Abstract
Objectives
An aberrant right hepatic artery (aRHA) is the most frequently encountered vascular anomaly during pancreatoduodenectomy (PD). This study was performed to investigate the incidence of aRHA in a large series of PDs and to explore its relationship with complications and survival.
Methods
In a consecutive series of 790 PDs, aRHA could be identified or ruled out in 758 patients by reviewing operation reports. Patients with and without aRHA were compared. Main outcome measures were complications and survival (only in patients with a malignancy).
Results
The aRHA group consisted of 143 patients (19%). Characteristics of patients in the aRHA and normal RHA groups were comparable. There were no differences in surgical complications. The aRHA was preserved without damage in 130 patients (91%). Two patients in whom the aRHA was either sacrificed or damaged suffered complications (haemorrhage and intra-abdominal abscess in the right upper quadrant) that may have been related. Longterm survival in patients with a malignancy and an aRHA was not compromised.
Conclusions
An aRHA is frequently encountered during PD. Preservation is generally feasible without compromising survival in patients with malignant tumours. Surgical morbidity is not higher in patients with an aRHA. Preservation is technically possible in most patients and does not negatively impact on outcomes.
Keywords: pancreatic neoplasia, adenocarcinoma, periampullary tumours, resection, outcomes, vascular anomalies
Introduction
Pancreatoduodenectomy (PD) represents the only chance for cure in patients with a pancreatic or periampullary tumour and is therefore the procedure of choice.1 It is a complex surgical procedure that is associated with high morbidity rates of up to 50%. Some of the most feared postoperative complications are anastomotic leakage at the site of pancreaticojejunostomy or hepaticojejunostomy, and post-pancreatectomy haemorrhage.2–6
Morbidity after PD may be even higher in the presence of aberrant hepatic arterial supply, which is reported to occur in up to 49% of patients.7–9 Anatomic variations in the hepatic arterial supply increase the risk of complications through several mechanisms. There is a higher risk of intraoperative vascular injury, especially when aberrant arteries are encountered unexpectedly or are not recognized promptly.10 Damage or ligation of an aberrant artery may induce bile duct or liver ischaemia, which can lead to breakdown of the bilioenteric anastomosis and liver dysfunction or abscesses.11 Excessive manipulation while trying to preserve an aberrant artery may result in damage to the vessel's adventitia and thus make it more prone to the formation of pseudoaneurysms, especially in the presence of pancreaticojejunostomy leakage.12 This implies a higher risk for life-threatening bleeding complications. Attempts to preserve aberrant vessels may also hinder radical oncological resections.
Normal hepatic arterial supply involves a common hepatic artery (CHA) arising from the coeliac trunk (Fig. 1). The section of artery subsequent to the branching off of the gastroduodenal and right gastric arteries is referred to as the proper hepatic artery; this bifurcates into the right and left hepatic arteries (RHA and LHA).
Figure 1.
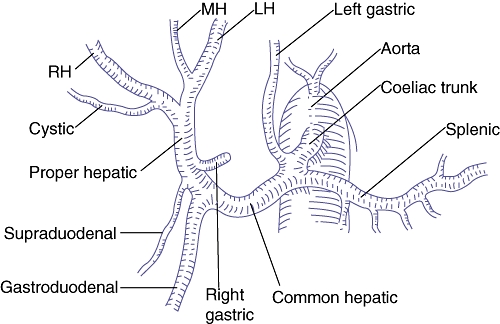
Normal hepatic arterial anatomy. RH, right hepatic artery; MH, middle hepatic artery; LH, left hepatic artery. (Source: Blumgart et al.25)
The two most widely accepted classifications of hepatic arterial variations are those by Michels, based on 200 autopsies, and Hiatt, based on 1000 angiographic analyses (Table 1).7,9 In both series, the most common reported vascular anomaly is an aberrant RHA (aRHA) (Fig. 2). Michels makes a distinction between a replaced and accessory aRHA. A replaced RHA (rRHA) arising from the superior mesenteric artery (SMA) is classified as Michels type III. It may course posterior to the pancreas, as well as within the pancreatic parenchyma or along the ventral side of the pancreas. Reported incidences vary from 8% to 14%.8,9,13,14 An accessory RHA (accRHA) follows the same course as a rRHA, in addition to a normal RHA. It is classified as Michels type VI and is reported to occur in up to 7% of patients.8,9 In the Hiatt classification, both rRHA and accRHA are classified as type III and reported incidences vary from 7% to 21%.7,15–17
Table 1.
