Abstract
During retinal development, the cell-fate of photoreceptors is committed long before maturation, which entails the expression of opsins and functional transduction of light. The mechanisms that delay the maturation of photoreceptors remain unknown. We have recently reported that immature photoreceptors express the LIM domain transcription factors Islet2 and Lim3, as well as the cell-surface glycoprotein axonin1 (Fischer et al., 2008a). As the photoreceptors mature to form outer segments and express photopigments, the expression of the Islet2, Lim3 and axonin1 is diminished. The purpose of this study was to investigate whether Thyroid Hormone (TH) influences the maturation of photoreceptors. We studied the maturation of photoreceptors across the gradient of maturity that exists in far peripheral regions of the postnatal chicken retina (Ghai et al., 2008). We found that intraocular injections of TH down-regulated Islet2, Lim3 and axonin1 in photoreceptors in far peripheral regions of the retina. By contrast, TH stimulated the up-regulation of red-green opsin, violet opsin, rhodopsin and calbindin in photoreceptors. We found a correlation between the onset of RLIM (RING finger LIM-domain binding protein) and down-regulation of Islet2 and Lim3 in maturing photoreceptors; RLIM is known to interfere with the transcriptional activity of LIM-domain transcription factors. We conclude that TH stimulates the maturation of photoreceptors in the avian retina. We propose that TH inhibits the expression of Islet2 and Lim3, which thereby permits photoreceptor maturation and the onset of photopigment-expression.
Keywords: retina, photoreceptor, thyroid hormone, Islet2, Lim3, RLIM
Introduction
The neural retina is an exceptionally sensitive light-detector and efficient image-processor. There are two classes of vertebrate photoreceptors: rods and cones. Cones function in bright, day-light and are responsible for color vision. Rods are sensors of dim light and do not discern color. Cone photoreceptors can be segregated into different categories based on their preferential sensitivity to different wavelengths of light. Humans have 3 types of cones that respond to blue, green or red light. Similar to the human retina, the avian retina has cone photoreceptors that preferentially respond to red, green, and blue light, but also has violet-sensitive cones. In the avian retina, the cones out-number the rods by 6-to-1 (Morris, 1970), and the cones are concentrated in a rod-free area centralis (Bruhn and Cepko, 1996).
During development of the chick, the rod and cone photoreceptors are generated between embryonic day 4 (E4) and E7 in central regions of the retina (Prada et al., 1991). After becoming post-mitotic, cells committed to become photoreceptors undergo differentiation. The differentiation of photoreceptors involves many different processes that are highly ordered (Sears et al., 2000, Hendrickson and Hicks, 2002, Cornish et al., 2004, Cornish et al., 2005). In the chick retina, the differentiation of photoreceptors begins at E6 with the onset of expression of visinin, rod and cone transducins, and rod and cone phosphodiesterases (Bruhn and Cepko, 1996, Bradford et al., 2005). However, red and green opsins are first expressed in central retina at about E14, rhodopsin at E15, and blue and violet opsins at E16 (Bruhn and Cepko, 1996, Bradford et al., 2005). The expression of opsins by photoreceptors occurs 8–10 days after the photoreceptors become post-mitotic. The mechanisms that delay the maturation of developing photoreceptors remain entirely unknown. At the time of hatching, between E21 and E22, the chicks are able to see, indicating that the retinas are fully functional with mature, active photoreceptors. Here we define photoreceptor maturity as the point in cellular development when photopigment expression begins to enable phototransduction. Interestingly, the differentiation of photoreceptors is greatly slowed in far peripheral regions of the retina compared to the rate of differentiation of photoreceptors in central regions of the retina (Ghai et al., 2008).
Recent studies have indicated that the LIM-domain transcription factors Islet2 and Lim3 are transiently expressed by differentiating photoreceptors in the embryonic chick retina (Edqvist et al., 2006, Fischer et al., 2008a). We found that photoreceptors in the embryonic chick retina express Islet2 and Lim3 between E7 and E16, about 7–9 days after becoming postmitotic (Fischer et al., 2008a). In parallel to the patterns of expression of Islet2 and Lim3, the cell-surface glycoprotein axonin1 is transiently expressed by differentiating photoreceptors (Fischer et al., 2008a). The expression of Islet2, Lim3 and axonin1 is maintained by immature photoreceptors in far peripheral retinal regions during the first 2 weeks of postnatal development (Fischer et al., 2008a). The photoreceptors in far peripheral regions of the retina do not express red/green opsins, rhodopsin or calbindin until at least P14, more than 21 days after terminal mitosis (Ghai et al., 2008). In the far peripheral retina there are expression gradients of rhodopsin, calbindin and red-green opsin that increase with increasing distance from the circumferential marginal zone (CMZ), and these markers are up-regulated as Islet2 and Lim3 are down-regulated (Fischer et al., 2008a). The maturation of photoreceptors in far peripheral regions of the retina is greatly slowed compared to the rates of maturation of photoreceptors in central regions of the retina, and the slowed maturation of photoreceptors coincides with the prolonged expression of Islet2, Lim3 and axonin1. The gradient of photoreceptor maturity in the far peripheral retina provides a unique experimental system in which to study the process of maturation across a spatial gradient that corresponds to changes in temporal maturation.
The purpose of this study was to investigate how thyroid hormone (TH) influences the maturation of photoreceptors in the chick retina. In the rodent retina, TH and thyroid hormone receptor β2 (TRβ2) are known to influence the commitment of cone photoreceptors to express blue-sensitive versus red/green-sensitive opsins (Ng et al., 2001, Roberts et al., 2006). A recent study in the rodent demonstrated that retinal levels of TH are regulated by deiodinase 3 and signaling through TRβ2 to control photoreceptor survival and patterning of types of cones in dorsal and ventral regions of the retina (Ng et al., 2010). By comparison, TH is known to regulate the degeneration and generation of UV-sensitive cones in the retinas of salmonid fish during transitions between fresh and saltwater environments through the normal life-cycle (Allison et al., 2006, Raine and Hawryshyn, 2009). In addition, genes related to TH-signaling are expressed in patterns that dynamically change in progenitor cells and photoreceptors during the embryonic development of the chick retina (Trimarchi et al., 2008). In this study, we investigate how TH influences photoreceptor maturation across the temporal-spatial gradient of maturity that persists at the far peripheral edge of the postnatal chick retina.
Methods and Materials
Animals
The use of animals was in accordance with the guidelines established by the National Institutes of Health and the Ohio State University. Newly hatched leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University and kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am). During the subjective day, chicks were maintained under standard, broad spectrum fluorescent lights at approximately 350 lux. Chicks were housed in a stainless steel brooder at about 28°C and received water and Purinatm chick starter ad libitum.
