Abstract
Several different pharmacological effects have been described for Nigella sativa (Siah-Daneh), including an anti-inflammatory effect. In the present study, the effect of the extract of N. sativa on lung pathology and blood interleukin-4 (IL-4) and interferon-γ (IFN-γ) of sensitized guinea pigs was examined. Three groups (n=8 for each group) of guinea pigs sensitized to ovalbumin (OA) were given drinking water alone, and drinking water containing low and high concentrations of the plant extract, respectively. The animals of the control group (n=8) were treated with saline instead of OA and were given drinking water. The pathological changes of the lung, including infiltration of eosinophils and lymphocytes, local epithelial necrosis, the presence of oedema, thickening of the basement membrane, smooth muscle layer hypertrophy, mucosal secretion, and the presence of mucosal plug, and blood IL-4 and IFN-γ of sensitized guinea pigs were evaluated. The lungs of the sensitized group showed significant pathological changes (P<0.001). Blood IL-4 and IFN-γ were increased in sensitized animals compared to the controls (P<0.01 and P<0.001, respectively). Treatment of sensitized animals with the extract led to a significant decrease in pathological changes of the lung (P<0.01 to P<0.001), except for the oedema in the sensitized group treated with low concentration of the extract, but an increased IFN-γ. These results confirm a preventive effect of N. sativa extract on lung inflammation of sensitized guinea pigs.
Keywords: Nigella sativa, Asthma, Sensitization, Inflammation, Cytokine
1. Introduction
Asthma is an inflammatory disorder of the airways (Busse et al., 1995). Many inflammatory cells, including eosinophils, mast cells, macrophages, and neutrophils, are involved in the pathogenesis of airway inflammation in asthma (Kelly et al., 1988). These inflammatory cells produce more reactive oxygen species (superoxides, hydrogen peroxides, hypohalites, etc.) than those of normal subjects (Cluzel et al., 1987).
The pathological changes of the respiratory system include: (1) thickening of the airway wall (subepithelial basement membrane), (2) infiltration of inflammatory cells in the lung parenchyma involving mainly eosinophils, as well as lymphocytes and mast cells, (3) increased smooth muscle mass, (4) mucous gland hypertrophy and vascular congestion (leading to edema or swelling of the airway wall), and (5) collagen deposition in the basement membrane leading to a thickened airway wall and markedly reduced airway caliber, airway epithelial shedding, epithelial desquamation and necrosis, and mucus plugs occluding medium and small bronchi (Barrios et al., 2006).
There are different asthma phenotypes including: (1) symptom-based: age at onset, natural history, and severity; (2) defined by triggers: allergic vs. non-allergic, exercise induced, viral triggered vs. multi-triggered wheeze. The most important asthma phenotypes are intrinsic (non-allergic) and extrinsic (allergic) asthma, classified on the basis of positive skin tests to common allergens or the presence of antibodies in the blood (Handoyo and Rosenwasser, 2009; Henderson et al., 2009).
Any T cell that has the ability to suppress the immune response is known as a regulatory T cell. These cells are classified as natural T regulatory cells and induced or adaptive T regulatory cells. The natural T regulatory (nTreg) cells are self antigen specific CD4+ T cells that express CD25 in high levels and Foxp3. The induced or adaptive T regulatory (aTreg) cells are the type 1 regulatory T (Tr1) cells and T helper 3 (Th3) cells. The Tr1 cells have both Th1 and Th2 phenotypic markers (Nandakumar et al., 2009). In allergy and asthma models, evidence suggests that both nTreg and aTreg cells control asthma, which is dependent on both interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Allergen challenge leads to stimulation of CD4+ effector T cells to produce considerable amounts of IL-10 in the lung by allergen specific Treg cells that are able to control asthma. However, the suppression of Th2-driven response to allergens in vivo by Treg cells is dependent on IL-10, and the production of IL-10 by Treg cells themselves is not required for the suppression observed. In fact, it has been shown that dendritic cells (DCs) from Treg cell-depleted mice had an increased capacity to stimulate T cell proliferation and Th2 cytokine production, which was concomitant with reduced IL-12 expression. These data suggest that resistance to allergen-driven airway hyperresponsiveness (AHR) is mediated in part by CD4 CD25 Treg cell suppression of DC activation (Vignali et al., 2008).
In allergic phenotypes of asthma, two principal immune mechanisms affecting airway obstruction depend on Th1 and Th2 cells. The Th2 cells secrete highly characteristic cytokines including IL-4, IL-5, IL-9, and IL-13, all of which contribute to manifestations of allergic inflammation and disease (Cohn et al., 2004). However, Th1 cells produce IL-2 and interferon-γ (IFN-γ), which can inhibit the inflammatory process of asthma (Romagnani, 1999).
The anti-inflammatory activities of both systemic and local administrations of essential oil from Nigella sativa have been demonstrated (Hajhashemi et al., 2004). It has been shown that thymoquinone (TQ), the main constituent of N. sativa seeds, inhibits the production of 5-hydroxyeicosatetraenoic acid, as well as 5-lipoxygenase (El-Dakhakhny et al., 2002).
