Abstract
Purpose
PF299804 is a potent, orally available, irreversible inhibitor of tyrosine kinase human epidermal growth factor receptors (HER) 1 (EGFR), HER2, and HER4. This first-in-human study investigated the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF299804 in patients with advanced solid malignancies.
Experimental Design
PF299804 was administered once daily continuously (schedule A), and intermittently (schedule B). Dose escalation proceeded until intolerable toxicities occurred. Skin biopsies were taken pre-dose and after 14 days of treatment, to establish a pharmacokinetic/pharmacodynamic relationship. Tumor response was measured once every 2 cycles. Efficacy was correlated with tumor genotypes in non-small cell lung cancer (NSCLC) patients.
Results
121 patients were included (111 in schedule A, 10 in schedule B). The maximum tolerated dose (MTD) was 45 mg/day. Dose-limiting toxicities included stomatitis and skin toxicities. Most adverse events were mild and comprised skin toxicities, fatigue, and gastrointestinal side-effects including diarrhea, nausea, and vomiting. Pharmacokinetic analyses revealed dose-dependent increases in PF299804 exposure associated with target inhibition in skin biopsy samples. Fifty-seven patients with non-small cell lung cancer (NSCLC) were treated in this study. Four patients, all previously treated with gefitinib or erlotinib (2 with exon 19 deletions, 1 with exon 20 insertion, 1 mutational status unknown), had a partial response to PF299804.
Conclusions
The MTD of PF299804 is 45 mg/day. Both continuous and intermittent treatment schedules were well tolerated, and encouraging signs of antitumor activity were observed in gefitinib/erlotinib treated NSCLC patients.
Keywords: Phase I clinical trial, Non-small cell lung cancer, epidermal growth factor receptor, mutation, kinase inhibitor
Introduction
The human epidermal growth factor receptor (HER) family of receptor tyrosine kinases comprises four members: epidermal growth factor receptor (EGFR, also called HER1 or ErbB1), HER2 (ErbB2/neu), HER3 (ErbB3), and HER4 (erbB4) (1). EGFR tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, have an established role in the treatment of non-small cell lung cancer (NSCLC) (2, 3). NSCLC patients with EGFR mutant cancers derive the greatest degree of clinical benefit from EGFR TKI therapy (3). Ultimately, all responding patients develop resistance (acquired resistance) to these agents. EGFR T790M, detected in 50% of patients, is the most common mechanism of acquired resistance (4).
PF299804 is a potent, highly selective, irreversible small-molecule inhibitor of EGFR, HER2, and HER4 (5, 6). PF299804 offers potential advances in targeting the HER signaling pathways. PF299804 achieves irreversible inhibition via covalent modification of nucleophilic cysteine residues in the catalytic domains of the HER family receptors. Irreversible inhibitors induce prolonged suppression of tyrosine kinase activity compared with reversible inhibitors, leading to improved antitumor activity in preclinical models (7). The T790M mutation leads to an increase in the affinity of EGFR for ATP, thus dramatically reducing the efficacy of reversible quinazoline inhibitors like gefitinib and erlotinib (8). Irreversible inhibitors achieve greater occupancy at the ATP site leading to inhibition of EGFR T790M despite the increased affinity of the receptor for ATP. PF299804 inhibits not only wild-type and the common activating mutations of the EGFR, but also demonstrates significant preclinical activity against tumors bearing the T790M mutation both in vitro and in vivo (5). PF299804 is also highly effective in lung cancer models with activating EGFR mutations that are resistant to gefitinib alone such as the EGFR exon 20 insertion mutations (5, 9). Several irreversible EGFR inhibitors are currently under evaluation in preclinical models and/or in clinical trials (10–12).
In contrast to gefitinib or erlotinib, PF299804 inhibits all three kinase-active members of the HER family. This may be advantageous in treating cancers, such as NSCLC, where genomic alterations involving multiple HER family have been described (13, 14). In fact pre-clinical studies have demonstrated that PF299804 is also effective against NSCLC models harboring either HER2 amplifications or mutations(5).
Pre-clinically, PF299804 shows promising pharmacokinetic properties across species and has a higher bioavailability, longer half-life, and larger volume of distribution than CI-1033, a first-generation, irreversible, pan-HER inhibitor (6). This first-in-human study investigated the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF299804 in patients with advanced solid malignancies. In addition, a large cohort of molecularly characterized NSCLC patients, previously treated with gefitinib or erlotinib, were included in order to explore the activity of this drug in the intended Phase II target population.
