Abstract
The T-type Ca2+ channel Cav3.1 subunit is present in pulmonary microvascular endothelial cells (PMVECs), but not in pulmonary artery endothelial cells (PAECs). The present study sought to assess the role of Cav3.1 in thrombin-induced Weibel-Palade body exocytosis and consequent von Willebrand factor (VWF) release. In PMVECs and PAECs transduced with a green fluorescent protein (GFP)-tagged VWF chimera, we examined the real-time dynamics and secretory process of VWF-GFP-containing vesicles in response to thrombin and the cAMP-elevating agent isoproterenol. Whereas thrombin stimulated a progressive decrease in the number of VWF-GFP-containing vesicles in both cell types, isoproterenol only decreased the number of VWF-GFP-containing vesicles in PAECs. In PMVECs, thrombin-induced decrease in the number of VWF-GFP-containing vesicles was nearly abolished by the T-type Ca2+ channel blocker mibefradil as well as by Cav3.1 gene silencing with small hairpin RNA. Expression of recombinant Cav3.1 subunit in PAECs resulted in pronounced increase in thrombin-stimulated Ca2+ entry, which is sensitive to mibefradil. Together, these data indicate that VWF secretion from lung endothelial cells is regulated by two distinct pathways involving Ca2+ or cAMP, and support the hypothesis that activation of Cav3.1 T-type Ca2+ channels in PMVECs provides a unique cytosolic Ca2+ source important for Gq-linked agonist-induced VWF release.
Keywords: endothelial cells, thrombin
The endothelium plays an essential role in regulating blood flow through its dynamic interaction with circulating blood cells. Under physiological conditions, individual endothelial cells produce an anticoagulant and antiadhesive surface that facilitates passage of plasma and cellular components through the vasculature. Changes in the local extracellular environment, as may take place at the site of inflammation, disrupt this homeostasis and stimulate endothelial cells to create a procoagulant and proadhesive microenvironment, thus initiating the adhesion process. Although the molecular mechanisms involved in circulating cell-endothelium adhesion are not yet fully identified, emerging evidence indicates that an essential step in endothelial cell membrane transformation from an anticoagulant, antiadhesive to a proadhesive, prothrombotic surface is the induction of Weibel-Palade body (WPB) exocytosis (8, 47).
WPBs are endothelial cell-specific, regulated secretory organelles (13, 54, 56) that contain a number of components including the adhesive protein von Willebrand factor (VWF) (39, 40, 55), the leukocyte adhesion molecule P-selectin (4, 28), and the chemotactic cytokine IL-8 (48, 57). The exocytosis of WPBs delivers VWF, P-selectin, and IL-8 to the cell surface (4, 18, 19, 28, 48, 57) where they contribute to hemostasis and inflammation. The most biologically active VWFs are high-molecular-weight multimers stored within the luminal space of WPBs (43, 44). These multimers are important ligands for platelet receptor glycoprotein Ibα and integrin αIIbβ3, which are involved in platelet adhesion and aggregation during vascular injury (14, 38). P-selectin is a WPB membrane protein whose regulated appearance at the apical plasma membrane of endothelial cells initiates the binding and rolling of leukocytes on the endothelium and the consequent recruitment into interstitial tissue at sites of inflammation (7, 15, 21, 27). IL-8 is colocalized with VWF within the luminal space of WPBs (48, 57). The presentation of IL-8 on the endothelial cell surface provides an effective means for controlling local leukocyte extravasation (33). Thus regulated exocytosis of WPBs furnishes endothelial cells with an active capacity to rapidly and selectively change the microenvironment of each individual vascular bed and modulate the interrelated processes of coagulation and inflammation. In spite of this importance, mechanisms underlying WPB exocytosis are still poorly understood.
Stimulation of WPB exocytosis can be triggered by a variety of naturally occurring inflammatory mediators that act by two distinct signaling pathways, elevating cytosolic Ca2+ ([Ca2+]i) or intracellular cAMP levels (12, 16, 19, 22, 37, 41, 52, 53). The exocytosis stimulated by Gq-linked neurohumoral inflammatory agonists is a Ca2+-dependent process that requires Ca2+ entry from the extracellular space (6, 12, 16, 18, 25). Although specific Ca2+ entry pathways that promote WPB exocytosis are not known, it has been established that in the inflamed circulation, Gq-linked agonists, e.g., thrombin, increased [Ca2+]i, and the resultant [Ca2+]i transitions are sufficient to trigger WPB exocytosis, causing VWF secretion and P-selectin expression at the membrane surface (1, 2, 16, 25, 32, 49). We previously reported that pulmonary microvascular endothelial cells (PMVECs) express a Cav3.1 (α1G) voltage-gated T-type Ca2+ channel, whereas pulmonary macrovascular (i.e., pulmonary artery) endothelial cells (PAECs) do not express voltage-gated Ca2+ channels. Thrombin-induced transitions in membrane potential activate the Cav3.1 channel, resulting in a physiologically relevant rise in [Ca2+]i. Furthermore, activation of the Cav3.1 channel induces a procoagulant endothelial phenotype, i.e., channel inhibition attenuates increased retention of sickled erythrocytes in the inflamed pulmonary circulation (58). In the present study, we sought to resolve whether PMVECs and PAECs differ in mechanisms regulating WPB exocytosis, and, furthermore, to determine whether Ca2+ entry through Cav3.1 T-type Ca2+ channels is an important amplification step in promoting the exocytosis of WPBs from lung microvascular endothelium.
MATERIALS AND METHODS
Isolation and culture of rat lung endothelial cells
Rat PAECs and PMVECs were isolated, cultured, and characterized as described previously (11, 23, 45). The protocol was approved by the Institutional Animal Care and Use Committee of the University of South Alabama (Protocol 02010). Cells used in all experiments were below passage 12.
Generation of stably Cav3.1-transduced PAECs
PAECs were transfected with the expression vector pcDNA3 (Invitrogen, Carlsbad, CA) encoding the rat Cav3.1a (31) (GenBank accession no. AF027984), i.e., pcDNA3-Cav3.1a (24), kindly provided by Dr. Edward Perez-Reyes (Univ. of Virginia). Cells were transfected with FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN). Positively transfected cells were selected with neomycin (400 µg/ml; Sigma, St. Louis, MO) and further analyzed by RT-PCR, Western blotting, voltage clamp electrophysiology recordings, and fluorometric assessments.
Construction of small hairpin RNAs targeting on Cav3.1
Oligonucleotides coding for small hairpin RNAs (shRNAs) that target on rat Cav3.1 and an unrelated (scrambled) shRNA were designed. The complementary oligonucleotides were annealed and cloned into a retroviral shRNA expression vector, RNAi-Ready pSIREN-RetroQ-ZsGreen Vector (BD Biosciences Clontech, Mountain View, CA), or into an HRST lentiviral vector (29) derivative encoding the reporter mCherry (GenBank accession no. AY678264) (42). Individual transduction of each of the shRNAs into cultured PMVECs was achieved by retro- or lentiviral delivery. Five designed oligonucleotide sequences for Cav3.1 shRNA and scrambled shRNA used in this study are listed in Table 1.
Table 1.
Oligonucleotide sequences for Cav3.1 small hairpin RNAs
| Identification | Target Sequence (Bases) | Antisense | Sense |
|---|---|---|---|
| #1 | 877–894 | TGGTGGTGAAGATGGTGG | CCACCATCTTCACCACCA |
| #2 | 1646–1664 | CCTGTGCCTGGTGGTGATT | AATCACCACCAGGCACAGG |
| #3 | 1820–1837 | AGCAGCCCGAAGGCTGGC | GCCAGCCTTCGGGCTGCT |
| #4 | 3263–3281 | TAAAGTCCTCTACAACGGC | GCCGTTGTAGAGGACTTTA |
| #5 | 3498–3516 | CACGCGGAACTACGAAAGA | TCTTTCGTAGTTCCGCGTG |
| Scrambled | Scrambled | CGTTGATTATGGTGCTAG | CTAGCACCATAATCAACG |
Construction of VWF-GFP expression vector
The VWF-green fluorescent protein (GFP) expression vector was constructed by replacing the VWF A2 domain with a sequence encoding GFP as described previously (34, 35). A recombinant retroviral vector for delivery of the VWF-GFP cDNA was constructed using the dicistronic retroviral LZRS vector as described previously (20).
