Abstract
The pathways by which painful stimuli are signaled within the human medial temporal lobe are unknown. Rodent studies have shown that nociceptive inputs are transmitted from the brainstem or thalamus through one of two pathways to the central nucleus of the amygdala. The indirect pathway projects from the basal and lateral nuclei of the amygdala to the central nucleus, while the direct pathway projects directly to the central nucleus. We now test the hypothesis that the human ventral amygdala (putative basal and lateral nuclei) exerts a causal influence upon the dorsal amygdala (putative central nucleus), during the application of a painful laser stimulus.
Local field potentials (LFPs) were recorded from depth electrode contacts implanted in the medial temporal lobe for the treatment of epilepsy, and causal influences were analyzed by Granger causality (GRC). This analysis indicates that the dorsal amygdala exerts a pre-stimulus causal influence upon the hippocampus, consistent with an attention-related response to the painful laser. Within the amygdala, the analysis indicates that the ventral contacts exert a causal influence upon dorsal contacts, consistent with the human (putative) indirect pathway. Potentials evoked by the laser (LEPs) were not recorded in the ventral nuclei, but were recorded at dorsal amygdala contacts which were not preferentially those receiving causal influences from the ventral contacts. Therefore, it seems likely that the putative indirect pathway is associated with causal influences from the ventral to the dorsal amygdala, and is distinct from the human (putative) indirect pathway which mediates LEPs in the dorsal amygdala.
Keywords: human, pain, amygdala, hippocampus, causality, fear conditioning
Introduction
Structures in the medial temporal lobe, including the amygdala, the hippocampus, and associated cortices, are well known to be involved in emotional learning, such as fear conditioning (Davis, 1992; LeDoux, 1992; Squire, 1992). Fear conditioning occurs when a neutral conditioned stimulus, such as a light, is paired with an unconditioned aversive stimulus, such as a foot shock. After conditioning, the conditioned stimulus itself acquires the ability to evoke a conditioned response, such as autonomic arousal. Although any stimulus that produces pain is inherently an unconditioned aversive stimulus, there has only recently been direct evidence of nociceptive processing in the primate medial temporal lobe (Liu et al., 2010).
A model of nociceptive processing in the amygdala was recently described by Neugebauer, as being composed of two nociceptive pathways (Neugebauer et al., 2009) see also (Pare et al., 2004; Dong et al., 2010). The direct pathway transmits nociceptive inputs from the pontine parabrachial nucleus and the thalamus directly to the central nucleus of the amygdala (Neugebauer et al., 2009; Slugg and Light, 1994; Bernard et al., 1996; Neugebauer, 2007; Lanuza et al., 2008). The indirect pathway transmits polymodal, associative inputs from the brainstem or the thalamus through the ventral amygdala (lateral and basal nuclei) to the dorsal amygdala (central nucleus)(Lanuza et al., 2008; Neugebauer, 2007; Neugebauer et al., 2009). These two pathways can synapse on the same cells so that they may jointly process nociceptive inputs as a local network (Dong et al., 2010; Neugebauer et al., 2009).
Another feature of nociceptive processing within the amygdala may involve inhibitory circuits. Specifically, the lateral nucleus projects to the central nucleus through two serial inhibitory connections in the inhibitory intercalated cell groups (Pare et al., 2004). In this pathway, increased activity in the lateral nucleus could activate the central nucleus by disinhibition. A better understanding of these pathways and their interaction in humans could be used to design therapies targeting anxiety and the emotional dimension of pain.
We have now tested the hypothesis that neuronal activity in the ventral amygdala exerts a causal influence or driver role (Granger causality - GRC, see Methods: ERC) upon neuronal activity in the dorsal amygdala (Granger, 1969). Recordings of local field potentials (LFPs) in the amygdala and hippocampus were made during application of a painful cutaneous laser stimulus over the week between implantation and removal of depth electrodes for the investigation of epilepsy. The results suggest that the putative indirect pathway mediates nociceptive transmission through the amygdala which is separate from the pathway mediating laser evoked potentials (LEPs).
Materials and Methods
The protocol for these studies was reviewed and approved annually by the Institutional Review Board of Johns Hopkins Medicine. These studies were carried out after implantation of depth electrodes in the amygdala and hippocampus for the investigation of medically intractable epilepsy in four subjects. We have previously published patient characteristics, laser evoked potentials and changes in ongoing LFPs in these subjects (Liu et al., 2010). Preoperative evaluation by a neurologist and neurosurgeon, including standard somatic sensory testing, disclosed no neurological abnormality except epilepsy (Lenz et al., 1993). All subjects gave informed consent for participation in these studies.
Surgical Procedures and Electrode Location
Depth electrodes were implanted in the frontal lobe, amygdala and hippocampus by a stereotactic procedure using the Leksell frame as previously described (Liu et al., 2010). Briefly, targeting was carried out using a coronal MRI scan of the head to determine the predicted location of implantation of the electrodes in the amygdala and hippocampus. The location of the electrode contacts was confirmed to be at the stereotactic target by intraoperative fluoroscopy, as demonstrated in the companion paper (Figure 1 in (Liu et al., 2010)). Along electrodes through the amygdala, contacts 1 and 2 were in the ventral amygdala, while 4 and 5 were in the dorsal amygdala; along hippocampal electrodes, contacts 4 and 5 were in the body of the hippocampus (Schaltenbrand and Bailey, 1959).