Methods
Study Design
The primary objectives of this Phase I study (NCT00225121) were to evaluate the safety and tolerability, and to define the maximum tolerated dose (MTD) of PF299804. Accelerated dose escalation proceeded in 100% increments until 1 patient experienced a dose limiting toxicity (DLT) and/or two patients experienced the same drug-related adverse event (AE) during the first treatment cycle. From this point, dose escalation continued according to a modified Fibonacci scheme. If a DLT was observed in one of the three initial patients treated at a dose level, up to three additional patients were enrolled into that cohort. Dose escalation continued until at least two of the three to six patients treated at that dose level experienced a DLT. The next lower dose was considered to be the MTD, defined as the highest dose associated with DLTs in less than 33% of patients receiving PF299804 administered on a continuous daily dosing (CDD) schedule. A DLT was defined as any of the following events as classified according to the National Cancer Institute Common Terminology Criteria (NCI-CTC) for AEs, version 3.0, which was attributed to PF299804 and occurred during the first 21 days of treatment (cycle 1): grade 3 or 4 nausea, vomiting, or diarrhea despite the use of adequate/maximal medical intervention and/or prophylaxis; any other grade 3 or greater non-hematologic toxicity; delayed recovery from toxicity related to PF299804 treatment which delays scheduled retreatment for > 14 days; grade 4 neutropenia (absolute neutrophil count (ANC) < 500 cells/mm3) for five or more consecutive days or febrile neutropenia (ie, fever > 38.5°C with ANC < 1,000 cells/mm3); grade 4 thrombocytopenia (< 25,000 platelets/mm3) or bleeding requiring a platelet transfusion. Intra-patient dose escalation was permitted if the maximum toxicity experienced during prior cycles of therapy was grade ≤2 and three patients receiving the next higher dose had completed 3 weeks of therapy without experiencing DLT.
Following the dose-escalation portion of the trial (schedule A), PF299804 was evaluated in a series of expansion cohorts as follows: (1) schedule B: at a dose 33% higher than the continuously dosed maximum tolerated dose (MTD) administered on an intermittent schedule comprising oral, once-daily (QD) dosing for 2 weeks followed by 1 week off therapy, repeated in 21-day cycles; (2) schedule A, food effect: to investigate the effect of food on PF299804 pharmacokinetics at the schedule A MTD; (3) schedule A, gastric pH effect: to investigate the effects of stomach pH on PF299804 pharmacokinetics at the schedule A MTD; (4) schedule A, loading dose: a loading dose of PF299804 at the schedule A MTD administered twice daily for 3 days followed by QD dosing at the schedule A MTD for the rest of cycle 1 and subsequent cycles. The loading dose schedule was explored as a way to achieve faster steady state levels of PF299804 than achieved with continuous dosing.
The Schedule A MTD expanded cohort was planned to include patients with a pretreatment biopsy of the primary tumor or a metastatic site showing increased copy number of EGFR, HER2 and/or HER3, or, for patients with NSCLC, showing somatic mutations of EGFR and/or HER2; and patients with NSCLC with known KRAS wild type tumors that were refractory (no initial response or stabilization) or resistant (response or stabilization followed by growth while on treatment) to previous treatment with erlotinib or gefitinib. There was no requirement for a specific tumor genotype for patients enrolling in Schedule B. Archived tumor samples, where available, for all patients treated within either the dose-escalation phase or the expanded cohorts were collected to examine for gene copy number alterations and/or mutations in EGFR, HER2 and KRAS. In the absence of archived biopsies, a fresh biopsy sample was collected at baseline, where feasible and agreed to by the patient.
The study was approved by the institutional review board or independent ethics committee at each of the participating centers, and was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and applicable local regulatory requirements and laws. All patients gave their written informed consent.
Study Population
Adults ≥ 18 years of age with histologically or cytologically confirmed malignant solid tumors unresponsive to currently available therapies and for which there is no currently approved treatment likely to be tolerated or acceptable were included in this study. Additional inclusion criteria included Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 and adequate cardiac, renal and hepatic functions. Exclusion criteria included prior chemotherapy, radiotherapy, biological or investigational agents ≤ 4 weeks before starting study treatment, apart from EGFR-inhibitors which could be taken until up to 2 weeks before starting PF299804; history of grade 3 or 4 toxicity related to treatment with an inhibitor of EGFR (apart from grade 3 skin rash); or requirement for treatment with drugs highly dependent on CYP2D6 for metabolism.
Efficacy and Safety assessments
The primary endpoints of this study were the determination of the MTD, and the safety and tolerability of continuous and intermittent daily dosing as assessed by monitoring adverse events (AEs) and laboratory abnormalities. Monitoring of vital signs, electrocardiograms, and laboratory analyses were performed at specified timepoints throughout the study. Left ventricular ejection fraction (LVEF) was determined at baseline and at the end of treatment. An ophthalmic slit lamp eye examination was performed at baseline and when indicated clinically. Tumors were assessed at baseline, every two cycles, and at the end of treatment. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST, v 1.0) (15).
