Abstract
Introduction
Gemcitabine, the current standard of care for pancreatic ductal adenocarcinoma (PDA), has a less than 10% partial response rate. Genexol-PM, a modified form of paclitaxel, has been shown to have antitumour effects in clinical trials of metastatic breast and small-lung-cell carcinoma. The aim of the present study was to determine if Genexol would be a beneficial treatment for gemcitabine-resistant PDA.
Materials and methods
We measured the in vitro IC50s of gemcitabine and genexol in cell lines sensitive and resistant to gemcitabine. In vivo, animals with orthotopic pancreatic tumours, resistant to gemcitabine, were treated with phosphate-buffered saline (PBS), gemcitabine, Genexol or gemcitabine+Genexol. Tumour progression was monitored using red fluorescent protein imaging.
Results
We showed equivalent IC50s for gemcitabine-sensitive and gemcitabine-resistant cell lines when treated with genexol. In vivo treatment with genexol resulted in a greater per cent reduction in tumour size, less metastatic spread and longer survival compared with treatment with gemcitabine.
Discussion
Genexol proved to be an effective treatment for gemcitabine-resistant PDA. These data combined with the successful clinical use of genexol in Phase II trials of other malignancies suggests it maybe an effective treatment for pancreatic cancer, specifically for those patients resistant to gemcitabine.
Keywords: chemotherapy, metastases, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDA) is an exceptionally lethal disease, with approximately 80% of patients presenting with unresectable disease as a result of metastases and local invasion.1 While survival in many cancers, including breast and colon, have increased as the result of advances in solid tumour therapies, it has been estimated that the overall 5-year survival rate for patients with pancreatic cancer is only 5%.2
The main line of defence against pancreatic cancer is gemcitabine, a nucleoside analogue, which stops tumour growth by blocking DNA synthesis. Although gemcitabine is a relatively well-tolerated drug, acquired as well as an initial non-response in PDA patients is a major limitation of this line of therapy.3 The median survival with advanced disease remains less than 6 months, the overall tumour response rate is less than 25% and mean survival benefit when given post-surgical resection is 6–8 months.3,4 Taken together the data suggests that a novel treatment that acts through a different pathway would offer advantages, as it could potentially work on those individuals that do not respond to gemcitabine, as well as those that acquire a resistance to gemcitabine.
One potential novel drug is Genexol. Genexol is a modified form of paclitaxel, which does not have the same toxicity problems that are encountered when treating with paclitaxel. The mechanism of action of Genexol at the cellular level is the stabilization of microtubules, thus preventing cell division. To date clinical trials with Genexol for the treatment of advanced malignancies, advanced small-cell-lung cancer and metastatic breast cancer have been promising.5–7
In terms of pancreatic cancer, there is preliminary data in vitro and in an animal model that suggests Genexol could be advantageous as a treatment regimen for pancreatic cancer.8 The results of a Phase II trial for the treatment of locally invasive and metastatic PDA patients was recently published, with a median overall survival of 6.5 months.9 Therefore, the aim of the present study is to examine Genexol as a potential treatment for gemcitabine-resistant pancreatic cancer in vitro and with an orthotopic animal model.
Materials and methods
Cell culture
The human pancreatic cancer cell line MiaPaCa-2 was obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA). Cells were cultured at 37°C in a 5% CO2 incubator. The MiaPaCa-2 cells were engineered to stably express red fluorescent protein (Clontech, Palo Alto, CA), as previously described.10,11 After stable transfection of red fluorescent protein (RFP), gemcitabine-resistant cells (GEM-MiaPaCa-2) were generated.12–14 We have consistently shown that repeated passages of our MiaPaCa-2 cells results in a gemcitabine-resistant cell line, without exposure to gemcitabine.15 We initially conducted an in vitro study to demonstrate that our cell line is resistant to gemcitabine.