Production of recombinant retro- and lentivirus and endothelial cell transduction
The helper-free recombinant retrovirus was produced after transfection of the retroviral vector DNA into Phoenix-A cells (20) using Lipofectamine 2000 (Invitrogen). Lentivirus was produced using a 293T producer cell line and ViraPower Lentiviral Expression System (Invitrogen) according to the manufacturer’s instructions. Viral supernatant was collected at consecutive times following the 24 or 36 h transfection of retro- or lentiviral vector, respectively. The collected supernatant was filtered through a 0.45-µm filter. For endothelial cell transduction, PMVECs or PAECs were grown to 20–40% confluency and subsequently infected with the harvested virus supernatant of the packaging cells. The transduction efficiency was tested by determining the percentage of GFP or mCherry positive cells using flow cytometry analysis with a BD FACSVantage SE flow cytometry system (Becton Dickinson, San Jose, CA).
Real-time quantitative RT-PCR analysis of Cav3.1 mRNA expression
Quantitative RT-PCR (qRT-PCR) analysis was performed using total RNA isolated from cells with TRIzol reagent (Invitrogen) and digested with DNase I (Ambion, Austin, TX). The primers and probes for rat Cav3.1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and synthesized by Applied Biosystems (Foster City, CA). qRT-PCR was conducted with a 7500HT Real-Time PCR system (Applied Biosystems). The reaction was conducted in triplicate (n = 3). The gene expression ΔCt value (the difference in threshold cycles for target and reference) of Cav3.1 from each sample was calculated by normalizing with internal housekeeping gene GAPDH, and relative quantitation values of gene expression were plotted.
Western blot analysis
Whole cell protein extracts were analyzed by SDS-PAGE and Western blotting. Cav3.1 was detected with an anti-Cav3.1 antibody (Alomone Labs, Jerusalem, Israel; diluted 1:200). Protein bands were visualized using SuperSignal West Pico Chemiluminescent System (Pierce Biotechnology, Rockford, IL). Actin was probed in the same membrane to ensure equal protein loading.
Electrophysiology, data acquisition, and analysis
Patch-clamp recordings were performed in whole cell configuration as previously described (58).
[Ca2+]i measurement
[Ca2+]i was estimated using fura 2-AM (Molecular Probes, Eugene, OR) fluorometric assessment according to the method described previously (30, 58, 59).
VWF secretion assay
Cultured endothelial cells were grown to confluence in 35-mm dishes. Cells were gently washed three times with PBS and were incubated at 37°C and 5% CO2 either with serum-free medium (500 µl/well) alone or accompanied by thrombin or isoproterenol, with or without mibefradil (10 µmol/l, IC100). The media were collected for VWF measurement after 10 and 60 min of incubation. The VWF-GFP levels present in the medium were measured by sandwich-style ELISA using paired capture and detecting anti-VWF antibodies (Cedarlane Laboratories, Hornby, Ontario, Canada) on triplicate medium samples (100 µl each) according to the manufacturer’s instructions. The standard curve was generated by using standard normal reference human plasma with verified VWF concentration (Precision Biologic, Dartmouth, Nova Scotia, Canada). The quantity of VWF secretion was normalized to the number of endothelial cells present in each of the original 35-mm dishes. Data are expressed in relative values as human VWF concentration equivalence (means ± SE). One-way ANOVA followed by Newman-Keuls tests were used, and P < 0.05 was considered significant.
Real-time imaging and confocal microscopy
Real-time imaging was performed on living VWF-GFP-expressing PAECs and PMVECs to measure the decrease of the number of VWF-GFP-containing vesicles in response to thrombin or isoproterenol using an Ultraview RS confocal microscope (PerkinElmer Life and Analytical Sciences, Boston, MA). Briefly, the VWF-GFP-transduced PAECs or PMVECs were grown on 25-mm glass coverslips for 3–4 days. Cells were then mounted in an Attofluor cell chamber (Molecular Probes) in 500 µl of HEPES-buffered physiological salt solution (HPSS) containing (in mmol/l) 107 NaCl, 6 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2 CaCl2, 11.5 d-glucose, 25 HEPES, pH 7.40, with NaOH. A ×60, 1.20 numerical aperture water-immersion objective along with 488-nm excitation and 525-nm emission filters and a 488-nm single wavelength dichroic mirror were used for GFP imaging. A set of serial optical sections (Z-stacks) was taken from apical to basal cell aspects at a 0.2- to 0.3-µm interval. Time-lapse images of GFP were generated by taking Z-stacks every 3 min during the 60-min experiments. Selected cells were first imaged for 6 min in the initial medium of HPSS. Subsequently, thrombin or isoproterenol (10 µl in 500 µl of media; EC100 concentration, both from Sigma) was gently applied, without disturbing the cells or changing the focal plane, and the recordings were continued for the rest of the experiment. All of the experiments were performed at room temperature (22–25°C).
Time-lapse image processing and analysis
Changes in total GFP following the stimulations were determined from time-lapse image recordings using the image processing and analysis software ImageJ (Research Services Branch, National Institute of Mental Health Sciences; http://rsb.info.nih.gov/ij/) with voxel counter plug-in that counts the thresholded voxels, i.e., the volume equivalent of pixels, in each of the Z-stacks. Background voxels and the voxels of GFP fluorescent vesicles were discriminated by applying a threshold to each Z-stack. For each time-lapse recording, data were normalized to the voxel count at time 0 and expressed as means ± SE. One-way ANOVA followed by Newman-Keuls tests were used, and P < 0.05 was considered significant.
