Abstract
Seed treatment with endophytic fungi has been regarded as an effective method for plant parasitic nematode control. Endophytic fungi from cucumber seedlings were isolated and screened for their potential to be used as seed treatment agents against Meloidogyne incognita. Among the 294 isolates screened, 23 significantly reduced galls formed by M. incognita in greenhouse test. The 10 most effective isolates were Fusarium (5), Trichoderma (1), Chaetomium (1), Acremonium (1), Paecilomyces (1), and Phyllosticta (1). Their control efficacies were repeatedly tested and their colonizations as well as in vitro activity against M. incognita were studied. They reduced the number of galls by 24.0%–58.4% in the first screening and 15.6%–44.3% in the repeated test, respectively. Phyllosticta Ph511 and Chaetomium Ch1001 had high colonizations on both the roots and the aboveground parts of cucumber seedlings. Fusarium isolates had colonization preference on the roots, their root colonizations ranging from 20.1% to 47.3% of the total root area. Trichoderma Tr882, Paecilomyces Pa972, and Acremonium Ac985 had low colonizations on both the roots and the aboveground parts. Acremonium Ac985, Chaetomium Ch1001, Paecilomyces Pa972, and Phyllosticta Ph511 produced compounds affecting motility of the second stage juveniles of M. incognita. Based on these results, Chaetomium Ch1001 was considered to have the highest potential as a seed treatment agent for M. incognita biocontrol.
Keywords: Cucumber endophytic fungi, Meloidogyne incognita, Control efficacy, Colonization, In vitro activity
1. Introduction
Cucumber (Cucumis sativus L.) is a vegetable crop produced at a large scale. However, its production is seriously threatened by root-knot nematodes Meloidogyne spp. (Sikora and Fernández, 2005). As the greenhouse production pattern becomes more popular, the damage caused by root-knot nematodes becomes even more serious. Four Meloidogyne species were frequently found in China, and M. incognita is the most dominant species (Wang et al., 2001; Liao et al., 2003). In many cases, nematicides are required to reduce damage and increase yield.
Interest in biological control of plant parasitic nematodes has increased because of the high cost and the environmental problems caused by nematicides. Using fungal endophytes against plant parasitic nematodes has been implicated in previous studies. Grasses infected by Neotyphodium endophytes inhibited some species of migratory and sedentary endoparasites (West et al., 1988; Bacetty et al., 2009). Non-pathogenic Fusarium strains isolated from plant roots were capable of reducing Radopholus similis on banana (Niere, 2001), Meloidogyne incognita on tomato (Hallmann and Sikora, 1994), and M. graminicola on rice (Le et al., 2009). It is very likely that certain endophytes can be used for nematode protection in agriculture (Zabalgogeazcoa, 2008).
It has been suggested that endophytic fungi can be used as seed treatment agents, thereby reducing the level of inoculum needed for biological control (Athman, 2006). Moreover, seed treatment provides endophytic fungi opportunities to colonize and protect the plants at the young seedlings stage, enhancing seedlings health, and as a result being beneficial to the whole crop production cycle. However, to date the research on using endophytic fungi as a seed treatment agent for plant parasitic nematode control is still rare. Therefore, the aim of the current study was to screen entophytic fungi from young cucumber seedlings for the potential to be used as a seed treatment agent against M. incognita.
2. Materials and methods
2.1. Isolation of cucumber endophytic fungi from young seedlings
A total of 87 soil samples were collected from Bonn (Germany), Hangzhou (China), and Hainan (China), representing temperate, subtropical, and tropical climate regions, respectively.
Each sample consisted of 300 g of 5–30 cm deep rhizosphere soil. The collected soil samples were placed in 400 cm3 pots. One seed (cultivar Chinesische schlange) was sowed in each pot approximately 1 cm below the surface and covered with top soil. Before sowing, seeds were surface-sterilised in 75% (v/v) ethanol for 30 s and 5 g/L NaClO for 3 min, and rinsed three times in sterile tap water. Seven to ten days after seed germination, endophytic fungi were isolated from symptomless plants. The plants were washed free of soil particles. The roots, leaves, and stems were segregated. Roots were surface-sterilised by shaking in 5 g/L NaClO solution for 3 min, followed by several rinses in sterile water (Dababat et al., 2008). Leaves and stems were surface-sterilised in 70% (v/v) ethanol for 30 s followed by rinsing (Mu et al., 2010). Roots and stems were cut into 1-cm sections while leaves were cut into 0.5 cm×0.5 cm pieces. The fragments were first pressed onto 10% (v/v) potato dextrose agar (PDA) amended with 150×10−6 g/ml streptomycin sulfate and 150×10−6 g/ml chloromycetin plates for sterility checks and then mounted on new plates (Le et al., 2009). All plates were incubated at 25 °C, and inspected for fungal growth at a 3-d intervals for two weeks.
