Abstract
Tissue factor (TF) is a single polypeptide integral membrane glycoprotein composed of 263 residues and is essential to life in its role as the initiator of blood coagulation.
Objective
Previously we have shown that the activity of the natural placental TF (pTF) and the recombinant TF (rTF) from Sf9 insect cells is different (Krudysz-Amblo, J. et al(2010) J. Biol. Chem. 285, 3371–3382). In this study, using mass spectrometry, we show by quantitative analysis that the extent of glycosylation varies on each protein.
Results
Fractional abundance, of each glycan composition at each of the three glycosylation sites, reveals the most pronounced difference to be at asparagine (Asn) 11. This residue is located in the region of extensive TFfactor VIIa (FVIIa) interaction. Carbohydrate fractional abundance at Asn11 revealed that glycosylation in the natural placental TF is much more prevalent (~76%) than in the recombinant protein (~20%). The extent of glycosylation on Asn124 and Asn137 is similar in the two proteins, despite the pronounced differences in the carbohydrate composition. Additionally, 77% of rTF exists as TF des-1, 2 (missing the first two amino acids from the N-terminus). In contrast, only 31% of pTF is found in the des-1, 2 form.
Conclusion
These observations may attribute to the difference in the ability of TF-FVIIa complex to activate factor X (FX). Structural and functional comparison of the recombinant and natural protein advances our understanding and knowledge on the biological activity of TF.
Keywords: mass spectrometry, fractional abundance, carbohydrates, tissue factor
1. Introduction
Tissue factor (TF) is an integral membrane glycoprotein, which in complex with factor VIIa (FVIIa), initiates the blood coagulation process. Tissue factor is expressed in the vascular adventitia, astroglial cells, organ capsules, central nervous system, lungs and placenta at relatively low concentrations [1–3]. Various forms of recombinant (r)TF including the mature protein (residues 1-263), the extracellular and transmembrane domain (residues 1-243) and the soluble extracellular domain (residues 1-219) have been expressed. Specific expression systems contribute to different levels of posttranslational modifications of the translated protein [4–8]. Structural and mutagenesis studies have been determined using rTFs [9–11]. Although these rTFs have been used extensively as surrogates for the natural protein, it has been well established that functional and structural limitations exist in the recombinant analogs natural human proteins [12]. Comparison of the natural and recombinant form, if possible, becomes important in biological assays and elucidation of structure-function relationships [13–15].
The most abundant and complex posttranslational modifications are asparagine (Asn-linked, N-linked) and serine/threonine (Ser/Thr-linked, O-linked) glycosylations [16, 17]. Specific cell lines, including the Sf9 insect cells, are preferred expression systems for recombinant human proteins due to their ability to carry out more complex glycosylation and provide better protein folding machineries than those of other organisms [12, 18, 19]. Despite the great potential of insect cells to produce human proteins, recognized differences between the mammalian and insect cell-derived proteins exist [20–22]. Notwithstanding few limitations of recombinant proteins in their exploitation as surrogates for natural forms, the recombinant proteins serve as valuable tools for elucidation of biological and structural roles of their natural counterparts.
The goal of the present study was to map similarities and differences in the extent of glycosylation and fractional abundances of each glycan in the natural and recombinant TF. By using mass spectrometry based proteomics [23], we have focused on the quantitative analysis of the micro and macro heterogeneity of carbohydrate composition in rTF and natural human pTF. We previously reported the composition of the carbohydrates at each glycosylated site in natural and recombinant TF [14]. Using a fluorogenic lipid-independent assay, we showed that the amidolytic activity of the TF-FVIIa complex increased in the order rTF1-243(E.coli)<rTF1-263(Sf9 insect cells)<pTF for the non-glycosylated and increasingly glycosylated forms, respectively. Deglycosylation had no effect on the amidolytic activity. In FXase, a lipid dependent FX activation assay, the Km of FX for rTF1-263–FVIIa and pTF-FVIIa remained minimally altered after deglycosylation, whereas the kcat decreased slightly for rTF and significantly, 4-fold, for pTF upon deglycosylation. In this study, our findings show that one of the three glycosylated sites in pTF, Asn11, has the majority or ~76% of that site glycosylated. In contrast, Asn11 in rTF is partially glycosylated, having only 20% occupied by carbohydrate. We have also determined that 31% of pTF exists as a truncated form in which the first two amino acid residues are missing from the N-terminal end of the protein. In rTF the fractional abundance of the truncated form is much higher or 77%. Moreover, fractional abundances of the glycans demonstrate that the majority of carbohydrates of pTF are sialylated and fucosylated, whereas the carbohydrates of rTF predominate in high-mannose glycans. No mutational studies on Asn11, Asn124 and Asn137 have been carried out in the past. The present analysis enables us to apply structural data to the understanding of functional properties of the TF protein. Our observations suggest that differences between the two TFs may influence the interaction of TF with FVIIa and FX.
2. Materials and methods
2.1 Proteins and Materials
rTF (mature rTF 1-263 residues) expressed in Sf9 insect cells, was a gift from Dr. R. Jenny (Haematologic Technologies Inc., Essex Junction, VT). The source of pTF (Thromborel S) was a gift from Dr. D. Barrow (Behring Diagnostics Inc., Liederbach, Germany). Anti-TF-5 monoclonal antibody (αTF-5 mAb) was produced in-house and characterized as previously described [24]. Proteomics grade trypsin, DL-Dithiothreitol (DTT), and iodoacetamide were purchased from Sigma (St.Louis, MO). HPLC-grade water, isopropanol and acetonitrile were purchased from Thermo Fisher Scientific (Pittsburgh, PA). Formic acid was purchased from VWR (West Chester, PA). 3-Compa-1-propanesulfonate (CHAPS) was purchased from Thermo Fisher Scientific (Rockford, IL).
2.2 Enzymatic digestion of TF proteins
Human pTF was purified from the commercial thromboplastin, Thromborel S®, by a procedure previously described [24]. Three µg of rTF and 1.5 µg of pTF in 50 mM NH4HCO3 at pH 8.3 were reduced with 10 mM DTT and alkylated with 17 mM iodoacetamide. Trypsin was added at a 1:50 enzyme to protein ratio and incubated at 37 °C overnight.
2.3 Liquid chromatography-mass spectrometry (LC-MS) analysis of TF proteolytic peptides
Liquid chromatography/mass spectrometry (LC-MS) analysis of 1 pmol of TF peptides was carried out using a Shimadzu SIL-20AC auto sampler with two LC-20AD pumps (Columbia, MD) and a Thermo LTQ mass spectrometer (San Jose, CA). The LC was connected to a 100 µm ID by 150 mm length nanospray column that was pulled and packed in-house with Magic C18AQ reverse phase material (5µ beads, Michrom, Auburn, CA). We used a slightly modified form of the two-split configuration previously described [25]. We used water (solvent A) and acetonitrile (solvent B) with 0.1% formic acid in both solvents to load and elute the sample from the nanospray column. The LC gradient consisted of the following: 97% A and 3% B at 20 min, 40% B at 57 min, 60% B at 59 min. Total time for the sample analysis was 65 min. Due to the presence of CHAPS in the TF samples, each sample analysis was followed by a 5 µL injection of 50% isopropanol to “wash” the column. The isopropanol injection was carried out in 60% B followed by a decrease to 3% B in 5 min; total time for the column wash was 25 min. The LTQ-MS was tuned according to the manufacturer’s recommended procedure. The nanospray column was placed in front of the mass spectrometer using an in-house constructed stage. The position of and voltage applied to the nanospray column was optimized to achieve a consistent spray. We applied 1.9 kV to the vent line on the tee at the head of the column. The transfer tube temperature was set to 150 °C. We viewed in Qualbrowser the tandem mass spectrometry (MS/MS) spectra after collision induced disassociation of the glycopeptides to identify glycan composition based on specific mass losses of different sugars as previously described [14, 26]. We determined the glycopeptide fractional abundance by running each sample three times and calculating the average relative abundance for each glycan composition from the three analyses using the LC-MS peak areas. For glycopeptides detected by more than one charge state or a miscleave by trypsin, we summed the peak areas of different forms [27, 28]. We used one standard deviation of the averages to represent the error in the figures. The triplicate injections were carried out using only a full MS scan from 250 to 2000 Da in centroid mode. LC-MS data files were viewed using Xcalibur Qualbrowser, version 2.0. Individual glycopeptides LC-MS areas were determined by manual integration using the add peaks tool in Qualbrowser. We extracted the peak areas by using the three most intense isotopes (i.e. M, M+1, M+2) coming from each glycopeptide ion.