Intraocular injections
Chickens were anesthetized by inhalation of 2.5% isoflurane in oxygen at a flow rate of 1.5 l/min. Injections were made using a 25-μl Hamilton syringe and a 26-gauge needle with a beveled, curved tip. Penetration of the needle was consistently made through the upper eyelid into the dorsal quadrant of the vitreous chamber. In all experiments, 20 μl of vehicle containing the test compound was injected into the experimental (left) eye, and 20 μl of vehicle alone was injected into the control (right) eye. The vehicle was sterile saline containing bovine serum albumin, 50 μg/ml, as carrier. We tested doses of TH (T4; L-Thyroxine sodium salt pentahydrate T35009; Sigma-Aldrich) between 200 ng and 5 μg per dose. Triiodothyronine (T3) is about four times more potent (biologically active) than thyroxine prohormone (T4), which is converted to T3 by deiodinases, which are known to be expressed by retinal cells (Trimarchi et al., 2008, Ng et al., 2010).
We used 2 different injection paradigms: (1) Paradigm A – on post-hatch day 0 (P0) the left eye received a single injection of test compound and the right eye received vehicle. Retinas were harvested 24 hrs later. (2) Paradigm B – on P0, P1, P2 and P3 the left eye received an injection of test compound and the right eye received vehicle. Retinas were harvested 24 hrs after the last injection on P4. Intraocular injections of TH were used to permit comparisons between control and treated conditions within individuals and to avoid the metabolic dysfunction that might occur from a systemic delivery. At least 8 individuals were used in each experimental paradigm or data set.
Reverse Transcriptase PCR
Retinas were pooled from 3 individuals from different stages of development. mRNA was harvested from central peripheral regions of the retinas by using the RNeasy Kit (Quiagen) as per the manufacturer’s instructions. Purified RNA was re-suspended in 100 μL RNAse-free water and DNA removed by using DNAse I (Ambion). cDNA was synthesized from mRNA by using oligo dT primers and Superscript™ III First Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol. Control reactions were performed by excluding the reverse transcriptase to assess whether primers were amplifying genomic DNA. PCR primers were designed by using the web-based program NCBI Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences for RLIM are as follows: for RT-PCR - RLIM forward 5′ CAG CCT GGA TCA GAA AGG AG 3′, RLIM reverse 5′ GAT TTG GAG TTG GGC CAC TA 3′; for qRT-PCR – RLIM forward 5′ GTT CCT CAA GCA GCA GGT TC 3′, RLIM reverse 5′ CCT CCC CGT TCA CTT TCA TA 3′. Predicted product sizes (in base pairs) for RLIM fragments were 748 for RT-PCR and 206 for qRT-PCR. Standard PCR reactions were performed, in which cDNA species were amplified for 35 cycles using standard protocols, Platinum™ Taq (Invitrogen) or TITANIUM™ Taq (Clontech) and an Eppendorf thermal cycler. The PCR products were run on an agarose gel and stained with ethidium bromide to determine the predicted product sizes. PCR products were cloned into TOPO TA Vector (pCR-II; Invitrogen) and sequenced to confirm the specificity of the reaction.
Quantitative RT-PCR
mRNA species were amplified from pools of cDNA by using real-time PCR for 40 cycles. Real-time PCR was performed using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Reactions were performed in triplicates, in 25 μl volumes with 0.5 μM primers and MgCl2 concentration optimized between 2–5 mM. Nucleotides, Taq DNA polymerase, and buffer were included in the SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Amplification protocols were designed on StepOne Software v2.0 (Applied Biosystems, Foster City, CA, USA) and involved a holding stage for 10 min at 95°C, 15 s denaturation step followed by 40 cycles with a 95°C denaturation for 15 s and 60°C annealing for 1 min. The Melt Curve stage included 95°C for 15 s, 60 °C annealing for 1 min and 95 °C for 15 s. Measurements of the fluorescence were carried out at the end of the 60°C annealing period. Ct values obtained from real-time PCR were normalized to GAPDH and the fold difference between control and treated samples was determined using the ΔCt method and represented as a percentage change from baseline.
Fixation, sectioning and immunocytochemistry
Enucleated eyes were hemisected equatorially and the gel vitreous removed from the posterior eye cup. Eye cups and anterior segments were fixed (4% paraformaldehyde plus 3% sucrose in 0.1 M phosphate buffer, pH 7.4, 30 min at 20°C), washed three times in PBS (phosphate-buffered saline; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4), cryoprotected in PBS plus 30% sucrose, immersed in embedding medium (OCT-compound; Tissue-Tek), and mounted onto sectioning blocks. Transverse sections, nominally 12 μm thick, were cut consistently from the posterior pole of the eye (for posterior eye cups) or through the pupil (for anterior segments) in the nasotemporal plane, and thaw-mounted onto SuperFrost Plustm slides (Fisher Scientific). Sections from control and treated eyes from the same individual were placed consecutively on slides to ensure equal exposures to reagents. Sections were air-dried and stored at −20°C until use.
Sections were thawed, ringed with rubber cement, washed three times in PBS, covered with primary antibody solution (200 μl of antiserum diluted in PBS plus 5% normal goat serum, 0.2% Triton X-100, and 0.01% NaN3), and incubated for about 24 h at 20°C in a humidified chamber. The slides were washed three times in PBS, covered with secondary antibody solution, and incubated for at least 1 hour at 20°C in a humidified chamber. Finally, samples were washed three times in PBS, rubber cement removed from the slides, and coverglass mounted on 4:1 (v:v) glycerol to water.