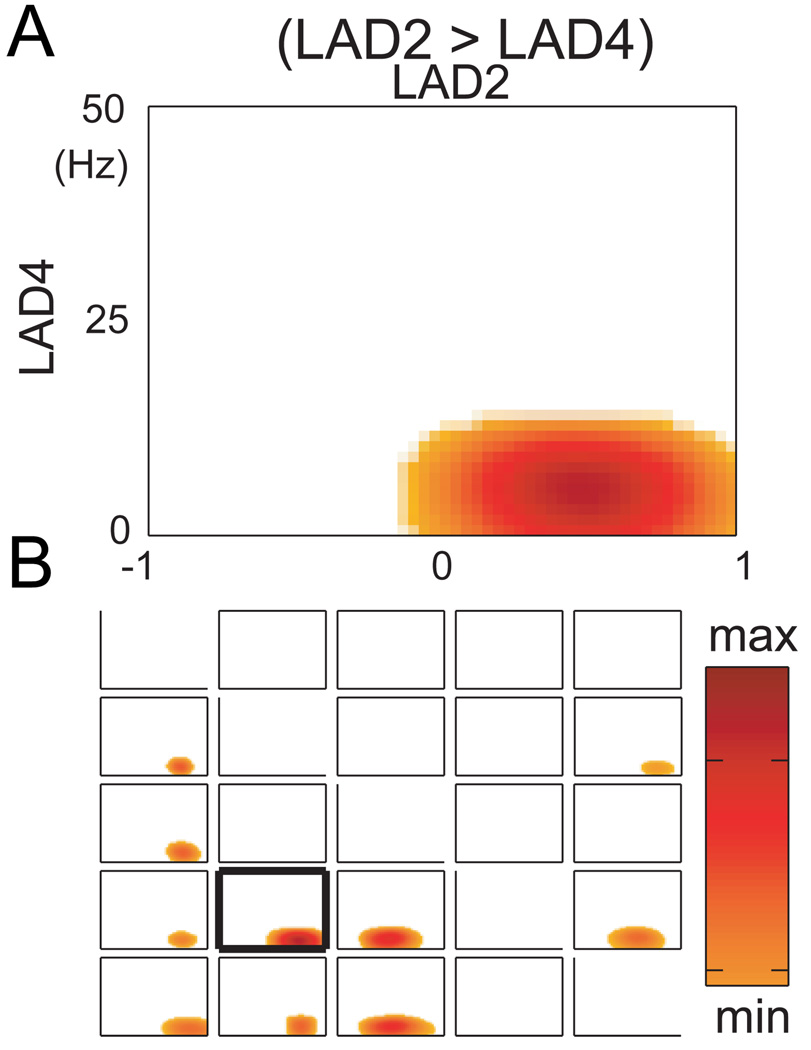
Figure 1.
A. causality between a pairs of electrodes within the amygdala as displayed in time frequency plots for subject 3. Specifically, the causal influence (GRC) of the signal for amygdala contact 2 upon contact 4 for subject 3, is shown with time and frequency represented along x and y axes. The presence of a significant increase in the GRC is indicated by hot colors while white indicates time frequencies which are subthreshold for GRC. B the grid of all time frequency plots for the patient 3, where the contacts numbered along the horizontal indicate contacts exerting a causal influence or driver role (GRC) upon contacts numbered along the vertical axis. The time frequency plot in A is shown in B with a heavy margin around the plot.
Laser Protocols
During the laser study, the patient wore protective glasses and reclined in bed with his/her eyes open, quietly wakeful. Noxious cutaneous heat stimulation, that the patient expected could be painful, was delivered by a Thulium YAG laser (Neurotest, Wavelight Inc, Starnberg, Germany). Stimuli were applied to the dorsum of the left or right forearm with a ~6 sec interval in between stimuli in order to avoid sensitization or fatigue of primary nociceptive afferents (Meyer et al., 1994). The laser beam was moved at random to a slightly different position for each stimulus.
The average energy level for laser stimulation, the patients’ pain intensity and unpleasantness ratings were measured at the end of each block of stimuli. The ratings were made with an intensity scale with 0 as no pain, and 10 as the most intense pain imaginable. A total of 25 laser pulses were applied during a single run and the inter-run interval time was 2 min. The signal was first amplified (12A5, Astro-Med Grass, Inc., West Warwick, RI), then band-pass filtered at 0.1–300 Hz and finally, digitized at 1000 Hz.
Granger Causality (GRC)
To evaluate the directional interactions between brain areas, the concept of Granger causality was used in this study (Granger, 1969). For two observed time series, variables X and Y, it is said that X is Granger causal of Y if the past knowledge of X significantly reduces the prediction error of Y. The number of coefficients of past knowledge is characterized by the auto-regressive model order which optimizes the description of the system. We have now used this technique to address the directional causal influences between structures in the medial temporal lobe.
The Granger causality analysis for the multichannel LFP data was realized using a multivariate auto-regressive (MVAR) modeling-based technique named short-time direct directed transfer function (SdDTF) (Korzeniewska et al., 2003; Korzeniewska et al., 2008; Liu et al., 2011). The SdDTF measures the directions, intensities, and spectral contents of direct causal interactions among acquired signals and is also adapted for the signals with short durations.
In this study, the GRC analysis was applied to signals recorded from electrode contacts located over different cortical structures to evaluate the strength of GRC interactions between these structures. The GRC was calculated in the 6–14 Hz frequency band which was based on the frequencies of peaks in ensemble averages of the LFP autopower, coherence and GRC (Liu et al., 2010). These frequencies were consistent with those of other studies of intracranial recordings (Ohara et al., 2001; Ohara et al., 2006; Tallon-Baudry et al., 2001), and were higher than those of recordings from the scalp (Andres et al., 1999; von Stein et al., 1999).
To determine causality in the frequency domain, we used a multivariate autoregressive modeling (MVAR) approach. The signals acquired during the experimental trials were treated as if they were produced by a common stochastic process, and were used to estimate the MVAR model coefficients for that process (Ding et al., 2000). In this study, a windowing approach was applied for the GRC analysis, and the length of the sliding window was set to 0.1 sec and the slide was set to 0.04 sec for consecutive windows. The MVAR model order was determined by the Akaike information criteria (AIC)(Akaike, 1974) as an estimate for the number of coefficients which would optimize the MVAR analysis.