Mycelia at the edge of the each colony growing from the fragments were transferred to new plates and purified (Huang and Xin, 2008). Purified isolates were stored at 4 °C. Morphologies of the fungi colonies were recorded. Mycelia and fruit bodies picked up from the colonies were mounted on glass slides and observed under light microscope. Identification of the fungi to genera level was based on the morphological characteristics of the fungi (Wei, 1979; Domsch et al., 1980; Stone et al., 2000).
2.2. Nematode culture
A population of M. incognita (Race 3) was maintained on a highly susceptible tomato variety (cultivar Superstar) in the greenhouse. Nematode eggs were extracted from infected tomato roots using 15 g/L NaOCl solution (Hussey and Barker, 1973). Second-stage juveniles were obtained by incubating eggs in extraction trays at 25 °C for 14 d. The J2 suspension was then adjusted to 200 nematodes per ml.
2.3. Screening cucumber endophytic fungi against Meloidogyne incognita
Only isolates grew and sporulated well on potato dextrose agar were screened against M. incognita. Isolates were incubated on potato dextrose agar plates at 25 °C for 15 d, then 5 ml of sterile water were pipetted onto the surface of the plates, and the spores were scrapped off from the plates. The spore suspension was then filtered through a fine mesh screen (Φ 0.15 mm) to separate spores from hyphae. Spore suspension concentration was determined by Neubauer hemacytometer and was then adjusted to 1×107 spores per ml and stored at 4 °C until use.
The soil substrate (sand:loam=1:1, v/v) was sterilised at 121 °C for 2 h and fixed into 400 cm3 pots. A total of 3 ml of nematode suspension (600 J2s) was mixed well with the soil. Surface-sterilised cucumber seeds were placed into a 1 cm deep hole in the soil substrate, and 1 ml spore suspension of the tested isolate was pipetted on top of the seed and covered with top soil. The control seed was pipetted with 1 ml water around. Six replicates were prepared for each fungal endophyte and control. The plants were kept in the greenhouse (25 °C, artificial light for 16 h), watered daily, and fertilized weekly with 3 g/L of Poly Fertisol (N:P:K=14:10:14). The pots were set up as a randomized complete block. Five weeks after seed germination, the plants were harvested and the number of galls was counted. All data were subjected to one-way analysis of variance (ANOVA) with statistical product and service solutions (SPSS) 14.0. Means were then separated using the least significant difference (LSD) test. Ten isolates with the highest significant differences were selected and their control efficacies were calculated (Abott, 1925).
2.4. Repeated control efficacy test and colonization ratio of the 10 selected fungi isolates
Control efficacies of the 10 selected fungi isolates were repeatedly tested following the same procedure as the first screening. After gall counting, three plant replicates of each isolate were used for re-isolation to assess the colonization ability. Thirty underground and aboveground fragments from each plant replicate were pressed onto 5–6 PDA plates. After 14 d, the number of pieces (N) with fungal colony growth was counted, and the colonization ratio was calculated as N/30×100%.
2.5. In vitro activity of the potato dextrose broth culture filtrates of the 10 selected fungi isolates towards J2 of Meloidogyne incognita
Agar discs from the 14 d-old PDA cultures were inoculated into 300 ml flasks containing 200 ml potato dextrose broth and shaken at 100 r/min at 25 °C for 7 d. The culture filtrates were obtained by separating the liquid from the mycelia using Whatman filter paper (No. 1) and micropore filter (Ф 0.22 μm). To test the in vitro toxicity of the culture filtrates against J2, 5 ml culture filtrates of each isolate and 1 ml J2 suspension containing 200 J2s were pipetted into Petri dishes. The control petri dishes were pipetted with 5 ml potato dextrose broth and 1 ml J2 suspension. After 48 h, the percentage of immotile nematodes was calculated, and immotile nematodes were allowed to recover in tap water for 5 h. Those that remained inactive were considered dead (Meyer et al., 2004). Six replicates were used for both treatment and control. One-way ANOVA and subsequent LSD test were used to test for significant differences. The test was conducted twice.
