;
p<0.05 Sham vs FPI.
Basal levels of plasma ACTH, prior to restraint-induced stress, were analyzed. An anesthesia effect was observed in the controls for ACTH at PID 1 compared to the other post injury days (F2,50 = 11.55, p < 0.0005). Baseline ACTH values were significantly higher at PID 1 compared to PID 7 (p < 0.005) and PID 14 (p < 0.005). Given this anesthesia effect in the Control group, only the ACTH values for PID 7 and 14 were pooled for comparisons with the Sham and FPI groups.
At PID 1, a significant group effect was observed (F2,65 = 6.52, P < 0.005). This effect was due to significantly higher ACTH levels in the FPI group compared to the Control (p < 0.05) and Sham (p < 0.05) groups. A significant group effect was also observed at PID 7 (F2,80 = 7.03, P < 0.0005); which was attributed to a significant ACTH increase in the FPI rats compared to Controls (p < 0.005). No significant group effects were observed at PID 14 for basal ACTH prior to restraint-induced stress (Table 1).
CORT Response to Restraint Stress
At PID 1, a significant group effect (F2,29 = 6.47, p < 0.005) was observed for the CORT levels following restraint-induced stress. Further analysis indicated that this effect was due to significantly higher levels of CORT in the Control group compared to the Sham group at 30-min (p = 0.005), 60-min (p = 0.05) and 90-min (p = 0.05) after restraint (Fig. 1A). However when the stress response, for PID 1, was analyzed as percent increase from baseline CORT levels (Fig. 1b) or as AUC, no group differences were observed (Fig. 2A).
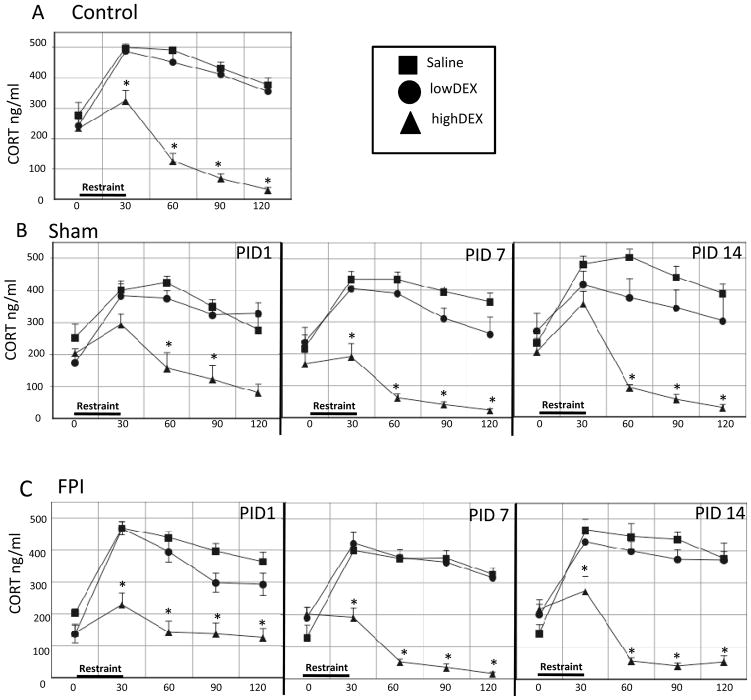
Fig. 1.
Plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH) responses to 30-min restraint stress in Control, Sham, and FPI rats at post-injury days (PID) 1, 7 and 14. Each value represents the mean ± SEM of 6–9 rats per group. Time pre- (0 min) and post-stress onset (30, 60, 90 and 120 min) is indicated on the X-axis.
(A) Effects of stress on plasma CORT levels. (B) Effects of stress on CORT percent increase from pre-restraint baseline levels.
(C) Effects of stress on plasma ACTH levels. (D) Effects of stress on ACTH percent increase from pre-restraint baseline levels. *p<0.05 vs Control, # p<0.05 vs Sham.
Fig. 2.
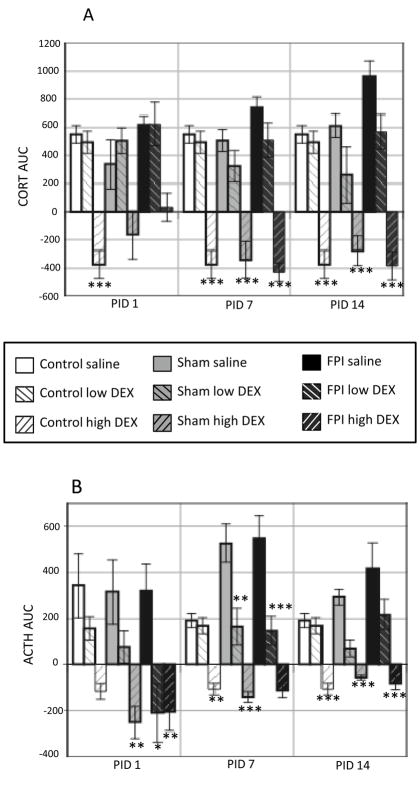
Area under the curve (AUC) for corticosterone (CORT) and adrenocorticotropic hormone (ACTH) levels normalized to pre-stress basal levels in Control, Sham and FPI rats following 30-min restraint stress at post-injury days (PID) 1, 7, and 14. Each histogram represents the mean ± SEM of 6–9 rats per group.