Overview of the Michels and Hiatt classifications of hepatic artery types
| Description | Michels type | Hiatt type |
|---|---|---|
| Normal anatomy | I | I |
| Replaced LHA from LGA | II | II |
| Replaced RHA from SMA | III | III |
| Replaced RHA + LHA | IV | IV |
| Accessory LHA | V | II |
| Accessory RHA | VI | III |
| Accessory RHA + LHA | VII | IV |
| Replaced RHA + accLHA or replaced LHA + accRHA | VIII | IV |
| CHA from SMA | IX | V |
| CHA from LGA | X | – |
| CHA from aorta | – | VI |
LHA, left hepatic artery; LGA, left gastric artery; RHA, right hepatic artery; SMA, superior mesenteric artery; acc, accessory; CHA, common hepatic artery
Figure 2.
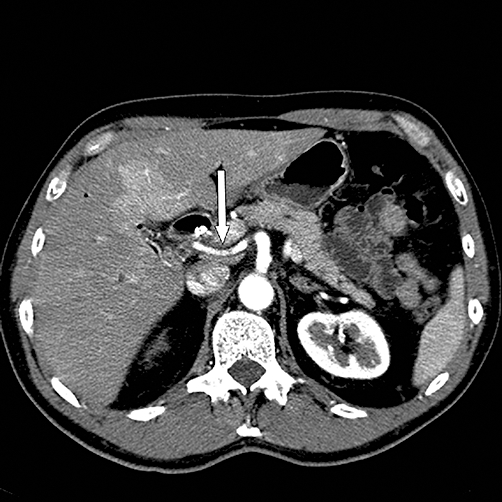
Computed tomography scan showing a replaced right hepatic artery (white arrow) arising from the superior mesenteric artery
An aRHA represents the vascular anomaly encountered most frequently during PD and, because of its course, is the hepatic arterial anomaly that is most susceptible to intraoperative damage and tumour involvement.14
Therefore, the aim of the present study was to investigate the incidence of aRHA in a large cohort of patients undergoing PD and to explore its relationships with the occurrence of complications and with longterm survival in patients with a malignancy.
Materials and methods
Patients and study outline
In a consecutive series of 790 PDs performed from 1992 to 2009, clinicopathological data, perioperative outcomes and longterm follow-up were prospectively recorded. Patients with an aRHA were identified by reviewing operation reports. In cases of doubt, preoperative computed tomography scans were reviewed to determine aberrant or normal RHA anatomy. The present study involved a retrospective analysis of anonymized data, for which Dutch ethical review board regulations do not require informed consent.
It was possible to reliably identify or rule out an aRHA in 758 patients. According to these findings, patients were divided into aberrant (n= 143, 19%) and normal (n= 615, 81%) RHA groups.
Outcome measures
For each study group, the following surgical complications were analysed: delayed gastric emptying; pancreaticojejunostomy leakage and post-pancreatectomy haemorrhage (each according to its consensus definition by the International Study Group of Pancreatic Surgery, grades B or C); hepaticojejunostomy leakage; primary intra-abdominal abscess; wound infection, and other surgical complications.18–20 Other short-term outcome measures were re-laparotomy, hospital mortality and length of hospital stay. In patients undergoing surgery for a malignancy, survival time was an additional outcome measure.
Surgical procedure
The standard surgical procedure was a pylorus-preserving PD. A classic Whipple procedure was reserved for patients with tumour ingrowth in the pylorus or duodenum. The standard procedure has been described earlier.21 In short, after resectability had been assessed and an extensive Kocher manoeuvre to evaluate local tumour ingrowth in the vena cava, aorta or SMA had been carried out, the hepatoduodenal ligament was explored. This is the surgical step during which an aRHA was usually encountered. Upon discovery, the aRHA was generally followed caudally in the direction of its origin. However, it was not always followed to its origin if there was clearly no tumour involvement of the aRHA.
In cases of limited tumour ingrowth in the portal or superior mesenteric vein, a segmental or wedge resection was carried out.22 Reconstruction was performed by retrocolic hepaticojejunostomy and pancreaticojejunostomy and retrocolic or antecolic duodenojejunostomy, without Roux-en-Y reconstruction. One silicone drain was left in the foramen of Winslow near the hepaticojejunostomy and pancreaticojejunostomy.21 A feeding jejunostomy procedure was standard until 2000, since when it has been performed only for indications of severe weight loss or malnutrition.23 Octreotide was routinely administered subcutaneously until 2002, since when it has been administered only in cases of soft pancreas or non-dilated pancreatic duct.