Working dilutions and sources of antibodies used in this study included; (1) Mouse anti-Islet2 was raised to recombinant full-length chicken Islet2 fused to GST and used at 1:50 (51.4H9; DSHB). (2) Mouse anti-Lim3 was raised to recombinant murine Lim3 (amino acids 249–402) fused to GST and used at 1:50 (67.4E12; DSHB). (3) Mouse anti-axonin1 (TAG-1) was raised to E5 chick spinal cord membranes and used at 1:300 (23.4-5; DSHB). (4) Rabbit anti-red/green opsin was raised to recombinant human red/green opsin (the last 42 amino acids at the C-terminus) and used at 1:400 (AB5405; Millipore). (5) Rabbit anti-blue opsin was raised to recombinant human blue opsin (the last 38 amino acids at the C-terminus) and used at 1:500 (AB5408; Millipore). (6) Mouse anti-rhodopsin was raised to purified bovine rhodopsin and used at 1:200 (rho4D2; Dr. R. Molday, University of British Columbia). (7) Mouse anti-calbindin was raised to calbindin D28k purified from chicken gut and used at 1:400 (300; Swant Immunochemicals). (8) Mouse anti-glutamine synthetase (GS) was raised to purified sheep GS and used at 1:5000 (MAB302; Millipore). (9) Mouse anti-Lim1/2 was raised to full-length recombinant rat Lim2 and used at 1:50 (4F2; DSHB). (10) Mouse anti-PCNA was raised to rat PCNA fused to protein A and used at 1:1000 (PC10; Dako immunochemicals). (11) Mouse anti-transitin was raised to transitin purified from E9 chick brain and used at 1:100 (EAP300; DSHB). (12) Rabbit anti-Sox9 was raised to synthetic peptide (VPSIPQTHSPQHWEQPVYTQLTRP) from human Sox9 and used at 1:2000 (AB5535; Millipore) (Poche et al., 2008). (13) Mouse anti-visinin was raised to purified bovine visinin and used at 1:100 (7G4; DSHB). (14) Goat anti-RLIM (RING finger LIM-domain binding protein) was raised to the N-terminus of mouse RLIM and used at 1:100 (sc-55392; Santa Cruz immunochemicals). (15) mouse anti-Brn3a was raised to amino acids 186-224 of Brn-3a fused to the T7 gene 10 protein (Xiang et al., 1995) and used at 1:400 (Chemicon). (16) Mouse anti-protein kinase C (PKC) was raised to a synthetic peptide, aa. 270–427 of human PKCα and used at 1:100 (clone 3; BD Pharmingen). (17) Mouse anti-cellular retinoic acid binding protein (CRABP) was raised to purified bovine CRABP and used at 1:1000 (C1: Dr. J. Saari, U. of Washington). (18) Mouse anti-PSD-95 (post-synaptic density 95) was raised to amino acids 77-299 of human PSD-95/SAP-90 and used at 1:50 (K28/43; NeuroMab).
None of the observed labeling appeared was due to secondary antibody or endogenous fluoresence because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies included donkey-anti-goat-Alexa488, goat-anti-rabbit-Alexa488, goat-anti-mouse-Alexa488/568, rabbit anti-goat Alexa488 and goat-anti-mouse-IgM-Alexa568 (Invitrogen) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Photography, measurements, cell counts, and statistical analyses
Photomicrographs were taken by using a Leica DM5000B microscope equipped with epifluorescence and a 12 megapixel Leica DC500 digital camera. Confocal microscopy was done by using a Zeiss LSM 510. Images were optimized for color, brightness and contrast, and double-labeled images overlaid by using Adobe Photoshop™ 6.0. Images chosen for the figures are representative of the means of the data sets. Cell counts were made from at least 8 different animals, and means and standard errors calculated on data sets. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the same region of retina for each data set.
Distances were quantified by using ImagePro 6.2. 5.4 megapixel digital images provided a spatial resolution of 3.7 pixels per micrometer. This method of measurement provides highly accurate and reproducible measurements (Fischer et al., 2007b, Fischer et al., 2008a, Fischer et al., 2008b, Ghai et al., 2009).
Results
Immature photoreceptors continue to express Islet2, Lim3 and axonin1, whereas red/green opsin, rhodopsin, calbindin and synaptic proteins are not expressed (Fischer et al., 2008a). Over the first 2 weeks of post-hatch development the photoreceptors gradually mature to express photopigments and calbindin (Fischer et al., 2008a). We found that the maturation of photoreceptors was greatly accelerated by L-Thyroxine (T4). Triiodothyronine (T3) is about four times more biologically active than thyroxine prohormone (T4), which is converted to T3 by deiodinases; enzymes that are known to be expressed by retinal cells (Trimarchi et al., 2008, Ng et al., 2010).
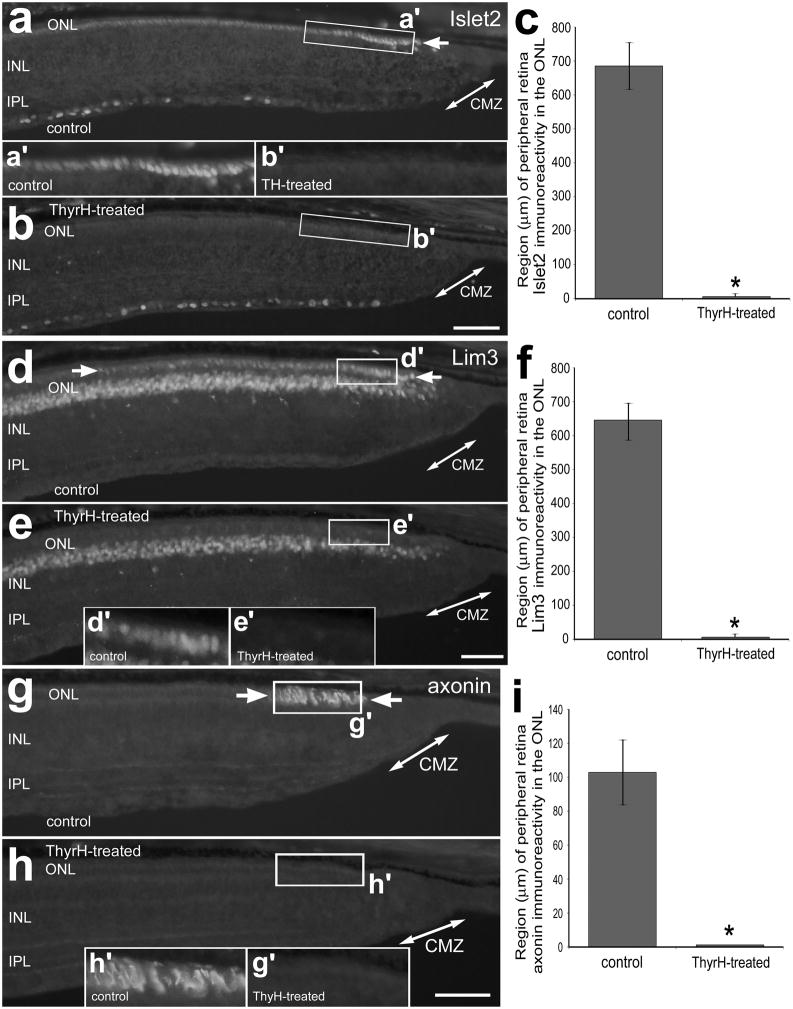
We found that 4 consecutive daily injections of TH from P0 through P3 (paradigm B) caused a down-regulation of Islet2, Lim3 and axonin1 in far peripheral regions of the retina (Fig. 1). Levels of Islet2 and Lim3 were reduced below detectable levels in the nuclei of photoreceptors in far peripheral regions of the outer nuclear layer (ONL) by TH-treatment (Figs. 1a–f). Similarly, levels of axonin1-immunofluoresence in photoreceptors fell below detectable levels in far peripheral regions of the ONL with TH-treatment (Figs. 1g–i).
Figure 1.