(A) AUC for CORT. Bracket indicates that fluid percussion injury (FPI) rats had significantly larger overall AUC values compared to Controls (p < 0.005) and Sham rats (p < 0.05).
(B) AUC for ACTH, **p <0.005 and ***p<0.0005 for bracketed groups.
At PID 7, a significant group effect (F2,27 = 9.67, p < 0.005) was observed for plasma CORT levels following restraint-induced stress. Further analysis indicated that this effect was due to significantly lower levels of CORT in the FPI rats compared to controls (p < 0.0005) at 30, 60 and 90 min post-restraint (Fig. 1A). It should be noted that this corresponds with the lower basal value of CORT that was observed in the FPI rats (Table 1). Consequently, when the stress response was analyzed as percent increase from basal CORT levels, the FPI rats had significantly higher overall increases in CORT as a response to stress compared to both the Control (p < 0.005) and Sham rats (p < 0.05) (Fig. 1B). These findings were supported by a significant group effect (F2,26 = 9.89, p < 0.005). Analysis of AUC levels of CORT, at PID 7, did not show significant effects. Compared to controls, FPI CORT increases only approached significance (p = 0.07) (Fig. 2A).
At PID 14, no significant effects were observed for plasma CORT values (Fig. 1A). Analysis of the percent change from baseline showed a significant group effect (F2,30 = 6.36, P < 0.005). This effect was attributed to a significantly higher restraint-induced increase of CORT in the FPI group compared to both the Control (p < 0.05) and Sham (p < 0.05) rats across all time points (Fig 1B). Likewise a significant group effect (F2,29 = 4.69, P < 0.05) was observed for CORT AUC at PID 14. AUC for CORT was significantly higher in the FPI group compared to Controls (p < 0.05) (Fig 2B).
AUC for CORT was also analyzed across PIDs 1, 7 and 14. Group differences for CORT AUC values were observed (F2,17 = 7.96, p < 0.005). FPI rats had significantly larger AUC CORT values compared to Controls (P < 0.005) and Sham rats (p < 0.05), (Fig 2A).
ACTH Response to Restraint Stress
No significant effects were observed for ACTH at PID1. In contrast, at PID 7, a significant group effect was found for plasma ACTH levels (F2,23 = 13.45, p < 0.0005). This effect was due to significantly higher levels of ACTH in the FPI (p < 0.005) and Sham (p < 0.005) rats compared to the Control group at 30, 60 and 90 min post-restraint time points (Fig 1C). Analysis of percent increase of ACTH indicated a significant increase in both FPI and Sham groups compared to Controls (p < 0.05) at 60 min post restraint at PID 7. Analysis of AUC values for ACTH, at PID 7, also indicated significant increases in FPI (F2,23 = 9.3, p < 0.0005) compared to Controls (Fig 2B). ACTH values in the Sham group at PID 7 appeared elevated compared to the Control group, although this effect did not attain significance (p=0.07).
At PID14 ACTH plasma levels indicated significantly higher elevations in the FPI group compared to Control (p < 0.005) at 30, 60 and 90 min post restraint. This was supported by a significant group effect (F2,20 = 9.49, p < 0.005) (Fig 1C). Similar findings were observed for ACTH percent increase from baseline (F2,17 = 9.94, p < 0.005), in that FPI values were significantly higher than Control p < 0.005) and Sham (p < 0.05) rats (Fig 1D). Further analysis indicated that ACTH levels in the FPI group were significantly increased at all time points compared to Controls; and compared to Shams they were significantly increased at 30 min post restraint (Fig 1D). Similarly a significant group effect (F2,21 = 9.64, P < 0.005) was observed for ACTH AUC values at PID 14. The AUC for ACTH was significantly higher in the FPI group compared to Controls (p < 0.005). (Fig 2B).