The therapeutic effect of the oil of this plant on patients with allergic diseases (allergic rhinitis, bronchial asthma, atopic eczema) has also been documented (Kalus et al., 2003). Salem (2005) summarized the immunomodulatory and therapeutic properties of the N. sativa L. seed and emphasized the potent immunomodulatory effects of this plant. Our previous studies have also shown different pharmacological effects of N. sativa on guinea pig tracheal chains, including relaxant and functional antagonistic effects on muscarinic receptors (Boskabady and Shahabi, 1997), inhibitory effect on histamine (H1) receptors (Boskabady and Shiravi, 2002) and calcium channels (Boskabady and Shirmohammadi, 2002), opening effect on potassium channels (Boskabady et al., 2004b), and stimulatory effect on β-adrenoceptors (Boskabady et al., 2004c). The antitussive effect of this plant on the guinea pig has also been demonstrated (Boskabady et al., 2004a).
N. sativa is widely used in traditional medicine, including in the treatment of respiratory disorders such as tightness and asthma (Ave-Sina, 1990). Therefore, in the present study, the protective effect of hydro-ethanolic extract of N. sativa, containing both water and lipid constituents of the plant, on lung inflammation (lung pathology and cytokines) of sensitized guinea pigs was examined.
2. Materials and methods
2.1. Plant and extract
N. sativa was collected from Torbat Heydarieh (northeast Iran), and its seeds were dried at room temperature in the absence of sunlight. The plant was identified by botanists in the herbarium of the Ferdowsi University of Mashhad; and the specimen number of the plant is 293-0303-1. The hydro-ethanolic extract was prepared as follows: 500 g of chopped N. sativa seeds were mixed with 450 ml 50% ethanol for 72 h at 40 °C and the solution was separated by maceration method. This process was repeated three times. The solution was dried by rotary evaporator at 50 °C.
2.2. Animal sensitization and animal groups
Thirty two adult Dunkin-Hartley guinea pigs (400–700 g, 13 females and 17 males) were used throughout the study. They were allowed to get into the habit of the new situation for 10 d. The animals were group-housed in individual cages in climate-controlled animal quarters and given water and food ad libitum, while a 12-h on/12-h off light cycle was maintained. After 10 d, sensitization of animals to ovalbumin (OA) was performed using the method previously described (McCaig, 1987; McCaig and de Jonckheere, 1993; Boskabady and Ziaei, 2003; Neamati et al., 2009). Briefly, guinea pigs were sensitized to OA (Sigma Chemical Ltd., UK) by injecting 100 mg i.p. and 100 mg s.c. on Day 1 and a further 10 mg i.p. on Day 8. From Day 14, sensitized animals were exposed to an aerosol of 0.04 g/ml OA for (18±1) d, 4 min daily. The aerosol was administered in a closed acrylic chamber, dimensions 30 cm×20 cm×20 cm, coupled to the nebulizer (CX3, Omron Healthcare Europe B.V., the Netherlands). The study was approved by the ethical committee of the Tabriz University of Medical Sciences, Iran.
The study was performed in control animals (C group, treated the same as the sensitized group, but normal saline was used instead of OA and they were given drinking water alone) and three different groups of sensitized animals, which were given various types of drinking water during the sensitization period as follows (n=8 for each group): (1) drinking water alone (S group, sensitized group), (2) drinking water containing 1.25 g/L N. sativa extract (S+LNS group), (3) drinking water containing 2.50 g/L N. sativa extract (S+HNS group).
2.3. Pathological evaluation
Guinea pigs were sacrificed by means of cervical dislocation, and the lungs and tracheas were removed and placed into the 10% (v/v) buffered formalin (Merck, Germany). Seven days later, tissues were dried using Autotecnicon apparatus by passage of tissues through 70%–100% ethanol and xylol to clear the tissues, and paraffin blocks of the tissues were prepared. The specimens were cut in 4-µm slices and were stained with hematoxylin-eosin (H&E). The tissues were then evaluated under a light microscope.
The pathologic changes in the lung of sensitized and treated groups with the extract included infiltration of eosinophils and lymphocytes, local epithelial necrosis, the presence of oedema, thickening of basement membrane, smooth muscle layer hypertrophy, mucosal secretion, and the presence of mucosal plug. The pathological changes were scored according previous studies (Boskabady et al., 2006; 2010b) as follows: (1) no pathologic changes, 0; (2) patchy changes, 0.5; (3) local changes, 1; (4) scattered changes, 2; (5) severe changes (in the most parts of the lung), 3.
2.4. Evaluations of blood IL-4 and IFN-γ
A total of 5 ml peripheral blood was obtained immediately after sacrificing the animals and placed at room temperature for 1 h. The samples were then centrifuged at 3 500×g at 4 °C for 10 min. The supernatant was collected and immediately stored at 70 °C until analyzed. Finally blood IL-4 and IFN-γ were measured using the enzyme-linked immunosorbent assay (ELISA) Sandwich method.