If the first channel is a driver of the second channel, then this causal influence will be identified by the GRC method, However, he inverse of this statement is not necessarily true. In addition, all signals relevant to system under study must be observed before final conclusions can be drawn from this analysis. All selected channels are included in the MVAR model used for the computation of GRC so that it is not computed for each of the cortical areas separately. Therefore, effects within modules, such as SI, will not determine GRC influences across the network.
In general, the significant GRC influences are observed for pairs of channels which are both active and correlated. However, larger activation or correlation within an area like the ventral amygdala does not necessarily influence the level or directionality of GRC due to the normalization of the signals before the computation of GRC. In fact, the coefficients of the MVAR model used in this analysis are calculated using correlation matrices for all observed channels. Therefore, the correlation may influence the magnitude of the GRC but will not determine the directionality of the GRC effect. This additional information about directionality of influences is determined by the GRC analysis.
The programs for the event-related causality analysis were written in C language and developed in the Linux environment and run on a cluster of sixteen computers implemented as a distributed system. The number of the knots was operationally chosen in order to obtain adequate time and frequency resolution.
Statistical Testing
To test the significant changes in the causal influences, a baseline statistical test was applied in this study. The significant level was set to α=0.05. For each paired electrode combination, this test compared the causal influence in every frequency and time between baseline and the interval of interest using a semi-parametric regression model. The durations for the interval were set to 1 second. The number of the knots used in this study was set to 20 for both time and frequency and was operationally chosen in order to obtain adequate time and frequency resolution. In addition, a formal bivariate smoothing model that takes into account both the frequency and time was used to reduce the effect of inhered noise in the recorded signals. The significant causal influence in the post-stimulus interval was declared if the causal influence was significantly greater than all those occurred in the pre-stimulus interval. In this study, the significant causal influences were color coded and shown in a time-frequency plot. The computer programs for the statistical test were written in R language and has been previously tested and used in similar multichannel human intracranial recordings (Korzeniewska et al., 2008; Boatman-Reich et al., 2010).
Results
This study was carried out in four subjects with medically intractable complex partial seizures, but without tonic clonic seizures. Scalp monitoring suggested the possibility of temporal lobe onsets in all subjects leading to further investigation by bilateral implantation of depth electrodes in the hippocampus and amygdala. Seizure monitoring was carried out over a one week period starting on the day after implantation. All seizure medications were discontinued for 36 hours after the implantation of the electrodes, so that all subjects had substantial blood levels of these drugs at all points relevant to this study (Levy et al., 2002). No subject had any medical or psychiatric condition other than epilepsy, or chronically took medications other than anti-epileptic drugs. These studies were carried out over a one week period starting the day after implantation. Laser stimulations evoked painful, pin-prick sensations in all four patients.
The ensemble autopower and coherence (not partial coherence) spectra were first computed from estimated MVAR models for each subject across electrode contacts that were associated with significant GRC. The coherence was averaged across all pairs of electrodes with significant GRC. The statistical threshold for ensemble coherence cannot be given since it varies with each pair of signals studied. Table 1 shows the ensemble autopower, coherence, and GRC spectral peaks for all such electrodes in the amygdala and hippocampus in each of four subjects. The autopower of a signal is the square of the magnitude of the signal as a function of frequency (Oppenheim and Schafer, 1975). For all four subjects, the average autopower spectra showed that peak oscillatory activities during the experimental task period occurred within the alpha band (8–13 Hz). Synchrony was assessed by coherence. The averaged coherence spectra showed that the alpha band oscillatory activities were often synchronous overall. Based upon these results, we examined GRC in the alpha/ beta range.
Table 1.
Summary for spectral quantities. Columns show the peak values and frequencies in the average power/coherence/ERC spectra for the electrode sites with significant ERC, within patients (rows).
| Patient | Power | Coherence | GRC | |||
|---|---|---|---|---|---|---|
| db | hz | value | Hz | value | Hz | |
| 1 | 48.3 | 7.2 | 0.316 | 8.2 | 0.126 | 8.7 |
| 2 | 52.4 | 7.5 | 0.261 | 7.4 | 0.312 | 9.4 |
| 3 | 47.2 | 6.8 | 0.288 | 6.9 | 0.174 | 8.3 |
| 4 | 53.1 | 6.6 | 0.214 | 8.7 | 0.255 | 9.1 |
| mean | 50.25 | 7.025 | 0.270 | 8.7 | 0.216 | 8.9 |
GRC Estimates of Causality within the Amygdala
Figure 1A shows the plot of time from one second before the stimulus (at time 0) to one second after. We examined the statistical significance of directional causal influences among electrode contacts in the amygdala. We tested the null hypothesis that the causality for prestimulus interval and poststimulus interval causality did not differ from that during baseline (1 sec before the prestimulus interval). Specifically, bivariate smooth SdDTF estimates in the pre-stimulus (baseline) and post-stimulus intervals were compared using the statistical testing procedures described in the “Statistical Testing” section. The threshold for significant level is different, based upon different electrode site paired combinations.
The onset and offset of the response is not precisely interpretable because the sliding window approach is used in GRC analysis for the data in this figure. Decreases in the causal influences were not taken into consideration because the physiological interpretation of decreases in GRC during task performance is unclear.
The relationship for the directional causal influences within the amygdala can be interpreted from the time-frequency plots in a 5×5 grid of time-frequency plots in the Figure 1B. In this grid, the time frequency plots show the GRC exerted by the signal at the contact (numbered across the top of the grid) upon the signal at the contact (numbered along the left side of the grid). Therefore, the plot in Figure 1B is identified as the plot with the heavy black margin in Figure 2, subject 3. The diagonal of the grid of the time frequency plots from top left to lower right indicates the comparison of the contact with itself, and so is blank.