All patients provided blood samples for pharmacokinetic analyses. The pharmacokinetics of a single dose of PF299804 were assessed for each dose-escalation cohort by administration of a single lead-in dose on an empty stomach at least 4 days prior to the initiation of CDD (doses up to 16 mg), or at least 10 days prior to the initiation of CDD (30, 45, and 60 mg dose levels). Blood samples were collected pre-dose and at intervals up to 3 days (doses up to 16 mg) or up to 9 days (30, 45, and 60 mg) post-dose. Steady-state pharmacokinetics were assessed on day 14 of the first treatment cycle. Blood samples were collected prior to dosing and at intervals up to 24 hours post-dose. Additional pre-dose samples were collected during cycle 2. The effect of food and pH on PF299804 pharmacokinetics was assessed prior to the initiation of CCD in patients enrolled into schedule A MTD expansion cohorts. Patients received PF299804 orally on an empty stomach 20 days prior to the initiation of CCD and a second dose 10 days later following either a standard typical breakfast or immediately after administration of Maalox® Maximum Strength. Blood samples for pharmacokinetic analyses were collected immediately prior to dosing and at intervals up to 9 days. Patients then began schedule A CDD of PF299804 on an empty stomach at the MTD (Day 1). The pharmacokinetic effects of a 45 mg twice-daily loading dose administered during the first 3 days of cycle 1 were assessed in patients enrolled into the schedule A MTD expansion cohort. Serial blood samples were collected pre-and up to 24 hours post-dose on the initiation of QD dosing (cycle 1 day 4) and at steady state (cycle 2 day 1). Additional pre-dose samples were collected at intervals up to the beginning of cycle 3.
Biomarker analyses
Skin biopsies were obtained at baseline and at steady state (cycle 1 day 14). Potential pharmacokinetic/pharmacodynamic relationships were investigated by evaluating the correlation between pharmacokinetic exposure parameters at steady state (Cmax, AUCtau, and Ctrough) on cycle 1 day 14 (cycle 2 day 1 for loading-dose cohort) and changes of pharmacodynamic biomarker endpoints (pERK1/2, pSTAT3, Ki67, and p27) from baseline to steady state.
Immunohistochemistry analysis was performed by QualTek (Newtown, PA) and was automated throughout using a TechMate 500 or TechMate 1000 (BioTek Solutions/Ventana Medical Systems). Antibodies used were: pERK 1/2 clone 20G11 (Cell Signaling Cat # 4376); p27 clone SX53G8 (Dako Cat # M7203); pSTAT3 (Cell Signaling Cat # 9131); Ki67 clone MIB-1 (Dako Cat # M7240). Data were reported as percentage of positive nuclei of the cells of the epidermis only. Whenever possible, flat areas of the epidermis devoid of invaginations from hair follicles were evaluated.
Tumor mutational (EGFR and KRAS) and copy number analyses (EGFR and HER2) were carried out by laboratories at the clinical site using local methods, by Pfizer or by Genzyme, depending on availability of resources (13, 16).
Statistical Considerations
The primary goal of this study was to determine MTD of PF299804. No specific statistical hypothesis tests with regard to safety, PK, and efficacy were planned. The sample size of the study was determined empirically based on the development of DLTs in the dose escalation cohorts. The minimum number of patients treated on this study would have been 6. For the expansion cohorts, at least 10 evaluable patients were required for each cohort in order to evaluate the effect of food, gastric pH and the loading dose on PF299804 pharmacokinetics. Descriptive statistics were used for the analysis of pharmacokinetics, pharmacodynamics, safety, and tumor response.
Results
Patient Characteristics and Drug Exposure
One hundred twenty-one patients (Table 1) were enrolled and treated with PF299804 (111 in schedule A [expansion cohorts: food effect, n = 11; stomach pH, n = 13; loading dose, n = 19], 10 in schedule B). Common tumor types were NSCLC (47%), colorectal cancer (20%), and breast cancer (7%). The characteristics and prior therapy for the 57 NSCLC patients entered on this study are detailed in Table S1. Patients received a total of 507 cycles of PF299804 with a median of 2 cycles per patient (range, 1 to 29).
Table 1.
Baseline patient characteristics .Abbreviations: SD, standard deviation; NSCLC, non-small cell lung cancer.