3. Results
3.1. Diversity of endophytic fungi from cucumber seedlings
A total of 514 isolates were recovered from 7–10 d old cucumber seedlings growing in 87 soil samples collected from tropical, subtropical, and temperate areas (Table 1). Among the 514 isolates, 143 were sterile and 77 could not grow well on PDA, with colonies <5 cm in diameter after 15 d. The remaining 294 isolates were distributed across the genera Acremonium, Actinomucor, Aspergillus, Aureobasidium, Cercospora, Chaetomium, Cladosporium, Colletotrichum, Curvularia, Fusarium, Humicola, Paecilomyces, Phyllosticta, Stagonospora, Trichoderma, Ureobasidium, and unknown genera based on their morphological characteristics of the colonies of mycelia, as well fruit bodies. Fusarium was the most frequently recovered genus (Table 2).
Table 1.
Geographical origins of the cucumber endophytic fungi
| Climate | Location | n1 | n2 |
| Tropical | Hainan, China | 65 | 391 |
| Subtropical | Hangzhou, China | 15 | 78 |
| Temperate | Bonn, Germany | 7 | 45 |
| Total | 87 | 514 | |
n 1: number of collected soil samples; n 2: number of isolated cucumber endophytic fungi
Table 2.
Diversity of endophytic fungi isolated from cucumber seedlings
| Genus | Number of isolates |
||
| Roots | Stems | Leaves | |
| Not grown well on PDA | 33 | 14 | 30 |
| No spore production | 57 | 24 | 62 |
| Acremonium spp. | 2 | 4 | |
| Actinomucor spp. | 9 | ||
| Aspergillus spp. | 7 | ||
| Aureobasidium spp. | 5 | ||
| Cercospora spp. | 5 | ||
| Chaetomium spp. | 6 | 2 | |
| Cladosporium spp. | 2 | ||
| Colletotrichum spp. | 4 | ||
| Curvularia spp. | 7 | ||
| Fusarium spp. | 64 | 15 | 12 |
| Humicola spp. | 1 | ||
| Paecilomyces spp. | 10 | ||
| Phyllosticta spp. | 2 | ||
| Stagonospora spp. | 1 | ||
| Trichoderma spp. | 20 | 5 | |
| Ureobasidium spp. | 6 | ||
| Unknown genera | 68 | 17 | 20 |
| Total | 275 | 88 | 151 |
3.2. Screening of cucumber endophytic fungi against Meloidogyne incognita
Amongst the 294 isolates tested, 23 (7.8%) significantly reduced the number of galls formed by M. incognita on cucumber seedlings. Ten fungi that exhibited the highest M. incognita control were Fusarium (5), Trichoderma (1), Chaetomium (1), Acremonium (1), Paecilomyces (1), and Phyllosticta (1) (Table 3). Their control efficacies ranged from 24.0% to 58.4%.
Table 3.