RESULTS
Thrombin-induced Ca2+ entry via the T-type Ca2+ channel in PMVECs
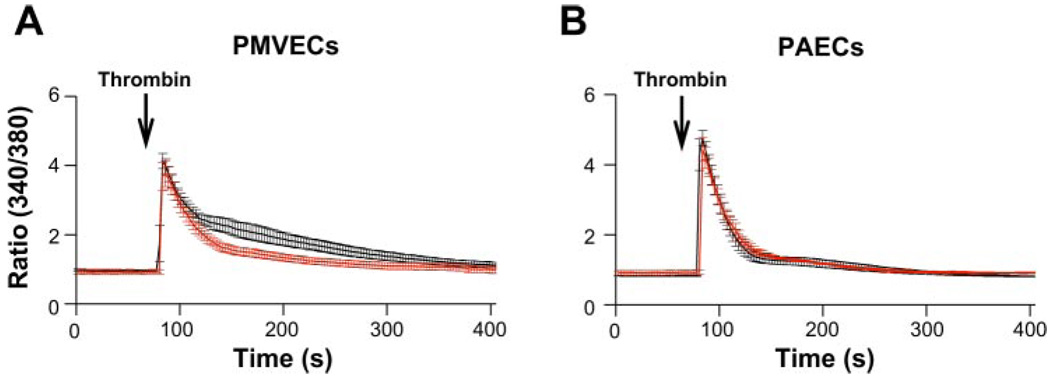
Lung macro- and microvascular endothelial cells exhibit distinct Ca2+ entry pathways that govern [Ca2+]i transitions in response to Gq-linked agonists (46, 58). The typical global [Ca2+]i response of endothelial cells to Gq-linked agonists, e.g., thrombin, comprises the initial immediate [Ca2+]i rise (first phase) that reflects release of Ca2+ from inositol 1,4,5-trisphosphate-sensitive intracellular Ca2+ stores and the second phase of [Ca2+]i elevation that reflects Ca2+ entry across the cell membrane after Ca2+ store depletion due to activation of store- and receptor-operated Ca2+ channels. Prior work revealed that the concentration of thrombin, which maximally activated both Ca2+ release and Ca2+ entry, was 1 U/ml in PAECs and 10 U/ml in PMVECs (9). To evaluate the role of Cav3.1 T-type Ca2+ channels in thrombin-stimulated [Ca2+]i transitions, we performed fluorometric assay using fura 2-AM-loaded cells to assess changes in [Ca2+]i following stimulation with thrombin in the presence and absence of the T-type Ca2+ channel blocker mibefradil [IC100 concentration; 10 µmol/l (58)]. In both cell types, thrombin stimulation triggered a typical biphasic [Ca2+]i response, yet only the Ca2+ entry phase in PMVECs was attenuated by mibefradil pretreatment (Fig. 1A), suggesting a major portion of Ca2+ entry is sensitive to T-type Ca2+ channel blockade. Mibefradil at the same concentration had no effect on the thrombin-induced [Ca2+]i transitions in PAECs (Fig. 1B), which lack expression of T-type Ca2+ channels. These data suggest that the reduction of thrombin-induced Ca2+ entry caused by mibefradil in PMVECs is due to the inhibitory effect on T-type Ca2+ channels. To confirm that expression of T-type Ca2+ channels contributes to thrombin-induced Ca2+ entry, similar studies were performed in PAECs overexpressing Cav3.1. The Cav3.1 stably transduced PAECs were generated by transient transfection of an expression vector encoding rat Cav3.1a (pcDNA3-Cav3.1a) (31) and selected with neomycin. Expression of Cav3.1 in transduced PAECs was confirmed by RT-PCR (data not shown) and Western blot analysis (Fig. 2A). Utilizing step depolarizations along with a two-step voltage protocol, where we could detect the maximally evoked T-type current (58), with the holding potential set to −90 mV, we demonstrated that expression of Cav3.1 resulted in a typical T-type current in PAECs. The current displayed low-threshold voltage activation, activated at −60 mV and above, rapid activation and inactivation kinetics that produce a criss-crossing pattern between successive traces of stepwise current-voltage (I-V) protocol (Fig. 2, B and C) (31a). In these Cav3.1 functionally overexpressing PAECs, we consistently observed that thrombin-induced Ca2+ entry was remarkably elevated (Fig. 2D), and the resultant elevation of Ca2+ entry was completely abolished by mibefradil (Fig. 2, E and F). These results further support that activation of the Cav3.1 T-type Ca2+ channel contributes to thrombin-induced physiological [Ca2+]i transition in PMVECs.
Fig. 1.
Mibefradil blockade of T-type Ca2+ channel inhibits the thrombin-induced rise in cytosolic Ca2+ ([Ca2+]i) in pulmonary microvascular endothelial cells (PMVECs). A: [Ca2+]i response to thrombin (10 U/ml) in PMVECs with (red trace) or without (black trace) 10-min pretreatment of mibefradil (10 µmol/l) (n = 7 and 9). Mibefradil significantly reduced the 2nd phase, but not the 1st phase, of the thrombin-induced rise in [Ca2+]i (P < 0.05). B: [Ca2+]i response to thrombin (1 U/ml) in pulmonary artery endothelial cells (PAECs) with or without 10-min pretreatment of mibefradil (10 µmol/l) (n = 4 and 5). Mibefradil had no effect on [Ca2+]i response to thrombin [P = not significant (ns)] in PAECs. Extracellular [Ca2+] = 2 mmol/l.
Fig. 2.
Functional expression of Cav3.1 T-type Ca2+ channel elevates thrombin-induced Ca2+ entry in PAECs. A: Cav3.1 protein expression appeared in Cav3.1-transduced PAECs, PAECs (NT), PAECs transduced with Cav3.1a (Trans), PMVECs (MV), rat brain. B: a representative set of macroscopic current traces in Cav3.1-transduced PAECs. Currents were recorded by increasing depolarization from −70 to +50 mV with 10-mV increments from a holding potential of −90 mV. Ca2+ (10 mmol/l) was used as a charge carrier. C: averaged current-voltage (I-V) relationship of peak currents obtained from 5 Cav3.1-transduced PAECs (■) and 12 nontransduced PAECs (□). Vm, membrane potential. D: [Ca2+]i response to thrombin (1 U/ml) in Cav3.1-transduced and nontransduced PAECs (n = 4 and 5, respectively). Functional expression of Cav3.1 considerably elevated the 2nd phase (P < 0.05), but not the 1st phase, of thrombin-induced rise in [Ca2+]i. E: [Ca2+]i response to thrombin (1 U/ml) in Cav3.1-transduced PAECs with or without 10-min pretreatment of mibefradil (10 µmol/l) (n = 4 each). Mibefradil reduced the 2nd phase of [Ca2+]i responses to thrombin (P < 0.05) but had no effect on the 1st phase of [Ca2+]i response to thrombin (P = not significant) in Cav3.1-transduced PAECs. F: superimposition of the thrombin-induced [Ca2+]i responses in Cav3.1-transduced PAECs with pretreatment of mibefradil and nontransduced PAECs without pretreatment of mibefradil demonstrates that mibefradil completely abolished elevated Ca2+ entry resulting from functional expression of Cav3.1 in PAECs. Extracellular [Ca2+] = 2 mmol/l in D–F.
Distinct regulation of Ca2+ - and cAMP-dependent exocytosis of WPBs from PMVECs and PAECs
In addition to Gq-linked agonists that trigger exocytosis of WPBs through elevating [Ca2+]i, the exocytosis of WPBs can be triggered by agonists that increase intracellular cAMP as well, e.g., β-adrenergic agonists (1, 49). We next assessed the Ca2+- and cAMP-dependent regulated exocytosis of WPBs in endothelial cells. A GFP-tagged VWF chimera (VWF-GFP) was stably introduced into cultured PMVECs and PAECs by retroviral infection. It has been previously demonstrated that this VWF-GFP chimera is targeted to WPBs together with endogenous VWF and colocalizes with P-selectin (35). Transduction of VWF-GFP resulted in a central cytosolic appearance of fluorescent vesicles in both PMVECs and PAECs (Fig. 3, A and B, Video 1. Supplemental data for this article is available online at the AJP-Lung web site). This is consistent with the distribution pattern observed in VWF-GFP-expressing endothelial cells in prior studies utilizing the same VWF-GFP chimeras (17, 35). Accordingly, fluorescent vesicles revealed by confocal microscopy in VWF-GFP-expressing PMVECs and PAECs resembled WPBs. By exploiting the intrinsic GFP fluorescence, this approach enables direct visualization of the translocation and dynamics of WPBs in living endothelial cells.
Fig. 3.
Von Willebrand factor (VWF)-green fluorescent protein (GFP)-containing vesicles resemble Weibel-Palade bodies (WPBs) in VWF-GFP-expressing endothelial cells. A: PMVECs were transduced with the VWF-GFP chimera. VWF-GFP-containing vesicles were observed in the cytoplasm. B: images of serial apical-to-basal optical sections (Z-stacks) taken in a VWF-GFP-expressing PMVEC at 0.3-µm intervals revealed that the vesicles were distributed throughout the cytoplasm, with the majority situated in the middle and lower sections of the cell.