| Patient Characteristics | Schedule A (n = 111) | Schedule B (n = 10) |
|---|---|---|
| Mean (SD) age, years | 56.9 (11.3) | 57.6 (13.4) |
| Male/female, n (%) | 52/59 (47/53) | 1/9 (10/90) |
| Race, n, (%) | ||
| White | 99 (89) | 9 (90) |
| Black | 2 (2) | 0 |
| Asian | 8 (7) | 1 (10) |
| Other | 2 (2) | 0 |
| Primary tumor, n, (%) | ||
| NSCLC | 50 (45) | 7 (70) |
| Colorectal | 24 (22) | 0 |
| Breast | 7 (6) | 1 (10) |
| Ovarian | 5 (5) | 0 |
| Biliary | 4 (4) | 1 (10) |
| Other | 21 (19) | 1 (10) |
| Smoking history | ||
| Current smoker | 15 (14) | 2 (20) |
| Ex-smoker | 37 (33) | 4 (40) |
| Never-smoker | 59 (53) | 4 (40) |
| Prior treatment | ||
| Chemotherapy, n (%) | 93 (84) | 7 (70) |
| 1 regimen | 18 (16) | 1 (10) |
| 2 regimens | 25 (23) | 2 (20) |
| ≥3 regimens | 50 (45) | 4 (40) |
| Surgery, n (%) | 99 (89) | 10 (100) |
| Radiotherapy, n (%) | 59 (53) | 4 (40) |
Dose limiting toxicities
Patients received doses of PF299804 ranging from 0.5 to 60 mg QD (Table 2). Initially, no DLTs were observed below 60 mg. At 60 mg, three of six patients experienced DLT (stomatitis, palmar–plantar erythema, and dehydration; all grade 3, n = 1 each). Consequently, this dose was considered intolerable as the DLT rate exceeded 33%. The next lowest dose, 30 mg, was then expanded and one of 13 patients experienced DLT (grade 3 oral mucositis). The dose was then escalated to 45 mg. At this dose, one of six patients experienced DLT (grade 3 rash, n = 1), and the MTD was established at 45 mg. Only one additional DLT (grade 3 acne) was observed in the MTD expansion cohort, making the total number of DLTs at 45 mg two of 52 (4%) treated patients (Table 2). Additional DLTs were observed in the 45 mg loading-dose MTD expansion cohort; two of 19 patients experienced grade 3 rash (n = 1) and grade 2 mucositis (n = 1), making the total number of DLTs at 45 mg four of 71 (6%) treated patients. One patient receiving 60 mg on schedule B experienced grade 3 stomatitis (Table 2).
Table 2.
Dose-limiting toxicities during treatment cycle 1 and median number of treatment cycles started
| Dose, mg/day | Evaluable patients, n |
DLTs (n) | Even ts, n/N |
Total cycles started per cohort |
Cycles started per patient, median (range) |
|---|---|---|---|---|---|
| Schedule A | |||||
| 0.5 | 3 | None | 0/3 | 8 | 2 (2–4) |
| 1 | 3 | None | 0/3 | 9 | 2 (2–5) |
| 2 | 3 | None | 0/3 | 8 | 2 (2–4) |
| 4 | 5 | None | 0/5 | 47 | 4 (2–28) |
| 8 | 3 | None | 0/3 | 12 | 4 (2–6) |
| 16 | 4 | None | 0/4 | 16 | 3 (1–10) |
| 30 | 13 | Stomatitis [oral mucositis] (1) | 1/13 | 38 | 2 (1–8) |
| 45 | 52 | Rash (1), acne (1) | 2/52 | 226 | 2 (1–29) |
| 45 (loading dose) | 19 | Rash (1), mucositis (1) | 2/19 | 80 | 4 (2–17) |
| 60 | 6 | Stomatitis (1), palmar-plantar erythema (1), dehydration (1) | 3/6 | 12 | 2 (1–4) |
| Schedule B | |||||
| 60 | 10 | Stomatitis (1) | 1/10 | 51 | 3 (1–28) |
Safety and Tolerability
The safety population comprised all (n = 121) patients who received at least one dose of PF299804. All 121 patients experienced a total of 1,416 AEs. Of these, 709 AEs, experienced by 111 patients, were considered to be related to study drug (schedule A: 102 patients, 626 AEs; schedule B: nine patients, 83 AEs). The most frequently occurring non-hematologic treatment-related AEs (all cycles) are summarized in Table 3. On both schedules A and B, frequently observed grade 1–3 AEs attributed to study drug included diarrhea, rash, fatigue, and nausea. Treatment-related eye disorders were infrequent, but included two grade 3 events at the 45 mg dose (conjunctival irritation and eyelid pruritus; n = 1 each). Ten patients discontinued due to AEs, of which four were considered treatment-related. Thirty-two patients had dose interruptions as a result of treatment-related AEs (29 in schedule A and 3 in schedule B), and eight patients continued with a reduced dose due to AEs (seven in schedule A, one in schedule B). The observed non-hematologic laboratory abnormalities were mainly grade 1/ 2. Grade 3 and 4 non-hematologic laboratory abnormalities reported across all cohorts were reversible and comprised changes in liver enzymes and electrolytes (Table S2). Hematologic laboratory abnormalities were mainly grade 1/2. Grade 3 hematologic abnormalities comprised: lymphocytopenia (n = 16), and hemoglobin toxicity, leukopenia, and neutropenia (n = 1 each). Grade 4 hematologic abnormalities comprised lymphocytopenia (n = 3), hemoglobin toxicity (n = 2), thrombocytopenia (n = 1), and leukopenia (n = 1).
Table 3.