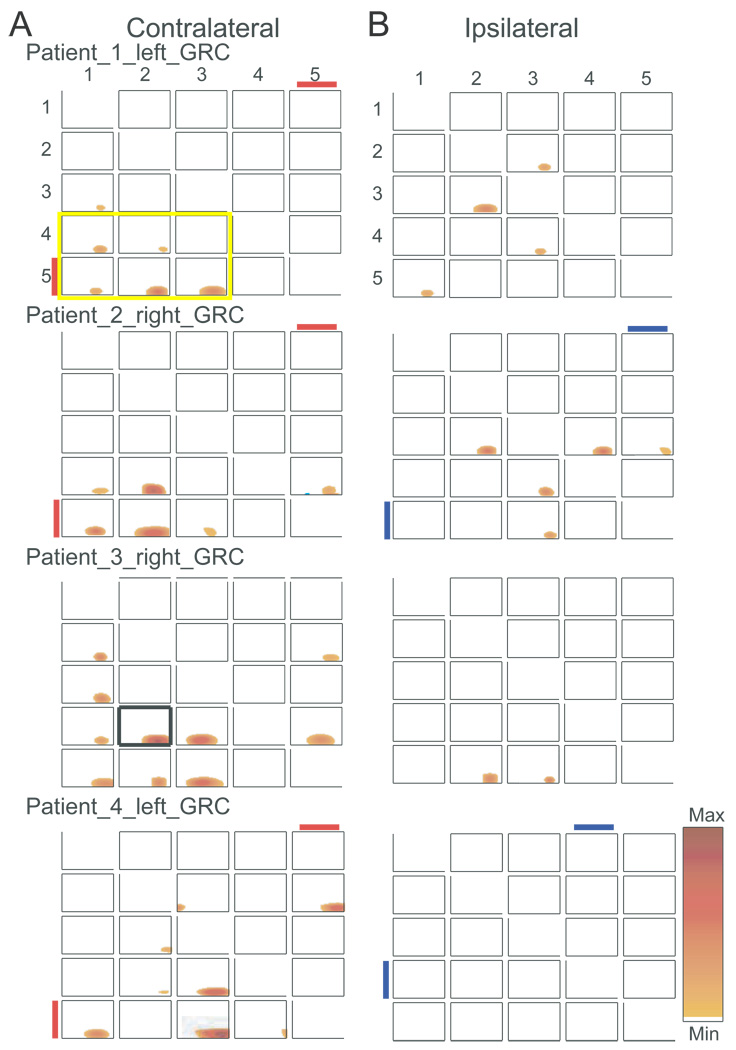
Figure 2.
Time frequency plots of GRC for all patients studied, where each row indicates results contralateral and ipsilateral to the stimulus, as labeled. For each patient the side of the recording is indicated for each patient on the contralateral side in A. The red or blue lines under the contact number indicate the presence of a reproducible LEP in response to a contralateral or ipsilateral laser stimulus. The time frequency plot in Figure 1A is shown in 2B with a heavy margin around the plot in patient 3, left grid.
Increases in GRC are seen from signals at contacts 1, 2, 3 upon those at contacts 4 and 5. In this study, it is clear that the laser stimulation induced significant oscillatory activities in low frequency range up to Beta band range (<30 Hz) during the experimental period. In addition, the event-related increase in signal energy had already been demonstrated at these frequencies (Liu et al., 2010).
GRC Estimates of Causality between Contacts within the Amygdala
Figure 2 shows the consistency across subjects of the pattern of significant GRC pairs following the laser stimulus seen in the putative indirect pathway. These pairs are indicated by contact pairs within the six plots enclosed by the heavy yellow margin at the lower left hand corner of Figure 2, subject 1. The proportion of contact pairs with significant GRC pairs consistent with putative indirect pathway (1-2-3 > 4–5, maximum of 6 contacts/subject), was significantly more likely than that for all other causal pairs by subject (20 pairs/subject minus 6 in the putative indirect pathway = 14). On this basis, analysis by subject revealed evidence for the putative indirect pathway in subjects 1 & 2 (5/6 vs. 1/14, P=0.002), subject 3 (6/6 vs. 4/14, P=0.01), and subject 4 (4/6 vs. 2/14, P<0.03). Therefore, significant GRC pairs were more common for the pathway from the ventral (1-2-3) to the dorsal electrodes (4–5), consistent with the putative indirect pathway. The time frequency plots indicate that significant GRC occurred after the stimulus, which is consistent with the response to a sensory stimulus.
We next tested the possibility that the proportion of GRC pairs from 4–5 > 1-2-3 are greater than the proportion from 1-2-3 > 4–5. The proportion of contacts with significant GRC 1-2-3 > 4–5 pairs was significantly more likely versus 4–5 > 1-2-3 for subjects 1 & 2 (5/6 vs. 0/6, P<0.02), and 3 (6/6 vs. 0/6, P=0.001), but not for subject 4 (4/6 vs. 1/6, P=0.24). The numbers of contacts across all four subjects with significant GRC was greater for amygdala contacts 1-2-3 > 4–5 vs. 4–5 > 1-2-3 (19/24 vs. 5/24, P=0.0001, Fisher).
Contralateral stimuli led to causal pairs consistent with the putative indirect pathway more frequently versus ipsilateral stimuli (contacts consistent with putative indirect pathway 6 per subject × 4subject, 20/24 vs. 6/24, P=0.0001), which is consistent with Figure 2. We next examined the effect of the laterality of recordings and found that among contralateral electrodes the number of activated contacts was not different between the left versus the right side (9/12 vs. 11/12, P=0.59). Differences between left and right were significant neither for ipsilateral contacts nor for contacts overall.