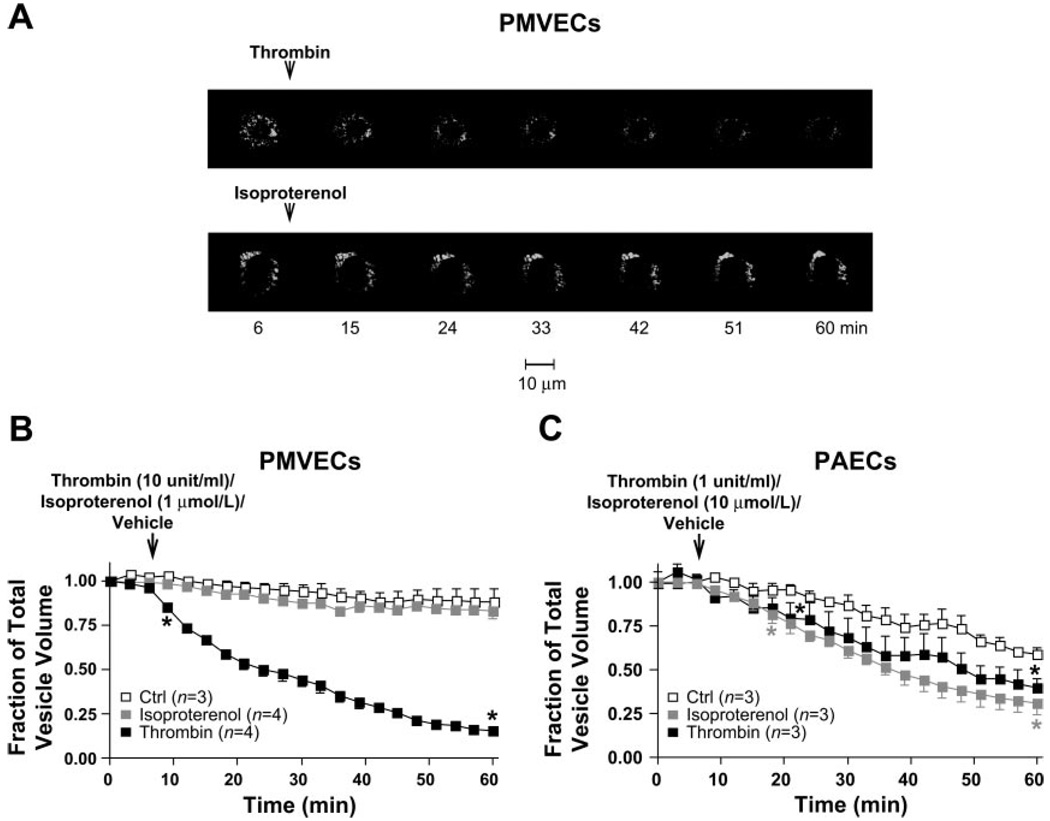
We next examined the real-time secretory process of WPBs in living endothelial cells in response to thrombin or the β-adrenergic agonist isoproterenol. In each experiment, the EC100 concentration of thrombin (10 U/ml for PMVECs and 1 U/ml for PAECs) or isoproterenol (1 µmol/l for PMVECs and 10 µmol/l for PAECs) was applied to PMVECs and PAECs to induce maximal Ca2+ entry across the cell membrane (the 2nd phase of thrombin-induced elevation in [Ca2+]i) (9) or maximal elevation in intracellular cAMP levels (45). The GFP fluorescence of the cells was monitored in real time during 60 min of stimulation at intervals of 3 min using time-lapse confocal microscopy. Cells subjected to vehicle application served as control. In VWF-GFP-expressing PMVECs or PAECs with no stimulation, a slow, random movement of VWF-GFP-containing vesicles was observed throughout the cell body, suggesting the occurrence of arbitrary trafficking of WPBs. Additionally, a slight decrease in the number of VWF-GFP-containing vesicles was detected during the 60-min period, possibly due to spontaneous release of fluorescent vesicles or photobleaching of green fluorescence. It appears that some photobleaching is indeed occurring with 21 measurements over 60 min because no decrease in fluorescence was observed with just two measurements over 60 min, i.e., at the beginning and at the end of the experiment. Nonetheless, in PMVECs and PAECs, following thrombin stimulation, a dramatic movement of VWF-GFP-containing vesicles was observed, and a rapid, progressive decrease in the number of fluorescent vesicles was detected, i.e., 85% (±2% SE) decrease in PMVECs and 60% (±5% SE) decrease in PAECs (Fig. 4, A and B) during 60 min of stimulation. A similar pattern of movement and decrease in number of fluorescent vesicles were also observed following isoproterenol stimulation in VWF-GFP-transduced PAECs (Fig. 4C) but not in PMVECs (Fig. 4, A and B). These results imply that Ca2+- and cAMP-dependent pathways regulating exocytosis of WPBs may differ in PMVECs and PAECs.
Fig. 4.
Activated exocytosis of VWF-GFP-containing vesicles by thrombin and isoproterenol in PMVECs and PAECs. A: Representative sets of merged time-lapse GFP fluorescent images of all serial sections (Z-stacks) taken from apical-to-basal cell aspects at 0.25-µm intervals every 3 min in live VWF-GFP-expressing PMVECs during 60-min experiments. A rapid, progressive decrease in total number of VWF-GFP-containing vesicles following thrombin (top, and Video 2) stimulation was observed. Arrow denotes when thrombin (10 U/ml) was applied. A, bottom: images taken in the same experimental setting in live VWF-GFP-expressing PMVECs following isoproterenol (1 µmol/l) stimulation, illustrating virtually no decrease in total number of VWF-GFP-containing vesicles. B and C: the time-course summary of changes in total pixel volume of GFP fluorescence, normalized to the fluorescence value at the beginning of the recording (time 0) in VWF-GFP-expressing PMVECs (B) and PAECs (C) over 60 min. Arrows denote when thrombin or isoproterenol (at the concentrations depicted) was applied. Controls (□) were cells without thrombin or isoproterenol exposure. Note thrombin induced a remarkable decrease in total number of vesicles in both PMVECs and PAECs (■), whereas isoproterenol only induced a decrease in total number of VWF-GFP-containing vesicles in PAECs, not PMVECs (B and C, gray squares). *Time point where the data from 2 groups start to have significant difference (P < 0.01) through the end of observation. Data were acquired on a PerkinElmer UltraView RS confocal microscope with a ×60 objective.