TH down-regulates the expression of Islet2, Lim3 and axonin1 in the outer nuclear layer (ONL) in far peripheral regions of the postnatal chicken retina. Treated eyes received injections of 2 μg of TH at P0, P1, P2 and P4, whereas control eyes received injections of vehicle. Sections of the peripheral retina and CMZ were labeled with antibodies to Islet2 (a and b), Lim3 (d and e) and axonin1 (g and h). Double-ended arrows indicate the domain of the CMZ, and arrows indicate the domain of cells in the ONL that are positive for Islet2, axonin1 or Lim3. Areas indicated by rectangular boxes are enlarged 2-fold in the in-sets between panels a and b, and within panels e and h. The calibration bar (50 μm) in h applies to a, b, d, e, g and h. Histograms illustrate the means (± standard deviation) of the expression domains within the far peripheral ONL (c, f and i). The “expression domain” was measured as the linear, radial distance of retina (beginning at the CMZ) that contains cells with detectable levels of immunofluoresence. Significance of difference (*p<0.0001) was determined using a two-tailed student’s t-test. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, CMZ – circumferential marginal zone.
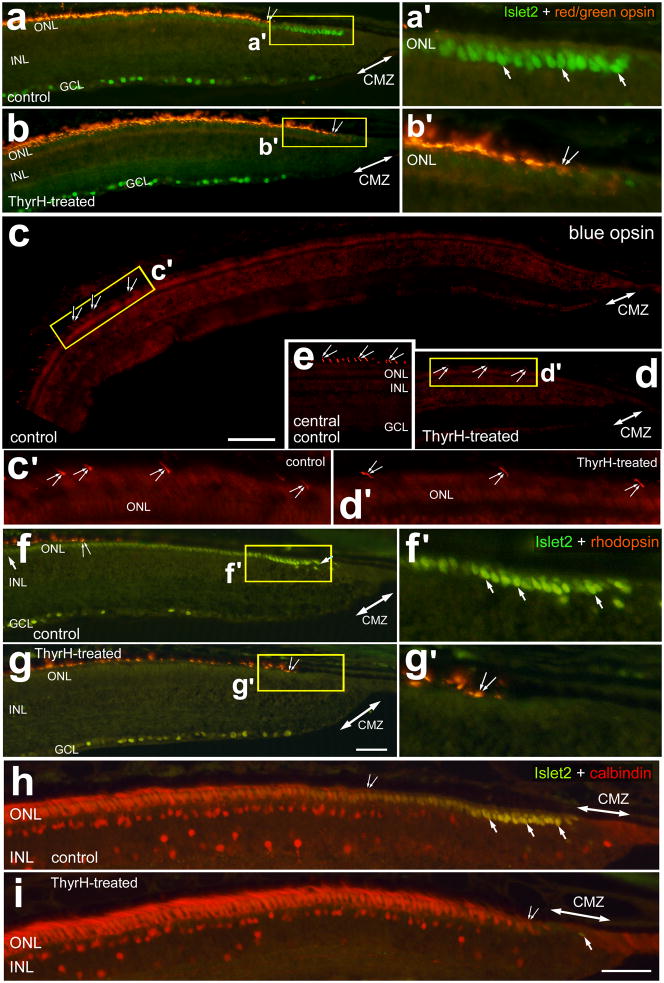
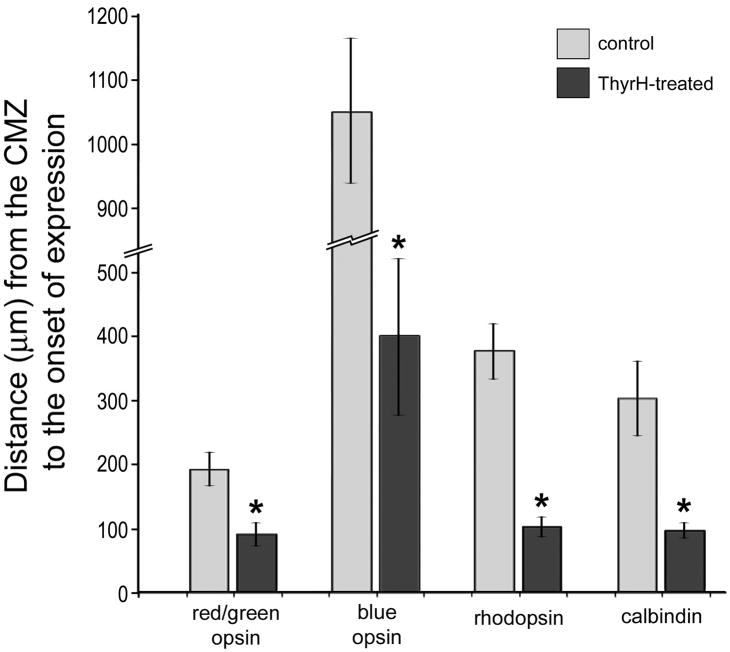
To assess whether photoreceptor maturation occurred with TH-mediated down-regulation of Islet2, Lim3 and axonin1 we probed for the expression of opsins and calbindin. We found that TH stimulated the maturation of cone photoreceptors in far peripheral regions of the retina, as indicated by the up-regulation of red-green opsin (Figs. 2a and 2b) and blue/violet opsin (Figs. 2c–e). Compared to chick blue opsin, chick violet opsin shares greater sequence homology to mammalian blue opsin. Therefore, the antibody to mammalian blue opsin likely recognizes chick violet opsin. The domain of immature photoreceptors that do not express cone opsins was significantly reduced in TH-treated eyes (Figs. 2a–d and 3). Similarly, the maturation of rod photoreceptors, as indicated by the onset of rhodopsin expression, was stimulated by TH (Figs. 2f, 2g and 3). Consistent with these findings, we found that TH-treatment stimulated the cone photoreceptors in far peripheral retinal regions to up-regulate calbindin (Figs. 2h, 2i and 3). Calbindin is known to be expressed by most, if not all, types of cone photoreceptors in the mature chick retina (Fischer et al., 2007a, Fischer et al., 2008a).
Figure 2.
TH stimulates the expression of opsins and calbindin in photoreceptors in far peripheral regions of the postnatal chick retina. Treated eyes received injections of 2 μg of TH at P0, P1, P2 and P4, whereas control eyes received injections of vehicle. Sections of the peripheral retina and CMZ were labeled with antibodies to Islet2 (green; a, b and f–i), red/green opsin (red; a and b), violet opsin (red; c–e), rhodopsin (red; f and g), and calbindin (h and i). Panel e is a representative image of immunolabeling for violet opsin in the outer segments of cone photoreceptors in a central region of retina, approximately 3 mm from the CMZ. Double-ended arrows indicate the domain of the CMZ, arrows Islet2-positive cells in the ONL, and small double-arrows indicate the onset of expression photoreceptor markers. Areas indicated by yellow boxes are enlarged 3-fold in the panels immediately to the right (a′, b′, f′ and g′). Panels c′ (approximately 1000 μm) and d′ (approximately 300 μm) are 4-fold enlargements of the areas indicated by yellow boxes in c and d. The calibration bar (50 μm) in panel c applies to panels c–e, the bar in g applies to a, b, f and g, and the bar in i applies to h and i. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Figure 3.