Ten fungi isolates with the highest inhibition to gall formation by M. incognita
| Isolate | Genus | Origin | Control efficacy (%) |
Colonization (%) |
||
| First test | Repeated test | Underground | Aboveground | |||
| Fu7 | Fusarium sp. | Bonn, Germany | 48.1c | 29.5b | 20.1 | 2.1 |
| Fu9 | Fusarium sp. | Hangzhou, China | 33.6bc | 21.4b | 23.0 | 5.0 |
| Fu546 | Fusarium sp. | Hainan, China | 24.0b | 25.3b | 47.3 | 0.0 |
| Fu234 | Fusarium sp. | Hainan, China | 58.4c | 44.3c | 32.4 | 15.0 |
| Fu654 | Fusarium sp. | Hainan, China | 36.9bc | 35.1b | 37.9 | 3.5 |
| Ph511 | Phyllosticta sp. | Hainan, China | 30.8b | 22.3b | 44.0 | 34.8 |
| Ch1001 | Chaetomium sp. | Hainan, China | 47.3c | 42.1c | 70.5 | 73.5 |
| Tr882 | Trichoderma sp. | Hainan, China | 25.4b | 19.2ab | 14.8 | 2.0 |
| Pa972 | Paecilomyces sp. | Hainan, China | 49.2c | 34.9bc | 12.4 | 10.2 |
| Ac985 | Acremonium sp. | Hainan, China | 33.0bc | 15.6ab | 17.9 | 10.4 |
The control efficacies of the 10 isolates were subjected to one-way ANOVA analysis followed by LSD test. Mean values with the same superscript letters within the same column are not significantly different (P≤0.05; n=6)
3.3. Repeated control efficacy tests of the 10 selected fungi isolates and their colonization ratios on cucumber seedlings
In the repeated test for the 10 strains, the control efficacies ranged from 15.6% to 44.3%. Fusarium strain Fu234, Paecilomyces strain Pa972, and Chaetomium strain Ch1001 demonstrated the highest gall reduction in both tests.
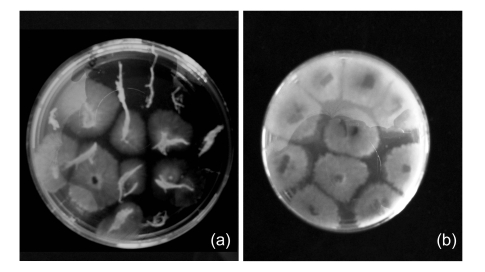
Re-isolation showed that the five Fusarium isolates preferred colonizing roots with colonization ratios of 20.1%–47.3% of the total root area. Colonization ratios of Trichoderma strain Tr882, Paecilomyces strain Pa972, and Acremonium strain Ac985 were low in the underground (12.4%–17.9%) and aboveground (2.0%–10.4%) parts of the cucumber seedlings. Phyllosticta strain Ph511 had 44.0% and 34.8% underground and aboveground colonizations, respectively. Ch1001 had the highest colonization both on the underground (70.5%) and aboveground (73.5%) parts (Table 3, Fig. 1).
Fig. 1.
Colonization of Chaetomium Ch1001 on cucumber seedlings
(a) Underground part; (b) Aboveground part
3.4. In vitro activity of potato dextrose broth culture filtrates of the 10 selected fungi isolates towards J2 of Meloidogyne incognita
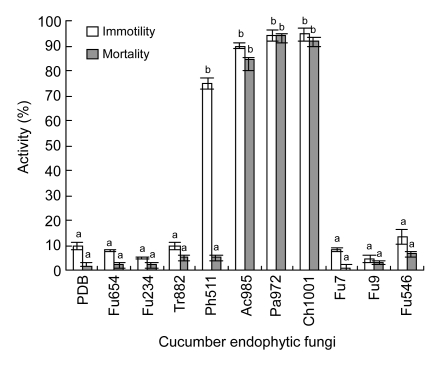
After 48 h of exposure to culture filtrates of Ch1001, Pa972, and Ac985, J2 mortality reached 92.0%, 94.0%, and 84.5%, respectively, indicating that nematicidal compounds were produced in the potato dextrose broth. After a 48-h exposure to culture filtrates of Ph511, 75.3% of J2s was inactivated; however, 90% of the immobilised J2s was recovered in tap water, indicating that nematistatic compounds were produced (Fig. 2). The culture filtrates of other six isolates were not active against the second stage juveniles of M. incognita.
Fig. 2.