CORT Response to Dexamethasone
PIDs 1, 7 and 14 were pooled for the Control group given that no significant differences were observed in the Control group’s response to either dose of DEX or saline on the different days. Analysis of CORT levels indicated that highDEX suppressed CORT compared to Saline-treated controls (p < 0.0005) at all post-restraint time points. This was supported by a significant group effect (F2,38 = 129.36, P < 0.0005) (Fig 3A).
Fig. 3.
Effects of high (0.1 mg/kg) or low (0.01 mg/kg) dose dexamethasone (DEX) or saline injected 2 hr prior to 30-min restraint on stress-induced corticosterone (CORT) responses in Control (A), Sham (B) and FPI (C) rats on post-injury days (PID) 1, 7 and 14. Each value represents the mean ± SEM of 6–9 rats/injection/group. Time pre-DEX (0 min) and post-stress onset (30, 60, 90 and 120 min) is indicated on the X-axis. *p<0.05 vs corresponding saline- treated group.
The sham rats, receiving highDEX, also had significant decreases of CORT compared to SAL-treated rats (F2,15 = 58.02, p < 0.0005). The degree of highDEX suppression in Sham rats varied across PIDs. On PID1, CORT decreases were observed at 60 (p < 0.005) and 90 (p< 0.05) min post restraint, on PID 7 at all time points (p < 0.0005), and on PID 14, at 60 (p < 0.0005), 90 (p < 0.0005) and 120 (p < 0.05) min. Within the Shams, a significant effect for lowDEX compared to the SAL-treated was found when CORT levels were analyzed across all time points on PID 14 (p < 0.05) (Fig 3B).
Analysis of plasma CORT levels indicated a significant DEX suppression in the FPI groups (F2,15 = 151.66, p < 0.0005). FPI rats receiving highDEX had a significant decrease in CORT at all time points following restraint compared to FPI rats that received SAL (F2,15 = 151.66, p < 0.0005). This effect was observed at PID 1, 7 and 14 (p < 0.0005). Administration of lowDEX did not reduce CORT levels at any time point compared to SAL-treated FPI rats (Fig 3C).
Analysis of CORT AUC values showed that on PID 1 highDEX CORT values were lower compared to SAL-treated Controls (P < 0.0005). This was supported by a significant group effect (F8,86 = 11.47, p < 0.0005). HighDEX-induced decreases in CORT only approached significance in Sham and FPI rats (p = 0.06) at PID 1. At PID7 and PID 14, AUC values for highDEX CORT were lower compared to the SAL-treated in all groups (p < 0.0005). This was supported by significant group effects (F8,85 = 20.02 and F8,91 = 17.69, p < 0.0005, respectively). These significant group effects for CORT AUC levels are indicative of CORT suppression by high DEX (p < 0.0005) (Fig 5A).
Fig. 5.
Area under the curve (AUC) for plasma CORT and ACTH after injection of high (0.1 mg/kg) or low (0.01 mg/kg) dose dexamethasone (DEX) or saline 2 hr prior to restraint on post-injury days (PID) 1, 7 and 14 in Control, Sham and FPI rats. Histograms indicate AUC normalized to pre-injection basal levels, for (A) corticosterone (CORT) and (B) adrenocorticotropic hormone (ACTH); Each histogram represents the mean ± SEM of 6–9 rats/injection/group. *p<0.05, **p<0.005 and ***p<0.0005 vs corresponding saline-treated group.
ACTH Response to Dexamethasone
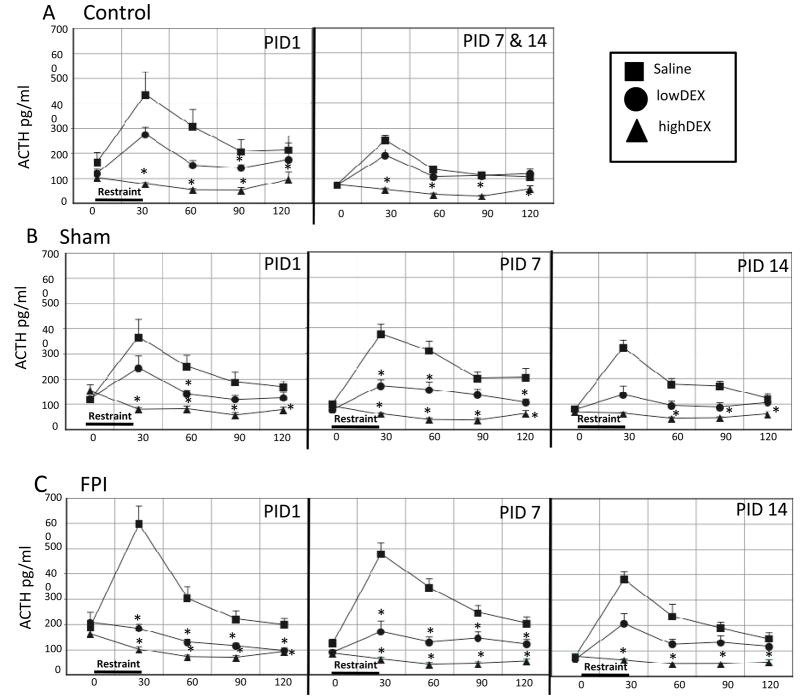
Only PIDs 7 and 14 were pooled for the control group given that anesthesia effects were observed at PID1. Analysis of ACTH at PID1 indicated ACTH decreases in control rats receiving highDEX compared to those receiving SAL at all post-restraint time points (F2,8 = 65.03, p < 0.0005). LowDEX also decreased ACTH significantly in controls at 90 min post restraint (p < 0.005) but only approached significance at 120 min (p < 0.07). Analysis of ACTH values for PIDs 7 and 14, indicated that Controls receiving highDEX had significant CORT suppression, compared to SAL counterparts at all time points (p < 0.0005). This was supported by a significant group effect (F2,27 = 87.25, p < 0.0005) (Fig 4A).