Thrombin-induced exocytosis of WPBs from PMVECs is sensitive to pharmacological blockade of T-type Ca2+ channels
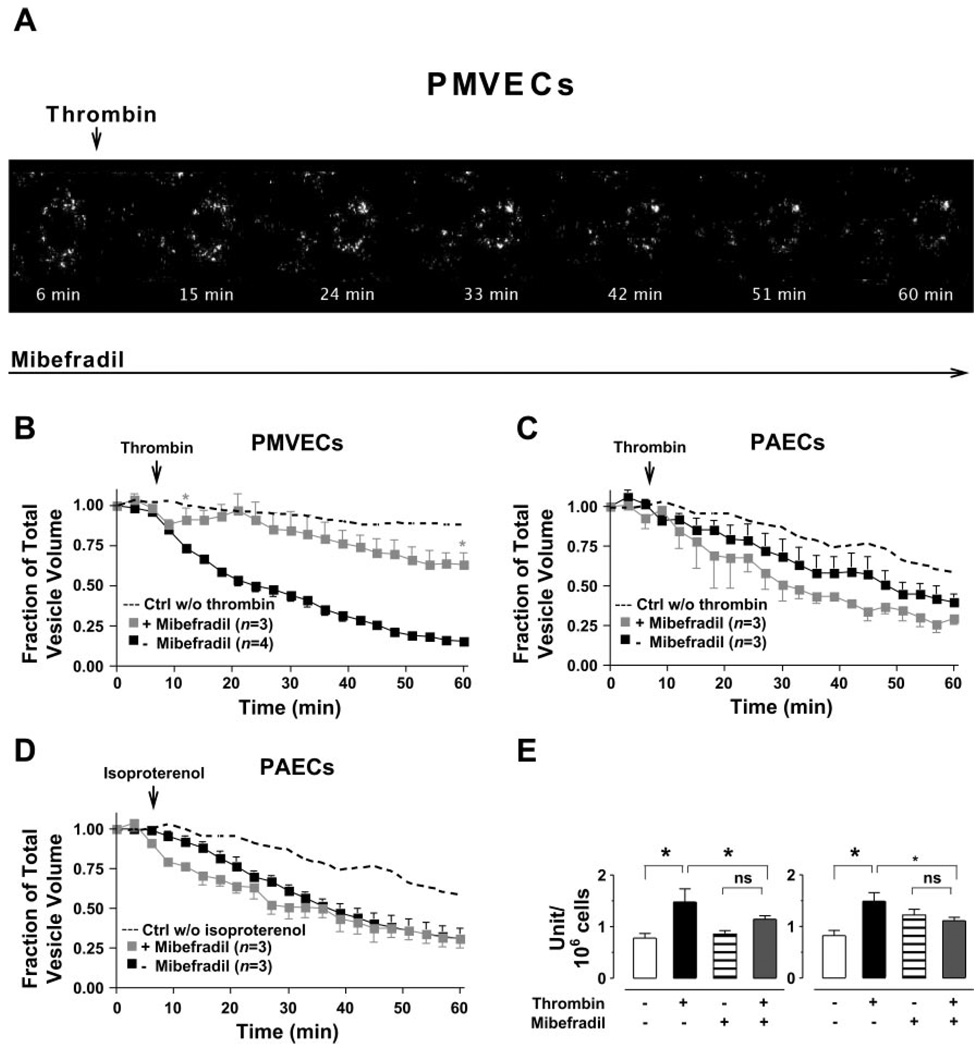
To test whether Ca2+ entry through T-type Ca2+ channels is required for the exocytosis of WPBs, we first applied mibefradil to endothelial cells. The VWF-GFP-expressing PMVECs or PAECs were subsequently stimulated with thrombin or isoproterenol. Exposure of cells to mibefradil alone did not evoke exocytosis of VWF-GFP-containing vesicles (data not shown). However, mibefradil nearly abolished the thrombin-induced decrease in the number of VWF-GFP-containing vesicles in PMVECs (Fig. 5, A and B) but not in PAECs (Fig. 5C). Additionally, the effect of isoproterenol on the decrease in number of fluorescent vesicles in PAECs was not affected by application of mibefradil (Fig. 5D).
Fig. 5.
Mibefradil inhibits thrombin-activated exocytosis of VWF-GFP-containing vesicles from PMVECs but not from PAECs. A: a set of merged time-lapse GFP fluorescent images of all parts of Z-stacks taken from apical-to-basal cell aspects at 0.25-µm intervals in live VWF-GFP-expressing PMVECs during experiments in the presence of mibefradil (10 µmol/l). The decrease in total number of VWF-GFP-containing vesicles activated by thrombin was nearly abolished by mibefradil. Arrow denotes when thrombin (10 U/ml) was applied. B–D: the time-course summary for changes in total pixel volume of GFP fluorescence, normalized to the fluorescence value at time 0 in VWF-GFP-expressing PMVECs (B) and PAECs (C and D) during the 60-min real-time image recordings with (gray squares) or without (■) mibefradil (10 µmol/l). Controls (dashed lines) were cells without thrombin or isoproterenol exposure. Note mibefradil markedly prevented thrombin-induced decrease in total number of VWF-GFP-containing vesicles in PMVECs (B). *Time point where the data from 2 groups (+mibefradil group and −mibefradil group) in PMVECs start to have significant difference (P < 0.01) to the end of the experiments. Mibefradil did not affect either thrombinor isoproterenol-stimulated changes in total number of VWF-GFP-containing vesicles in PAECs (C and D). Arrows denote when thrombin or isoproterenol (at the concentrations depicted) was applied. Data were acquired on a PerkinElmer UltraView RS confocal microscope with a ×60 objective. E: the VWF release from PMVECs was determined by measuring the amount of VWF in extracellular media using ELISA following 10 and 60 min of thrombin (10 U/ml) stimulation of cells in the presence and absence of mibefradil (10 µmol/l). Mibefradil attenuated VWF release at each time point following thrombin stimulation but had no effect on basal VWF release. *Significantly different between the 2 sample sets depicted; P < 0.05.
The effect of T-type Ca2+ channel blockade on exocytosis of WPBs was further investigated by assessing the amount of VWF-GFP released in the medium following stimulation of PMVECs with thrombin (10 U/ml) by ELISA. As expected, we observed a rapid increase in VWF levels in the supernatant of thrombin-stimulated VWF-GFP-expressing PMVECs (Fig. 5E), which paralleled the thrombin-induced decrease in VWF-GFP-containing vesicles observed with confocal live cell imaging (Fig. 4C). While T-type Ca2+ channel blockade with mibefradil did not change the basal level of VWF in the supernatant of nonstimulated PMVECs, it indeed nearly abolished the increase in VWF in the supernatant of thrombinstimulated PMVECs (Fig. 5E).
Effects of Cav3.1 RNA interference on thrombin-stimulated [Ca2+]i transitions and WPB exocytosis
To confirm that the inhibition caused by mibefradil on thrombin-stimulated exocytosis of VWF-GFP-containing vesicles was indeed due to T-type Ca2+ channel blockade, the functional role of Cav3.1 in thrombin-stimulated WPB exocytosis was further investigated utilizing RNA interference approach to specifically silence the Cav3.1 gene in PMVECs. Five shRNA coding oligonucleotides were designed to target sequences within the coding region of the Cav3.1 gene. Each oligonucleotide was ligated into a retroor lentiviral expression vector allowing for individual expression of the shRNA into cultured PMVECs through viral delivery. We initially employed GFP-expressing retrovirus for delivery of shRNAs to determine efficiency in Cav3.1 gene silencing. An mCherry-expressing lentiviral vector was used for delivery of a selected shRNA and the scrambled shRNA for fura 2 fluorometric [Ca2+]i assessments and the studies requiring VWF-GFP coexpression. The shRNAs in transduced cells were processed into small interfering RNA-like molecules capable of carrying out Cav3.1 gene-specific silencing. Since the expression vector constitutively expresses GFP or mCherry, positively transduced PMVECs were easily identified, and the stable transfectants were selected to virtually 100% purity. This approach resulted in a remarkable reduction of Cav3.1 gene expression in virtually all cells, allowing for the use of a large number of cells for patch-clamp electrophysiology, Ca2+ epifluorescence studies, as well as real-time imaging and confocal microscopy studies on living VWF-GFP coexpressing PMVECs.