3. Results
Figure 1 presents three of four potential sites found to be glycosylated by N-linked carbohydrates on rTF and includes Asn9/11, Asn124 and Asn137 (Figure 1a, 1b, 1c respectively). The presence of carbohydrates on these three sites in TF protein was previously observed [14, 29]. We analyzed the sites of glycosylation and the composition of the carbohydrates by a method described previously [27]. All N-linked carbohydrates share a trimannosyl diacetylchitobiose core structure composed of two asparagine linked N-acetylglucosamine (GlcNAc) molecules and three mannose (Man) residues (-Asn-GlcNAc2Man3). To eliminate redundancies, we discuss in this manuscript the glycans that extend from and do not include this core.
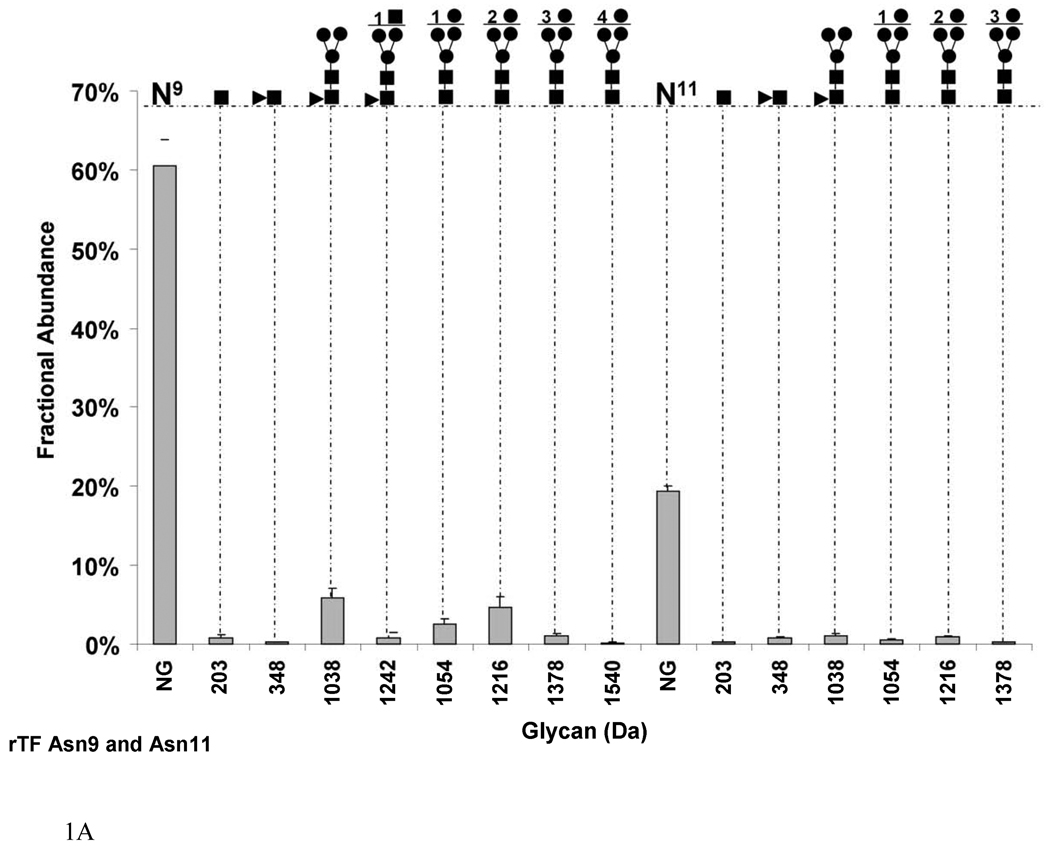
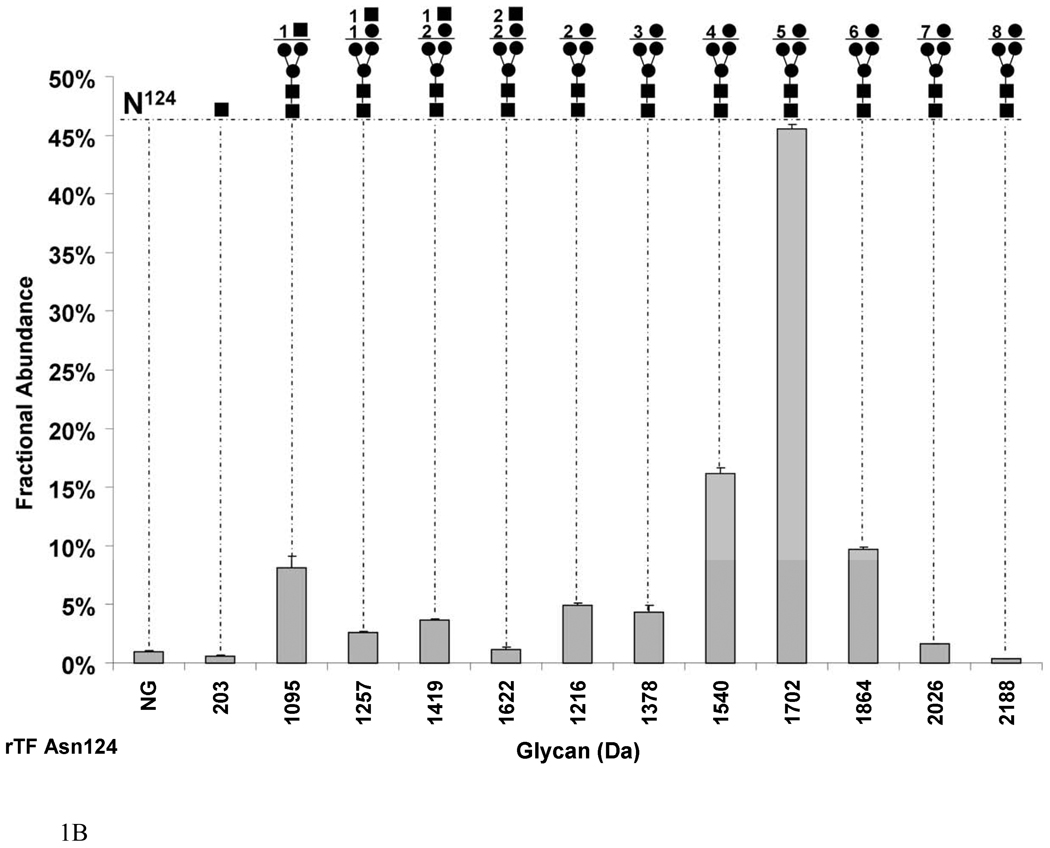
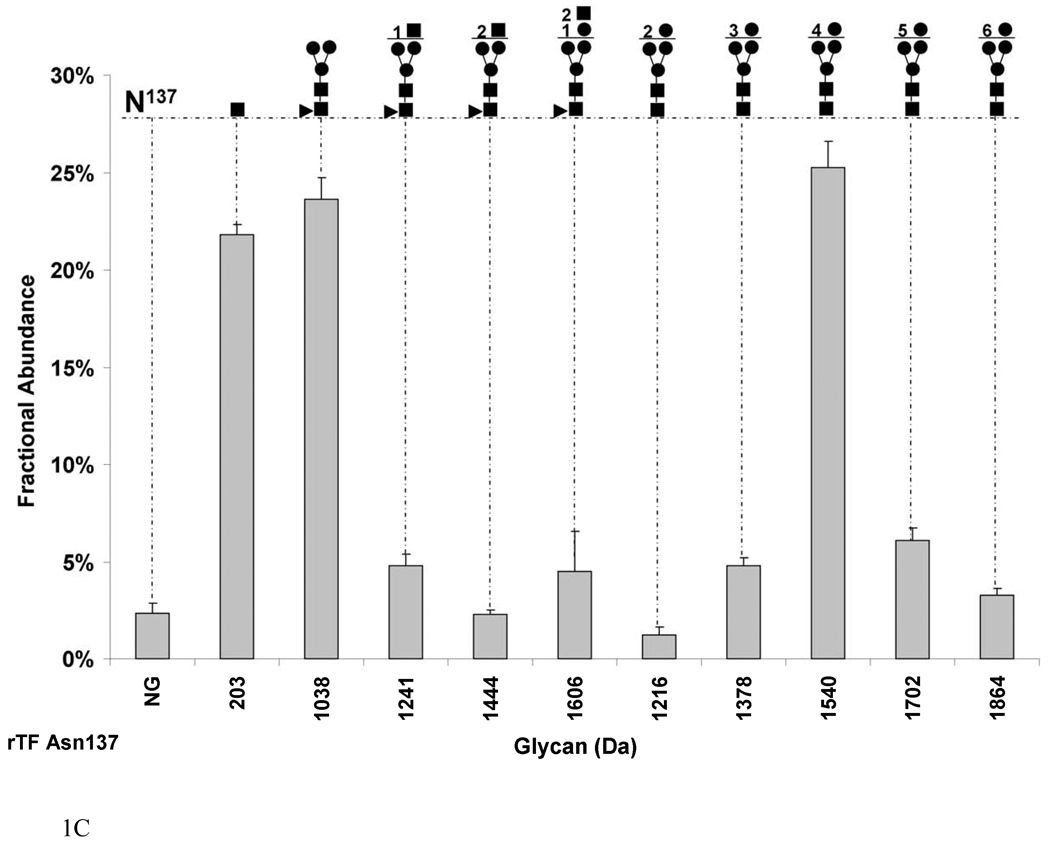
Fig. 1.
Graphs showing the fractional abundances of the non-glycosylated (NG) and glycosylated peptides versus glycan mass for the three N-glycosylation sites on rTF: (a) Asn9 and Asn11 (truncated protein and full-length protein, respectively), (b) Asn124 and (c) Asn137. One standard deviation of the mean of three measurements is shown as an error bar for each fractional abundance. The figures above the graph define the glycan composition where the symbol (●) represents Man/Hex, (■) represents GlcNAc/HexNAc and (▲) represents Fuc. The numerical coefficients in front of each symbol designate the number of molecules of each sugar in the glycan beyond the core.
In rTF, we detected by LC-MS/MS that the first glycosylation site existed on two slightly different peptide sequences. One peptide contained the expected amino acids after digestion with trypsin (1-SGTTNTVAAYNLTWK-15), and the other peptide was missing serine and glycine from the N-terminus of the protein (1-TTNTVAAYNLTWK-13). The absence or truncation of the two amino acids in TF protein was previously reported [4, 7]. We observed that 76.8±0.1% of rTF exists as a truncated protein. Both protein forms, mature and truncated, show comparable potential for glycosylation. We refer to the glycosylation sites as Asn11 for the mature protein and Asn9 for the truncated protein. We treated the mature and truncated protein as well as their glycosylation as a unique form representing this first glycosylation site. Hence we calculated the relative abundance of each form relative to the abundance of all forms added together. Figure 1a shows that the overall fractional abundance of glycosylation on Asn9 and Asn11 is 20.0±0.02% in rTF. Therefore the majority of that site exists as nonglycosylated in the recombinant protein. The nonglycosylated peptide accounts for 60.5±3.3% on Asn9 and 19.4±0.6% on Asn11. In the glycosylated form, the most abundant glycan on both Asn9 and Asn11 is a fucosylated core (1038 Da) with a fractional abundance of 5.8±1.1% and 1.0±0.2% respectively. However, the