Studies of LEPs in these patients indicate that contralateral responses to laser stimuli occur exclusively at amygdala contact 5, as described in the companion paper (Liu et al., 2010), and as indicated by the red line adjacent to the contact number in Figure 2. Table 2 shows the contacts at which reproducible LEPs and significant causal pairs from 1-2-3 > 4–5 were found. This table shows that LEPs and causal pairs were found together for a pair (bold font) as commonly as either one was found separately (plain or italic font)(8/20 versus 12/20, P=0.21, Chi square), although this analysis does not prove the lack of the association. In view of this finding and the lack of basolateral electrodes with LEPs, it seems unlikely that the pathway from ventral to dorsal amygdala is the same as that transmitting LEPs to the dorsal amygdala.
Table 2.
Summary of the LEPs and GRCs within for amgdala > amygdala contact pairs. The number in the column indicates the electrode contact which exerts a causal influence upon the electrode indicated by the number in the row, as in Figures 1 and 2. Within the table the number indicates the patient identifier, the italic font indicates that only LEPs were found at that electrode > electrode pair, the plain font indicates significant GRC only were found, and the bold font indicates that both LEPs and GRC were found. A maximum of four numbers (patients) could be found at contact pair; a missing number indicates that neitherLEP or GRC was found.
| Amygdala > Amygdala |
Driver 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Receiver 1 | 1, 2, 4 | ||||
| 2 | 3 | 4 | 1, 2, 3, 4 | ||
| 3 | 1, 3 | 4 | 1, 2, 4 | ||
| 4 | 1, 2, 3 | 1, 2, 3, 4 | 3, 4 | 2, 3 | |
| 5 | 1, 2, 3, 4 | 1, 2, 3, 4 | 1, 2, 3, 4 | 1, 2, 4 | 1, 2, 4 |
GRC Estimates of Causality between the Amygdala and the Hippocampus
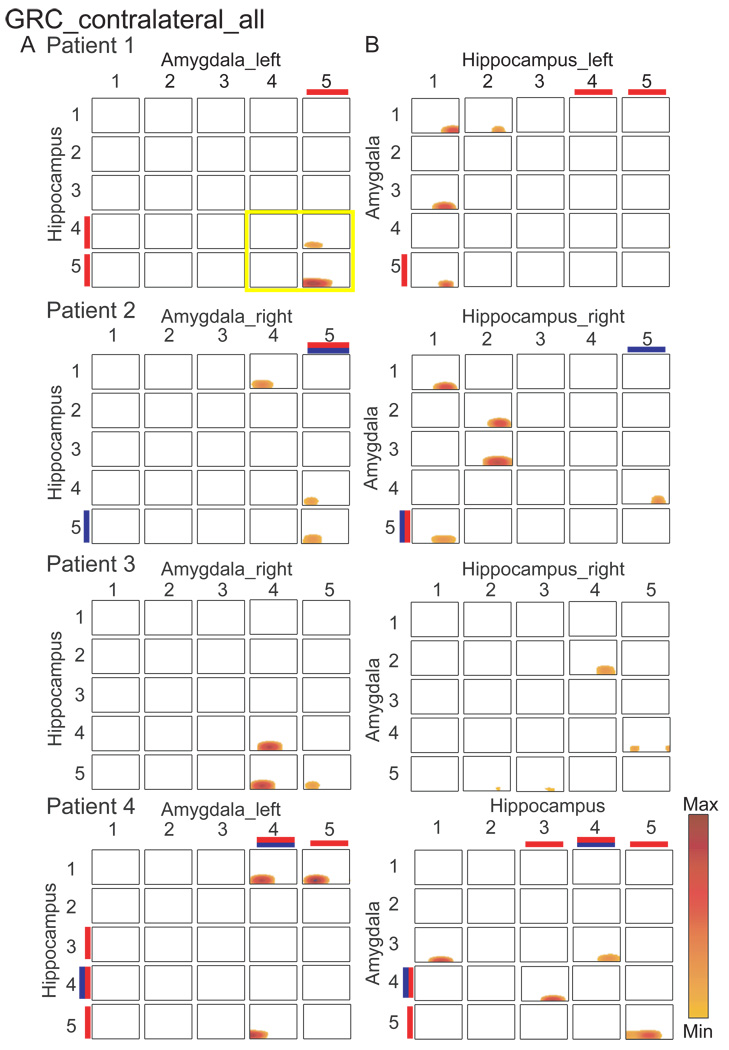
Figure 3 (left) shows significant GRC pairs between the amygdala and hippocampus contralateral to the side of the stimulus. Amygdala contacts 4 or 5 or both exert a causal influence upon hippocampal electrodes 4 or 5. The number of causal pairs of contacts with this pattern varied from one to four, and significant GRC occurred consistently before the stimulus. The four contact pairs involved in this amygdala to hippocampus connection are the four time frequency plots in Figure 3 (patient 1), which are enclosed in the heavy yellow margin.
Figure 3.
The GRC in time frequency plots between pairs of contacts with analysis of amygdala > hippocampus (A), and hippocampus > amygdala in each of the four patients (B), as labeled. A. the time frequency plots in the grid show causal influence of amygdala contacts numbered along the horizontal axis, while those for the hippocampus numbered along the vertical axis. B. the time frequency plots in the grid show causal influence of hippocampus contacts numbered along the horizontal axis, while those for the amygdala are numbered along the vertical axis, i.e. axes reversed from A.
The proportion of contacts with significant GRC from among the 4 amygdala 4–5 > hippocampal 4–5 contact pairs, (both contralateral), were significantly more likely than for all other pairs (21 contacts outside the box with the heavy yellow margin in Figure 3) for subject 1 (2/4 vs. 0/21, P<0.02), subject 2 (2/4 vs. 1/21, P=0.05), subject 3 (3/4 vs. 0/21, P=0.002), but not for subject 4 (1/4 vs. 2/21, P=0.42). The proportion of significant GRC pairs for contralateral amygdala 4–5 > hippocampal 4–5 (4 contacts × 4 subjects = 16 contacts) was significantly greater (8/16 vs. 3/84, P<0.00001) versus the proportions at all other GRC pairs (21 contacts × 4 subjects + 84). Significant GRC from amygdala upon hippocampus uniformly occurred before the stimulus, which may be related to vigilance or anticipation of the stimulus.