2.5. Statistical analysis
The data are expressed as mean±standard error of the mean (SEM). Comparisons between sensitized animals and the control, between two groups treated with different doses of the extract, and between extract treated and sensitized guinea pigs were performed using unpaired t-test. Significance was accepted at P<0.05.
3. Results
3.1. Pathology
All pathological changes were significantly higher in the S group than in the control group (P<0.001 for all cases; Figs. 1 and 2).
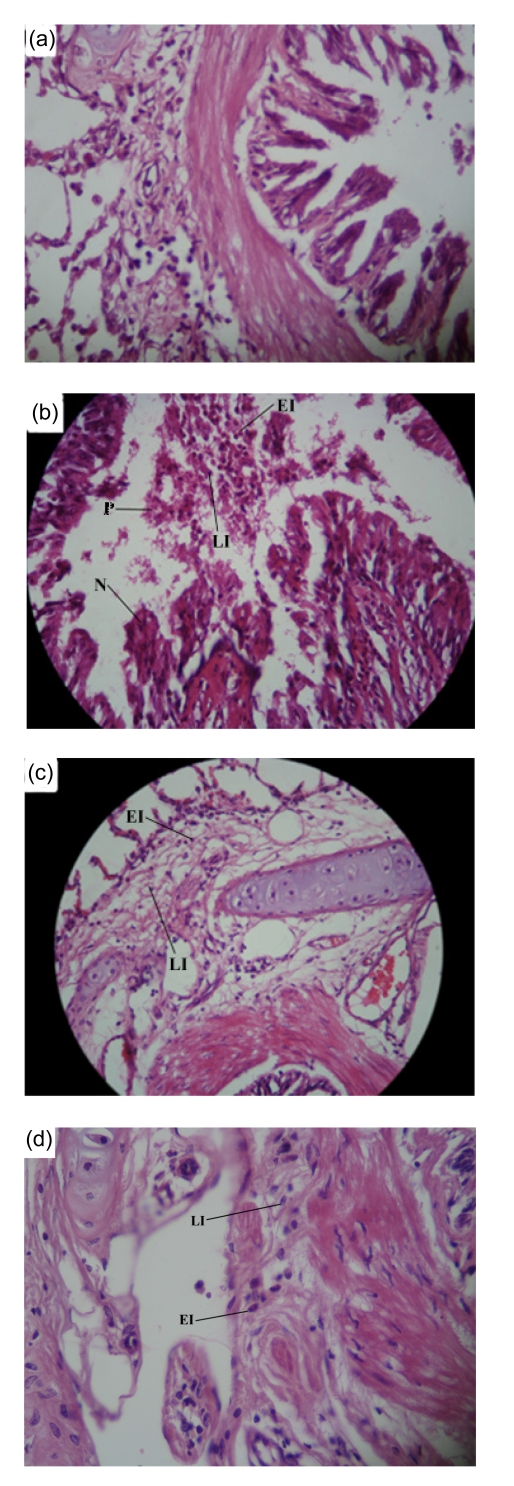
Fig. 1.
Photographs of a lung specimen in the control (a), sensitized (b), and low (c) and high (d) doses ofN. sativa extract treated sensitized guinea pigs
The photograph of the sensitized group showed local epithelial necrosis (N), mucosal plug (P), severe eosinophil infiltration (EI), and lymphocyte infiltration (LI), but these changes were improved in the treated animals with both doses of the extract. Magnification: (a, b, c) 10×40; (d) 10×60
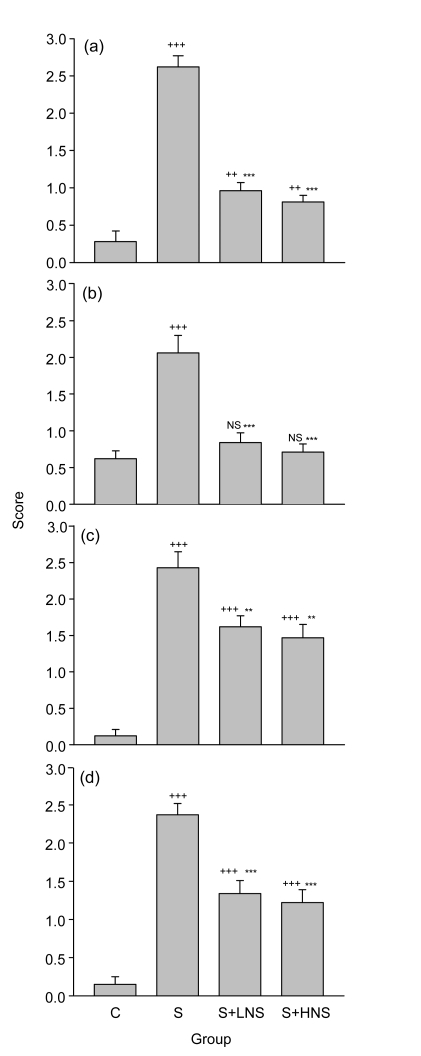
Fig. 2.