Frequency of non-hematologic treatment-related AEs occurring in ≥15% of patients in each treatment arm* (all treatment cycles). Abbreviation: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events.
| AE | CTCAE Grade* |
|||
|---|---|---|---|---|
| Grade 1, n (%) |
Grade 2, n (%) |
Grade 3, n (%) |
Total, n (%)† |
|
| Schedule A (n = 111) | ||||
| Diarrhea | 43 (38.7) | 20 (18.0) | 11 (9.9) | 74 (66.7) |
| Rash | 30 (27.0) | 16 (14.4) | 4 (3.6) | 50 (45.0) |
| Fatigue | 20 (18.0) | 15 (13.5) | 3 (2.7) | 38 (34.2) |
| Nausea | 28 (25.2) | 7 (6.3) | 1 (0.9) | 36 (32.4) |
| Dry skin | 29 (26.1) | 1 (0.9) | 0 | 30 (27.0) |
| Stomatitis | 17 (15.3) | 9 (8.1) | 4 (3.6) | 30 (27.0) |
| Anorexia | 12 (10.8) | 9 (8.1) | 2 (1.8) | 23 (20.7) |
| Dermatitis acneiform | 11 (9.9) | 3 (2.7) | 6 (5.4) | 20 (18.0) |
| Vomiting | 14 (12.6) | 3 (2.7) | 1 (0.9) | 18 (16.2) |
| Schedule B (n = 10) | ||||
| Diarrhea | 2 (20.0) | 5 (50.0) | 2 (20.0) | 9 (90.0) |
| Rash | 4 (40.0) | 2 (20.0) | 0 | 6 (60.0) |
| Fatigue | 0 | 3 (30.0) | 1 (10.0) | 4 (40.0) |
| Nausea | 1 (10.0) | 2 (20.0) | 1 (10.0) | 4 (40.0) |
| Anorexia | 0 | 3 (30.0) | 0 | 3 (30.0) |
| Dermatitis acneiform | 0 | 3 (30.0) | 0 | 3 (30.0) |
| Dry eye | 1 (10.0) | 2 (20.0) | 0 | 3 (30.0) |
| Dry skin | 1 (10.0) | 2 (20.0) | 0 | 3 (30.0) |
| Skin fissures | 2 (20.0) | 1 (10.0) | 0 | 3 (30.0) |
| Stomatitis | 1 (10.0) | 1 (10.0) | 1 (10.0) | 3 (30.0) |
| Vomiting | 2 (20.0) | 1 (10.0) | 0 | 3 (30.0) |
| Alopecia | 2 (20.0) | 0 | 0 | 2 (20.0) |
| Eye pain | 2 (20.0) | 0 | 0 | 2 (20.0) |
| Pruritus | 2 (20.0) | 0 | 0 | 2 (20.0) |
| Skin exfoliation | 1 (10.0) | 0 | 1 (10.0) | 2 (20.0) |
Highest National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade reported for each subject.
There were no treatment-related grade 5 AEs and 1 treatment-related grade 4 AE (pulmonary embolism) on schedule A, and no treatment-related grade 4 or grade 5 AEs on schedule B.
Pharmacokinetics
Pharmacokinetic parameters after single- and multiple-dose PF299804 are summarized in Table S3 and Fig. S1. The terminal half-life (t1/2) could not be adequately characterized due to insufficient sampling time in the 2 to 16 mg cohorts. With the extension of pharmacokinetics collection times, the t1/2 was 59 to 85 hours over the 30 to 60 mg dose range (coefficient of variation, 29 to 47%). This long t1/2 also led to the discontinuation of additional exploration of schedule B as it was felt that a 7 day washout period would be insufficient to avoid overlapping toxicities.
In the food-effect cohort (n = 4), the geometric mean of Cmax was similar between patients with food (22.5 ng/mL) and without (25.6 ng/mL). In the antacid-effect cohort (n = 13), no effects were observed, with similar geometric means of Cmax (20.6 and 21.8 ng/mL) and AUC0–inf (1,582 ng·hr/mL and 1,533 ng·hr/mL) in patients with and without antacid, respectively. The geometric mean of Cmax after the loading dose (cycle 1 day 4) was comparable to the Cmax on cycle 2 day 1 (104 ng/mL and 76.9 ng/mL, respectively).