In vitro activity of culture filtrates of 10 cucumber endophytic fungi towards M. incognita
Columns with the same letters are not significantly different in the mean numbers of immotile and dead nematodes based on LSD test (P≤0.05, n=6). Bars represent the standard errors of the means. PDB: potato dextrose broth
4. Discussion
The number of endophytic species potentially associated to a plant species is often estimated in several hundreds (Sánchez Márquez et al., 2007). However, plant age has an effect upon endophyte diversity, and older plant parts may harbour more endophytes than younger ones (Arnold et al., 2003). Some studies have shown that seeds and seedlings are virtually endophyte-free, and the incidence of fungal endophytes increases as symbiotic lifestyles leaves or seeds grow older (Gallery et al., 2007). In our study, the endophytic fungi recovered from 7–10 d old cucumber seedlings were very diversified, and other isolates were distributed across more than 16 genera. The diversity might be correlated to the fact that large numbers of endophytic fungi were recovered from the roots. Fungi from the rhizosphere soil can colonize roots in a relative short time, and most of the soil samples collected in this study were from a tropical area. It has been suggested that endophytic assemblages appear to be richer in tropical than in temperate or cold zones of the world (Fisher et al., 1995). Fusarium was the most frequently recovered genus from cucumber seedlings, and this result was consistent with studies on other crops (Sikora et al., 2008).
In the screening test, 7.9% of the isolates significantly reduced the number of galls formed by M. incognita. The 10 most effective isolates were Fusarium (5), Trichoderma (1), Chaetomium (1), Acremonium (1), Paecilomyces (1), and Phyllosticta (1). Endophytic Fusarium isolates were proved again to be useful candidates for plant parasitic nematode control. Fungi from the genera Acremonium, Paecilomyces, Trichoderma, and Chaetomium have been extensively documented as antagonistic to plant parasitic nematodes (Sharon et al., 2001; Nitao et al., 2002; Kalele et al., 2007; Goswamia et al., 2008). In this study, it was shown that part of their life cycle can also be endophytic. For the first time, one isolate from genus Phyllosticta was found to be antagonistic to M. incognita.
Re-isolation showed that the colonization ratios of Acremonium, Paecilomyces, and Trichoderma on cucumber seedlings were very low. It was likely that they suppressed nematodes mainly before the nematode penetration (Kiewnick and Sikora, 2006). Fusarium strains prefer colonizing roots, where the colonizing level (20.1%–47.3%) was similar to that reported by Hallmann and Sikora (1994) on tomato, which was 12%–50%. Chaetomium Ch1001 and Phyllosticta Ph511 had colonizations on both the roots and the aboveground parts of the cucumber seedlings, with Ch1001 exhibiting the highest colonization. Despite their good colonization abilities, the growth of the plants was not negatively affected by Ch1001 and Ph511. At harvest, the fresh weight of the plants was not significantly different from that of the control plants (data not shown), indicating that Ch1001 and Ph511 were not pathogenic to young seedlings.
In an in vitro study, Acremonium Ac985, Paecilomyces Pa972, Chaetomium Ch1001, and Phyllosticta Ph511 produced compounds in potato dextrose broth affecting root-knot nematode motility. Other studies also demonstrated that fungi from the genera Acremonium, Paecilomyces, and Chaetomium produced nematicidal compounds (Sharon et al., 2001; Nitao et al., 2002; Goswamia et al., 2008), Phyllosticta strain was for the first time found to produce nematicidal compounds in our study. Toxic activities of the culture filtrates of Fusarium isolates and Trichoderma Tr882 were not detected. Their control efficacies may be attributed to the indirect interaction mediated by the plants. Vu et al. (2006) demonstrated that some Fusarium isolates induced systemic resistance in banana against burrowing nematodes Radopholus similis. Dababat and Sikora (2007a; 2007b) reported that non-pathogenic Fusarium Fo162 changed the chemical composition of tomato root exudates; thus, reducing the attraction and invasion of M. incognita and induced systemic resistance against M. incognita.
Root-knot nematodes continuously attack the crop during the whole production cycle. Endophytic fungi producing nematicidal compounds, and with the ability to trigger plant defense, are considered to be most likely to be used as seed treatment agents for the biocontrol of root-knot nematodes. At the young seedlings stage, when the root is small, the direct contact between the fungi and nematodes around the roots is enough. Nematicidal compounds from the fungi can play the major role suppressing nematode penetration. As the plants grow bigger, and the root system develops, the direct contact between fungi and nematodes is reduced, but then the triggered plant defense can continue to reduce nematode invasion. It was supposed that colonization ability is correlated to induced resistance. Therefore, based on the results of the study, Chaetomium Ch1001 was considered to have the most potential to be used as a seed treatment agent for root-knot nematode control. Further studies need to be conducted to address: (1) identification of the fungi to species, (2) biological characteristics of the fungi, (3) triggered plant defense, (4) optimum dose to be used on seeds, and (5) its influence on the life cycle of M. incognita in cucumber.