Scores of pathological changes in the control (C), sensitized (S), and low and high doses of N. sativa extract treated sensitized (S+LNS and S+HNS, respectively) guinea pigs
The pathological changes include eosinophil infiltration (a), lymphocyte infiltration (b), epithelial necrosis (c), and mucosal plug (d) of the lung. Values are expressed as mean±SEM (n=8 for each group). Statistical differences between the control and other groups: NS Non-significant difference, ++ P<0.01, +++ P<0.001; Statistical differences between the extract-treated and sensitized groups: * P<0.05, ** P<0.01, *** P<0.001
Pretreatment with both concentrations of N. sativa extract caused a significant improvement in all pathological changes of the sensitized animals (P<0.01 to P<0.001), except for oedema in the treated group with a low concentration of N. sativa extract (Fig. 2). However, there were still significant differences in pathological changes between the control and treated groups, except for lymphocyte infiltration in the treated groups with both concentrations of N. sativa extract and for mucosal secretion in the treated group with the high dose of N. sativa extract (P<0.01 to P<0.001; Fig. 2). Eosinophil infiltration, which is a specific finding for allergic asthma, was mostly affected by the extract of N. sativa (reduced to one third of that of S group) (Table 1).
Table 1.
Pathological finding scores in the control (C), sensitized (S), and low and high doses of N. sativa extract treated sensitized (S+LNS and S+HNS, respectively) guinea pigs
| Pathological finding | Score |
|||
| C | S | S+LNS | S+HNS | |
| Eosinophil infiltration | 0.28±0.14 | 2.62±0.15 | 0.96±0.11 | 0.81±0.09 |
| Lymphocyte infiltration | 0.62±0.11 | 2.06±0.24 | 0.84±0.13 | 0.71±0.11 |
| Local epithelial necrosis | 0.12±0.09 | 2.43±0.22 | 1.62±0.15 | 1.47±0.18 |
| Mucosal plug | 0.15±0.10 | 2.37±0.15 | 1.34±0.17 | 1.22±0.17 |
| Oedema | 0.65±0.11 | 1.90±0.06 | 1.62±0.12 | 1.47±0.12 |
| Basement membrane thickening | 0.03±0.03 | 1.68±0.20 | 0.93±0.10 | 0.87±0.15 |
| Muscular hypertrophy | 0.03±0.03 | 2.90±0.06 | 2.31±0.18 | 1.90±0.12 |
| Mucosal secretion | 0.62±0.11 | 1.75±0.13 | 0.90±0.04 | 0.84±0.04 |
Values are presented as mean±SEM (n=8 for each group). There was not statistical difference between effects of two concentrations of the extract
The lung pathological changes in the animals treated with the low concentration of N. sativa extract were also not significantly greater than those of the animals treated with high concentration (Table 1).
3.2. Blood IL-4 and IFN-γ
The mean value of blood IL-4 of the S group [(4.90±0.15) pg/ml] was significantly higher than that of the C group [(3.52±0.37) pg/ml] (P<0.01; Fig. 3a). Blood IL-4 levels in the treated groups with low [(3.91±0.31) pg/ml] and high [(3.56±0.35) pg/ml] concentrations of the extract were significantly low when compared with the S group (P<0.05 and P<0.01, respectively; Fig. 3a). However, the mean values of IL-4 in these groups were not significantly higher than that in the C group (Fig. 3a).
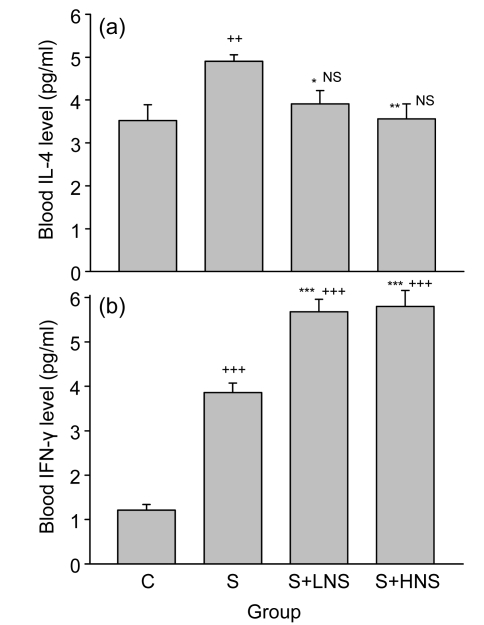
Fig. 3.