The histogram indicates the mean (± standard deviation) distance (μm) from the anterior edge of the CMZ to the onset of expression of photoreceptor markers. The distances were measured as the linear, radial distance beginning at the CMZ to the first appearance of immunofluorescence in photoreceptors. Significance of difference (*p<0.0001) was determined using a two-tailed student’s t-test. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Consecutive daily doses of TH of at least 2 μg/dose stimulated the maturation of photoreceptors, whereas doses of 1 μg TH or less had no effect (not shown). A single large dose (5 μg) of TH on the day of hatching had no significant influence upon the expression of Islet2, Lim3, axonin1, photopigments or calbindin in photoreceptors in far peripheral regions of the retina (not shown). Taken together these findings suggest that injections of TH greatly accelerate the maturation of both rod and cone photoreceptors in the far peripheral retina.
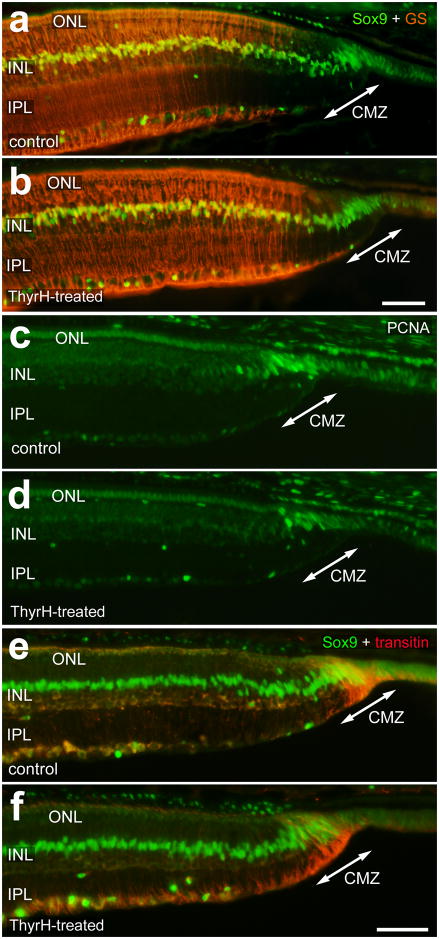
To assess whether the maturation of amacrine and bipolar cells is influenced by TH, we probed for the expression of different markers for inner retinal neurons in control and TH-treated eyes. TH had no obvious effect upon the maturation of bipolar cells, as assessed by immunolabeling for PKC, CRABP and PSD-95 (not shown). We next examined whether TH influenced the maturation of Müller glia in far peripheral regions of the retina. In control retinas, Sox9-expressing Müller glia immediately posterior to the CMZ were negative for glutamine synthetase (GS) (Fig. 4a). The zone of GS-negative Müller glia was approximately 150 μm wide at the temporal edge of the retina (Fig. 4a). In retinas treated with TH, the Müller glia in far peripheral regions appeared to up-regulate GS, narrowing the “gap” in expression (Figs. 4a and 4b).
Figure 4.
TH does not influence the abundance of progenitors in the CMZ, but stimulates the maturation of Müller glia in far peripheral regions of the retina. Treated eyes received injections of 2 μg of TH at P0, P1, P2 and P4, whereas control eyes received injections of vehicle. Vertical sections of the far peripheral retina were labeled with antibodies to Sox9 and glutamine synthetase (GS; a and b), PCNA (c and d), and Sox9 and transitin (e and f). Retinas were obtained from eyes treated with vehicle (a, c and e) or 4 consecutive daily injections of TH (b, d and f). The calibration bar (50 μm) in a applies to a alone, and the bar in e applies to c–e. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
We next examined whether TH influenced the retinal progenitors within the CMZ. We assayed for progenitors within the CMZ by probing for Proliferating-Cell Nuclear Antigen (PCNA), Sox9 and transitin, markers that are known to be expressed by retinal progenitors (Fischer and Omar, 2005, Fischer et al., 2009). We found that there was no significant influence of TH on the number of PCNA-positive cells within the CMZ (Figs. 4c and 4d). In addition, there was no significant influence of TH on the number of Sox9/transitin progenitors in the CMZ (Figs. 4e and 4f). These findings suggest that TH does not enhance nor diminish the pool of progenitors in the CMZ.
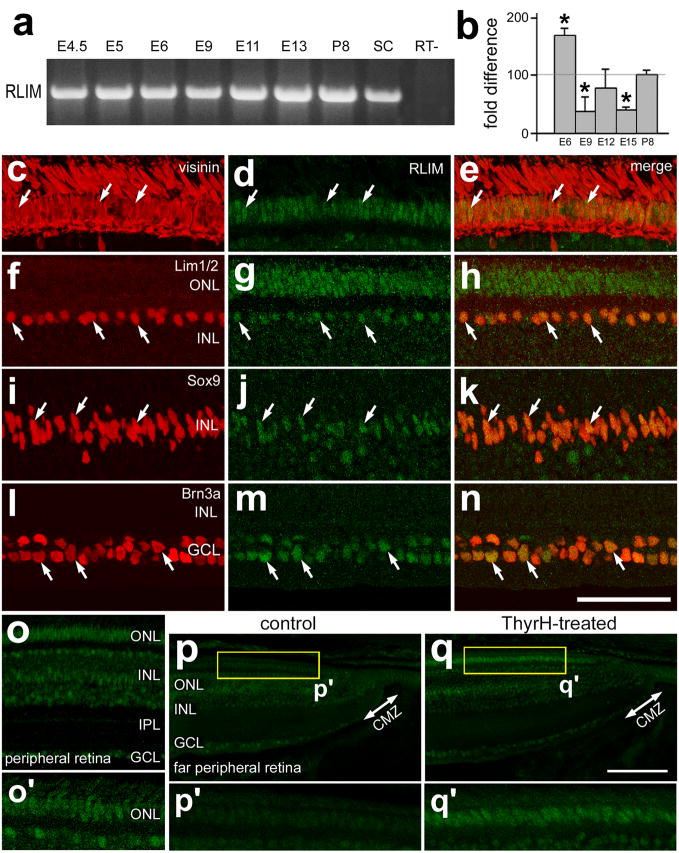
The mechanisms that control the down-regulation of Islet2 and Lim3 in photoreceptors are unknown, but could involve LIM-domain binding proteins such RLIM (RING finger LIM-domain binding protein). RLIM is known to inhibit the activity of LIM-domain transcription factors, whereas interactions with CLIM proteins promotes the activity of LIM-domain transcription factors (reviewed by (Retaux and Bachy, 2002). RLIM functions as a ubiquitin protein ligase that targets LIM-domain factors for degradation through the 26S proteasome pathway (Ostendorff et al., 2002). To better understand whether RLIM could be involved in retinal development, we probed for the expression of RLIM in the embryonic retina and in far peripheral regions of the postnatal retina. By using RT-PCR, we found that RLIM is expressed throughout retinal development, starting at E4 and continuing through P8 (Fig. 5a). By using qRT-PCR, we found that levels of RLIM expression are dynamic during development. Levels of RLIM were highest at E6, decreased at E9 through E15, and remained stable at an elevated level during postnatal development (Fig. 5b). Levels of RLIM in central regions of the postnatal retina did not change significantly from P1 through P28 (not shown). In central regions of the retina, we found that RLIM was expressed by distinct types of cells. RLIM-immunofluoresence was present at high levels in the nuclei of visinin-positive photoreceptors (Figs. 5c–e), in Lim1/2-positive horizontal cells (Figs. 5f–g), in Sox9-positive Müller glia (Figs. 5i–k), and Brn3a-positive ganglion cells (Figs. 5l–n). These patterns of RLIM expression were maintained in peripheral regions of the retina, between 1 and 3 mm of the CMZ (Fig. 5o). By comparison, far peripheral regions of the retina, within 1 mm of the CMZ, contained very low levels of RLIM-immunofluoresence (Figs. 5o and 5p). In particular, there was no detectable RLIM in the ONL in far peripheral regions of the retina at P1 (Fig. 5p). Intraocular injections of TH resulted in the appearance of RLIM in the ONL in far peripheral regions of the retina (Fig. 5q).