types of glycans that predominate are high mannose sugars which constitute the majority of Asn9 and Asn11 sugars (fractional abundance of 8.3±0.01% and 1.9±0.03% respectively). On the truncated form of the rTF, the greatest number of mannose residues attached to the core is 4 residues (1540 Da) with a fractional abundance of 0.1±0.06% of the total. On the mature form, the maximum number of mannose residues is 3 (1378 Da) with a fractional abundance of 0.2±0.06% of total. For clarity, we refer to the next two glycosylated sites as Asn124 and Asn137, following the numbering of the mature protein.
Figure 1b shows that Asn124 of rTF undergoes almost complete glycosylation, where 99.0±0.1% of the site is modified. The dominating glycan on this site is the 5 mannose sugar (1702 Da) with a 45.5±0.4% fractional abundance. The largest high mannose glycan by mass and the least abundant carbohydrate on this site is composed of 8 mannose residues (2188 Da) with a fractional abundance of 0.3±0.03% of the total. Asparagine 124 is also modified by hybrid carbohydrates. Hybrid and complex carbohydrates are described as containing hexoses (Hex), including mannose, galactose and glucose (162 Da) and N-acetylhexosamines (HexNAc), including N-acetylglucosamine and N-acetylgalactosamine (203 Da). The most abundant, although the smallest among the hybrid glycans (1095 Da) on Asn124, is HexNAc1 attached to the core with 8.1±0.9% by fractional abundance. A glycan composed of Hex2HexNAc2 is the largest glycan ion by mass among the hybrid sugars (1622 Da) but also the least abundant with 1.2±0.1% by fractional abundance. A small portion, 0.6±0.05%, of Asn124 is modified by one Asn-linked GlcNAc. Interestingly, Asn124 lacks fucosylated glycans.
Figure 1c shows the glycosylation pattern on Asn137 in rTF. Glycosylation of this site is almost complete, with a fractional abundance of 97.6±0.1%. Asparagine137 is found on both a correctly cleaved peptide as predicted from the trypsin digest, and on a miscleaved fragment, where arginine (Arg) 136 is attached to Asn137 (RAsn) due to incomplete cleavage by trypsin. We calculated the fractional abundance of each glycopeptide on Asn137 with the denominator being the total population of the properly cleaved plus the miscleaved ion abundances [27]. The 4 mannose (1540 Da) sugar is the most abundant glycan on this site constituting 25.2±1.3% of total. The largest high mannose carbohydrate by mass is composed of 6 mannose (1864 Da) residues attached to the core with 3.3±0.3% by fractional abundance. Fucosylated glycans make 35.2±0.1% of the sugars at this site with the majority, 23.6±1.0%, being a fucosylated core (1038 Da). The largest fucosylated carbohydrate by mass at this site is HexHexNAc2 (1606 Da) with 4.5±2.0% by fractional abundance. Interestingly, 21.8±0.5% of Asn137 is modified by one GlcNAc (203 Da) molecule attached to the Asn.