Blood levels of IL-4 (a) and IFN-γ (b) in the control (C), sensitized (S), and low and high doses ofN. sativa extract treated sensitized (S+LNS and S+HNS, respectively) guinea pigs
Values are presented as mean±SEM (n=8 for each group). Statistical differences between the control and other groups: NS Non-significant difference, ++ P<0.01, +++ P<0.001; Statistical differences between the extract-treated and sensitized groups: * P<0.05, ** P<0.01, *** P<0.001. There was no statistical difference between the effects of two concentrations of the extract
The mean value of blood IFN-γ of the S group [(3.86±0.21) pg/ml] was significantly higher than that of the C group [(1.21±0.13) pg/ml] (P<0.001; Fig. 3b). Treatment with both concentrations of the extract caused a significant increase in blood IFN-γ level [low, (5.68±0.28) pg/ml; high, (5.80±0.36) pg/ml], compared with that of the S group (P<0.001 for both cases; Fig. 3b). In addition, the mean values of IFN-γ in these groups were significantly higher than that in the C group (P<0.001 for all cases; Fig. 3b).
Blood IL-4 level in the animals treated with the low concentration of the extract was not significantly greater than that in the high concentration group. However, blood IFN-γ level in the treated group with low concentration of the extract was lower than that in the high concentration group (Fig. 3).
4. Discussion
In the present study, the preventive effects of long-term administration of hydro-ethanolic extract of N. sativa (during sensitization period, i.e., (32±1) d) on pathological changes of the lung and the level of cytokines (IL-4 and IFN-γ) were examined. The results showed increased IL-4 and IFN-γ in sensitized guinea pigs. Histological evaluations of the lung tissues also showed the infiltration of eosinophils and lymphocytes in the lung parenchyma, epithelial damage, mucosal plug, oedema, basement membrane thickening, and muscular hypertrophy in sensitized guinea pigs, similar to the results of previous studies (Boskabady and Kiani, 2007), which, especially eosinophil infiltration, are compatible with pathological changes seen in asthma (Barrios et al., 2006).
Pretreatment of sensitized animals with N. sativa extract prevented increased IL-4 and almost all lung histological changes of sensitized guinea pigs. In addition, pretreatment of sensitized animals with N. sativa increased IFN-γ. There were no significant differences between the preventive effects of different doses of N. sativa extract on lung pathologic changes.
The preventive effects of long-term administration of the extract of N. sativa on pathological changes of the lungs in the sensitized animals are perhaps due to its suppressing effects on inflammation. In fact, the inhibitory effects of the essential oil of N. sativa have been shown in both cyclooxygenase and 5-lipoxygenase pathways of arachidonic acid metabolism and also in membrane lipid peroxidation (Houghton et al., 1995; Boskabady and Farhadi, 2008). The anti-inflammatory activity effects of both systemic and local administrations of the essential oil from this plant have also been shown (Hajhashemi et al., 2004). The therapeutic effects of N. sativa oil on patients with allergic diseases (allergic rhinitis, bronchial asthma, atopic eczema) have also been demonstrated (Kalus et al., 2003). Salem (2005) summarized the immunomodulatory and therapeutic properties of N. sativa seeds and emphasized the potent immunomodulatory effects of this plant. Ali and Blunden (2003) also summarized different pharmacological effects of N. sativa including effects on asthma disease, inflammation, and the immune system, and indicated its different constituents.
One proposed mechanism of action for the extract of N. sativa on cytokines and pathological changes of the sensitized guinea pigs is its regulation of Th1 and Th2 balance. It has been shown that the inflammatory condition, such as airway inflammation, seen in asthma is regulated by the balance of two T helpers, Th1, and Th2 cells. Th2 cells promote the activities of macrophages and regulate the pro-inflammatory response, whereas Th1 cells inhibit the activity of Th2 (Romagnani, 1999). Th1 cells produce IL-2 and IFN-γ, whereas Th2 cells produce IL-4 and IL-10 (Randolph et al., 1999). In fact, the results of the present study showed that the extract from N. sativa caused inhibitory effect on IL-4 but enhanced the production of IFN-γ, indicating the inhibitory effect on Th2 cells and the stimulatory effect on Th1 cells.
Another possible mechanism of action for N. sativa may be due to its antioxidant effect. In fact, the antioxidant effects of N. sativa (Burits and Bucar, 2000; Thippeswamy and Naidu, 2005) and its main constituent TQ (Badary et al., 2003; El-Mahdy et al., 2005; El-Mahmoudy et al., 2005a; 2005b) have been demonstrated. The antioxidant effects of other ingredients of N. sativa oil such as α-lipoic acid have also been shown (Ibrahim et al., 2008). Oxidative stress plays an important role in asthmatic airway inflammation and α-lipoic acid may be useful in adjuvant therapy for bronchial asthma (Cho et al., 2004). Significant decreases of various components of both enzymatic and nonenzymatic antioxidant defenses have also been shown in asthma (Sackesen et al., 2008). Oxidative stress plays a role in the production of airway responsiveness in mice (Talati et al., 2006). The uptake of antioxidants may slow down the Th1-type immune response and thereby favors an overproduction of Th2-type cytokines, and foods rich in antioxidants may cause a lack of triggers for the Th1-type immune response (Murr et al., 2005). In addition, a relatively powerful preventive effect of vitamin C on increased tracheal responsiveness of the sensitized guinea pigs with similar method of sensitization has been shown in our previous study (Boskabady and Ziaei, 2003; Neamati et al., 2009).