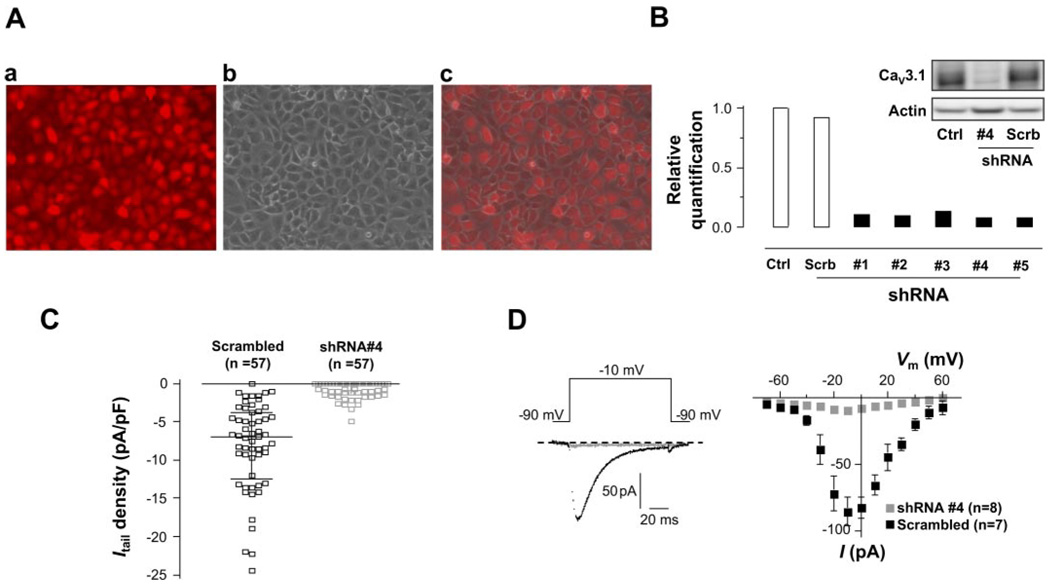
We first demonstrated the high efficacy of retro- and lentivirus-mediated delivery of shRNA to PMVECs (Fig. 6A). qRT-PCR analysis revealed that in shRNA retrovirally infected PMVECs, all five shRNAs markedly decreased the Cav3.1 mRNA level by a minimum of 86%, with shRNA #4 being the most efficient (92%) (Fig. 6B). The Cav3.1 gene silencing with shRNA #4 significantly attenuated the functional T-type Ca2+ channel in PMVECs as evidenced by the virtual disappearance of the deactivating tail current elicited at the peak of + 80-mV pulse, where the maximally evoked T-type current could be measured, in transduced PMVECs (Fig. 6C). The T-type currents elicited in scrambled shRNA-transduced cells retained a similar current amplitude as well as current-voltage relationship (Fig. 6D) as we previously reported in normal PMVECs, i.e., currents were consistently activated at −60 mV, maximal current activation was observed at −10 mV (58). Elimination of the functional T-type Ca2+ channel by Cav3.1 gene silencing caused a significant attenuation of thrombin-stimulated Ca2+ entry as demonstrated by the decrease in the second phase of [Ca2+]i elevation, but not Ca2+ release, in mCherry-labeled shRNA #4-transduced PMVECs (Fig. 7, A and B). This result is consistent with the data in Fig. 1A where pharmacological T-type Ca2+ channel blockade with mibefradil reduced thrombin-stimulated Ca2+ entry.
Fig. 6.
Silencing Cav3.1 expression by RNA interference (RNAi). A: representative fluorescence image of mCherry-tagged small hairpin RNA (shRNA) #4-transduced PMVECs (a), phase-contrast image of the same cell monolayer (b), and merged image of the 2 (c) showing the high efficiency of virus-mediated delivery of shRNA to PMVECs. B: quantitative RT-PCR analysis of Cav3.1 gene expression by relative quantification. Rat GAPDH was used as internal standard for expression normalization. Cav3.1 gene expression was knocked down by 86–92% by 5 different shRNAs (n = 3 each). Inset: Western blotting analysis of Cav3.1 protein levels. Cav3.1 protein expression was markedly suppressed by shRNA #4. Scrambled shRNA had no effect on Cav3.1 protein expression. C: individual data points of the deactivating tail current (ICa, tail) elicited at the peak of + 80 mV pulse in PMVECs transduced with shRNA #4 or scrambled shRNA. Lines in each data group are at median with interquartile range, i.e., for shRNA #4-transduced group (in pA/pF): median 0.0, 25th percentile −1.5, 75th percentile 0.0; for scrambled shRNA transduced group: median −6.9, 25th percentile −12.5, 75th percentile −3.8 (P < 0.0001, Mann-Whitney test). D, left: representative traces of the current elicited at −10 mV from shRNA #4- (gray) and scrambled shRNA- (black) transduced PMVECs, respectively. D, right: I-V relationships of peak currents at each depolarizing pulse from shRNA #4- (n = 8, gray squares) and scrambled shRNA- (n = 7, ■) transduced PMVECs, showing shRNA #4-induced Cav3.1 gene silencing nearly eliminated T-type current in PMVECs. Ca2+ (10 mM) was used as a charge carrier.
Fig. 7.
Effects of shRNA-induced Cav3.1 gene silencing on thrombin-stimulated PMVEC [Ca2+]i transitions and WPB exocytosis. A: [Ca2+]i responses to thrombin (10 U/ml) in PMVECs transduced with shRNA #4 (red trace, n = 4) and PMVECs transduced with scrambled shRNA (black trace, n = 4) measured in buffer containing 2 mmol/l extracellular Ca2+. Cav3.1 silencing with shRNA #4 considerably reduced the thrombin-stimulated Ca2+ entry, i.e., 2nd phase of [Ca2+]i elevation (*P < 0.05 vs. scrambled shRNA) with no evidence of a decrease in Ca2+ release, i.e., no difference in the peak of thrombin-induced Ca2+ release phase between groups (P > 0.05). B: [Ca2+]i responses to thrombin (10 U/ml) in PMVECs transduced with shRNA #4 (red trace, n = 4) and PMVECs transduced with scrambled shRNA (black trace, n = 3) measured in Ca2+-free buffer. It was further confirmed that Cav3.1 silencing with shRNA #4 did not change the thrombin-stimulated Ca2+ release (P > 0.05 vs. scrambled shRNA). C: the time-course summary of changes in total pixel volume of GFP fluorescence, normalized to the fluorescence value at the beginning of the recordings (time 0), in PMVECs transduced with shRNA #4 (red squares, n = 6) and scrambled shRNA (■, n = 5) during the real-time image recordings. The rapid, progressive decrease in total number of VWF-GFP-containing vesicles activated by thrombin seen in PMVECs transduced with scrambled shRNA was nearly abolished in PMVECs transduced with shRNA #4. Arrow denotes when thrombin (10 U/ml) or isoproterenol (1 µmol/l) was applied. Top black dashed line shows the time control of GFP fluorescence changes in normal nonstimulated nontransduced PMVECs. Middle gray dashed line shows the time course of thrombin-stimulated decrease in the number of GFP-fluorescent vesicles when PMVECs were pretreated with mibefradil before thrombin stimulation (see Fig. 5C). Bottom red dashed line represents the time course of decrease in GFP-fluorescent vesicles during 60-min interval when normal PMVECs were stimulated with thrombin (see Fig. 5C). Note the shRNA #4 mimics the effect of mibefradil in Fig. 6B, and PMVECs transduced with scrambled shRNA retain the same response as normal nontransduced PMVECs to thrombin in release of VWF-GFP-containing vesicles. Data were acquired on a PerkinElmer UltraView RS confocal microscope with a ×60 objective. *P < 0.01 between shRNA #4 and scrambled shRNA.
Real-time imaging and confocal microscopy studies were finally performed on mCherry-labeled shRNA- and VWF-GFP-transduced living PMVECs to measure the decrease of the number of WPBs in response to thrombin stimulation. Notably, transduction of VWF-GFP with either shRNA #4 or scrambled shRNA exhibited the same distribution pattern and dynamics of VWF-GFP-containing vesicles in resting status as in PMVECs transduced with VWF-GFP alone. A rapid and progressive decrease in VWF-GFP-containing vesicles in response to thrombin was observed in scrambled shRNA-transduced PMVECs, virtually identical to that in nontransduced cells. However, the decrease in VWF-GFP-containing vesicles in response to thrombin was nearly abolished in shRNA #4-transduced PMVECs, similar to that of mibefradil-treated PMVECs (Fig. 7C).