Figure 5.
RLIM is dynamically expressed during retinal development, and is up-regulated in mature photoreceptors and in response to treatment with TH. RT-PCR (a) and quantitative RT-PCR (b) were used to detect and measure levels RLIM in embryonic retina from E4.5 through P8. E8 spinal cord (SC) was used as a positive control. Vertical sections of central (c–n) and peripheral (o–q) retina were labeled with antibodies to RLIM (green) and visinin (red: c and e), Lim1/2 (red; f and h), Sox9 (red; i and k), and Brn3a (red; l and n). Retinas were obtained from control (c–p) and TH-treated (q) eyes. Treated eyes received injections of 2 μg of TH at P0, P1, P2 and P4, whereas control eyes received injections of vehicle. Arrows indicate cells labeled for RLIM and other markers. The calibration bar (50 μm) in n applies to c–n, and the bar in e applies to c–e. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, CMZ – circumferential marginal zone, SC – spinal cord.
Discussion
The data provided here demonstrate that TH stimulates the maturation of photoreceptors in the far peripheral retina. This maturation was characterized by the onset of expression of red-green opsin, blue/violet opsin, rhodopsin and calbindin. Further, TH-mediated upregulation of photopigments and calbindin in photoreceptors is coincident with the down-regulation of axonin1, Islet2 and Lim3. However, evidence indicates that one large dose of TH into the eyes of newly hatched chicks does not significantly influence the maturation of photoreceptors in far peripheral regions of the retina. Thus, it is possible that larger doses are required or sustained exposure, over several days, to TH is required to stimulate the maturation of photoreceptors. We propose the TH-mediated down-regulation of the transcription factors Lim3 and Islet2 permits the maturation of photoreceptors. However, it remains unknown whether TH-mediated maturation of photoreceptors is a function of the down-regulation of Islet2 and Lim3.
It is likely that TH influences the differentiation of photoreceptors in central retinal regions during the normal course of embryonic development. Interestingly, levels of serum TH fluctuate dynamically through embryonic and postnatal development (Lu et al. 2007). In chicks, there are systemic increases in TH that occur during late stages of embryonic development between E15 and E20 (Lu et al. 2007). Before hatching, between E19 and E21, levels of TH are decreased, remain low from P1 through P5, and slowly become elevated from P6 through P21 (Lu et al. 2007). In our studies, 2 μg of T4 thyroxine were injected into the vitreous chamber at P0 through P3, when serum levels of T4 are relatively low. This dose should have provided a transient, initial maximum concentration, assuming a vitread chamber volume of 1ml, approximately 50-fold above serum T4 levels, but should have been cleared quickly from the eye given the hydrophilic nature of the hormone. The increases in TH during embryonic development coincide with when photoreceptors mature to express photopigments (Bruhn and Cepko, 1996). Together, these findings suggest that TH coordinates photoreceptor maturation with late-stage embryonic development to prepare the animal for vision at the time of hatching. Thus, we propose that increases in systemic TH down-regulate levels of Islet2 and Lim3 in photoreceptors and, thereby, permit expression of opsins and calbindin. We attempted to test whether TH stimulates precocious maturation of photoreceptors in the embryo. However, we found that the delivery of between 20 to 50 μg of TH into yolk-sac of embryos between E10 and E12 was nearly always lethal, with embryos perishing within 2 days of treatment (data not shown).
It is likely that the effects of TH on the maturation of chick photoreceptors are mediated by TRβ2, a nuclear hormone receptor for TH. TRβ2 is known to be expressed at high levels in the ONL (but not in inner retinal cells) after E6 and through E16 in the embryonic chick retina, but is down-regulated by the time of hatching (Sjoberg et al., 1992). However, it remains unknown whether TRβ2 is expressed by all types of photoreceptors in the embryonic and far peripheral regions of the post-hatch retina. It remains uncertain how photoreceptors in far peripheral regions of the postnatal retina fail to mature during embryonic surges in TH levels. The photoreceptors in far peripheral regions of the retina are known be generated between E12 and E13 (Ghai et al., 2008) before elevations in systemic TH (Lu et al., 2007). It is possible that Deiodinase 3 inactivates TH locally to keep the photoreceptors in far peripheral regions of the retina from maturing during systemic increases of TH during development. Deiodinases are dynamically expressed during development and are thought to locally regulate levels of active hormone within tissues (reviewed by (Galton, 2005, Bianco and Kim, 2006). For example, Deiodinase 3 removes iodine from either T4 prohormone or T3 active hormone to render these compounds incapable of activating receptors. Deiodinase 3 is expressed by mitotically active progenitor cells, and perhaps immature Müller glia (Trimarchi et al., 2008); cell-types that persist in peripheral regions of late-stage chick embryos (Ghai et al., 2008).