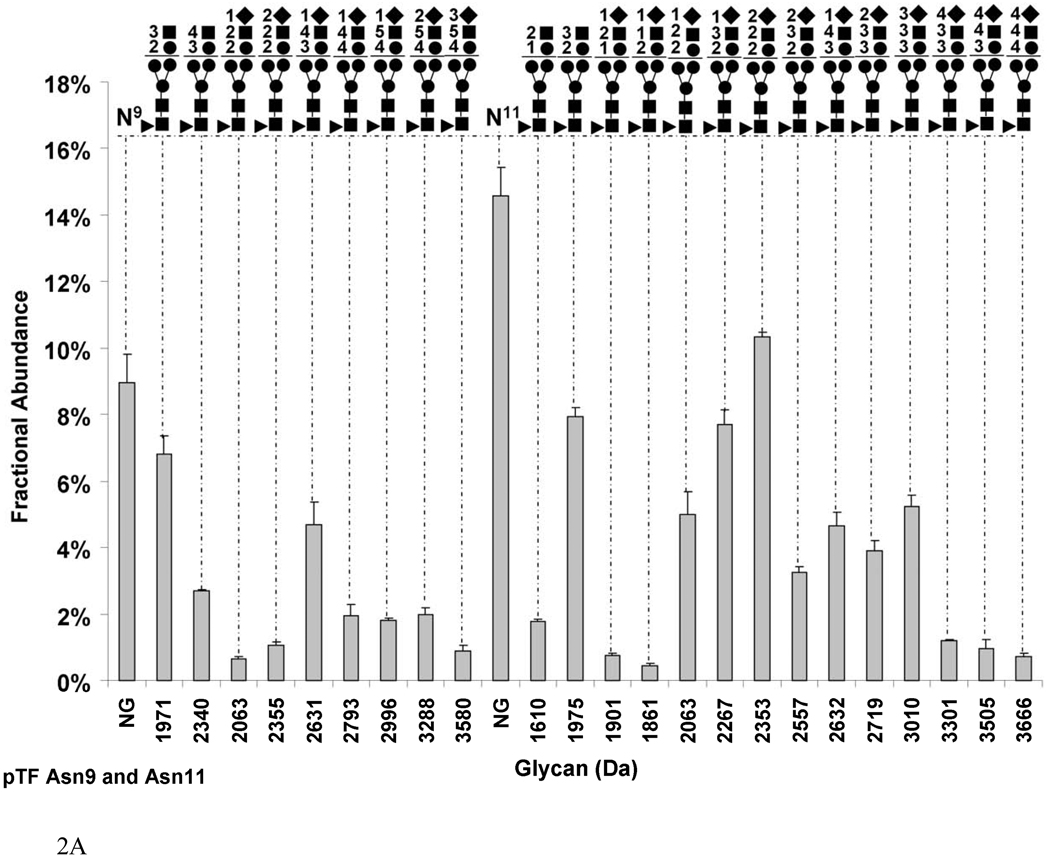
Figure 2 presents the carbohydrate analysis of pTF. Similar to rTF, the natural protein is glycosylated on Asn9/11, Asn124 and Asn137. We analyzed the sites of glycosylation and the composition of the carbohydrates by a method described previously and discussed for rTF [27]. Detailed analyses of the proteolytic fragments revealed that pTF exists in two forms, analogous to rTF. Most of pTF exists as a mature form with a fractional abundance of 68.4±0.4%, whereas the truncated form, TF des-1,2, constitutes 31.6±0.2%. Figure 2a shows that glycosylation on Asn9 and Asn11 of pTF occupies 76.3±0.5% of that site. All of the glycans on this site are fucosylated. Sialylated carbohydrates comprise 13.0±0.01% on Asn9 and of 44.1±0.03% on Asn11. The most abundant sialylated glycan on Asn9 is Hex3HexNAc4SA1 (2631 Da) with a fractional abundance of 4.6±0.7% and on Asn11 Hex2HexNAc2SA2 (2353 Da) with a fractional abundance of 10.3±0.1% of total. Combined, the most abundant sialylated carbohydrate on Asn9 and Asn11 is Hex2HexNAc2SA2 (2355 Da) with 11.4±0.1% by fractional abundance. When we sum the fractional abundances based on glycan composition, on both the mature and truncated form, the most abundant composition is the non-sialylated Hex2HexNAc3 (1971 Da) constituting 14.7±0.01% of total. The remaining carbohydrates have a quite heterogeneous composition with varying numbers of Hex, HexNAc and sialic acid (SA) molecules.
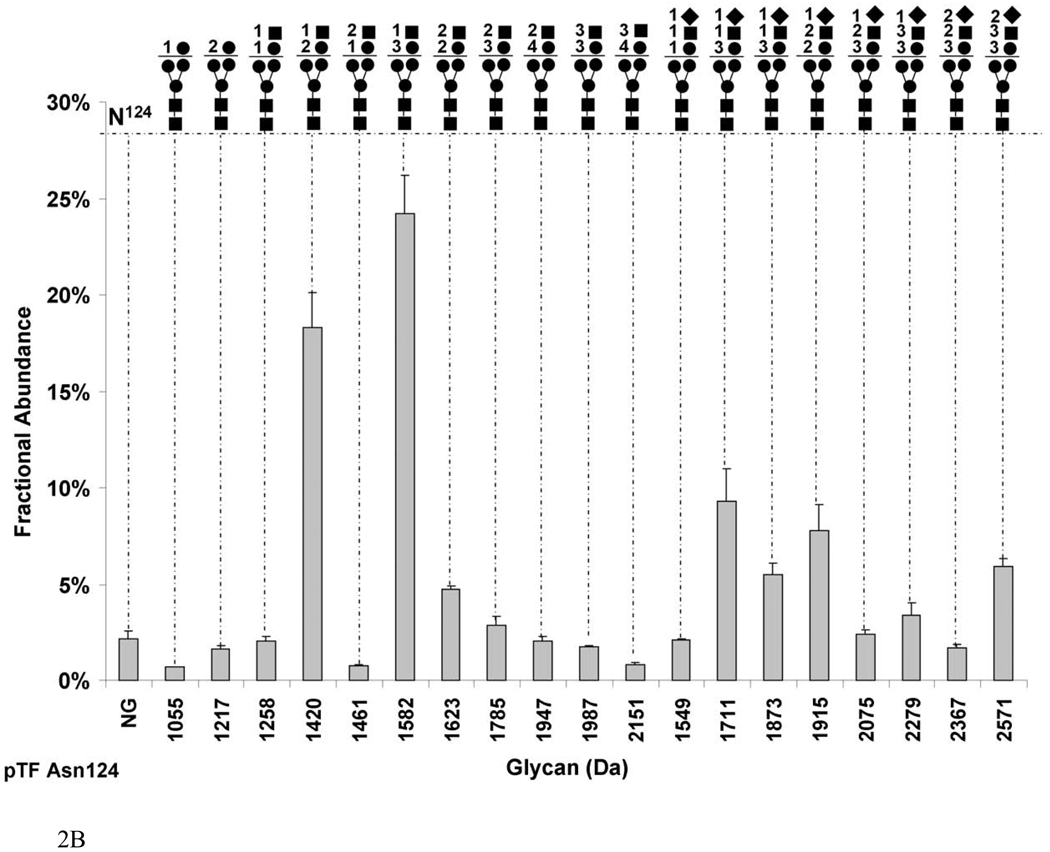
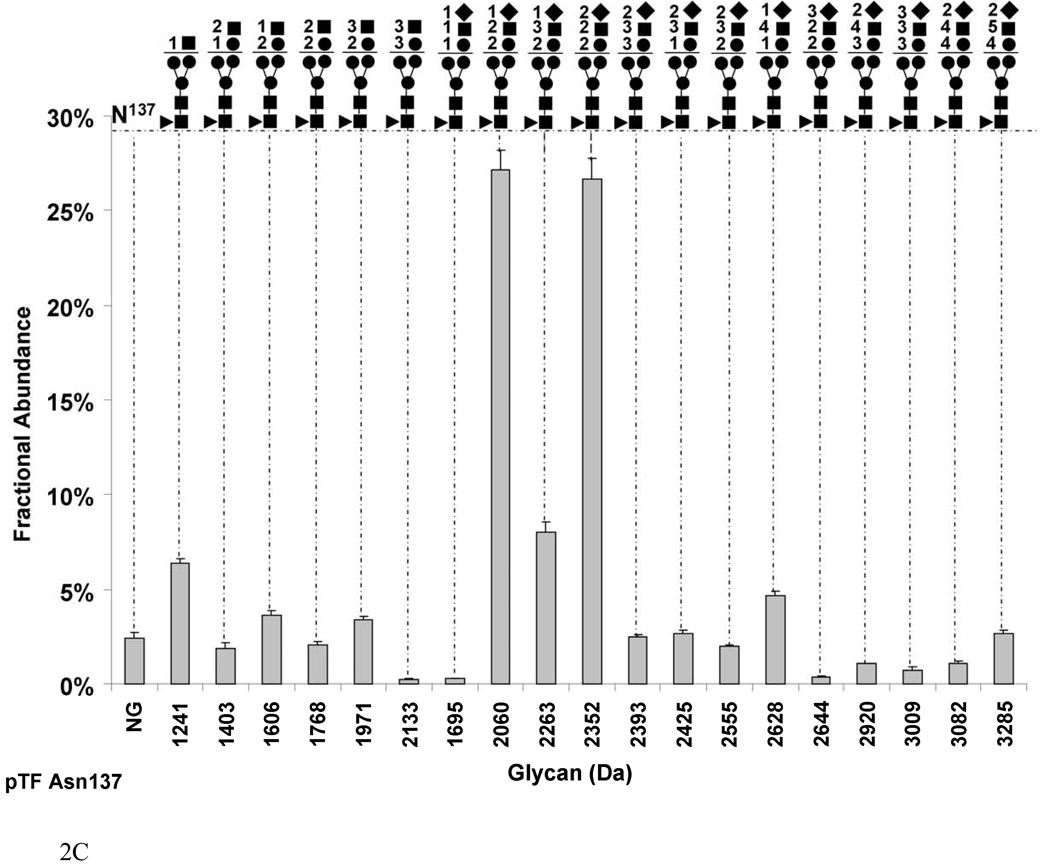
Fig. 2.
Graphs showing the fractional abundances of the non-glycosylated (NG) and glycosylated peptides versus glycan mass for the three N-glycosylation sites on pTF: (a) Asn9 and Asn11 (truncated protein and full-length protein, respectively), (b) Asn124 (c) Asn137. One standard deviation of the mean of three measurements is shown as an error bar for each fractional abundance. The figures above the graph define the glycan composition where the symbol (●) represents Man/Hex, (■) represents GlcNAc/HexNAc, (▲) represents Fuc and (♦) represents SA. The numerical coefficients in front of each symbol designate the number of molecules of each sugar in the glycan.