Fig. 4.
Effects of high (0.1 mg/kg) or low (0.01 mg/kg) dose dexamethasone (DEX) or saline injected 2 hr prior to 30-min restraint on stress-induced adrenocorticotropic hormone (ACTH) responses in Control (A), Sham (B) and FPI (C) rats on post-injury days (PID) 1, 7 and 14. Each value represents the mean ± SEM of 6–9 rats/injection/group. Time pre-DEX (0 min) and post-stress onset (30, 60, 90 and 120 min) is indicated on the X-axis. *p<0.05 vs corresponding saline-treated group.
Sham rats, receiving highDEX or lowDEX had overall significant decreases of ACTH compared to Sham-Saline treated rats (F2,17 = 44.86, P < 0.0005). The degree of highDEX suppression in Sham rats varied across PIDs. At PID1, ACTH decreases by highDEX were observed at all post-restraint time points (p < 0.0005) and lowDEX ACTH suppression was significant at 60 min post-restraint p < 0.05). At PID 7, highDEX decreased ACTH all time points (p < 0.0005) and lowDEX significantly decreased ACTH at 30 (p < 0.0005), 60 (p < 0.05) and 120 p < 0.05) min post-restraint. At PID 14, highDEX decreased ACTH at all time points p < 0.0005) except 30 min post-restraint (Fig 4B).
FPI rats, receiving highDEX or lowDEX had overall significant decreases of ACTH compared to FPI SAL-treated rats (F2,16 = 88.54, p < 0.0005). Administration of highDEX suppressed ACTH, compared to SAL at all post-restraint time points and days. The degree of lowDEX suppression in FPI rats varied across PIDs. At PID1, ACTH decreases by lowDEX were observed at all post-restraint time points (p < 0.005). At PID 7, lowDEX also decreased ACTH at all time points (p < 0.0005). However lowDEX did not decrease ACTH at any PID 14 time point (Fig 4C).
Analysis of ACTH AUC values showed that on PID 1, highDEX ACTH values were lower compared to corresponding SAL-treated groups in the Sham (p < 0.005) and FPI rats (p < 0.0005). A significant decrease of ACTH in FPI rats treated with lowDEX was found at PID 1. This was supported by a significant group effect (F8,58 = 6.71, p < 0.0005). At PID7, highDEX ACTH values were lower compared to SAL in all groups (p < 0.0005). Significant ACTH decreases following lowDEX were found in the Sham (p < 0.005) and FPI (p< 0.0005) rats compared to corresponding SAL-treated groups. This was supported by a significant group effect (F8,71 = 22.8, p < 0.0005). At PID14, highDEX ACTH values were lower compared to SAL in all groups (p < 0.0005). A significant group effect for ACTH AUC levels at PID 14 (F8,71 = 14.79, p < 0.0005) was also observed (Fig 5B).
Discussion
Dissociation of ACTH and CORT was observed after a mild FPI
Basal post-injury measurements indicated that FPI elevated ACTH levels and decreased CORT. These effects on ACTH and CORT are indicative of their dissociation. The HPA response to stress initially involves hypothalamic activation of the parvocellular region of the paraventricular nucleus resulting in the release of corticotropin releasing hormone (CRH) that consequently leads to the release of ACTH from the anterior pituitary. ACTH increases normally elicit CORT release from the adrenals. Instead, these findings indicated significant increases of basal ACTH and significant decreases of basal CORT levels after FPI. This dissociation between CORT and ACTH was most pronounced at 7 days after FPI and subsided by PID 14. Dissociation of ACTH and glucocorticoids is a frequently observed phenomenon and the degree of this dissociation has been linked with the level of surgery complications, for example (Bornstein et al., 2008).
