Abstract
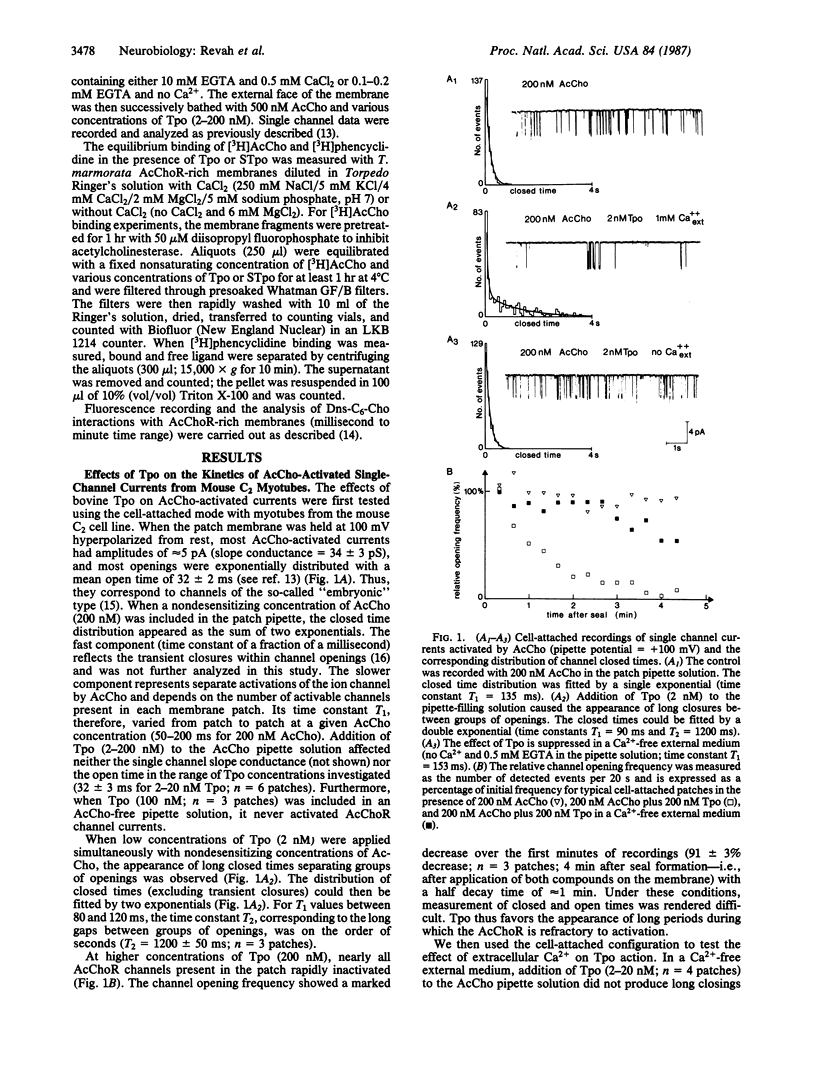
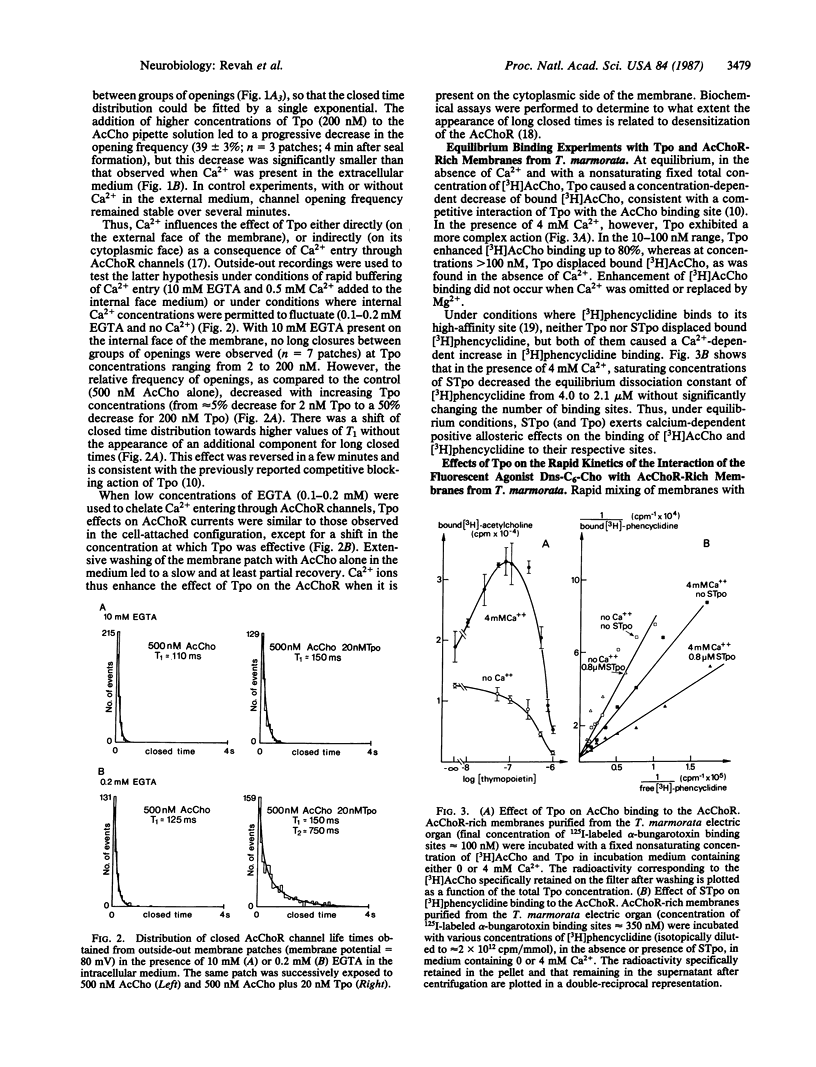
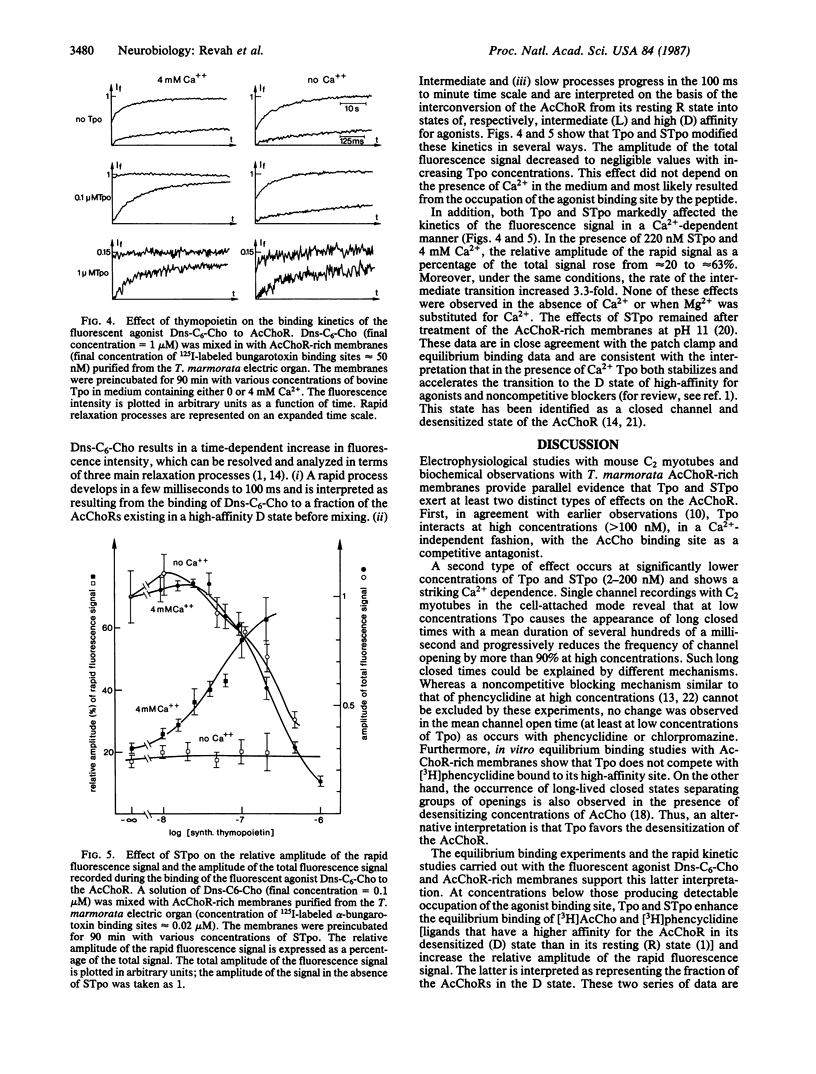
The effects of the thymic polypeptide thymopoietin (Tpo) on the properties of the nicotinic acetylcholine receptor (AcChoR) were investigated by patch clamp techniques on mouse C2 myotubes and by biochemical assays on AcChoR-rich membrane fragments purified from the Torpedo marmorata electric organ. At high concentrations (greater than 100 nM), Tpo inhibits the binding of cholinergic agonists to the AcChoR in a Ca2+-insensitive manner. At lower concentrations (2 nM), Tpo applied on C2 myotubes simultaneously with nondesensitizing concentrations of acetylcholine results in the appearance of long closed times separating groups of openings. This effect depends on the presence of Ca2+ in the external medium. Outside-out recordings, performed with various concentrations of EGTA in the intracellular medium, suggest that Ca2+ acts on the cytoplasmic face of the membrane after entry through acetylcholine-activated channels. Parallel studies with T. marmorata AcChoR-rich membranes show that in the presence of Ca2+ Tpo causes a decrease in the apparent equilibrium dissociation constant of the noncompetitive blocker [3H]phencyclidine, enhances, at low concentrations, the binding of [3H]acetylcholine, and also alters the binding kinetics of the fluorescent agonist 6-(5-dimethylamino-1-naphthalenesulfonamido)-n-hexanoic acid beta-(N-trimethylammonium bromide) ethyl ester to the AcChoR. It was concluded that, in the presence of Ca2+, Tpo displaces the conformational equilibrium of the AcChoR towards