Figure 2b shows that Asn124 in pTF undergoes almost complete glycosylation with 97.8±0.06% by fractional abundance. The most abundant hybrid glycan, and at the same time the most abundant glycan among the sialylated and non-sialylated sugars on this site is Hex3HexNAc1 (1582Da) comprising 24.1±2.0%. The biggest hybrid glycan (2151 Da) and the least prevalent glycan on this site (0.8±0.1%) are composed of Hex4HexNAc3. The Hex2HexNAc1SA1 (1711 Da) is the predominant sialylated group composing 9.2±1.7% of the carbohydrates. The largest of the sialylated sugars by mass is Hex3HexNAc3SA2 (2571 Da) with a fractional abundance of 5.9±0.4% of total. None of the glycans on Asn124 of pTF are fucosylated.
Figure 2c shows that Asn137 in pTF exists mainly in the glycosylated form with a fractional abundance of 97.5±0.08%. There are two almost equally abundant sialylated glycans. One composition is Hex2HexNAc2SA1 (2060 Da) with a fractional abundance of 27.1±0.9%. The second composition is Hex2HexNAc2SA2 (2352 Da) with a fractional abundance of 26.6±1.0%. The largest glycan by mass is Hex4HexNAc5SA2 (3285 Da) which constitutes 2.4±0.8% of total. All of the carbohydrates on Asn137 in pTF are fucosylated.
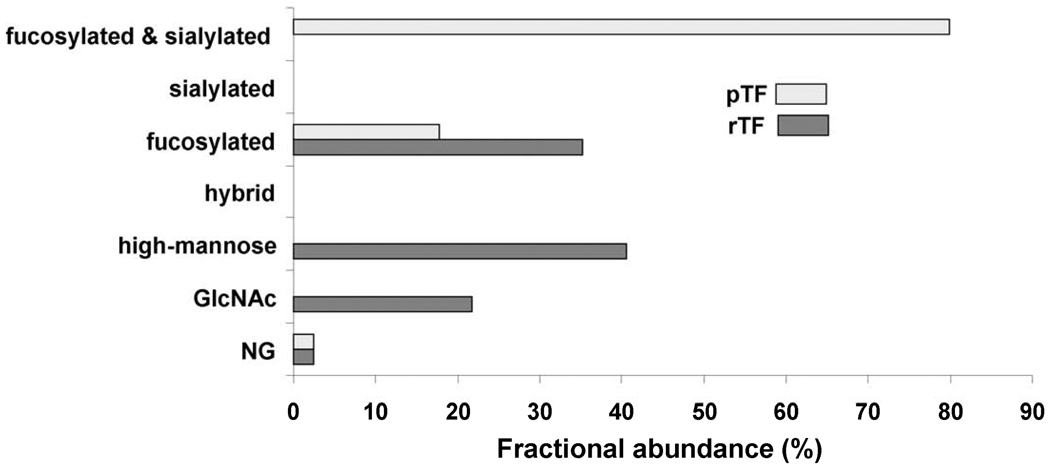
Figures 3, 4 and 5 compare the fractional abundances of the different types of carbohydrates found at each site on rTF and pTF. The carbohydrate composition of rTF predominates in high mannose glycans on all three sites with a fractional abundance of 10.0% on Asn9 and Asn11, 82.8% on Asn124 and 40.6% on Asn137. High mannose glycans are absent in pTF. Figures 3 and 5 show that fucosylation is extensive in the natural pTF with a fractional abundance of 76.4% on Asn9 and Asn11 and 97.5% on Asn137. In contrast, Figures 3 and 5 show that small fucosylated sugars constitute only 8.8% of all glycans on Asn9 and Asn11 and 35.2% on Asn137 in the rTF. A feature of pTF is the presence of SA on all three sites, Asn11, Asn124, and Asn137, whereas sialylation does not exist in the insect-expressed rTF. Figures 3, 4 and 5 demonstrate that sialylated carbohydrates comprise the majority of human pTF glycans with fractional abundances of 57.2% on Asn9 and Asn11, 38.0% on Asn124 and 79.8% on Asn137. A difference between the two TF proteins is also observed in the level of glycosylation. Figure 3 shows that the majority of rTF Asn9 and Asn11 is non-glycosylated with a fractional abundance of 80.0±4.0%, whereas Asn9 and Asn11 in pTF exist as non-glycosylated with a fractional abundance of only 23.6±1.7%. Figure 4 shows that nonglycosylated Asn124 comprises a minute fraction of total in both rTF and pTF with a fractional abundance of 0.9±0.06% and 2.1±0.3% respectively. Likewise, Figure 5 shows that a small fraction of Asn137 is found as nonglycosylated in rTF and pTF, with a fractional abundance of 2.3±0.5% and 2.4±0.2%, respectively.