Pharmacodynamics
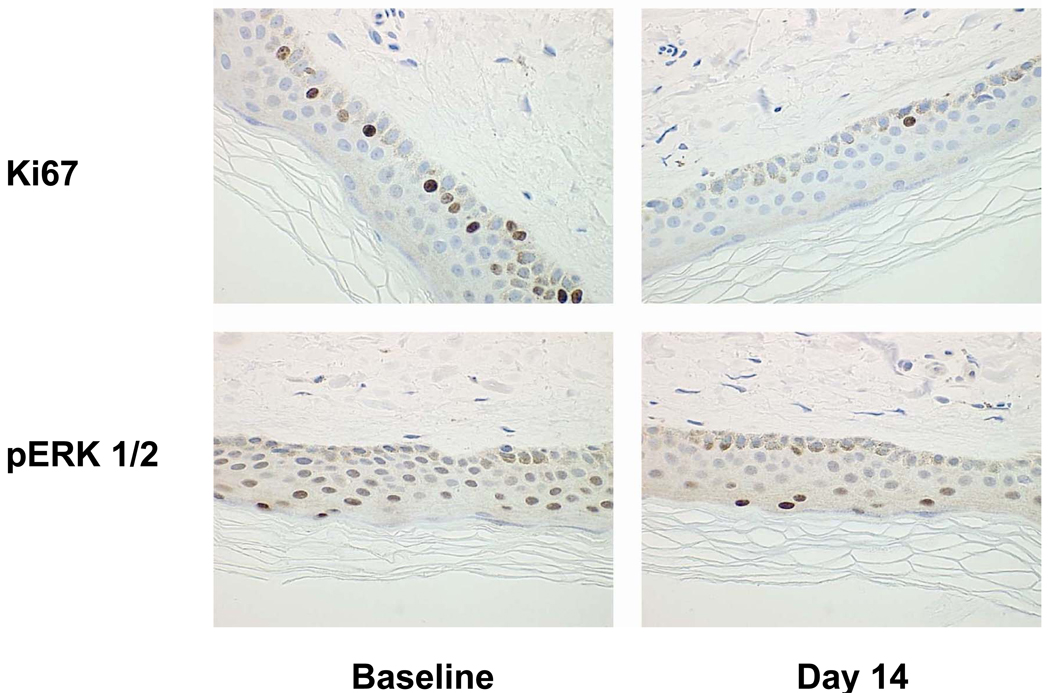
Ninety-nine patients provided pre- and post-dose skin biopsy samples. A representative example of paired skin biopsy samples demonstrating a reduction in Ki67 and pMAPK following PF299804 treatment is shown in Figure 1. Modulation of Ki67 and pMAPK appeared to have significant association with pharmacokinetic exposure (P < 0.05). Levels of Ki67 were significantly decreased with increased dose and steady-state predose concentration (Ctrough) and levels of pMAPK were significantly decreased with increased dose, Cmax, AUCtau, and Ctrough (Table S4, Figures S2 and S3).
Figure 1.
Decrease in Ki67 (top) and pERK1/2 (bottom) in skin biopsies following PF299804 treatment. Left: Skin biopsies taken at baseline. Right: Skin biopsies taken at cycle 1 day 14. These samples were taken from a patient treated at the 45 mg dose level
Antitumor Activity
In total, 110 patients had at least one response evaluation following PF299804 treatment. No complete responses were observed but four patients (3.6%), all with NSCLC, had a PR. These patients were treated on schedule A at doses of 16 mg (n = 1) and 45 mg (n = 2), and on schedule B at 60 mg (n = 1). Over all treatment cohorts, 44 patients had stable disease as best response, 55 patients had progressive disease at their first evaluation, and eight patients (7.3%) had clinical benefit, defined as complete response, partial response, or stable disease for at least 24 weeks.
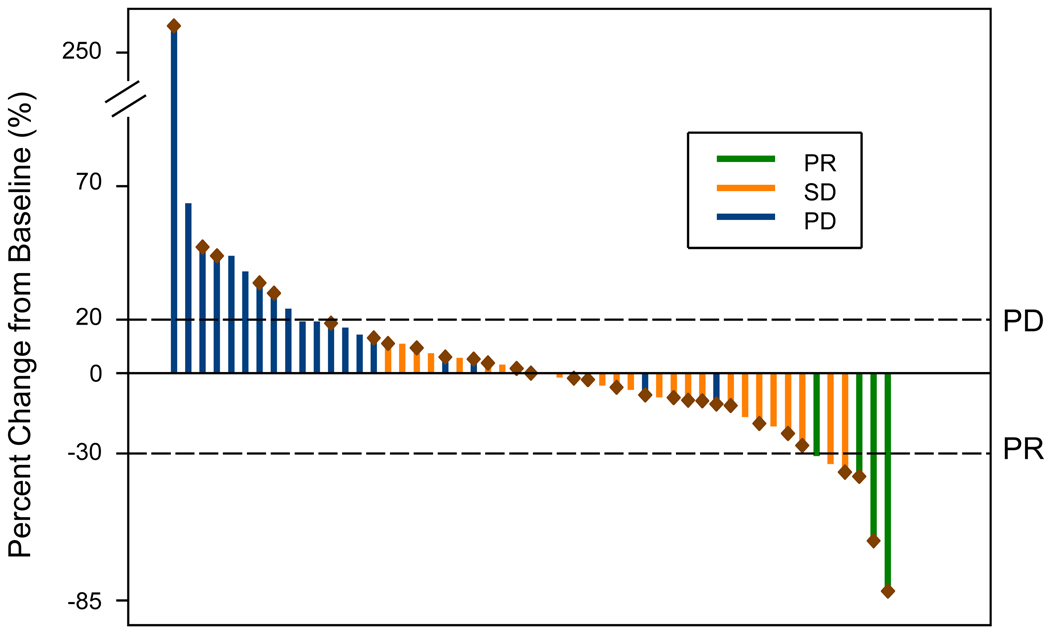
Among the NSCLC patients, an additional 28 patients exhibited stable disease (SD) of ≥6 weeks duration. Responses achieved by patients with measurable disease and associated durations of therapy are presented in Figures 2 and S4. Representative scans from one responding patient are shown in Figure S5. All responding patients had been previously treated with gefitinib and/or erlotinib and one had a PR while the remaining three achieved SD. The durations of response to PF299804 were: 12.3, 18.9, 54.1 and 86.7+ weeks. In the patients who developed a PR, the intervals between ending gefitinib or erlotinib therapy and starting of PF299804 were 8.1, 91.6, 57.9, and 11.0 weeks, respectively.