Statistical analysis
Depending on the data distribution, results are reported as mean ± standard deviation (SD) or median with interquartile range (IQR). Independent samples t-test (for normally distributed data) and Mann–Whitney U-test (for abnormally distributed data) were used to compare continuous variables between the study groups. The chi-squared test was used for categorical data. Kaplan–Meier estimates of survival were obtained in patients undergoing surgery for a malignancy. Overall survival was compared between the normal and aberrant RHA groups, using log-rank test statistics.
P-values of <0.05 were considered statistically significant. All analyses were performed in spss Version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient and operation characteristics
Characteristics of patients in the aberrant and normal RHA groups are summarized in Table 2. There were no significant differences in terms of age, sex, American Society of Anesthesiologists class or co-morbidity. The pathological entity for which PD was performed did not differ between the two groups (Table 3). In both groups, 83% of patients were operated for a malignant lesion.
Table 2.
Characteristics of patients with and without aberrant right hepatic artery
| Patient characteristic | Aberrant RHA (n= 143) | Normal RHA (n= 615) | P-value | ||
|---|---|---|---|---|---|
| Mean age (SD), years | 60.7 | (11.4) | 62.3 | (11.0) | 0.14 |
| Males, n (%) | 79 | (55) | 344 | (56) | 0.88 |
| ASA classification, n (%) | |||||
| I | 35 | (25) | 126 | (21) | 0.30 |
| II | 87 | (61) | 372 | (61) | |
| III/IV | 20 | (14) | 116 | (19) | |
| Co-morbidity, n (%) | |||||
| Cardiac | 25 | (18) | 133 | (22) | 0.26 |
| Pulmonary | 11 | (8) | 78 | (13) | 0.09 |
| Diabetes mellitus | 18 | (13) | 95 | (16) | 0.38 |
| Hypertension | 28 | (20) | 129 | (21) | 0.70 |
RHA, right hepatic artery; SD, standard deviation; ASA, American Society of Anesthesiologists
Table 3.
Underlying disease in patients with and without aberrant right hepatic artery undergoing pancreatoduodenectomy
| Aberrant RHA (n= 143) | Normal RHA (n= 615) | P-value | |||
|---|---|---|---|---|---|
| Malignant disease, n (%) | 119 | (83) | 508 | (83) | 0.86 |
| Pathological entity, n (%) | |||||
| Pancreatic adenocarcinoma | 55 | (39) | 216 | (35) | 0.38 |
| Ampullary adenocarcinoma | 33 | (23) | 144 | (23) | |
| Distal common bile duct adenocarcinoma | 22 | (15) | 76 | (12) | |
| Duodenal carcinoma | 0 | (0) | 17 | (3) | |
| Other (pre)malignant disease | 14 | (10) | 66 | (11) | |
| Chronic pancreatitis | 13 | (9) | 55 | (9) | |
| Other benign disease | 6 | (4) | 41 | (7) | |
RHA, right hepatic artery
Operation variables did not differ between the two groups (Table 4). In eight patients (6%) with aRHA, the aberrant vessel was willingly sacrificed, for oncological (n= 6) or technical (n= 2) reasons. In one of these patients, reconstruction was considered, but was not performed after intraoperative Doppler ultrasonography showed normal antegrade arterial liver perfusion. In five patients (3%) the aberrant vessel was accidentally damaged (n= 2) or ligated (n= 3). This occurred mainly during bile duct dissection. The two damaged aRHAs were repaired by primary closure with prolene 6–0. Of the three accidentally ligated aRHAs, one was reconstructed by end-to-end anastomosis.
Table 4.
Surgical variables in patients with and without aberrant right hepatic artery
| Aberrant RHA (n= 143) | Normal RHA (n= 615) | P-value | |||
|---|---|---|---|---|---|
| Operation | |||||
| Pylorus preserved, n (%) | 128 | (90) | 535 | (87) | 0.41 |
| Mean duration of operation (SD), min | 299 | (91) | 300 | (86) | 0.96 |
| Median estimated blood loss (IQR), mla | 1100 | (963) | 1050 | (1200) | 0.88 |
| Aberrant right hepatic artery handling | |||||
| Preserved, n (%) | 130 | (91) | – | ||
| Sacrificed, n (%) | 8 | (6) | – | ||
| Accidentally damaged or ligated, n (%) | 5 | (3) | – | ||
Calculated in 99 patients with aberrant RHA and 398 patients with normal RHA
RHA, right hepatic artery; SD, standard deviation; IQR, interquartile range
Right hepatic arterial variations
Table 5 provides an overview of the RHA variations encountered in the aRHA group. The most common variations were rRHA and accRHA (Hiatt type III). In 45 of 127 (35%) patients with a Hiatt type III aRHA, whether the RHA was replaced or accessory could not be distinguished with certainty.