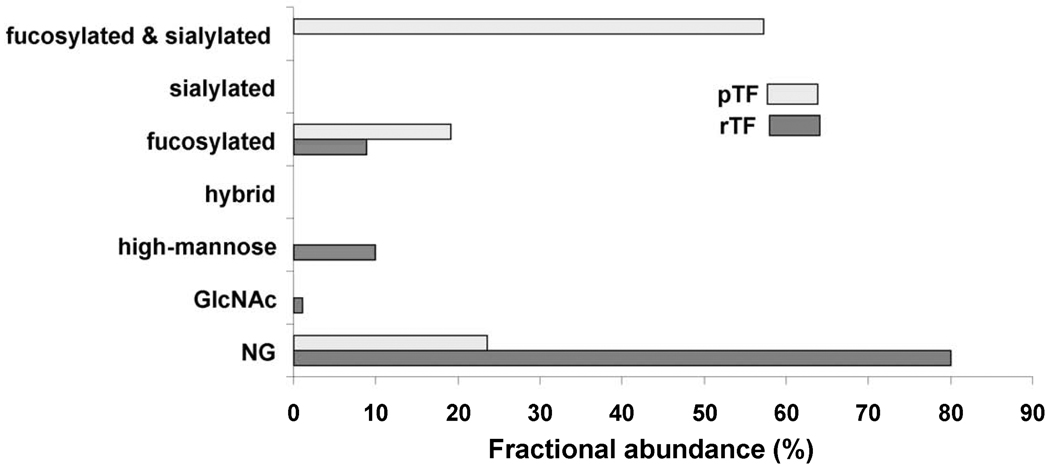
Fig. 3.
Comparison of the fractional abundances for the non-glycosylated (NG) and glycosylated peptides containing Asn9/11 (truncated protein/full-length protein) in pTF and rTF. The non-glycosylated peptide fractional abundance is 23.6% in pTF and 80.0% in rTF. Types of glycans found on glycopeptides containing Asn9/11 in pTF include 19.2% fucosylated and 57.2% sialylated. Glycans on rTF include 1.1% Asn-linked N-acetylglucosamine (GlcNAc), 8.8% fucosylated and 10.0% high-mannose.
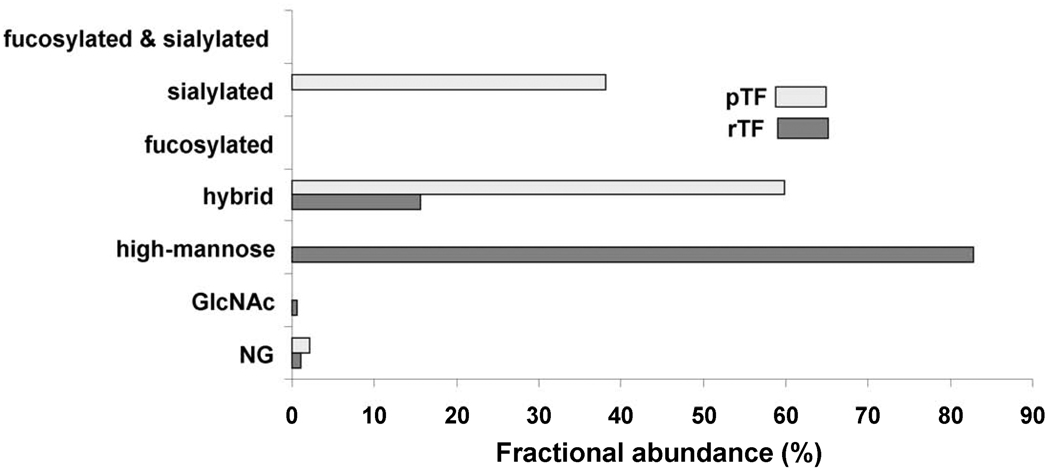
Fig. 4.
Comparison of the fractional abundances for the non-glycosylated (NG) and glycosylated peptides containing Asn124 in pTF and rTF. The non-glycosylated peptide fractional abundance is 1.2% in pTF and 0.9% in rTF. Types of glycans found on glycopeptides containing Asn124 in pTF include 59.7% hybrid and 38.0% sialylated. Glycans in rTF include 0.6% Asn-linked N-acetylglucosamine (GlcNAc), 15.6% hybrid and 82.8% high-mannose.
Fig. 5.
Comparison of the fractional abundances for the non-glycosylated (NG) and glycosylated peptides containing Asn137 in pTF and rTF. The non-glycosylated peptide fractional abundance is 2.4% in pTF and 2.3% in rTF. Types of glycans found on glycopeptides containing Asn137 in pTF include 17.6% fucosylated and 79.8% sialylated. Glycans on rTF include 21.8% Asn-linked N-acetylglucosamine (GlcNAc ), 35.2% fucosylated and 40.6% high-mannose.
4. Discussion
In the present study we used mass spectrometry to evaluate the relative quantitation and composition of glycosylation between recombinant insect expressed human TF and natural TF from human placenta. The type of analysis carried out in this study clearly defines differences between glycan compositions among proteins from different sources, in this case recombinant and natural human TF, but is limited to only glycan composition and can not be used to define the structure of the glycans. Glycan structure analysis is best carried out by releasing the glycan from the protein followed by permethylation and MSn analysis [30–32]. Our goal was to quantify any differences in the type of glycans attached to each glycosylation site in relation to the ability of mammalian and insect cells to incorporate Asn-linked carbohydrates [18]. The MS analysis that we performed is consistent with previously published work that focused on similar goals [26, 33].
We have chosen to convey the heterogeneity of glycosylation at each site for rTF and natural pTF as fractional abundance versus glycan mass. Recent reports by Rebecchi and Thaysen-Andersen demonstrate the validity of this approach for glycoprotein profiling [27, 28]. However, both reports emphasize the limitation when sialic acid is present in the glycopeptides. Neither report addresses the possibility of a fraction of the glycopeptide existing in the nonglycosylated form and how the fractional abundance will compare to the glycosylated forms. An earlier report by Jiang suggests that there will be a bias for detecting glycopeptides without sialic acid over glycopeptides with sialic acid when the LC-MS is set for positive ion mode [34]. This bias may extend to the nonglycosylated form of the glycopeptide as well. Hence, the fractional abundance values for the nonglycosylated form of the glycopeptides and those values for glycopeptides without sialic acid may be overestimated relative to their sialic acid containing forms. Relative to our current goal in this study, this overestimate does not detract from our ability to define the differences in glycan composition between the two sources of TF. The possible overestimate also does not underestimate the fact that our data convey the intensities of the different forms of the glycopeptides as measured by the mass spectrometer using nanoelectrospray ionization. Further work is required to define how much of a bias exists in ionization efficiency between non-glycosylated, neutral and sialic acid containing glycopeptides.