Figure 2.
Waterfall plot of patients with NSCLC with measurable disease. Fifty-one (51) had measurable disease as defined by RECIST. The best response is demonstrated in this waterfall plot. PR = partial response, SD = stable disease, PD = progressive disease. Patients with known EGFR mutant cancers are marked with a burgundy diamond.
Tumor genomic analyses in NSCLC patients and correlation with treatment outcome
Table 4 summarizes all genomic studies performed in the NSCLC patients. Thirty-three patients had EGFR activating mutations. Four patients had documented EGFR T790M mutations (all in conjunction with an exon 19 deletion) all of which were detected from tumor biopsies that had been obtained after development of clinical resistance to either gefitinib or erlotinib. All other tumor analyses were performed using tumor specimens obtained at diagnosis. Two of the patients with a PR had EGFR exon 19 deletion mutations, 1 patient had an exon 20 insertion mutation (D770delinsGY; Figure 2) and no tumor tissue was available for 1 patient. Of the other 5 patients with exon 20 insertion mutations, 3 (including 1 whose disease was not measurable) had SD (12, 14, and 104, weeks respectively) while 2 patients had progressive disease (PD) as their best response to treatment. None of the 4 patients with exon 19 deletions and documented T790M had an objective response to treatment (Table 4).
Table 4.
Summary of tumor genotypes and treatment responses for patients with NSCLC with EGFR or HER2 genomic alterations.
| Mutation (N=57) | No. of Patients | Treatment Outcome |
|---|---|---|
| EGFR mutant | 33 | |
| L858R | 4 | SD: 3; PD:1 |
| Exon 19 deletion* | 17 | PR: 2; SD: 8; PD:7 |
| Exon 20 insertion† | 6^ | PR: 1; SD:2; PD:2 |
| Exon 19 deletion/T790M | 4 | SD:2; PD:2 |
| Other | 2 | SD: 1; PD:1 |
| EGFR wild type | 12^ | SD:5, PD:5; IND: 1 |
| EGFR mutation unknown‡ | 12^ | PR:1, SD:7; PD:2; IND: 1 |
| KRAS mutant | 0 | |
| KRAS wild type | 45 | |
| KRAS unknown | 12 | |
| Amplification | ||
| HER2 amplified | 2 | SD:2 |
| HER2 non-amplified | 6 | |
| HER2 amplification unknown | 49 |
Durations of partial responses = 12.3 weeks and 86.7+ weeks, respectively
Duration of partial response = 54.1 weeks
Duration of partial response = 18.9 weeks
One patient not evaluable
IND = indeterminate; PD = progressive disease; PR = partial response; SD = stable disease
Discussion
This first-in-human study demonstrates that PF299804 is generally safe and well tolerated on both continuous and intermittent schedules. The MTD was established as 45 mg daily, with only two DLTs (rash and acne) among 52 patients. The most common treatment-related grade 3 AEs were skin and gastrointestinal disorders for both continuous and intermittent schedules. The treatment-related AEs and DLTs observed in this study are consistent with side effects observed with erlotinib and with inhibitors targeting multiple HER family members (2, 12, 17). Although preclinical studies of PF299804 identified epithelial atrophy in the cornea and kidney toxicity as potential side effects, these were not common side effects observed in the current clinical study (Table 3).
Pharmacokinetic analyses revealed a linear increase in PF299804 exposure with increasing dose. PF299804 demonstrated a long half-life, and a very large apparent volume of distribution, indicating extensive tissue penetration of the drug. PF299804 half-life is much longer than those observed for HKI-272, CI-1033, or EKB-569 (18–20). There was evidence of accumulation, as expected with a long half-life after multiple doses. However, based on the mean PF299804 t1/2, maximal accumulation is expected to occur during cycle 1. This may explain the absence of increased toxicity observed in patients who remained on study for many cycles.