Footnotes
Project supported by the German Academic Exchange Agency (DAAD), the China Scholarship Council (CSC), the Special Fund for Agro-scientific Research in the Public Interest (No. nyhezx07-050-6), the Science and Technology Department of Zhejiang Province of China (No. 2008C22062), and the Zhejiang Provincial Natural Science Foundation of China (No. R307396)
References
- 1.Abott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18(6):265–267. [Google Scholar]
- 2.Arnold AE, Mejía LC, Kyllo D, Rojas EI, Robbins N, Herre EA. Fungal endophytes limit pathogen damage in a tropical tree. PNAS. 2003;100(26):15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athman SY. Host-Endophyte-Pest Interactions of Endophytic Fusarium oxysporum Antagonistic to Radopholus similis in Banana (Musa spp.) Pretoria, South Africa: University of Pretoria; 2006. pp. 25–29. (PhD Thesis) [Google Scholar]
- 4.Bacetty AA, Snook ME, Glenn AE. Toxicity of endophyte-infected tall fescue alkaloids and grass metabolites on Pratylenchus scriberiri . Phytopathology. 2009;99(12):1336–1345. doi: 10.1094/PHYTO-99-12-1336. [DOI] [PubMed] [Google Scholar]
- 5.Dababat AA, Sikora RA. Induced resistance by the mutualistic endophyte, Fusarium oxysporum 162, toward Meloidogyne incognita on tomato. Biocontrol Sci Technol. 2007;17(9):969–975. doi: 10.1080/09583150701582057. [DOI] [Google Scholar]
- 6.Dababat AA, Sikora RA. Influence of the mutualistic endophyte Fusarium oxysporum 162 on Meloidogyne incognita attraction and invasion. Nematology. 2007;9(6):771–776. doi: 10.1163/156854107782331225. [DOI] [Google Scholar]
- 7.Dababat AA, Selim ME, Saleh AA, Sikora RA. Influence of Fusarium wilt resistant tomato cultivars on root colonization of the mutualistic endophyte Fusarium oxysporum strain 162 and its biological control efficacy toward the root-knot nematode Meloidogyne incognita . J Plant Dis Prot. 2008;115(6):273–278. [Google Scholar]
- 8.Domsch KH, Gams W, Anderson TH. Compendium of Soil Fungi. London, the United Kingdom: Academic Press; 1980. pp. 1–405. [Google Scholar]
- 9.Fisher PJ, Graf F, Petrinil LE, Sutton BC, Wookey PA. Fungal endophytes of Dryas octopetala from a high polar semidesert and from the Swiss Alps. Mycologia. 1995;87(3):319–323. doi: 10.2307/3760828. [DOI] [Google Scholar]
- 10.Gallery RA, Dalling JW, Arnold AE. Diversity, host affinity and distribution of seed-infecting fungi: a case study with Cecropia. Ecology. 2007;88(3):582–588. doi: 10.1890/05-1207. [DOI] [PubMed] [Google Scholar]
- 11.Goswamia J, Pandey RK, Tewaria JP, Goswami BK. Management of root knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum . J Environ Sci Health B. 2008;43(3):237–240. doi: 10.1080/03601230701771164. [DOI] [PubMed] [Google Scholar]
- 12.Hallmann J, Sikora RA. Influence of Fusarium oxysporum, a mutualistic fungal endophyte on Meloidogyne incognita infection of tomato. J Plant Dis Prot. 1994;101(4):475–481. [Google Scholar]
- 13.Huang XL, Xin MX. Experimental Technique in Microbiology. Beijing: Higher Education Press; 2008. pp. 55–79. (in Chinese) [Google Scholar]
- 14.Hussey RS, Barker KR. A comparison of methods of collecting inocula Meloidogyne spp. including a new technique. Plant Dis Rep. 1973;57(6):1025–1028. [Google Scholar]
- 15.Kalele DN, Affokpon A, Coosemans J. Efficacy of Paecilomyces lilacinus strain 251 against root knot nematodes in tomato under greenhouse conditions. Commun Agric Appl Biol Sci. 2007;72(1):209–213. [PubMed] [Google Scholar]