The extracellular domain of TF contains regions of interaction with FVIIa and FX (Figure 6). The sites of interaction between TF, FVIIa, FX and the membrane have previously been determined [9, 11, 35, 36]. Asparagine 9, Asn11 and Asn137 are located in the region of extensive FVIIa interaction. FX binding to the TF-FVIIa complex occurs close to the membrane and in close proximity to Asn124. How glycosylation affects the interaction of TF with its molecular substrates is still unclear, however, our data suggests that carbohydrates play a distinctive role. It is possible that the difference in the extent of glycosylation on Asn9 and Asn11 contributes to the difference in the activity of the rTF and natural pTF. The extent of glycosylation at this site may be affected by the degree of truncation. However, other factors such as the carbohydrate compositions, potential altered membrane distance and/or indirect effect on disulfide formation cannot be underestimated. Glycosylation of Asn124 especially may be important provided its close location to the membrane and FX recognition region. Further site directed studies are required to determine the contribution of each glycosylated site. This study suggests that both the composition and the extent of glycosylation may play a role in TF activity. In conclusion, various sources of human TF such as lungs, brain, placenta and monocytes may exploit processes of protein production and modification in the degree of protein truncation, the extent of glycosylation and the type of carbohydrates added, to control its biological activity. These structural differences, which result in different activities of TF proteins, could be significant in the evaluation of TF function in vivo. Tissue factor-triggered thrombin generation and consequential blood coagulation are threshold events, sensitive to minor changes in TF concentration/activity [37]. Thus seemingly trivial differences in the specific in vitro activity of recombinant and natural TF protein could extrapolate to notable differences in downstream events in vivo. The influence of posttranslational modifications in general and that of glycosylation in particular, on the activity of proteins, also becomes crucial in the study of proteins used therapeutically as well as for diagnostic and research purposes [21, 38]. Analyses of structural and functional differences and similarities of the recombinant and natural protein allow us to extrapolate our understanding and knowledge of tissue factor in a biological setting.
Fig. 6.
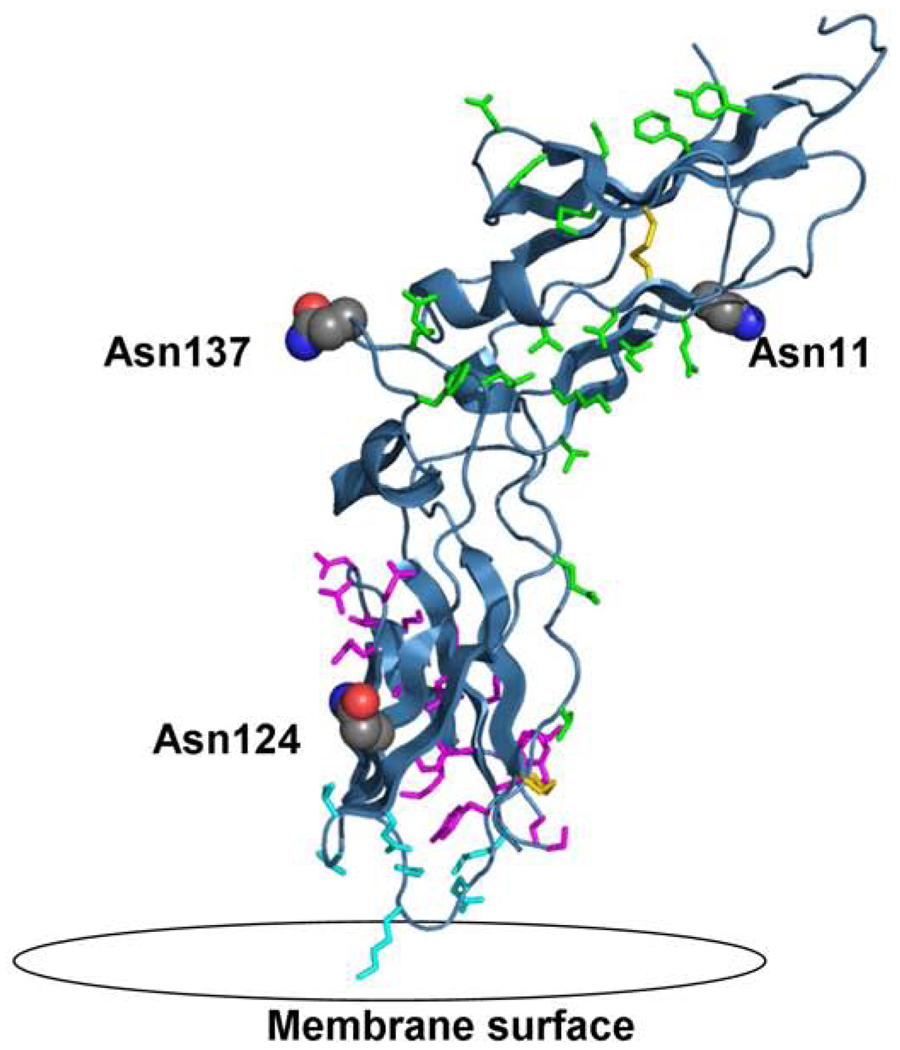
The extracellular domain of tissue factor (PBD 2HFT) on a modeled lipid membrane. The figure shows in red the three sites of glycosylation on TF including Asn11, Asn124 and Asn137. Highlighted in green are residues important for TF interaction with FVIIa including Thr17, Lys20, Ile22, Glu24, Gln37, Asp44, Lys46, Lys48, Asp58, Thr60, Phe76, Tyr78, Gln110, Leu133, Arg135, Phe140, Val207. Highlighted in magenta are residues important for the interaction with FX including Thr154-Glu174 and Tyr185. Highlighted in aqua are the residues important for TF interaction with the membrane including Gln118, Val119, Thr121, Lys159, Asp180, Lys181, Glu183. Also shown in yellow are the two disulfide bridges of TF at positions Cys49-Cys57 and Cys186-Cys209.