Although Büyüköztürk et al. (2005) showed that N. sativa (black seed) oil did not affect the Th1 and Th2-type cytokine productions from splenic mononuclear cells in allergen sensitized mice, the studied animals, the method of sensitization, the type of extract, and cytokine profile were different from our present study. Another important difference between the study of Büyüköztürk et al. (2005) and our study was the source of cytokine. In their study, the splenic mononuclear cytokines were measured, while in our study the peripheral blood cytokines were measured. The most important difference between the two studies was the evaluation of lung pathological changes in our present study, which was lacking in the study of Büyüköztürk et al. (2005). In addition, in the study of Büyüköztürk et al. (2005), the animals were sensitized just by i.p. injection of OA and the effect of N. sativa mainly on systemic inflammation was evaluated, whereas in our present study animals were sensitize by i.p. injection and inhalation of OA, and the effect of plant mainly on lung inflammation was examined.
The study of Talatt Abbas et al. (2005) in murine model of allergic asthma showed that treatment with N. sativa leads to significant reductions of peripheral blood eosinophil count, IgG1 and IgG2a levels, cytokine profiles, and inflammatory cells in the lung tissue, which was equivalent to the effects of dexamethasone, but IFN-γ level did not changed due to N. sativa and dexamethasone treatment. These data support the results of the present study. However, the results of our study show an increased IFN-γ level due to N. sativa treatment, indicating the stimulatory effect of N. sativa on Th1 cells. In addition, in the study of Talatt Abbas et al. (2005), only inflammatory cell infiltrations of lung tissues were examined, while in the present study more comprehensive pathological evaluations were performed. The method of sensitization and studied animals in the two studies were also different. Besides, in both the studies of Büyüköztürk et al. (2005) and Talatt Abbas et al. (2005), the effect of essential oil of the plant was examined, but in the present study hydro-ethanolic extract was used. In the study of Talatt Abbas et al. (2005), the effect of N. sativa was evaluated mainly on systemic inflammation.
The effect of N. sativa oil on murine cytomegalovirus infection showed the most striking inhibition of virus titers in the spleen and liver and an increase in the serum level of IFN-γ (Salem and Hossain, 2000). Although this study was done on murine virus infected model and the systemic effect of N. sativa was examined, the effect of the plant on serum IFN-γ supports the results of our study.
The bronchodilatory (Boskabady et al., 2010a) and preventive effects on asthmatic patients (Boskabady et al., 2007), as well as preventive effect on chemical warfare victims (Boskabady and Farhadi, 2008), have been shown in our previous studies for this plant. In a new series of experiments, including the present study, the cellular and molecular mechanisms of the effect of this plant on respiratory disorders, mainly asthma (sensitized animals), were investigated. In fact, several studies have indicated the anti-inflammatory and immunological effects of this plant. Kalus et al. (2003) examined the effect of orally administered N. sativa oil at a dose of 40 to 80 mg/(kg·d) on patients with allergic rhinitis, bronchial asthma, and atopic eczema. The severity of symptoms decreased in treated patients. These findings showed that N. sativa could be an effective adjuvant for treatment of allergic diseases. Salem (2005), in a review article, provided clear evidence that both the oil of N. sativa and its active ingredients, in particular TQ, possess reproducible anti-oxidant effects and potent anti-inflammatory effects on several inflammation-based models by suppression of the inflammatory mediators, prostaglandins, and leukotrienes. The oil and certain active ingredients also showed beneficial immunomodulatory properties, augmenting the T cell- and natural killer cell-mediated immune responses. El Gazzar et al. (2006) also examined the anti-inflammatory effect of TQ in a mouse model of allergic lung inflammation. The results showed a marked decrease in lung eosinophilia, Th2 cytokines, and OA specific IgE and IgG1 in TQ-treated sensitized mice. However, despite a high number of studies published evaluating the immune functions of N. sativa products so far, this plant is not yet in clinical use for treatment of asthma.
The goal of the treatment of asthma is reducing airway inflammation with anti-inflammatory drugs. However, the available anti-inflammatory drugs for asthma do not lead to complete cure of airway inflammation. In fact, GINA guideline (National Institutes of Health, 2006) recommended the use of complementary and alternative therapies, including herbal medicine. With regards to anti-inflammatory, immunomodulatory, and antioxidant effects of N. sativa and its constituents, this plant has a potential therapeutic effect on asthma. However, more studies are required for clinical application of N. sativa, including examining the effect of plant on IL-17 and IL-10 and comparing the effect of N. sativa with current therapy in humans.
A non-significant difference between the preventive effects of two concentrations of the extract on cytokines and pathological changes may indicate that the maximum preventive effect of the plant extract was obtained at lower concentration used.