a high-affinity desensitized state and increases the transition rate towards the same state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo L. G., Witkop B., Albuquerque E. X. Voltage- and time-dependent effects of phencyclidines on the endplate current arise from open and closed channel blockade. Proc Natl Acad Sci U S A. 1986 May;83(10):3523–3527. doi: 10.1073/pnas.83.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya T., Schlesinger D. H., Goldstein G. Complete amino acid sequences of bovine thymopoietins I, II, and III: closely homologous polypeptides. Biochemistry. 1981 Oct 13;20(21):6195–6200. doi: 10.1021/bi00524a044. [DOI] [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine to Torpedo postsynaptic membranes: association and dissociation rate constants by rapid mixing and ultrafiltration. Biochemistry. 1980 Nov 11;19(23):5353–5358. doi: 10.1021/bi00564a032. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Pinset C., Ribera A. B. Effects of chlorpromazine and phencyclidine on mouse C2 acetylcholine receptor kinetics. J Physiol. 1986 Sep;378:497–513. doi: 10.1113/jphysiol.1986.sp016232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Fuccello A., Audhya T., Talle M. A., Goldstein G. Immunoassay for bovine serum thymopoietin: discrimination from splenin by monoclonal antibodies. Arch Biochem Biophys. 1984 Jan;228(1):292–298. doi: 10.1016/0003-9861(84)90070-5. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Hofmann W. W. Endocrine function of the thymus affecting neuromuscular transmission. Clin Exp Immunol. 1969 Feb;4(2):181–189. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Manganaro A. Thymin: a thymic polypeptide causing the neuromuscular block of myasthenia gravis. Ann N Y Acad Sci. 1971 Sep 15;183:230–240. doi: 10.1111/j.1749-6632.1971.tb30754.x. [DOI] [PubMed] [Google Scholar]