DISCUSSION
Although Ca2+ entry has been recognized as the critical triggering mechanism in transducing thrombin- as well as other Gq-linked inflammatory agonist-stimulated exocytosis of WPBs (2, 12, 16, 26), the molecular identities mediating this response remain to be elucidated. Indeed, thrombin activates multiple pathways including store-operated and receptor-operated Ca2+ entry pathways along with other reported signaling cascades in both macro- and microvascular endothelial cell types (for review, see Ref. 3). Our present study sought to specifically determine the relevance of the Cav3.1 T-type Ca2+ channel in thrombin-induced exocytosis of WPBs. The major findings in this study are that PMVECs differ from their macrovascular counterparts, PAECs, in mechanisms regulating the exocytosis of WPBs and that Cav3.1 T-type Ca2+ channels play a critical role in controlling WPB exocytosis from microvascular endothelial cells, i.e., T-type Ca2+ channel activation provides a unique [Ca2+]i source important for thrombinstimulated WPB exocytosis.
To address the role of endothelial cell Cav3.1 T-type Ca2+ channels in thrombin-stimulated exocytosis of WPBs, we initially introduced a fusion protein consisting of VWF, a prominent WPB constituent, and GFP (VWF-GFP) to directly visualize the secretory process of WPBs in living cells. Using live cell imaging analysis to assess changes in the number of VWF-GFP-containing vesicles, we observed a rapid and progressive decrease in fluorescent vesicles following stimulation of PMVECs or PAECs with thrombin or PAECs with isoproterenol. Notably, isoproterenol had no effect on the dynamics of VWF-GFP-containing vesicles in PMVECs. These results suggest that PMVECs are distinct from PAECs in regulated exocytosis of WPBs. Whereas the exocytosis of WPBs is predominantly Ca2+ dependent in PMVECs, it is both Ca2+ and cAMP dependent in PAECs.
Prior studies reported heterogeneity of lung macro- and microvascular endothelial cells in both Ca2+ and cAMP signaling pathways (45, 46). Compared with PAECs, PMVECs exhibit a greater global [Ca2+]i response to thrombin (9), but an attenuated intracellular cAMP response to forskolin or β-adrenergic receptor agonist isoproterenol (45, 46), both activating transmembrane type 6 adenylyl cyclase. On the other hand, the intracellular cAMP response to the cAMP-specific type 4 phosphodiesterase inhibitor rolipram is more pronounced in PMVECs (45, 46). Such heterogeneity in Ca2+ and cAMP signaling may be closely related to our observation of the diversity in regulated exocytosis of WPBs between Ca2+-and cAMP-dependent pathways in the two endothelial cell types. Additionally, our results imply the possible existence of functionally distinct populations of WPBs whose exocytosis is differentially regulated. In support of this notion, a recent study revealed differential effects of thrombin, histamine, protease-activated receptor-activating peptides, and forskolin on secretion of VWF and cell surface expression of P-selectin in human umbilical vein endothelial cells (HUVECs), which was proposed to be due to the existence of distinct populations of WPBs containing VWF with or without P-selectin (10). Moreover, previous real-time studies performed with the VWF-GFP fusion protein transduced into HUVECs revealed striking differences in the dynamics of WPBs between [Ca2+]i and cAMP responses. For instance, stimulation with cAMP-elevating agonists, in addition to triggering fusion of WPBs with the plasma membrane, resulted in perinuclear clustering of a subset of WPBs that escaped exocytosis (35, 36). Thus cAMP mediates release of VWF from peripheral WPBs. In contrast, [Ca2+]i mediates release of both central and peripheral WPBs (51). These observations all suggest a selective coupling between [Ca2+]i- or cAMP-dependent regulation of WPB exocytosis to specific populations of WPBs. Consistent with this idea, our findings indicate that WPB exocytosis in pulmonary macro-and microvascular endothelial cells is differentially regulated by two distinct pathways involving [Ca2+]i and/or cAMP. Although not studied here in detail, the observation that the cAMP-mediated exocytosis of WPBs only occurred in PAECs, but not in PMVECs, is most likely caused by the existence of distinct populations of WPBs existing in the two cells types.
Based on our prior work, activation of Cav3.1 T-type Ca2+ channels by thrombin generates a physiologically relevant [Ca2+]i rise in microvascular endothelial cells via depolarization of the plasma membrane (58). The present study provided further evidence that the Cav3.1 T-type Ca2+ channel is a critical downstream effector for thrombin signaling in endothelial cells. Although the T-type Ca2+ channel blocker mibefradil had no effect on thrombin-induced rise in [Ca2+]i in PAECs, it did cause a remarkable decrease in the thrombin-induced rise in [Ca2+]i in PMVECs. Consistently, Cav3.1 gene silencing in PMVECs nearly eliminated functional T-type Ca2+ channels and markedly reduced thrombin-induced Ca2+ entry. Furthermore, expression of recombinant Cav3.1 in PAECs resulted in the emergence of a T-type current and augmented thrombin-induced Ca2+ entry. The latter response was completely reversed by pharmacological blockade of the T-type Ca2+ channels.
Despite the fact that WPB exocytosis in both PMVECs and PAECs involves the activation of thrombin/[Ca2+]i and/or cAMP signaling pathways, only in PMVECs was the thrombin-induced release of VWF-GFP-containing vesicles diminished by pharmacological blockade of the Cav3.1 T-type Ca2+ channels. In addition, T-type Ca2+ channel blockade had no effect on the response of isoproterenol-stimulated release of VWF-GFP-containing vesicles in PAECs. These results are consistent with the observation that there are no T-type Ca2+ channels in PAECs (58) and support the specificity of mibefradil on T-type Ca2+ channel blockade. Furthermore, Cav3.1 gene silencing nearly prevented PMVECs from releasing VWF-GFP-containing vesicles in response to thrombin stimulation and significantly attenuated thrombin-stimulated Ca2+ entry to a similar extent as mibefradil. Together, these results indicate that the Cav3.1 T-type Ca2+ channel is a specific downstream target of thrombin, important for regulated exocytosis of WPBs in PMVECs.
In summary, we have utilized two distinct endothelial cell phenotypes, PMVECs and PAECs, to evaluate the dynamic activity and the role of T-type Ca2+ channels in thrombin-stimulated WPB exocytotic process. We have demonstrated that in microvascular endothelial cells, thrombin-induced Ca2+ entry via the Cav3.1 T-type Ca2+ channel plays a crucial role in promoting exocytosis of WPBs. These findings establish physiological relevance for the T-type Ca2+ channel in non-excitable microvascular endothelial cells; namely, activation of the channel induces a procoagulant endothelial phenotype important for site-specific vascular endothelial activation. The significance of the findings can be exemplified by the observations in sickle cell anemia in which sickle erythrocytes adhere more readily to microvascular endothelium than to endothelium from conduit vessels (5). Indeed, our prior study also shows that inhibition of T-type Ca2+ channels attenuates increased retention of sickled erythrocytes in the inflamed pulmonary circulation (58). With regard to the question raised by Varghese and Weir (50) as whether targeted delivery of T-type blockers can be used as therapy for vaso-occlusive crisis in sickle cell anemia, we propose that specific T-type Ca2+ channel antagonists or alteration of the Cav3.1 channel is a prospective therapeutic strategy for regulating endothelial activation in thrombotic disorders, especially in lung microcirculation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Troy Stevens and Dr. Donna L. Cioffi for critical reading of the manuscript and Linn Ayers and Anna Penton for excellent assistance with cell culture studies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-74116 (to S. Wu).