Significant GRC pairs tended to be more common for the causal influence from amygdala 4–5 to hippocampus 4–5 versus from hippocampus 4–5 to amygdala 4–5 (11/16 vs. 5/16, P=0.076, Chi Square). Overall, these results suggested that signals at the dorsal contacts in the amygdala exerted a causal influence upon the dorsal contacts in the hippocampus (see companion paper (Liu et al., 2010)). In Figure 3B, the hippocampus does not seem to exert any consistent significant GRC upon the amygdala ipsilateral to the stimulus.
Contacts with both reproducible amygdala LEP and significant GRC from amygdala 4–5 > hippocampus 4–5 were not apparently more common versus those at which either one is found separately (Table 3)(5/11 vs. 6/11, P<1, Fisher)(Table 3). Therefore, the presence of input arising from nociceptors is not associated with evidence of causal influence from the amygdala upon the hippocampus, although this analysis does not prove the lack of this association.
Table 3.
Summary of the LEPs and GRCs within for amgdala > hippocampus contact pairs. These pairs were calculated for in response to contralateral laser stimuli. LEP recordings were those from the contralateral amygdala electrodes. Other conventions as in table 2.
| Amygdala > Hippocampus |
Driver Contact 1 |
2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Receiver contact 1 | Columns 1 to 3 are blank | 2, 4, | 1, 2, 4 | ||
| 2 | 4 | 1, 2, 4 | |||
| 3 | 4 | 1, 2, 4 | |||
| 4 | 3, 4 | 1, 2, 4 | |||
| 5 | 3, 4, | 1, 2, 3, 4 |
Pain Ratings
Table 4 indicates that the pain intensityand unpleasantness ratings were comparable across all patients during the experiment. In this study, the VAS ratings recorded from all four patients were not significantly correlated with the number of electrode contacts that were associated with significant GRC relative to other contacts in the amygdala by regression analysis (α=0.05).
Table 1.
Average pain intensity and unpleasantness ratings as well as energy level for laser stimulation should be reported
| Subject | VAS Intensity | VAS unpleasantness |
Laser energy |
|---|---|---|---|
| 1 | 5/10 | 6/10 | 720 mj |
| 2 | 5/10 | na | 720 mj |
| 3 | 3/10 | 3/10 | 640 mj |
| 4 | 7/10 | 4/10 | 720 mj |
Discussion
These results demonstrate that LFP activity recorded from the human ventral amygdala (putative basal and lateral nuclei) during a train of laser stimuli exerts a post-stimulus GRC effect upon that recorded from the dorsal amygdala (putative central nucleus). In rodents, there is good evidence of a nociceptive pathway from the spinal cord to the amygdala through the pontine parabarachial nucleus or the thalamic posterior intralaminar nucleus (Bernard et al., 1992; Neugebauer et al., 2009; Pare et al., 2004). This nociceptive pathway is based upon anatomical evidence of connections from the spinal dorsal horn via the parabrachial nucleus to the central nucleus of the amygdala (Saper, 1995; Ma and Peschanski, 1988; Bernard and Besson, 1990). An alternate nociceptive pathway is through the thalamic posterior intralaminar nucleus or parabrachial nucleus to the central nucleus of the amygdala via the basal and lateral nuclei of the amygdala (Lanuza et al., 2008; Neugebauer, 2007). Noxious stimuli activate neurons in the central nucleus (Slugg and Light, 1994; Bernard and Besson, 1990; Bernard et al., 1992) and may lead to synaptic plasticity such as hypersensitivity to subsequent noxious stimuli (Neugebauer et al., 2000; Neugebauer et al., 2009)(see also (Shi and Davis, 1999; Lanuza et al., 2008)).
Methodological Considerations
The subjects in the studies described here all have epilepsy with associated clinical, radiological and electrical abnormalities related to seizure onsets, and with significant blood levels of antiepileptic drugs (see Table 1 in Liu et al., 2010)(Williamson et al., 1993; Levy et al., 2002). As described previously, the occurrence of LEPs was not related to mesial temporal sclerosis (Liu et al., 2010), scarring of the medial temporal lobe which is associated with epilepsy (Williamson et al., 1993). Furthermore, the side of the brain where LEPS were recorded was not related to the side upon which seizure onsets were located. Therefore, differences in the side on which LEPs were recorded could not be explained by the absence of mesial temporal sclerosis or electrical seizure related activity.
There are limitations to GRC analysis which constrain the interpretation of causality. The observation of significant GRC between signals recorded from a pair of electrode contacts does not itself prove that neurons around one recording contact exert a causal influence over those at the other contact through an observed pathway. It is important to consider the possibility that causality may be the result of functional connections through other structures, which are ‘unobserved’, such as the intercalated cell group.
In addition, GRC influences might be detected between signals that are both active and correlated, but not truly causal. However, the coefficients of the MVAR model used in this analysis are calculated using correlation matrices for all observed channels. Therefore, the correlation may influence the magnitude of the GRC but not the directionality.
Nociceptive Connections of the Hippocampus
There is evidence of nociceptive input to the rodent hippocampus. Neurons in the rodent hypothalamus, which project to the hippocampus, respond to nociceptive stimuli applied in large receptive fields (Dutar et al. 1985). In rats, immersion of the tail in hot water produces hippocampal theta activity and prolonged modification of hippocampal synapses (Khanna and Sinclair, 1989). Similar changes have been observed following injection of formalin into the hind paw, and may be associated with pain-related behavior (Tai et al., 2006).