It should also be noted that there was a stress response to the craniotomy. When the response to restraint was analyzed, an elevation in ACTH was observed at PID 1 and 7 following sham injury. This stress response was no longer significant at PID 14. Activation of the HPA axis following surgical stress due to a craniotomy has been observed clinically (Shenkin et al., 1971, DeKeyser et al., 2000, Hirasawa et al., 2000). Surgical stress activates the HPA axis to promote intravascular fluid retention, modulation of the immune/inflammatory response and an increase in metabolic substrate availability (Johnson, 2006). Thus in the case of the FPI rats, it should be taken into account that these effects will be compounded with those of the brain injury.
The response from a mild FPI is likely to differ from that of a more severe injury. Clinical studies have demonstrated elevations in cortisol acutely after brain trauma and these elevations are correlated with the duration of coma and recovery after head trauma (Woolf et al., 1990). Similarly elevations in corticosterone have been observed within the first 12 hours following experimental brain injury (Shohami et al., 1995, McCullers et al., 2002). The occurrence of pituitary damage is proportional to injury severity (Cohan et al., 2005). However, it is not uncommon to also find pituitary damage following milder TBI (Niederland et al., 2007, Tanriverdi et al., 2010). Magnetic resonance imaging studies indicate that 37% of mild TBI patients, with a Glascow Coma Score ranging from 13–15, had pituitary abnormalities (Bondanelli et al., 2004).
There is a heightened stress response after a mild FPI
When CORT was analyzed in a manner that accounted for baseline levels, a hyper-responsiveness to stress was observed. CORT increases, resulting from restraint, were more pronounced and longer lasting in FPI rats compared to the Controls. This hyper-responsiveness was observed at PIDs 7 and 14. The ACTH response to stress was also heightened in the FPI rats at PIDs 7 and 14. A craniotomy effect was also observed in that ACTH levels in the Sham group were elevated compared to controls, particularly at PID 7.
Clinical studies have shown that alterations in the HPA axis may not be apparent unless there is a provocative manipulation. As such, that was the case in these experiments. At PID 14, when basal levels of ACTH and CORT approximated those of Controls, the FPI rats’ response to stress was heightened. Although a stress-induced elevation in ACTH was still observed at PID14, in the FPI group, it was not as pronounced as that observed at PID7. Thus, it appears that the hyper-responsiveness to stress may resolve over time, although we did not study later time-points. Indeed, a heightened stress response has also been observed following a mild CCI injury at PID 34 and 70 (Taylor et al., 2008). Future studies will determine if a similar temporal profile will occur after a mild FPI. This is also possible due the differences between these injury models. In contrast to the mild FPI, which has been utilized in these studies, the CCI injury is a less diffuse injury that results in marked cortical and hippocampal cell death (Morales et al., 2005, Hall et al., 2008). Moreover, the temporal CORT profile in the mild CCI model appears to be different in that CORT was decreased when measured at PID 7 (Taylor et al., 2008).
Although there is no gross neuronal death in our FPI model, we have observed that there are marked molecular changes related to neuroplasticity (Griesbach et al., 2002, Griesbach et al., 2004a). These effects are likely due to altered neuronal excitability and circuit function (Tran et al., 2006, Avramescu and Timofeev, 2008). Changes in neuronal properties can have an effect on hypothalamic and pituitary hormonal release. The paraventricular nucleus is an integrative region of the hypothalamus, receiving suprahypothalamic input with notable projections from the limbic system. Moreover, it also receives input from other hypothalamic nuclei that may relay other stress specific signals. Thus, alterations in function and connectivity are likely to have an influence on HPA regulation. Pronounced increases in both CORT and ACTH following FPI were observed in these experiments. These increases are indicative of hypothalamic disruptions in neuronal circuitry. Indeed CRH mRNA has been shown to increase after TBI (Roe et al., 1998, Grundy et al., 2001). There are multiple other possibilities that may facilitate post-TBI hyper-responsiveness such as changes in the refractory period of corticoptropes, the pituitary target cells, thus resulting in more hormone release; and injury-induced increases in arginine vasopressin (AVP). AVP, a major regulator of fluid homeostasis, is involved in the inflammatory response following TBI. AVP has a synergistic effect with CRH at pituitary corticotropes (Swanson and Sawchenko, 1983, Szmydynger-Chodobska et al., in press). AVP has been shown to increase after CCI (Pascale et al., 2006) and following subarachnoid hemorrhage in humans (Mather et al., 1981, Xu et al., 2007, Yuan et al., 2010). All these potential scenarios need to be further explored.