Our findings are consistent with reports that have demonstrated the influence of TH-signaling in different classes of vertebrates. In the rodent retina, studies have demonstrated the requirement for TH and TRβ2 in the differentiation of red/green cones (Ng et al., 2001, Roberts et al., 2006). During the normal course of retinal development, it has been proposed that a ventral-to-dorsal gradient of TH-signaling establishes domains of red/green opsin-expressing cone photoreceptors in dorsal retina and the transition to blue opsin-expressing cone photoreceptors in ventral retina (Roberts et al., 2006). A recent study has indicated that type 3 deiodinase limits retinal levels of TH to support survival of cone photoreceptors, excessive levels of TH cause the death of cones, and permit the patterning of red/green-cones to ventral retina and blue-cones to dorsal retina (Ng et al., 2010). By comparison, in salmonid fish, TH is known to induce the degeneration of UV-sensitive cones, whereas TH-withdrawal permits the regeneration of UV-sensitive cones (Allison et al., 2006, Raine and Hawryshyn, 2009, Raine et al., 2010). The degeneration and regeneration of UV-sensitive cones coincide with transitions between fresh and saltwater environments during metamorphosis and with return to rivers for spawning (Hawryshyn et al., 1989). In the avian retina, we provide evidence that TH signaling may regulate the delayed maturation of both rod and cone photoreceptors by down-regulating the transcription factors Islet2 and Lim3. Collectively, these findings indicate that TH-signaling has important functions in photoreceptor development. However, the specific roles of TH in photoreceptor development differ between vertebrate classes.
The functional significance of TH-mediated down-regulation of axonin1 remains uncertain. Axonin1 is a member of the immunoglobulin superfamily and is known to function as a cell adhesion molecule to guide the formation of neurites in the developing CNS (Stoeckli and Landmesser, 1995, Sonderegger et al., 1998, Stoeckli, 1998, Perrin et al., 2001, Stepanek et al., 2005). In the retina, axonin1 is expressed by differentiating amacrine and ganglion cells (Drenhaus et al., 2004), and immature photoreceptors (Fischer et al., 2008a). We propose that the expression of axonin1 in immature photoreceptors may facilitate the growth of photoreceptor axons, or may suppress the premature formation of outersegments prior to the onset expression of photopigments.
The regulation of expression of LIM-domain transcription factors is likely to be cell type-specific. The LIM-domain transcription factors are known to be expressed by different types of cells in the retina. For example, Islet1 is expressed by ganglion cells, cholinergic amacrine cells, many bipolar cells and a subset of horizontal cells early during development (Galli-Resta et al., 1997, Fischer et al., 2002, Fischer and Reh, 2003, Edqvist et al., 2006, Fischer et al., 2008a). A recent publication has demonstrated that Islet1 is required for the normal differentiation and survival of bipolar cells, cholinergic amacrine cells, and ganglion cells in the rodent retina (Elshatory et al., 2007). Islet2 is another LIM-domain homeotic transcription factor and is known to be expressed by a subset of ganglion cells in the retina. Loss of function mutations in Islet2 result in aberrant formation of visceral motor neurons in the spinal cord (Thaler et al., 2004) and pathfinding deficits in retinal ganglion cells (Pak et al., 2004). It remains unknown whether Islet2 contributes to aspects of retinal development in addition to axon-guidance in ganglion cells. Lim3, also known as Lim Homeobox Gene 3 (Lhx3), is normally expressed by post-mitotic bipolar cells in the developing (Edqvist and Hallbook, 2004) and mature retina (Fischer et al., 2008a). Lim3 is known to participate in the specification of motor neurons and interneurons in the developing spinal cord (Thaler et al., 2002). Currently, nothing is known about the roles of Lim3 in the retina. Lim1/2 is expressed by differentiating horizontal cells shortly after terminal mitotsis (Edqvist and Hallbook, 2004). The onset and patterns of expression of Islet1, Islet2, Lim3 and Lim1/2 in inner retinal neurons do not coincide with the expression of TH-receptors or fluctuations of systemic TH levels during development. Thus, TH-signaling likely does not influence the expression of LIM-domain transcription factors in inner retinal neurons; TH-mediated down-regulation of Islet2 and Lim3 is likely to be exclusive to photoreceptors in the retina.
It is possible that TH acts indirectly to down-regulate Islet2 and Lim3 by up-regulating RLIM in photoreceptors. RLIM is known to negatively regulate the activity of LIM-domain transcription factors (reviewed by (Retaux and Bachy, 2002). RLIM can directly interact with and ubiquitinate other LIM-domain proteins to target these proteins for degradation through the 26S proteasome pathway (Ostendorff et al., 2002). Thus, upregulation of RLIM is a possible mechanism to down-regulate LIM domain transcription factors. We found that injections of TH results in increased RLIM expression in photoreceptors in far peripheral regions of the retina. However, we found that levels of RLIM are decreased at E15, when Islet2 and Lim3 are down-regulated in photoreceptors, photopigments are upregulated in photoreceptors, and when systemic levels of TH become elevated. However, qRT-PCR was used to measure total retinal levels of RLIM, and decreased RLIM at E15 may not represent levels in photoreceptors. Immunofluoresence indicates that relative levels of RLIM are increased in mature photoreceptors in the postnatal retina and that exogenous TH stimulates RLIM expression in far peripheral regions of the postnatal retina. Thus, we propose that TH induces the expression of RLIM, which, in turn, may reduce levels Islet2 and Lim3 in photoreceptors by targeting these factors for degradation. Further, we propose that TH-induced expression of RLIM occurs through activation of TRβ2.
Our data suggest that the maturation of inner retinal neurons is unaffected by TH, whereas the maturation of Müller glia may be stimulated. We found that glial expression of glutamine synthetase is accelerated by TH-treatment in far peripheral regions of the retina. This could occur directly through activation of an unidentified TH receptor expressed by immature Müller glia. Alternatively, TH-mediated upregulation of glutamine synthetase could occur secondarily through the maturation of photoreceptors via activation of TRβ2. Interestingly, during embryonic retinal development the expression of glutamine synthetase in young, postmitotic Müller glia remains low until about E15 and increases sharply thereafter (Moscona and Moscona, 1979, Linser and Moscona, 1983, Vardimon et al., 1986). Developmentally, the elevated expression of glutamine synthetase coincides with the maturation of photoreceptors and increases in systemic TH. The expression of glutamine synthetase in Müller glia can be induced by activation of the glucocorticoid receptor with cortisol (Moscona and Moscona, 1979). It remains uncertain whether intraocular injections of TH somehow activate signaling through glucocorticoid receptors in immature Müller glia.
Acknowledgments
We thank Dr. Heithem El-Hodiri for providing comments that contributed to final form of the manuscript. We thank Christopher Zelinka for providing expert technical assistance. We also wish to thank Dr. J. Saari for kindly providing antibodies to CRABP. The antibodies developed by Drs T. Jessell (Islet2, Lim3, Lim1/2), J. Dodd (axonin1), and C. Cepko (visinin), respectively, were obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the NICHD and is maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Confocal microscopy was performed at the Hunt-Curtis Imaging Facility at the Department of Neuroscience of The Ohio State University. This work was supported by grants from the National Science Foundation (#0413795) and the National Institutes of Health (EY016043-05) to AJF.