In humans, hippocampal activation measured by BOLD signals has been observed in response to mild to moderate pain (Derbyshire et al., 1997). The hippocampus was activated during pain which occurred unexpectedly or randomly in a mismatch learning task (Ploghaus et al., 2000; Bingel et al., 2002). Pain may be used as an unconditioned stimulus in conditioned fear protocols.
Experimentally-induced anxiety in humans can evoke increased sensitivity to painful stimuli which is correlated with hippocampal activation (Ploghaus et al., 2001). The evidence of imaging studies indicates that the hippocampus is involved in all the procedural phases of fear conditioning, and that hippocampal BOLD activations occur in response to conditioned stimuli used in fear conditioning (Knight et al., 2004; Delgado et al., 2008). Hippocampal activity is often proposed to mediate the role of the environment or the context of fear conditioning, and hippocampal activations may be better related to context than to the unconditioned stimulus (Marschner et al., 2008).
In the present study, the pre-stimulus GRC of amygdala upon hippocampus may be related to vigilance as the subject attends the next painful stimulus in a random series of such stimuli. Involvement of the amygdala in attention is supported by studies demonstrating that anticipation leads to increased neuronal activity in the primate amygdala (Belova et al., 2008), and stimulation of the central nucleus leads to low voltage fast EEG activity (Kapp et al., 1994), as well as orienting responses (Applegate et al., 1983; Davis and Whalen, 2001). In addition, lesions of the central nucleus lead to decreased conditioned, but not unconditioned, attentional responses in rodents (Gallagher et al., 1990; Holland et al., 2000) so that the hippocampus may be involved in learning and memory processes related to painful stimuli.
Nociceptive Pathways through the Amygdala
In primates, anatomical studies have demonstrated that the spinothalamic tract (STT) projects to the amygdala, particularly the central nucleus (Newman et al., 1996). Imaging studies have shown that the amygdala or hippocampus or both show changes in blood flow and blood oxygen level dependent (BOLD) activity in response to a painful contact heat stimulus (Derbyshire et al., 1997; Becerra et al., 1999), or a painful laser stimulus (Bingel et al., 2002; Bornhovd et al., 2002; Apkarian et al., 2005). The companion paper demonstrates that these activations may be related to LEPs which are recorded from the dorsal amygdala (Liu et al., 2010).
These results are consistent with a putative indirect pathway through the amygdala, although the presence of LEPs at a contact does not correlate with involvement of that contact in a significant GRC pairs. However, LEPs were also recorded at contacts which often received causal influences from ventral contacts. Therefore, analysis of larger numbers of subjects might reveal the presence of a subpopulation of dorsal contacts characterized by inputs giving rise to both LEPs and causal influence from ventral electrodes (Dong et al., 2010; Neugebauer et al., 2009).
These results suggest that activity involved in 1-2-3 > 4–5 GRC pairs is not phase locked to the afferent volley evoked by the laser stimulus (Kenton et al., 1980; Kobayashi et al., 2009). Perhaps this volley is temporally dispersed after transmission from the spinal cord through the brainstem or medial thalamus, and the basolateral amygdala, to the central nucleus. This is consistent with the multiple sequential responses with wide dispersion of response latencies of neurons in the parabrachial pathway to the central nucleus in rodents (Bernard and Besson, 1990; Bernard et al., 1992; Bernard et al., 1994), or the spinothalamic pathway to the medial thalamus in primates (Whitlock and Perl, 1961; Casey, 1966), and cats (Albe-Fessard and Kruger, 1962; Dong et al., 1978). Similar dispersion of the afferent volley might occur by activation of the central nucleus after processing through circuits involving the basolateral amygdala or the intercalcated cell group (Pare et al., 2004).
Less temporal dispersion may result from transmission of the afferent volley through the direct pathway to the central nucleus via the parabrachial nucleus (Neugebauer, 2007). We recorded LEPs in the amygdala but only at dorsal contacts, which were not preferentially involved in pairs with ventral contacts having significant GRC. These findings suggest the presence of two separate nociceptive pathways in the human central nucleus. The putative direct pathway may lead to a synchronized volley which produces LEPs. The putative indirect pathway may be based upon a causal influence or driver role exerted by the basal and lateral nuclei, upon the central nucleus.
The putative direct and indirect nociceptive pathways have been associated with different pain-related pathological processes (Bernard et al., 1996; Neugebauer, 2007; Neugebauer et al., 2009). Specifically, different rodent models of chronic pain are distinguished by the presence of different excitatory amino acid receptors in different nuclei included in these pathways. Modulation of these receptors in the central nucleus of the amygdala will influence behaviors related to pain and emotion (Palazzo et al., 2008), and the relationship between them (McGaraughty and Heinricher, 2002) (Neugebauer, 2007; Ikeda et al., 2007; Carrasquillo and Gereau, 2007). These effects are mediated through widespread, reciprocal connections to structures mediating affective and autonomic emotional behaviors related to noxious stimuli and conditioned fear (Price and Amaral, 1981; Davis et al., 1994; Cassell et al., 1999; LeDoux, 1996). Therefore, these human pain-related pathways through the medial temporal lobe may subserve multiple different mechanisms of chronic pain, fear and anxiety.