Drug sensitivity
The two cell lines, GEM-MiaPaCa-2 and MiaPaCa-2, were subsequently exposed to serial dilutions of gemcitabine, paclitaxel, and Genexol to determine their respective IC50s. Specifically, 500 000 cell/well were plated onto a 96-well plate, allowed to adhere for 24 h and then exposed to fresh drug and media every day for 72 h. The dosages of gemcitabine were 0, 3.9 nM, 7.8 nM, 15.6 nM, 31.25 nM, 62.5 nM, 125 nM, 250 nM, 500 nM and 1000 nM. The serial dosages for paclitaxel (Sigma-Aldrich, St. Louis, MO, USA) and Genexol (Samyang Corp, Daejeon, South Korea) were 0, 0.625 nM, 1.25 nM, 2.5 nM, 5 nM, 10 nM, 20 nM, 40 nM and 80 nM. At 72 h cells were incubated in a 10% alamarBlue solution (Invitrogen, Carisbad, CA, USA) for 2 h at 37°C, and then fluorescence read (FLx800; BioTek, Winooski, VT, USA) according to the manufacturer's instructions. IC50s were then determined using commercially available software (CalcuSyn, Cambridge, UK). All experiments were done in triplicate.
Surgical procedures
Thirty male nude mice between 4 and 6 weeks of age were maintained in a barrier facility equipped with HEPA-filtered racks. All studies were conducted with the approval and guidance of the University of Utah Institutional Animal Care and Use Committee. The orthotopic tumour induction surgery was performed as previously described.10,11 Briefly, 1.5 × 106 RFP expressing GEM-MiaPaCa-2 cells suspended in 150 µl of serum-free media were injected into the tail of the pancreas via a left subcostal incision. The abdomen was closed using 6-0 silk sutures closing both skin and muscle simultaneously. All procedures were done under aseptic conditions.
Imaging
After the primary surgery, high resolution (3456 × 2304 pixels) whole body digital images (EOS Digital Rebel, Canon USA, Lake Success, NY, USA) of each mouse were obtained once a week to monitor primary tumour growth. We have previously showed that the RFP tumour area is linearly correlated with tumour mass.10,11 The RFP was visualized with an Illumatool Bright Light System that consisted of a 563-nm excitation filter and a 587-nm viewing filter (Model LT-9900, LightTools Research, Encinitas, CA, USA). Animals were imaged under nose-cone induced isoflurane general anaesthesia. Primary tumour area was quantified using public domain software (National Institutes of Health ImageJ; http://rsb.info.nih.gov/ij/).
Treatment
At 4 weeks post-tumour induction 27 of the 30 animals had confirmed tumours, as determined by RFP imaging. Animals with tumours were randomly assigned to one of four groups: Control (Saline), Gemcitabine (3.5 mg/200 µl/animal), Genexol (0.5 mg/200 µl/animal) and Gemcitabine+Genexol (3.5 mg/200 µl/animal and 0.5 mg/200 µl/animal, respectively). The animals were treated twice a week for 2 weeks, followed by 2 weeks of no treatment, and then subsequently retreated twice a week for 2 weeks (Fig. 1). Tumour size was monitored weekly and when the tumour reached roughly 2 cm in diameter the animal was necropsied.
Figure 1.
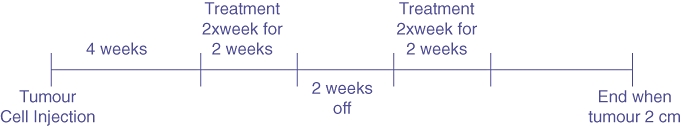
Experimental timeline. Animals were treated twice, both for two times a week for 2 weeks. The endpoint was defined as when the tumour reached 2 cm in diameter as measured with red fluorescent protein (RFP) imaging
At necropsy, after in vivo imaging, the primary tumour was removed and weighed. Additionally, all metastases were located and sites of metastases noted, as well as a sample removed. All removed tumours and metastases were fixed in 10%formalin, embedded in paraffin and stained for histological analysis. RFP imaging was utilized to confirm the presence and location of any metastasis.
Statistics
Group differences for final tumour mass, survival, number of metastatic sites and percent reduction in tumour size were compared utilizing an ANOVA followed by Tukey's post-hoc test. Weekly tumour size as determined by RFP imaging was compared between the groups with a repeated measure ANOVA followed by a post-hoc test. A P-value of <0.05 was considered significant.
Results
In vitro IC50s
As expected the IC50 for GEM-MiaPaCa-2 (331 ± 42 nM) was considerably higher, 10-fold, than our original wild-type MiaPaCa-2 cell lines (30 ± 2 nM) (Table 1). The IC50 for paclitaxel was significantly less than gemcitabine and Genexol, with no differences between the two cell lines. Additionally, the IC50 for Genexol was less than half the dose of gemcitabine, 13 nM compared with 30 nM. Thus, both cell lines appear to be equally sensitive to paclitaxel and Genexol and the IC50 dose for paclitaxel and Genexol is smaller than gemcitabine.