In conclusion, the results of the present study indicated that N. sativa extract prevents pathological changes of the lung.
Footnotes
Project (No. 85/2TU) supported by the Tuberculosis and Lung Research Centre and Drug Applied Research Centre of Tabriz University of Medical Sciences, Iran
References
- 1.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa . Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 2.Ave-Sina . In: Law in Medicine. Sharafkhandy A, translator. Theran: Ministry of Guidance Publication; 1990. p. 314. (in Farsi) [Google Scholar]
- 3.Badary OA, Taha RA, El-Din AMG, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26(2):87–98. doi: 10.1081/DCT-120020404. [DOI] [PubMed] [Google Scholar]
- 4.Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma, pathology and pathophysiology. Arch Pathol Lab Med. 2006;130(4):447–451. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- 5.Boskabady MH, Shahabi M. Bronchodilatory and anticholinergic effects of Nigella sativa on isolated guinea-pig tracheal chains. Iran J Med Sci. 1997;22(3):127–133. [Google Scholar]
- 6.Boskabady MH, Shiravi N. Inhibitory effect of Nigella sativa on histamine (H1) receptors of isolated guinea pig tracheal chains. Pharmac Biol. 2002;40(8):596–602. doi: 10.1076/phbi.40.8.596.14653. [DOI] [Google Scholar]
- 7.Boskabady MH, Shirmohammadi B. Effect of Nigella sativa on isolated guinea pig tracheal chains. Arch Iran Med. 2002;5(2):103–107. [Google Scholar]
- 8.Boskabady MH, Ziaei T. Effect of ascorbic acid on airway responsiveness in ovalbumin sensitized guinea pigs. Respirology. 2003;8(4):473–478. doi: 10.1046/j.1440-1843.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 9.Boskabady MH, Kiani S. The effect of exposure of guinea pigs to cigarette smoke and their sensitization with ovalbumin in tracheal responsiveness to histamine and histamine (H1) receptor blockade by chlorpheniramine. Pathophysiology. 2007;14(2):97–104. doi: 10.1016/j.pathophys.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Boskabady MH, Farhadi F. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14(9):1137–1144. doi: 10.1089/acm.2008.0049. [DOI] [PubMed] [Google Scholar]
- 11.Boskabady MH, Kiani S, Jandaghi P, Ziaei T, Zarei A. Antitussive of Nigella sativa . Pak J Med Sci. 2004;20(3):224–228. [Google Scholar]
- 12.Boskabady MH, Shirmohammadi B, Jandaghi P, Kiani S. Possible mechanisms for relaxant effect of aqueous and macerate extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4(1):3. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boskabady MH, Kiani S, Jandaghi P. Stimulatory effect of Nigella sativa on β2-adronceptors of guinea pig tracheal chains. Med J Islam Rep Iran. 2004;18(2):153–158. [Google Scholar]
- 14.Boskabady MH, Kiani S, Aslani MR. Tracheal responsiveness to both isoprenaline and β-adrenoreceptor blockade by propranolol in cigarette smoke exposed and sensitized guinea pigs. Respirology. 2006;11(5):572–578. doi: 10.1111/j.1440-1843.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- 15.Boskabady MH, Javan H, Sajadi M, Rakhshandah H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21(5):559–566. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 16.Boskabady MH, Mohsenpoor N, Takallo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17(10):707–713. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Eslamizade MJ, Tabatabaei A, Nemati H, Mansouri F. Effect of inhaled fluticasone on lung inflammation administered during and after guinea pig sensitization. Arch Bronconeumol. 2010;46(5):215–222. doi: 10.1016/j.arbres.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–328. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Busse W, Banks-Schlegel SP, Larson GL. Childhood versus adult-onset asthma. Am J Respir Crit Care Med. 1995;151(5):1635–1639. doi: 10.1164/ajrccm.151.5.7735626. [DOI] [PubMed] [Google Scholar]
- 20.Büyüköztürk S, Gelincik A, Özseker F, Genç S, Savran FO, Kiran B, Yillar G, Bilir A. Nigella sativa (black seed) oil does not affect the T-helper 1 and T-helper 2 type cytokine production from splenic mononuclear cells in allergen sensitized mice. J Ethnopharmacol. 2005;100(3):295–298. doi: 10.1016/j.jep.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Cho YS, Lee JC, Lee TH. α-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Allergy Clin Immunol. 2004;114(2):429–435. doi: 10.1016/j.jaci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Cluzel M, Damon M, Chanez P. Enhanced alveolar cell luminol-dependent chemiluminescence in asthma. J Allergy Clin Immunol. 1987;80(2):195–201. doi: 10.1016/0091-6749(87)90129-1. [DOI] [PubMed] [Google Scholar]
- 23.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22(1):789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 24.El-Dakhakhny M, Madi NJ, Lembert N, Ammon HP. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygenase products in polymorphonuclear leukocytes from rats. J Ethnopharmacol. 2002;81(2):161–164. doi: 10.1016/S0378-8741(02)00051-X. [DOI] [PubMed] [Google Scholar]