- 16.Kiewnick S, Sikora RA. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Control. 2006;38(2):179–187. doi: 10.1016/j.biocontrol.2005.12.006. [DOI] [Google Scholar]
- 17.Le TH, Padgham LJ, Sikora RA. Biological control of the rice root-knot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. Int J Pest Manage. 2009;55(1):31–36. doi: 10.1080/09670870802450235. [DOI] [Google Scholar]
- 18.Liao J, Jiang H, Sun L. Identification of species and race of root-knot nematodes on crops in southern China. J Huazhong Agric Univ. 2003;22(6):35–40. (in Chinese) [Google Scholar]
- 19.Meyer SLF, Huettel RN, Liu XZ, Humber RA, Juba J, Nitao JK. Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenlile motility. Nematology. 2004;6(1):23–32. doi: 10.1163/156854104323072883. [DOI] [Google Scholar]
- 20.Mu LX, Niu YC, Deng H. The endophytic mycobiota in summer growing cucumber in Beijing. Mycosystema. 2010;29(2):214–221. (in Chinese) [Google Scholar]
- 21.Niere BI. Significance of Non-pathogenic Isolates of Fusarium oxysporum Schlecht: Fries for the Biological Control of the Burrowing Nematode Radopholus similis (Cobb) Thorne on Tissue Cultured Banana. Bonn, Germany: University of Bonn; 2001. pp. 45–58. (PhD Thesis) [Google Scholar]
- 22.Nitao JK, Meyer SLF, Oliver JE, Schmidt WF, Chitwood DJ. Isolation of flavipin, a fungus compound antagonistic to plant-parasitic nematodes. Nematology. 2002;4(1):55–63. doi: 10.1163/156854102760082203. [DOI] [Google Scholar]
- 23.Sánchez Márquez S, Bills GF, Zabalgogeazcoa I. The endophytic mycobiota of the grass Dactylis glomerata . Fungal Diversity. 2007;27(3):171–195. [Google Scholar]
- 24.Sharon E, Bar-Eyal M, Chet I, Herrera-Estrella A, Kleifeld O, Spiegel Y. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum . Phytopathology. 2001;91(7):687–693. doi: 10.1094/PHYTO.2001.91.7.687. [DOI] [PubMed] [Google Scholar]
- 25.Sikora RA, Fernández E. Nematode Parasites of Vegetables. In: Luc M, Sikora RA, Bridge J, editors. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. Wallingford: CABI; 2005. pp. 319–392. [DOI] [Google Scholar]
- 26.Sikora RA, Pocasangre L, Zum Felde A, Niere B, Vu TT, Dababat AA. Mutualistic endophytic fungi and in-plant suppressiveness to plant parasitic nematodes. Biol Control. 2008;46(1):15–23. doi: 10.1016/j.biocontrol.2008.02.011. [DOI] [Google Scholar]
- 27.Stone JK, Bacon CW, White JF. An Overview of Endophytic Microbes: Endophytism Defined. In: Bacon CW, White JFJr, editors. Microbial Endophytes. New York: Marcel Dekker Inc; 2000. pp. 3–29. [Google Scholar]
- 28.Vu TT, Hauschild R, Sikora RA. Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology. 2006;8(6):847–852. doi: 10.1163/156854106779799259. [DOI] [Google Scholar]
- 29.Wang L, Yang B, Li C. Investigation of root-knot nematodes in East China. Forest Res. 2001;14(5):24–28. (in Chinese) [Google Scholar]
- 30.Wei J. Fungi Identification Manual Book. Shanghai: Shanghai Science and Technology Press; 1979. pp. 256–461. (in Chinese) [Google Scholar]
- 31.West CP, Oosterhuis DM, Robbins RT. The effect of Acremonium coenophialum on growth and nematode infestation of tall fescue. Plant Soil. 1988;112(1):3–6. doi: 10.1007/BF02181745. [DOI] [Google Scholar]
- 32.Zabalgogeazcoa I. Fungal endophytes and their interaction with plant pathogens. Span J Agric Res. 2008;6(Special Issue):138–146. [Google Scholar]