Acknowledgements
This work was supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke (NS38493 and NS40059 to FAL). We thank L.H. Rowland and J. Winberry for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akaike H. New Look at Statistical-Model Identification. IEEE Transactions on Automatic Control. 1974;AC19:716–723. [Google Scholar]
- Albe-Fessard D, Kruger L. Duality of unit discharges from cat centrum medianum in response to natural and electrical stimulation. J. Neurophysiol. 1962;25:3–20. doi: 10.1152/jn.1962.25.1.3. [DOI] [PubMed] [Google Scholar]
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain. 1999;122(Pt 5):855–870. doi: 10.1093/brain/122.5.855. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn. Res. Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J. Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog. Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol. 1994;71:1646–1660. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Boatman-Reich D, Franaszczuk PJ, Korzeniewska A, Caffo B, Ritzl EK, Colwell S, Crone NE. Quantifying auditory event-related responses in multichannel human intracranial recordings. Front Comput. Neurosci. 2010;4:4. doi: 10.3389/fncom.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, Gereau RW. Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J. Neurosci. 2007;27:1543–1551. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL. Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J. Neurophysiol. 1966;29:727–750. doi: 10.1152/jn.1966.29.4.727. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann. N. Y. Acad. Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone L. Pain processing during three levels of noxious stimulation produces different pattern of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol. Cybern. 2000;83:35–45. doi: 10.1007/s004229900137. [DOI] [PubMed] [Google Scholar]
- Dong WK, Ryu H, Wagman IH. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol. 1978;41:1592–1613. doi: 10.1152/jn.1978.41.6.1592. [DOI] [PubMed] [Google Scholar]
- Dong YL, Fukazawa Y, Wang W, Kamasawa N, Shigemoto R. Differential postsynaptic compartments in the laterocapsular division of the central nucleus of amygdala for afferents from the parabrachial nucleus and the basolateral nucleus in the rat. J. Comp Neurol. 2010;518:4771–4791. doi: 10.1002/cne.22487. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J. Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CW. Investigating causal relations by econometric models and cross spectral methods. Econometri. 1969;37:424–438. [Google Scholar]
- Holland PC, Han JS, Gallagher M. Lesions of the amygdala central nucleus alter performance on a selective attention task. J. Neurosci. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav. Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kenton B, Coger R, Crue B, Pinsky J, Friedman Y, Carmon A. Peripheral fiber correlates to noxious thermal stimulation in humans. Neurosci. Lett. 1980;17:301–306. doi: 10.1016/0304-3940(80)90040-3. [DOI] [PubMed] [Google Scholar]
- Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain. 1989;39:337–343. doi: 10.1016/0304-3959(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect. Behav. Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Winberry J, Liu CC, Treede RD, Lenz FA. A painful cutaneous laser stimulus evokes responses from single neurons in the human thalamic principal somatic sensory nucleus ventral caudal - Vc. J. Neurophysiol. 2009;101:2210–2217. doi: 10.1152/jn.91347.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Crainiceanu CM, Kus R, Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum. Brain Mapp. 2008;29:1170–1192. doi: 10.1002/hbm.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Manczak M, Kaminski M, Blinowska KJ, Kasicki S. Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J. Neurosci. Methods. 2003;125:195–207. doi: 10.1016/s0165-0270(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, LeDoux JE. Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neurosci. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Emotion. In: Mountcastle VB, editor. Handbook of Physiology. Section 1: The Nervous System. Volume: The Higher Functions. The American Physiologic Society; 1996. [Google Scholar]
- LeDoux JE. Emotion and the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992. pp. 339–351. [Google Scholar]
- Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J. Neurophysiol. 1993;70:200–212. doi: 10.1152/jn.1993.70.1.200. [DOI] [PubMed] [Google Scholar]
- Levy RH, Mattson RH, Melega W, Perucca E. Antiepilpetic Drugs. Lippincott, Williams and Wilkins; 2002. [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional connectivity between activity recorded directly from human pain-related cortical structures. Pain. 2011 doi: 10.1016/j.pain.2010.12.016. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Zagzoog N, Gallagher M, Lenz FA. Painful stimuli evoke potentials recorded from the medial temporal lobe in humans. Neurosci. 2010;165:1402–1411. doi: 10.1016/j.neuroscience.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Peschanski M. Spinal and trigeminal projections to the parabrachial nucleus in the rat: electron-microscopic evidence of a spino-ponto-amygdalian somatosensory pathway. Somatosens. Res. 1988;5:247–257. doi: 10.3109/07367228809144629. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Campbell JN, Raja SN. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh: Churchill Livingstone; 1994. pp. 13–44. [Google Scholar]
- Neugebauer V. The amygdala: different pains, different mechanisms. Pain. 2007;127:1–2. doi: 10.1016/j.pain.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J. Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res. Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates 'pain networks' defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ohara S, Mima T, Baba K, Ikeda A, Kunieda T, Matsumoto R, Yamamoto J, Matsuhashi M, Nagamine T, Hirasawa K, Hori T, Mihara T, Hashimoto N, Salenius S, Shibasaki H. Increased synchronization of cortical oscillatory activities between human supplementary motor and primary sensorimotor areas during voluntary movements. J. Neurosci. 2001;21:9377–9386. doi: 10.1523/JNEUROSCI.21-23-09377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Digital Signal Processing. Englewood Heights, NJ: Prentice-Hall; 1975. [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharm. 2008;55:537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J. Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The spinoparabrachial pathway: shedding new light on an old path. J. Comp Neurol. 1995;353:477–479. doi: 10.1002/cne.903530402. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Bailey P. Introduction to stereotaxis with an atlas of the human brain. Stuttgart: Thieme; 1959. [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J. Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slugg RM, Light AR. Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated with Phaseolus vulgaris leucoagglutinin. J. Comp Neurol. 1994;339:49–61. doi: 10.1002/cne.903390106. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tai SK, Huang FD, Moochhala S, Khanna S. Hippocampal theta state in relation to formalin nociception. Pain. 2006;121:29–42. doi: 10.1016/j.pain.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J. Neurosci. 2001;21:RC177. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb. Cortex. 1999;9:137–150. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- Whitlock DG, Perl ER. Thalamic projections of spinothalamic pathways in monkey. Exp. Neurol. 1961;3:240–255. doi: 10.1016/0014-4886(61)90015-2. [DOI] [PubMed] [Google Scholar]
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results and pathology. Ann. Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]