Dexamethasone suppression
Disruptions in the negative feedback of glucocorticoids are also likely to contribute to an elevated stress response. However, the DEX administration studies did not support this hypothesis. All groups, including the FPI, responded to the higher dose of DEX with suppressed ACTH and CORT release. Significant suppression of CORT was not observed with the lower dose of DEX. This lower dose of DEX did however reduce levels of ACTH in the Sham and FPI rats, particularly at PID 7. Similar nonresponsiveness to 0.01 mg/kg DEX has been reported at 5 weeks after mild CCI, although this dose suppressed CORT and ACTH release after moderate CCI at this time-point (Taylor et al., 2010).
These essentially negative DEX findings suggest that hyper-responsiveness to stress may not be associated with reduced sensitivity of CORT feedback regulation, but do not completely rule out the possibility that reduced negative feedback contributed to the hyper-responsiveness to stress. Because DEX does not cross the blood-brain barrier, it acts at the hypothalamic level, where the blood-brain barrier is incomplete, instead of other areas such as the hippocampus. Glucocorticoid receptors are widespread within the brain and are predominantly expressed within the hippocampus (McEwen, 1999, McEwen and Magarinos, 2001). Thus the hippocampus has been suggested as a key regulator of glucorticoid negative feedback. Basal levels of ACTH were significantly elevated in the Sham and FPI groups, compared to Controls, and DEX administration may have had an additive effect that approximated higher glucocorticoid levels triggering a suppressive response. An alternative possibility is that the response to the lower dose of DEX was indicative of increased negative feedback, which may also occur as a compensatory response to suprahypothalamic inputs (de Kloet, 2000).
Injury alters the stress response, which in turn may hinder neuroplasticity
The temporal profile of the dissociation in basal CORT and ACTH levels and the heightened stress response following mild TBI coincides with a period of non-responsiveness to rehabilitative exercise. Work from this laboratory has demonstrated that post-TBI voluntary exercise therapy promotes recovery of function and increases molecules that are critical for neuroplasticity when it occurs at the appropriate post-injury time. However, when exercise is provided too early during the first post-injury week there is no increase in molecular markers of plasticity and behavioral outcome worsens (Griesbach et al., 2004a). This introduces the possibility that certain rehabilitative efforts may be counterproductive when started too soon after injury. Events during the initial post-injury days may be perceived as stressful when they would normally not elicit a strong stress response. It is well known that glucocorticoids can downregulate molecular markers of plasticity both at the mRNA and protein levels (Smith, 1995, Hansson et al., 2000, Schaaf et al., 2000, Gronli et al., 2006, Hansson et al., 2006). Thus an enhanced stress response may hinder reparatory/compensatory mechanisms following injury.
Stress may contribute to post-TBI psychopathology
Stress is also likely to have negative affective consequences. It is plausible that a heightened stress response will influence affective well-being after TBI. For example, a recent cross-sectional study, evaluating serotonergic levels and cortisol responsiveness in depressive patients suggests that an elevated stress response interferes with serotonergic activity (Reimold et al., 2010). A considerable number of TBI patients present with a psychiatric disorder, of which depression is the most frequently diagnosed (Ashman et al., 2006, Bombardier et al., 2010). Also associated with TBI are anxiety disorders (Moore et al., 2006). Although these studies do not demonstrate that mild TBI will increase the risk of anxiety and/or depression, they do indicate that there is a disruption of glucocorticoid regulation and a heightened stress response that may have an effect on glucocorticoid regulation over time. Repeated stress leads to increases in CRH within the amygdala and has been associated with increased levels of anxiety in the rat (Santibanez et al., 2006). Likewise, depression has been repeatedly linked to cortisol increases in humans (Gold and Chrousos, 2002, Juruena et al., 2004). Future studies are necessary to determine the relationship between post-injury stress hyper-responsiveness and long-term neurological/psychological consequences of mild TBI.
Conclusions
In summary, these experiments, in an animal model of mild TBI, indicated that there is a heightened stress response during the first two post-injury weeks. Moreover this hyper-responsiveness to stress is evident when basal levels of ACTH and CORT are similar to those of controls. It should be noted that the time windows utilized in the animal models of TBI are most likely different to those found in humans. Specific time windows are more achievable in animal models of TBI because there is more subject and injury homogeneity, unlike clinical cases where injury characteristics vary greatly. For this reason it is important to understand the neuroendocrine correlates that are likely to influence molecular markers of synaptic plasticity following brain injury.