Table 1.
In vitro IC50s* for three different drug formulations
| Cell lines | Gemcitabine | Taxol | Genexol |
|---|---|---|---|
| MiaPaCa2 | 29.6 ± 1.7 | 5.5 ± 0.8 | 13.7 ± 3.8 |
| GEM-MiaPaCa2 | 330.5 ± 42.2 | 4.5 ± 0.8 | 13.1 ± 4.2 |
All dosages in nM.
In vivo weekly tumour area
There were no differences between the four groups in terms of initial tumour area as measured by RFP imaging (Table 2). However, at the end of the first 2 weeks of treatment there were marked differences in tumour RFP area between the groups (Fig. 2). Specifically, at week three both the Control (429 ± 113 mm2) and Gemcitabine (354 ± 76 mm2) groups had significantly larger tumours than the Genexol (44 ± 47 mm2) and Gemcitabine+Genexol (34 ± 49 mm2) groups. The size of the Control and Gemcitabine groups' tumours had increased 57 ± 17% and 57 ± 9% relative to their starting areas by week three, respectively. While the tumours in the Genexol group had shrunk by 64 ± 38% and the Gemcitabine + Genexol had decreased by 84 ± 17%, all of the tumours in the Control and Gemcitabine groups had reached 2 cm at 4 weeks, thus the animals in these two groups did not receive the second round of treatment. Although the Genexol-treated groups manifested significant tumour regression during the first 2 weeks of treatment, the second 2-week treatment did not cause regression of residual tumours (Fig. 2). By week 7 the tumours in both the Genexol and Gemcitabine+Genexol groups were increasing in size. There were no differences in terms of absolute, relative or per cent change in tumour area between the Genexol and Gemcitabine+Genexol groups at anytime point.
Table 2.
In vivo experimental endpoints for the different treatment regimens
| Group | Initial tumour area (mm^2) | Final tumour area (mm^2) | Final tumour mass (g) | Survival (days) | Per cent metastasize |
|---|---|---|---|---|---|
| Control | 148 ± 59 | 367 ± 89 | 4.0 ± 1.4 | 43 ± 5* | 100* |
| Gemcitabine | 151 ± 50 | 364 ± 82 | 3.4 ± 1.2 | 46 ± 4* | 100* |
| Genexol | 137 ± 50 | 317 ± 101 | 3.8 ± 1.5 | 107 ± 32 | 71 |
| Gemcitabine+Genexol | 175 ± 64 | 367 ± 43 | 3.9 ± 1.1 | 103 ± 15 | 84 |
Statistically different from Genexol and Gemcitabine+Genexol; P < 0.05.
Figure 2.
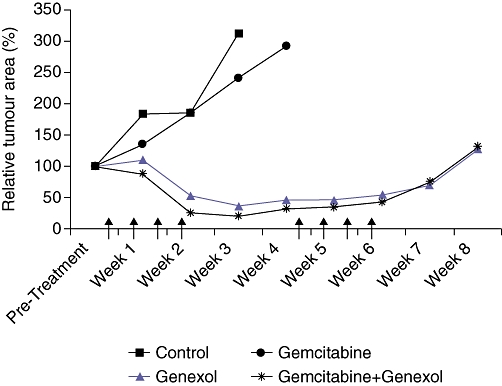
Graph of relative tumour area by week for the four treatment groups. The Control and Gemcitabine groups had statistically larger tumours by 2 weeks after the start of treatment. The Genexol and Gemcitabine+Genexol groups had dramatic decreases in tumour area during the first set of treatments, but tumours appeared to remain the same size during the second set of treatments. Arrows indicate when the animals were treated
Survival
There was a clear difference in the survival of the Control (43 ± 5 days) and Gemcitabine (46 ± 4 days) groups compared with the Genexol (107 ± 32 days) and Gemcitabine+Genexol groups (103 ± 15 days) (Table 2; Fig. 3). Four weeks after the initiation of treatment all of the tumours in the Control and Gemcitabine groups had reached 2 cm. The Genexol and Gemcitabine+Genexol groups had a mean survival of 15 weeks (Table 2).