REFERENCES
- 1.Birch KA, Ewenstein BM, Golan DE, Pober JS. Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. J Cell Physiol. 1994;160:545–554. doi: 10.1002/jcp.1041600318. [DOI] [PubMed] [Google Scholar]
- 2.Birch KA, Pober JS, Zavoico GB, Means AR, Ewenstein BM. Calcium/ calmodulin transduces thrombin-stimulated secretion: studies in intact and minimally permeabilized human umbilical vein endothelial cells. J Cell Biol. 1992;118:1501–1510. doi: 10.1083/jcb.118.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry. 2002;67:75–84. doi: 10.1023/a:1013904231324. [DOI] [PubMed] [Google Scholar]
- 4.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 5.Brittain HA, Eckman JR, Wick TM. Sickle erythrocyte adherence to large vessel and microvascular endothelium under physiologic flow is qualitatively different. J Lab Clin Med. 1992;120:538–545. [PubMed] [Google Scholar]
- 6.Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991;138:213–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 8.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 9.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol. 2002;157:1267–1278. doi: 10.1083/jcb.200204022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creighton JR, Masada N, Cooper DM, Stevens T. Coordinate regulation of membrane cAMP by Ca2+-inhibited adenylyl cyclase and phosphodiesterase activities. Am J Physiol Lung Cell Mol Physiol. 2003;284:L100–L107. doi: 10.1152/ajplung.00083.2002. [DOI] [PubMed] [Google Scholar]
- 12.de Groot PG, Gonsalves MD, Loesberg C, van Buul-Wortelboer MF, van Aken WG, van Mourik JA. Thrombin-induced release of von Willebrand factor from endothelial cells is mediated by phospholipid methylation. Prostacyclin synthesis is independent of phospholipid methylation. J Biol Chem. 1984;259:13329–13333. [PubMed] [Google Scholar]
- 13.Ewenstein BM, Warhol MJ, Handin RI, Pober JS. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987;104:1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federici AB, Bader R, Pagani S, Colibretti ML, De Marco L, Mannucci PM. Binding of von Willebrand factor to glycoproteins Ib and IIb/IIIa complex: affinity is related to multimeric size. Br J Haematol. 1989;73:93–99. doi: 10.1111/j.1365-2141.1989.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 15.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987;79:600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannah MJ, Skehel P, Erent M, Knipe L, Ogden D, Carter T. Differential kinetics of cell surface loss of von Willebrand factor and its propolypeptide after secretion from Weibel-Palade bodies in living human endothelial cells. J Biol Chem. 2005;280:22827–22830. doi: 10.1074/jbc.M412547200. [DOI] [PubMed] [Google Scholar]
- 18.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 19.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- 20.Jaleco AC, Stegmann AP, Heemskerk MH, Couwenberg F, Bakker AQ, Weijer K, Spits H. Genetic modification of human B-cell development: B-cell development is inhibited by the dominant negative helix loop helix factor Id3. Blood. 1999;94:2637–2646. [PubMed] [Google Scholar]
- 21.Jones DA, Abbassi O, McIntire LV, McEver RP, Smith CW. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993;65:1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann JE, Oksche A, Wollheim CB, Gunther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106:107–116. doi: 10.1172/JCI9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loesberg C, Gonsalves MD, Zandbergen J, Willems C, van Aken WG, Stel HV, Van Mourik JA, De Groot PG. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta. 1983;763:160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- 26.Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- 27.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 28.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Norwood N, Moore TM, Dean DA, Bhattacharjee R, Li M, Stevens T. Store-operated calcium entry and increased endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2000;279:L815–L824. doi: 10.1152/ajplung.2000.279.5.L815. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 31a.Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 32.Richardson M, Tinlin S, De Reske M, Webster S, Senis Y, Giles AR. Morphological alterations in endothelial cells associated with the release of von Willebrand factor after thrombin generation in vivo. Arterioscler Thromb. 1994;14:990–999. doi: 10.1161/01.atv.14.6.990. [DOI] [PubMed] [Google Scholar]
- 33.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 34.Romani de Wit T, de Leeuw HP, Rondaij MG, de Laaf RT, Sellink E, Brinkman HJ, Voorberg J, van Mourik JA. Von Willebrand factor targets IL-8 to Weibel-Palade bodies in an endothelial cell line. Exp Cell Res. 2003;286:67–74. doi: 10.1016/s0014-4827(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 35.Romani de Wit T, Rondaij MG, Hordijk PL, Voorberg J, van Mourik JA. Real-time imaging of the dynamics and secretory behavior of weibel-palade bodies. Arterioscler Thromb Vasc Biol. 2003;23:755–761. doi: 10.1161/01.ATV.0000069847.72001.E8. [DOI] [PubMed] [Google Scholar]
- 36.Rondaij MG, Bierings R, Kragt A, Gijzen KA, Sellink E, van Mourik JA, Fernandez-Borja M, Voorberg J. Dynein-dynactin complex mediates protein kinase A-dependent clustering of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:49–55. doi: 10.1161/01.ATV.0000191639.08082.04. [DOI] [PubMed] [Google Scholar]
- 37.Rondaij MG, Sellink E, Gijzen KA, ten Klooster JP, Hordijk PL, van Mourik JA, Voorberg J. Small GTP-binding protein Ral is involved in cAMP-mediated release of von Willebrand factor from endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1315–1320. doi: 10.1161/01.ATV.0000131267.13425.45. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 39.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 40.Sakariassen KS, Bolhuis PA, Sixma JJ. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979;279:636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- 41.Schlüter T, Bohnensack R. Serotonin-induced secretion of von Willebrand factor from human umbilical vein endothelial cells via the cyclic AMP-signaling systems independent of increased cytoplasmic calcium concentration. Biochem Pharmacol. 1999;57:1191–1197. doi: 10.1016/s0006-2952(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 42.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 43.Sporn LA, Marder VJ, Wagner DD. Differing polarity of the constitutive and regulated secretory pathways for von Willebrand factor in endothelial cells. J Cell Biol. 1989;108:1283–1289. doi: 10.1083/jcb.108.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 45.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol. 1999;277:L119–L126. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- 46.Stevens T, Fouty B, Hepler L, Richardson D, Brough G, McMurtry IF, Rodman DM. Cytosolic Ca2+ and adenylyl cyclase responses in phenotypically distinct pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 1997;272:L51–L59. doi: 10.1152/ajplung.1997.272.1.L51. [DOI] [PubMed] [Google Scholar]
- 47.Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JG, Hebbel RP, Tuder RM, Garfinkel S. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol. 2001;281:C1422–C1433. doi: 10.1152/ajpcell.2001.281.5.C1422. [DOI] [PubMed] [Google Scholar]
- 48.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Eijnden-Schrauwen Y, Atsma DE, Lupu F, de Vries RE, Kooistra T, Emeis JJ. Involvement of calcium and G proteins in the acute release of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:2177–2187. doi: 10.1161/01.atv.17.10.2177. [DOI] [PubMed] [Google Scholar]
- 50.Varghese A, Weir EK. T-type calcium current in sickle cell disease: a channel to therapy? Circ Res. 2003;93:274–276. doi: 10.1161/01.RES.0000089472.85758.8B. [DOI] [PubMed] [Google Scholar]
- 51.Vischer UM, Barth H, Wollheim CB. Regulated von Willebrand factor secretion is associated with agonist-specific patterns of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:883–891. doi: 10.1161/01.atv.20.3.883. [DOI] [PubMed] [Google Scholar]
- 52.Vischer UM, Wollheim CB. Epinephrine induces von Willebrand factor release from cultured endothelial cells: involvement of cyclic AMP-dependent signalling in exocytosis. Thromb Haemost. 1997;77:1182–1188. [PubMed] [Google Scholar]
- 53.Vischer UM, Wollheim CB. Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood. 1998;91:118–127. [PubMed] [Google Scholar]
- 54.Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–110. [PubMed] [Google Scholar]
- 55.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982;95:355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolff B, Burns AR, Middleton J, Rot A. Endothelial cell “memory” of inflammatory stimulation: human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J Exp Med. 1998;188:1757–1762. doi: 10.1084/jem.188.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res. 2003;93:346–353. doi: 10.1161/01.RES.0000087148.75363.8F. [DOI] [PubMed] [Google Scholar]
- 59.Wu S, Sangerman J, Li M, Brough GH, Goodman SR, Stevens T. Essential control of an endothelial cell ISOC by the spectrin membrane skeleton. J Cell Biol. 2001;154:1225–1233. doi: 10.1083/jcb.200106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.