Elevations in corticosterone have been demonstrated to negatively affect hippocampal plasticity and emotional health. Given the effects of stress on neuronal plasticity, it is likely that pronounced stress-induced increases in CORT may inhibit neuroplasticity responses to certain early rehabilitation paradigms, particularly if these are perceived as stressful. Given that DEX suppression was not differentially affected by FPI, alterations in suprahypothalamic inputs are likely to contribute to stress hyper-responsiveness and should be further explored.
Acknowledgments
This study was supported by NINDS award NS6190 (GSG) and the UCLA Brain Injury Research Center. We would also wish to thank David L. McArthur, PhD. for his statistical advice and David Garfinkel for his excellent technical help.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ANOVA
analysis of variance
- AUC
area under the curve
- AVP
arginine vasopressin
- CCI
controlled cortical impact
- CORT
corticosterone
- CRH
corticotropin releasing hormone
- DEX
dexamethasone
- FPI
fluid-percussion injury
- HPA
hypothalamic-pituitary-adrenal
- PID
postinjury day
- SAL
saline
- TBI
traumatic brain injury
- UCLA
University of California at Los Angeles
Footnotes
Author disclosure statement
No conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agha A, Rogers B, Mylotte D, Taleb F, Tormey W, Phillips J, Thompson CJ. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf) 2004a;60:584–591. doi: 10.1111/j.1365-2265.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- Agha A, Rogers B, Sherlock M, O’Kelly P, Tormey W, Phillips J, Thompson CJ. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004b;89:4929–4936. doi: 10.1210/jc.2004-0511. [DOI] [PubMed] [Google Scholar]
- Ashman TA, Gordon WA, Cantor JB, Hibbard MR. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J Med. 2006;73:999–1005. [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion--looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Benvenga S, Campenni A, Ruggeri RM, Trimarchi F. Clinical review 113: Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353–1361. doi: 10.1210/jcem.85.4.6506. [DOI] [PubMed] [Google Scholar]
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondanelli M, De Marinis L, Ambrosio MR, Monesi M, Valle D, Zatelli MC, Fusco A, Bianchi A, Farneti M, degli Uberti EC. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685–696. doi: 10.1089/0897715041269713. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Cernak I, Savic VJ, Lazarov A, Joksimovic M, Markovic S. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 1999;13:1005–1015. doi: 10.1080/026990599121016. [DOI] [PubMed] [Google Scholar]
- Cohan P, Wang C, McArthur DL, Cook SW, Dusick JR, Armin B, Swerdloff R, Vespa P, Muizelaar JP, Cryer HG, Christenson PD, Kelly DF. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358–2366. doi: 10.1097/01.ccm.0000181735.51183.a7. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- DeKeyser FG, Leker RR, Weidenfeld J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7:182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG N. C. f. I. P. a. Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004a;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Gomez-Pinilla F. Alterations in BDNF and synapsin I within the occipital cortex and hippocampus after mild traumatic brain injury in the developing rat: reflections of injury-induced neuroplasticity. J Neurotrauma. 2002;19:803–814. doi: 10.1089/08977150260190401. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004b;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Grundy PL, Harbuz MS, Jessop DS, Lightman SL, Sharples PM. The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J Neurotrauma. 2001;18:1373–1381. doi: 10.1089/08977150152725669. [DOI] [PubMed] [Google Scholar]
- Gurkoff GG, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur J Neurosci. 2000;12:2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J Neuroendocrinol. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Harris LK, Black RT, Golden KM, Reeves TM, Povlishock JT, Phillips LL. Traumatic brain injury-induced changes in gene expression and functional activity of mitochondrial cytochrome C oxidase. J Neurotrauma. 2001;18:993–1009. doi: 10.1089/08977150152693692. [DOI] [PubMed] [Google Scholar]
- Hirasawa K, Kasuya H, Hori T. Change in circulating blood volume following craniotomy. J Neurosurg. 2000;93:581–585. doi: 10.3171/jns.2000.93.4.0581. [DOI] [PubMed] [Google Scholar]
- Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol. 2001;13:875–886. doi: 10.1046/j.1365-2826.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Johnson JA. The hypothalamic-pituitary-adrenal axis in critical illness. AACN Clin Issues. 2006;17:39–49. doi: 10.1097/00044067-200601000-00006. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Pariante CM. The hypothalamic pituitary adrenal axis, glucocorticoid receptor function and relevance to depression. Rev Bras Psiquiatr. 2004;26:189–201. doi: 10.1590/s1516-44462004000300009. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Mather HM, Ang V, Jenkins JS. Vasopressin in plasma and CSF of patients with subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1981;44:216–219. doi: 10.1136/jnnp.44.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Saykin AJ. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol. 2001;23:775–791. doi: 10.1076/jcen.23.6.775.1026. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Moore EL, Terryberry-Spohr L, Hope DA. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20:117–132. doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Karelina K, Zhang N, Glasper ER, Owens MJ, Plotsky PM, Nemeroff CB, Devries AC. Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J Cereb Blood Flow Metab. 2009;29:1673–1682. doi: 10.1038/jcbfm.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederland T, Makovi H, Gal V, Andreka B, Abraham CS, Kovacs J. Abnormalities of pituitary function after traumatic brain injury in children. J Neurotrauma. 2007;24:119–127. doi: 10.1089/neu.2005.369ER. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of Microstructural White Matter Injury in Postconcussive Syndrome Correlates with Impaired Cognitive Reaction Time: A 3T Diffusion Tensor Imaging Study of Mild Traumatic Brain Injury. AJNR Am J Neuroradiol. 2008 doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Pascale CL, Szmydynger-Chodobska J, Sarri JE, Chodobski A. Traumatic brain injury results in a concomitant increase in neocortical expression of vasopressin and its V1a receptor. J Physiol Pharmacol. 2006;57(Suppl 11):161–167. [PubMed] [Google Scholar]
- Posener JA, DeBattista C, Williams GH, Chmura Kraemer H, Kalehzan BM, Schatzberg AF. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry. 2000;57:755–760. doi: 10.1001/archpsyc.57.8.755. [DOI] [PubMed] [Google Scholar]
- Rapoport M, McCauley S, Levin H, Song J, Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:123–132. [PubMed] [Google Scholar]
- Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Strohle A, Zimmer A, Schonknecht P, Smolka MN, Weinberger DR, Goldman D, Machulla HJ, Bares R, Heinz A. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe SY, McGowan EM, Rothwell NJ. Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur J Neurosci. 1998;10:553–559. doi: 10.1046/j.1460-9568.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Santibanez M, Gysling K, Forray MI. Desipramine prevents the sustained increase in corticotropin-releasing hormone-like immunoreactivity induced by repeated immobilization stress in the rat central extended amygdala. J Neurosci Res. 2006;84:1270–1281. doi: 10.1002/jnr.21023. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Shenkin HA, Gutterman P, Bouzarth WF. The adrenocortical response to craniotomy for brain tumor. J Neurosurg. 1971;34:657–664. doi: 10.3171/jns.1971.34.5.0657. [DOI] [PubMed] [Google Scholar]
- Shohami E, Bass R, Trembovler V, Weidenfeld J. The effect of the adrenocortical axis upon recovery from closed head injury. J Neurotrauma. 1995;12:1069–1077. doi: 10.1089/neu.1995.12.1069. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: evidence for multiple independent injury phenotypes. J Neurosci. 2004;24:3543–3553. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann NY Acad, Sci. 1995;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Thompson G. Serum cortisol abnormalities after craniocerebral trauma. Neurosurgery. 1979;5:559–565. doi: 10.1227/00006123-197911000-00003. [DOI] [PubMed] [Google Scholar]
- Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Szmydynger-Chodobska J, Zink BJ, Chodobski A. Multiple sites of vasopressin synthesis in the injured brain. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2010.188. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006;91:2105–2111. doi: 10.1210/jc.2005-2476. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146–153. doi: 10.1007/s11102-009-0215-x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma. 2008;25:311–323. doi: 10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Tio DL, Gardner SM, Kim CJ, Sutton RL. Injury severity differentially alters sensitivity to dexamethasone after traumatic brain injury. J Neurotrauma. 2010;27:1081–1089. doi: 10.1089/neu.2009.1252. [DOI] [PubMed] [Google Scholar]
- Tran LD, Lifshitz J, Witgen BM, Schwarzbach E, Cohen AS, Grady MS. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Woolf PD, Cox C, Kelly M, Nichols D, McDonald JV, Hamill RW. The adrenocortical response to brain injury: correlation with the severity of neurologic dysfunction, effects of intoxication, and patient outcome. Alcohol Clin Exp Res. 1990;14:917–921. doi: 10.1111/j.1530-0277.1990.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Su W, Huang WD, Lu YQ, Xu QP, Chen ZJ. Effect of AVP on brain edema following traumatic brain injury. Chin J Traumatol. 2007;10:90–93. [PubMed] [Google Scholar]
- Yuan XQ, Wade CE. Neuroendocrine abnormalities in patients with traumatic brain injury. Front Neuroendocrinol. 1991;12:209–230. [PubMed] [Google Scholar]
- Yuan ZH, Zhu JY, Huang WD, Jiang JK, Lu YQ, Xu M, Su W, Jiang TY. Early change of plasma and cerebrospinal fluid arginine vasopressin in traumatic subarachnoid hemorrhage. Chin J Traumatol. 2010;13:42–45. [PubMed] [Google Scholar]