Figure 3.
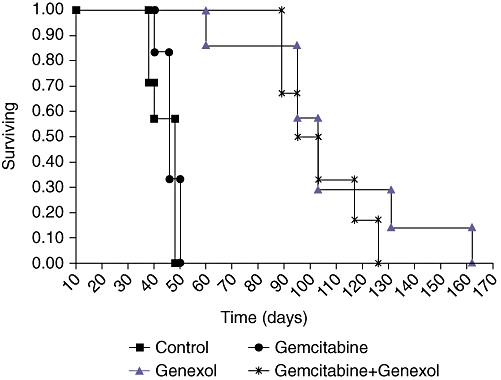
Survival graph for the four treatment groups. The Genexol and Gemcitabine+Genexol groups lived longer on average than the Control and Gemcitabine treatment groups
Tumour burden
The final tumour area as measured with RFP and the final tumour mass did not differ between the four groups. In terms of metastases, 100% of the Control and Gemcitabine animals had metastases at necropsy, compared with 71% of the Genexol and 84% of the Gemcitabine+Genexol. The most frequent sites of metastases, irrespective of group, were the mesentery, diaphragm, liver and kidney. The Control (3.7 ± 1) and Gemcitabine (3.8 ± 0.4) groups had statistically more sites of metastases compared with the Genexol (1.6 ± 1.3) and Gemcitabine+Genexol (2.2 ± 1.5) groups. Roughly 70% of the animals in the Control, Gemcitabine and Gemcitabine+Genexol groups had ascites at necropsy, whereas only 42% of the animals in the Genexol groups had ascites.
Discussion
We have shown in the present study that Genexol is a potential drug to treat gemcitabine-resistant pancreatic adenocarcinoma. Initially we confirmed that our GEM-MiaPaCa-2 cells were resistant to gemcitabine, as GEM-MiaPaCa-2 cells had an IC50 that was ten times greater than our original MiaPaCa-2 cells. Next, we demonstrated equivalent IC50s for Genexol in the two cell lines. To confirm our in vitro data we compared gemcitabine, Genexol and the two in combination as a treatment in an orthotopic animal model of pancreatic cancer. There were dramatic differences in the response to treatment with a per cent reduction in tumour size, as measured by RFP, number of metastases and survival all showing more positive results with Genexol treatment.
These results are important, as gemcitabine resistance is a major problem in the treatment of pancreatic cancer. Less than 10% of pancreatic adenocarcinoma patients undergoing gemcitabine treatment show a partial response.4 As gemcitabine is currently the gold standard of care, resistance is a dramatic problem. There are several mechanisms by which pancreatic cancer can acquire resistance to gemcitabine. Numerous studies have shown a positive correlation between hENT1, the main nuclear transporter that brings gemcitabine into the cell, and survival, as well as gemcitabine sensitivity.16–18 Other mediators of gemcitabine resistance include deoxycytidine kinase and ribonucleotide reductase.19,20 Reductions in the function of these kinases results in an increase in gemcitabine resistance, as both are important components in the metabolism of gemcitabine. Alhough the elucidation of the mechanism of gemcitabine resistance is beyond the scope of the present study, we show that cells resistant to gemcitabine are sensitive to Genexol, both in vitro and in vivo. Thus, Genexol maybe an important second line of defence in the treatment of pancreatic cancer, as the animals treated with Genexol survived on average three times as long as those treated with gemcitabine.