Research highlights
-
►
Natural human placental TF exhibits enhanced activity relative to a recombinant form
-
►
Major differences in carbohydrates and extent of glycosylation between the two forms
-
►
Extent and glycosylation type may augment TF activity in various systems/tissues
-
►
New insights into the biological contribution of carbohydrates on TF activity
Acknowledgment
This work was supported by grant P01 HL46703 (project 2) from the National Institutes of Health and by grant P20 RR16462. We would like to thank Matthew Gissel and Nick Johnson for technical assistance in purification of pTF. We would like to thank Dr. Stephen Everse for assistance in the generation of the figure presenting the extracellular domain of tissue factor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of Interests
Kenneth G Mann is the chairman of the board of Haematologic Technologies Inc.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 2.Fleck RA, Rao LVM, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;57:765–781. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, Delatorre JC, Oldstone MBA, Loskutoff DJ, Edgington TS, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system: a role for astrocytes in cerebral hemostasis. J Clin Invest. 1993;92:349–358. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, Kraus J, Lin TC, Nemerson Y, Konigsberg WH. Isolation of cDNA clones coding for human tissue factor: Primary structure of the protein and cDNA. Proc Natl AcadSci USA. 1987;84:5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach R, Nemerson Y, Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981;256:8324–8331. [PubMed] [Google Scholar]
- 6.Nemerson Y, Bach R. Tissue factor revisited. Prog Hemost Thromb. 1982;6:237–261. [PubMed] [Google Scholar]
- 7.Paborsky LR, Tate KM, Harris RJ, Yansura DG, Band L, McCray G, Gorman CM, Obrien DP, Chang JY, Swartz JR, Fung VP, Thomas JN, Vehar GA. Purification of recombinant human tissue factor. Biochemistry. 1989;28:8072–8077. doi: 10.1021/bi00446a016. [DOI] [PubMed] [Google Scholar]
- 8.Stone MJ, Ruf W, Miles DJ, Edgington TS, Wright PE. Recombinant soluble human tissue factor secreted by Saccharomyces cerevisiae and refolded from Escherichia coli inclusion bodies: glycosylation of mutants, activity and physical characterization. Biochemical Journal. 1995;310:605–614. doi: 10.1042/bj3100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller YA, Ultsch MH, de Vos AM. The Crystal Structure of the Extracellular Domain of Human Tissue Factor Refined to 1.7 Å Resolution. J Mol Biol. 1996;256:144–159. doi: 10.1006/jmbi.1996.0073. [DOI] [PubMed] [Google Scholar]
- 10.Kittur FS, Manithody C, Morrissey JH, Rezaie AR. The cofactor function of the N-terminal domain of tissue factor. J Bioll Chem. 2004;279:39745–39749. doi: 10.1074/jbc.M406628200. [DOI] [PubMed] [Google Scholar]
- 11.Harlos K, Martin DMA, Obrien DP, Jones EY, Stuart DI, Polikarpov I, Miller A, Tuddenham EGD, Boys CWG. Crystal structure of the extracellular region of human tissue factor. Nature. 1994;370:662–666. doi: 10.1038/370662a0. [DOI] [PubMed] [Google Scholar]
- 12.Demain AL, Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv. 2009;27:297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 14.Krudysz-Amblo J, Jennings ME, II, Mann KG, Butenas S. Carbohydrates and Activity of Natural and Recombinant Tissue Factor. J Biol Chem. 2010;285:3371–3382. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lie M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nature Medicine. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 16.Kornfeld R, Kornfeld S. Assembly of asparagine linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 17.PeterKatalinic J, Burlingame AL. Methods in Enzymology: O-Glycosylation of Proteins, Methods in Enzymology. Academic Press; 2005. pp. 139–171. [DOI] [PubMed] [Google Scholar]
- 18.Agathos SN. Production scale insect cell culture. Biotechnol Adv. 1991;9:51–68. doi: 10.1016/0734-9750(91)90404-j. [DOI] [PubMed] [Google Scholar]
- 19.Altmann F, Staudacher E, Wilson IBH, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 20.Altmann F. More than silk and honey - or, can insect cells serve in the production of therapeutic glycoproteins? Glycoconj J. 1997;14:643–646. doi: 10.1023/a:1018548812675. [DOI] [PubMed] [Google Scholar]
- 21.Staudacher E, Altmann F, Wilson IBH, Marz L. Fucose in N-glycans: from plant to man. Biochim Biophys Acta. 1999;1473:216–236. doi: 10.1016/s0304-4165(99)00181-6. [DOI] [PubMed] [Google Scholar]
- 22.Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: Sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 24.Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 25.Guzzetta AW, Basa LJ, Hancock WS, Keyt BA, Bennett WF. Identification of carbohydrate structures in glycoprotein peptide maps by the use of LC MS with selected ion extraction with special reference to tissue plasminogen activator and a glycosylation variant produced by site directed mutagenesis. Anal Chem. 1993;65:2953–2962. doi: 10.1021/ac00069a004. [DOI] [PubMed] [Google Scholar]
- 26.Iacob RE, Perdivara I, Przybylski M, Tomer KB. Mass spectrometric characterization of glycosylation of hepatitis C virus E2 envelope glycoprotein reveals extended microheterogeneity of N-glycans. J Am Soc Mass Spectrom. 2008;19:428–444. doi: 10.1016/j.jasms.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebecchi KR, Wenke JL, Go EP, Desaire H. Label-Free Quantitation: A New Glycoproteomics Approach. J Am Soci Mass Spectrom. 2009;20:1048–1059. doi: 10.1016/j.jasms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Thaysen-Andersen M, Mysling S, Hojrup P. Site-Specific Glycoprofiling of N-Linked Glycopeptides Using MALDI-TOF MS: Strong Correlation between Signal Strength and Glycoform Quantities. Anal Chem. 2009;81:3933–3943. doi: 10.1021/ac900231w. [DOI] [PubMed] [Google Scholar]
- 29.Paborsky LR, Harris RJ. Post-translational modifications of recombinant human tissue factor. Thromb Res. 1990;60:367–376. doi: 10.1016/0049-3848(90)90219-3. [DOI] [PubMed] [Google Scholar]
- 30.Reinhold VN, Reinhold BB, Costello CE. Carbohydrate molecular weight profiling, sequence, linkage, and branching data: ES-MS and CID. Anal Chem. 1995;67:1772–1784. doi: 10.1021/ac00107a005. [DOI] [PubMed] [Google Scholar]
- 31.Weiskopf AS, Vouros P, Harvey DJ. Electrospray ionization-ion trap mass spectrometry for structural analysis of complex N-linked glycoprotein oligosaccharides. Anal Chem. 1998;70:4441–4447. doi: 10.1021/ac980289r. [DOI] [PubMed] [Google Scholar]
- 32.Ashline D, Singh S, Hanneman A, Reinhold V. Congruent strategies for carbohydrate sequencing. 1. Mining structural details by MSn. Anal Chem. 2005;77:6250–6262. doi: 10.1021/ac050724z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go EP, Irungu J, Zhang Y, Dalpathado DS, Liao HX, Sutherland LL, Alam SM, Haynes BF, Desaire H. Glycosylation site-specific analysis of HIV envelope proteins (JR-FL and CON-S) reveals major differences in glycosylation site occupancy, glycoform profiles, and antigenic epitopes' accessibility. J Proteome Res. 2008;7:1660–1674. doi: 10.1021/pr7006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Desaire H, Butnev VY, Bousfield GR. Glycoprotein profiling by electrospray mass spectrometry. J Am Soci Mass Spectrom. 2004;15:750–758. doi: 10.1016/j.jasms.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Banner DW, Darcy A, Chene C, Winkler FK, Guh A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo YZ, Morrissey JH, Tajkhorshid E. Dynamical view of membrane binding and complex formation of human factor VIIa and tissue factor. J Thromb Haemost. 2010;8:1044–1053. doi: 10.1111/j.1538-7836.2010.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van't Veer C, Mann KG. Regulation of Tissue Factor Initiated Thrombin Generation by the Stoichiometric Inhibitors Tissue Factor Pathway Inhibitor, Antithrombin-III, and Heparin Cofactor-II. J Biol Chem. 1997;272:4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 38.Hancock K, Narang S, Pattabhi S, Yushak ML, Khan A, Lin SC, Plemons R, Betenbaugh MJ, Tsang VCW. False positive reactivity of recombinant, diagnostic, glycoproteins produced in High Five (TM) insect cells: Effect of glycosylation. J Immunol Methods. 2008;330:130–136. doi: 10.1016/j.jim.2007.08.002. [DOI] [PubMed] [Google Scholar]