Support: Start-up funds from The Ohio State University (to A.J.F.) and grant support NIH RO1 EY016043-05 (to A.J.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison WT, Dann SG, Veldhoen KM, Hawryshyn CW. Degeneration and regeneration of ultraviolet cone photoreceptors during development in rainbow trout. J Comp Neurol. 2006;499:702–715. doi: 10.1002/cne.21164. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford RL, Wang C, Zack DJ, Adler R. Roles of cell-intrinsic and microenvironmental factors in photoreceptor cell differentiation. Dev Biol. 2005;286:31–45. doi: 10.1016/j.ydbio.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J Neurosci. 1996;16:1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EE, Hendrickson AE, Provis JM. Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: early development of spatial order and density profiles. Vision Res. 2004;44:2019–2026. doi: 10.1016/j.visres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Cornish EE, Madigan MC, Natoli R, Hales A, Hendrickson AE, Provis JM. Gradients of cone differentiation and FGF expression during development of the foveal depression in macaque retina. Vis Neurosci. 2005;22:447–459. doi: 10.1017/S0952523805224069. [DOI] [PubMed] [Google Scholar]
- Drenhaus U, Morino P, Rager G. Expression of axonin-1 in developing amacrine cells in the chick retina. J Comp Neurol. 2004;468:496–508. doi: 10.1002/cne.10986. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Hallbook F. Newborn horizontal cells migrate bi-directionally across the neuroepithelium during retinal development. Development. 2004;131:1343–1351. doi: 10.1242/dev.01018. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Myers SM, Hallbook F. Early identification of retinal subtypes in the developing, pre-laminated chick retina using the transcription factors Prox1, Lim1, Ap2alpha, Pax6, Isl1, Isl2, Lim3 and Chx10. Eur J Histochem. 2006;50:147–154. [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Foster S, Scott MA, Sherwood P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. J Comp Neurol. 2008a;506:584–603. doi: 10.1002/cne.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Omar G. Transitin, a nestin-related intermediate filament, is expressed by neural progenitors and can be induced in Muller glia in the chicken retina. J Comp Neurol. 2005;484:1–14. doi: 10.1002/cne.20406. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259:225–240. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008b;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009;57:166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol. 2007a;500:1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Ghai K, Scott M, Omar G. Development of bullwhip neurons in the embryonic chicken retina. J Comp Neurol. 2007b;503:538–549. doi: 10.1002/cne.21404. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17:7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton VA. The roles of the iodothyronine deiodinases in mammalian development. Thyroid. 2005;15:823–834. doi: 10.1089/thy.2005.15.823. [DOI] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;1192:76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Serotonin released from amacrine neurons is scavenged and degraded in bipolar neurons in the retina. J Neurochem. 2009;111:1–14. doi: 10.1111/j.1471-4159.2009.06270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryshyn CW, Arnold MG, Chaisson DJ, Martin PC. The ontogeny of ultraviolet photosensitivity in rainbow trout (Salmo gairdneri) Vis Neurosci. 1989;2:247–254. doi: 10.1017/s0952523800001164. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Hicks D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res. 2002;74:435–444. doi: 10.1006/exer.2002.1181. [DOI] [PubMed] [Google Scholar]
- Linser P, Moscona AA. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983;96:529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Lu JW, McMurtry JP, Coon CN. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poult Sci. 2007;86:673–683. doi: 10.1093/ps/86.4.673. [DOI] [PubMed] [Google Scholar]
- Morris VB. Symmetry in a receptor mosaic demonstrated in the chick from the frequencies, spacing and arrangement of the types of retinal receptor. J Comp Neurol. 1970;140:359–398. doi: 10.1002/cne.901400308. [DOI] [PubMed] [Google Scholar]
- Moscona M, Moscona AA. The development of inducibility for glutamine synthetase in embryonic neural retina: inhibition by BrdU. Differentiation. 1979;13:165–172. doi: 10.1111/j.1432-0436.1979.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Lyubarsky A, Nikonov SS, Ma M, Srinivas M, Kefas B, St Germain DL, Hernandez A, Pugh EN, Jr, Forrest D. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30:3347–3357. doi: 10.1523/JNEUROSCI.5267-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorff HP, Peirano RI, Peters MA, Schluter A, Bossenz M, Scheffner M, Bach I. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature. 2002;416:99–103. doi: 10.1038/416099a. [DOI] [PubMed] [Google Scholar]
- Pak W, Hindges R, Lim YS, Pfaff SL, O’Leary DD. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell. 2004;119:567–578. doi: 10.1016/j.cell.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Perrin FE, Rathjen FG, Stoeckli ET. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron. 2001;30:707–723. doi: 10.1016/s0896-6273(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J Comp Neurol. 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Raine JC, Coffin AB, Hawryshyn CW. Systemic thyroid hormone is necessary and sufficient to induce ultraviolet-sensitive cone loss in the juvenile rainbow trout retina. J Exp Biol. 2010;213:493–501. doi: 10.1242/jeb.036301. [DOI] [PubMed] [Google Scholar]
- Raine JC, Hawryshyn CW. Changes in thyroid hormone reception precede SWS1 opsin downregulation in trout retina. J Exp Biol. 2009;212:2781–2788. doi: 10.1242/jeb.030866. [DOI] [PubMed] [Google Scholar]
- Retaux S, Bachy I. A short history of LIM domains (1993–2002): from protein interaction to degradation. Mol Neurobiol. 2002;26:269–281. doi: 10.1385/MN:26:2-3:269. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S, Erickson A, Hendrickson A. The spatial and temporal expression of outer segment proteins during development of Macaca monkey cones. Invest Ophthalmol Vis Sci. 2000;41:971–979. [PubMed] [Google Scholar]
- Sjoberg M, Vennstrom B, Forrest D. Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for alpha and N-terminal variant beta receptors. Development. 1992;114:39–47. doi: 10.1242/dev.114.1.39. [DOI] [PubMed] [Google Scholar]
- Sonderegger P, Kunz S, Rader C, Buchstaller A, Berger P, Vogt L, Kozlov SV, Ziegler U, Kunz B, Fitzli D, Stoeckli ET. Discrete clusters of axonin-1 and NgCAM at neuronal contact sites: facts and speculations on the regulation of axonal fasciculation. Prog Brain Res. 1998;117:93–104. doi: 10.1016/s0079-6123(08)64010-8. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET. Molecular mechanisms of commissural axon pathfinding. Prog Brain Res. 1998;117:105–114. doi: 10.1016/s0079-6123(08)64011-x. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Harpavat S, Billings NA, Cepko CL. Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev Biol. 2008;8:101. doi: 10.1186/1471-213X-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L, Fox LE, Moscona AA. Developmental regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci U S A. 1986;83:9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]