- Goldstein G. Thymitis and myasthenia gravis. Lancet. 1966 Nov 26;2(7474):1164–1167. doi: 10.1016/s0140-6736(66)90479-x. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Whittingham S. Experimental autoimmune thymitis. An animal model of human myasthenia gravis. Lancet. 1966 Aug 6;2(7458):315–318. doi: 10.1016/s0140-6736(66)92599-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur J Biochem. 1979 Feb 15;94(1):255–279. doi: 10.1111/j.1432-1033.1979.tb12893.x. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Un modèle moléculaire de régulation d'efficacité au niveau postsynaptique d'une synapse chimique. C R Seances Acad Sci III. 1982 Dec 6;295(12):665–670. [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Marlas G., Bon C. Relationship between the pharmacological action of crotoxin and its phospholipase activity. Eur J Biochem. 1982 Jun 15;125(1):157–165. doi: 10.1111/j.1432-1033.1982.tb06663.x. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian K., Audhya T., Goldstein G. Binding of thymopoietin to the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(10):3171–3174. doi: 10.1073/pnas.83.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Viamontes G. I., Audhya T., Goldstein G. Immunohistochemical localization of thymopoietin with an antiserum to synthetic Cys-thymopoietin28-39. Cell Immunol. 1986 Jul;100(2):305–313. doi: 10.1016/0008-8749(86)90031-6. [DOI] [PubMed] [Google Scholar]