The mechanism of action of gemcitabine differs from that of Genexol and paclitaxel. Gemcitabine acts by stabilizing DNA.3 In contrast, Genexol and paclitaxel stabilize microtubules.6,21 Thus, Gemcitabine acts at the genetic level by preventing the replication of DNA, where as Genexol and paclitaxel physically prevent the cells from dividing by stabilizing the microtubules. Genexol may be advantageous to use as a treatment regimen compared with paclitaxel. The micelle formulation of Genexol alleviates the need to use Cremophor EL, thus reducing the cytotoxicity of the drug.6 Paclitaxel has poor water solubility, so Cremophor EL is required to increase the solubility of the drug to allow for systemic delivery. However, Cremophor EL is also associated with toxicity.22 Genexol has a larger maximum tolerated dose than paclitaxel, probably the result of the different formulations.6
Given these benefits of Genexol there have been several clinical trials evaluating the efficacy of Genexol. In the Phase II trial for metastatic breast cancer 24 of the 41 patients (59.5%) were either complete or partial responders, with a median time to progression of 9 months. A Phase II trial for patients with advanced non-small-cell lung caner, reported a response rate of 37.7%.5 The clinical trials suggest that Genexol maybe a beneficial chemotherapy agent for the treatment of PDA, as such a Phase II trial has recently been published. Patients with metastatic or locally invasive PDA were treated with Genexol at a dose of 300 mg/m2 every 3 weeks.9 The disease control rate was 65%, with a medial overall survival of 6.5 months. The present investigation suggests the Genexol could also be a useful treatment option for PDA patients that are insensitive to gemcitabine.
Conclusion
Gemcitabine resistance is a major obstacle in the treatment of PDA. Genexol treatment resulted in a reduction in tumour mass, as measured by RFP, a reduction in the incidence of metastases and increased survival in a gemcitabine-resistant orthotopic animal model of PDA. Clinical trials for the treatment of metastatic breast cancer and non-small-cell lung cancer, accompanied by the data presented here suggest that Genexol could be a good second line of defence against pancreatic cancer, specifically for patients resistant to gemcitabine.
Acknowledgments
We would like to thank Patti Larrabee for her assistance with data collection. This manuscript was supported in part by grants from NIH (R01 EB1033) and the Director's Translational Research Initiative at Huntsman Cancer Institute, University of Utah.
Conflicts of interest
None declared.
References
- 1.Cancer Statistics. American cancer society. 2005.
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V. Gemcitabine: progress in the treatment of pancreatic cancer. Oncology. 2001;60:8–18. doi: 10.1159/000055290. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 6.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 8.Kim SO, Lee YH, Jeong SW, Jung YA, Kang SY, Kumar S, et al. Superior antitumor eficacy of Genexol-PM, a biodegradable polymeric micelle-based formulation of paclitaxel compared with Gemzar (gemcitabine) and Taxol in human pancreatic cancer cell in vitro and in vivo. Proc Am Assoc Cancer Res. 2005;46 Abstract 1440. [Google Scholar]
- 9.Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, et al. Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest. 2010;28:186–194. doi: 10.3109/07357900903179591. [DOI] [PubMed] [Google Scholar]
- 10.Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1074–1080. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- 11.Torgenson MJ, Shea JE, Firpo MA, Dai Q, Mulvihill SJ, Scaife CL. Natural history of pancreatic cancer recurrence following ‘curative’ resection in athymic mice. J Surg Res. 2008;149:57–61. doi: 10.1016/j.jss.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Nakai Y, Otsuka M, Hoshida Y, Tada M, Komatsu Y, Kawabe T, et al. Identifying genes with differential expression in gemcitabine-resistant pancreatic cancer cells using comprehensive transcriptome analysis. Oncol Rep. 2005;14:1263–1267. [PubMed] [Google Scholar]
- 13.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 14.Togawa A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Shimamura F, et al. Establishment of gemcitabine-resistant human pancreatic cancer cells and effect of brefeldin-a on the resistant cell line. Pancreas. 2003;27:220–224. doi: 10.1097/00006676-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jennings CS, Coursen JD, Dai Q, Firpo MA, Mulvihill SJ. Spontaneous development of gemcitabine resistance in pancreatic cancer cell lines. HPB. 2010;12:1–468. [Google Scholar]
- 16.Garcia-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clin Cancer Res. 2003;9:5000–5008. [PubMed] [Google Scholar]
- 17.Marechal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, et al. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913–2919. doi: 10.1158/1078-0432.CCR-08-2080. [DOI] [PubMed] [Google Scholar]
- 18.Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, et al. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 19.Nakahira S, Nakamori S, Tsujie M, Takahashi Y, Okami J, Yoshioka S, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 20.Sebastiani V, Ricci F, Rubio-Viqueira B, Kulesza P, Yeo CJ, Hidalgo M, et al. Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res. 2006;12:2492–2497. doi: 10.1158/1078-0432.CCR-05-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5(Suppl. 6):S3–S6. [PubMed] [Google Scholar]
- 22.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]