- 25.El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6(7):1135–1142. doi: 10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.El-Mahdy MA, Zhu Q, Wang QE, Wani G, Wani AA. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int J Cancer. 2005;117(3):409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 27.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, Nikami H, Takewaki T. Macrophage-derived cytokine and nitric oxide profiles in type I and type II diabetes mellitus: effect of thymoquinone. Acta Diabetol. 2005;42(1):23–30. doi: 10.1007/s00592-005-0170-6. [DOI] [PubMed] [Google Scholar]
- 28.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, El-Sayed M, Takewaki T. Successful abrogation by thymoquinone against induction of diabetes mellitus with streptozotocin via nitric oxide inhibitory mechanism. Int Immunopharmacol. 2005;5(1):195–207. doi: 10.1016/j.intimp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and anti-inflammatory drug. Phytother Res. 2004;18(3):195–199. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- 30.Handoyo S, Rosenwasser LJ. Asthma phenotypes. Curr Allergy Asthma Rep. 2009;9(6):439–445. doi: 10.1007/s11882-009-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson J, Granell R, Sterne J. The search for new asthma phenotypes. Arch Dis Child. 2009;94(5):333–336. doi: 10.1136/adc.2008.143636. [DOI] [PubMed] [Google Scholar]
- 32.Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipidperoxidation. Planta Med. 1995;61(1):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim SF, Osman K, Das S, Othman AM, Abdul Majid N, Abdul Rahman MP. A study of the antioxidant effect of α-lipoic acids on sperm quality. Clinics. 2008;63(4):545–550. doi: 10.1590/S1807-59322008000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, Kiesewetter H. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–1214. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 35.Kelly CA, Ward C, Stenton SC, Bird G, Hendrick DJ, Walters EH. Number and activity of inflammatory cells in bronchoalveolar lavage fluid in asthma and their relation to airway responsiveness. Thorax. 1988;43(9):684–692. doi: 10.1136/thx.43.9.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCaig D. Comparison of autonomic responses in the trachea isolated from normal and albumin-sensitive guinea-pig. Br J Pharmacol. 1987;92(4):809–816. doi: 10.1111/j.1476-5381.1987.tb11385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaig D, de Jonckheere S. Effect of two Ca2+ modulator in normal and albumin-sensitized guinea-pig trachea. Eur J Pharmacol. 1993;249(1-2):53–63. doi: 10.1016/0014-2999(93)90661-Z. [DOI] [PubMed] [Google Scholar]
- 38.Murr C, Schroecksnadel K, Winkler C, Ledochowski M, Fuchs D. Antioxidants may increase the probability of developing allergic diseases and asthma. Med Hypotheses. 2005;64(5):973–977. doi: 10.1016/j.mehy.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Nandakumar S, Miller CWT, Kumaraguru U. T regulatory cells: an overview and intervention techniques to modulate allergy outcome. Clin Mol Allergy. 2009;7(1):5. doi: 10.1186/1476-7961-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institutes of Health. Global Strategy for Asthma Management and Prevention: NHBLI Workshop Report. Bethesda, MD: 2006. p. 22.p. 35. Publication No. 02. [Google Scholar]
- 41.Neamati A, Boskabady MH, Tavakol Afshari J, Mohaghegh Hazrati S, Haeri Rohani A. The effect of natural adjuvants on tracheal responsiveness and cell count in lung lavage of sensitized guinea pigs. Respirology. 2009;14(6):877–884. doi: 10.1111/j.1440-1843.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 42.Randolph DA, Stephens R, Carruthers CJL, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104(8):1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5(4):285–294. doi: 10.1002/ibd.3780050410. [DOI] [PubMed] [Google Scholar]
- 44.Sackesen C, Ercan H, Dizdar E, Soyer O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol. 2008;122(1):78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int J Immunopharmacol. 2005;5(13-14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Inter J Immunopharmacol. 2000;22(9):729–740. doi: 10.1016/S0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 47.Talati M, Meyrick B, Peebles RS, Davies SS, Worski RD, Mernrugh R, Mitchell B, Boothby M, Roberts LJ, Sheller JR. Oxidant stress modulates murine allergic airway responses. Free Radical Biol Med. 2006;40(7):1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Talatt Abbas A, Abdel-Aziz MM, Zalata KR, Abd Al-Galel TE. Effect of dexamethasone and Nigella sativa on peripheral blood eosinophil count, IgG1 and IgG2a, cytokine profiles and lung inflammation in murine model of allergic asthma. Egypt J Immunol. 2005;12(1):95–102. [PubMed] [Google Scholar]
- 49.Thippeswamy NB, Naidu KA. Antioxidant potency of cumin varieties—cumin, black cumin and bitter cumin—on antioxidant systems. Eur Food Res Technol. 2005;220(5-6):472–476. doi: 10.1007/s00217-004-1087-y. [DOI] [Google Scholar]
- 50.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]