Systemic exposure at doses ≥30 mg/day exceeded the threshold concentrations for efficacy predicted from non-clinical studies in models harboring wild-type EGFR or common EGFR-activating mutations (5, 6). This was further supported by our skin PD studies (Figure 2) which demonstrated a dose dependent decrease in pERK1/2 and Ki67 expression. However, the concentrations achieved clinically with the regimens reported may be insufficient to achieve full inhibition of T790M based on pre-clinical studies (5). The IC50 for growth inhibition of NSCLC cell lines harboring EGFR T790M is 100 to 900 nM which is unlikely to be clinically achieved using the current dosing schedule (5). Furthermore, although the sample size was limited, no PRs were observed in the 4 patients with documented T790M mutations. A more formal phase II trial is required to get additional clarification on the efficacy of PF299804 in patients pretreated with gefitinib or erlotinib. Such trials are currently underway (NCT00548093; NCT00553254). An alternative strategy for treating drug resistance is preventing the emergence of drug resistance. This would be manifested by a greater time to disease progression than is currently achieved (8–10 months) with either gefitinib or erlotinib (3,21). This strategy is also currently being pursued in phase II clinical trials (NCT0081844; NCT00769067).
In the present study, one of the responding patients had an exon 20 insertion mutation, confirming the ability of PF299804 to inhibit this gefitinib/erlotinib resistance mutation in patients with NSCLC (5). Exon 20 insertions comprise up to 9% of all EGFR mutant cancers(22). However, there were 5 additional patients with exon 20 insertions that did not manifest objective responses to PF299804, although only 4 of these had measurable disease per RECIST (Table 4). The apparent heterogeneity of benefit among exon 20 mutations may be due to differences in the specific exon 20 mutations or other concurrent differences in the patients’ tumors. All 6 patients had different exon 20 insertion mutations. It is currently not known if there are any biologic differences among the different exon 20 insertion mutations. The exon 20 insertion mutation in the responding patient (D770DelInsGY) has not previously been described and it is possible that this specific mutation is uniquely sensitive to PF299804. Additional in vitro and structural studies of EGFR exon 20 insertion mutations will be required to determine why some but not all are sensitive to PF299804.
In summary, PF299804 can be safely administered up to 45 mg/day. This dose is undergoing further evaluation in phase II studies of gefitinib/erlotinib refractory and naïve NSCLC patients to further evaluate the clinical efficacy in these patient populations.
Statement of Translational Relevance.
PF299804 is a potent, highly selective, irreversible small-molecule tyrosine kinase inhibitor (TKI) of human epidermal growth factor receptor (EGFR/HER1), HER2, and HER4. Irreversible receptor inhibition has the potential to improve antitumor activity and overcome mechanisms of resistance to reversible EGFR TKIs gefitinib and erlotinib. Pre-clinical studies demonstrate that PF299804 is effective against gefitinib resistant (EGFR exon 20 insertion and T790M) mutations. This first-in-human study of PF299804 in patients with advanced solid malignancies showed that it has a manageable safety profile, is well tolerated on continuous and intermittent schedules, with dose-proportional pharmacokinetics and demonstrated a dose-dependent increase in target inhibition. This study included 57 NSCLC patients, majority of whom previously received gefitinib or erlotinib. We observe encouraging activity (including a PR in a patient with an exon 20 insertion mutation) in this previously treated subset of NSCLC patients, providing the rationale for further phase II clinical development of PF299804 in NSCLC patients
Supplementary Material
Acknowledgements
Supported in part by grants from the National Institutes of Health R01CA135257 (P.A.J., and J.A.E), and the National Cancer Institute Lung SPORE P50CA090578 (P.A.J. and J.A.E.). The investigators wish to thank the patients and their families, the study coordinators, and the physicians who referred their patients to this study. Editorial assistance was provided by Christine Arris (ACUMED® Tytherington, UK) and funded by Pfizer Inc. This study was funded by Pfizer Inc.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7:1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 7.Fry DW, Bridges AJ, Denny WA, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–4882. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 10.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008 doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 13.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 14.Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Janne PA, Borras AM, Kuang Y, et al. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006;12:751–758. doi: 10.1158/1078-0432.CCR-05-2047. [DOI] [PubMed] [Google Scholar]
- 17.Calvo E, Tolcher AW, Hammond LA, et al. Administration of CI-1033, an irreversible pan-erbB tyrosine kinase inhibitor, is feasible on a 7-day on, 7-day off schedule: a phase I pharmacokinetic and food effect study. Clin Cancer Res. 2004;10:7112–7120. doi: 10.1158/1078-0432.CCR-04-1187. [DOI] [PubMed] [Google Scholar]
- 18.Wong KK, Fracasso PM, Bukowski RM, et al. HKI-272, an irreversible pan erbB receptor tyrosine kinase inhibitor: Preliminary phase 1 results in patients with solid tumors. Journal of Clinical Oncology. 2006;24:3018. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 19.Zinner RG, Nemunaitis J, Eiseman I, et al. Phase I clinical and pharmacodynamic evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2007;13:3006–3014. doi: 10.1158/1078-0432.CCR-06-1958. [DOI] [PubMed] [Google Scholar]
- 20.Erlichman C, Hidalgo M, Boni JP, et al. Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2006;24:2252–2260. doi: 10.1200/JCO.2005.01.8960. [DOI] [PubMed] [Google Scholar]
- 21.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.