Table 5.
Right hepatic arterial variations observed in 758 pancreatoduodenectomies
| Variation | n (%) |
|---|---|
| Aberrant RHA | 127 (17) |
| Replaced RHA | 60 (8) |
| Accessory RHA | 22 (3) |
| Replaced OR accessory RHA | 45 (6) |
| Aberrant RHA and LHA | 9 (1) |
| Replaced RHA and LHA | 4 (1) |
| Accessory RHA and LHA | 4 (1) |
| Replaced OR accessory RHA and LHA | 1 (0) |
| Aberrant CHA | 7 (1) |
| Replaced CHA | 7 (1) |
| Total | 143 (19) |
RHA, right hepatic artery; LHA, left hepatic artery; CHA, common hepatic artery
Nine patients (1%) had both aRHA and aLHA (Hiatt type IV). Seven patients (1%) had a replaced CHA arising from the SMA (Hiatt type V). These were grouped in the aRHA group for the purpose of this analysis.
Short-term outcomes
There was no difference in overall surgical morbidity and no difference in rates of any of the surgical complications between the two groups (Table 6). Incidences of complications that might be related to the presence of an aRHA, such as haemorrhage, hepaticojejunostomy leakage or intra-abdominal abscess, were comparable in both groups. Hospital mortality, re-laparotomy rate and length of hospital stay did not differ between the aberrant and normal RHA groups.
Table 6.
Short-term outcomes after pancreatoduodenectomy in patients with and without aberrant right hepatic artery
| Aberrant RHA (n= 143) | Normal RHA (n= 615) | P-value | |||
|---|---|---|---|---|---|
| Surgical complications, n (%) | 80 | (56) | 303 | (49) | 0.15 |
| Pancreaticojejunostomy leakagea | 18 | (13) | 87 | (14) | 0.63 |
| Delayed gastric emptyinga | 48 | (34) | 193 | (31) | 0.61 |
| Post-pancreatectomy haemorrhagea | 11 | (8) | 44 | (7) | 0.82 |
| Hepaticojejunostomy leakage | 2 | (1) | 21 | (3) | 0.21 |
| Primary intra-abdominal abscess | 7 | (5) | 25 | (4) | 0.66 |
| Wound infection | 16 | (11) | 55 | (9) | 0.41 |
| Other | 18 | (13) | 70 | (12) | 0.71 |
| Re-laparotomy, n (%) | 10 | (7) | 68 | (11) | 0.15 |
| Hospital mortality, n (%) | 2 | (1) | 13 | (2) | 0.58 |
| Median hospital stay (IQR), days | 15 | (11) | 14 | (11) | 0.94 |
International Study Group of Pancreatic Surgery definition, grade B or C
RHA, right hepatic artery; IQR, interquartile range
In the 13 patients in whom the aRHA was either willingly sacrificed or accidentally damaged or ligated, two complications that may be related occurred: one patient suffered a delayed massive haemorrhage, and the other was readmitted with a large intra-abdominal abscess in the right upper quadrant after making an initially good recovery (Table 7). However, there were no differences between the aRHA and normal RHA groups in terms of surgical complications, hospital mortality, re-laparotomy rate or length of hospital stay.
Table 7.
Short-term outcomes after pancreatoduodenectomy in patients with ligated or damaged aberrant vs. normal right hepatic artery
| Ligated or damaged aberrant RHA (n= 13) | Normal RHA (n= 615) | P-value | |||
|---|---|---|---|---|---|
| Surgical complications, n (%) | 7 | (54) | 303 | (49) | 0.74 |
| Pancreaticojejunostomy leakage | 3 | (23) | 87 | (14) | 0.36 |
| Delayed gastric emptying | 4 | (31) | 193 | (31) | 0.96 |
| Post-pancreatectomy haemorrhage | 1 | (8) | 44 | (7) | 0.94 |
| Hepaticojejunostomy leakage | 0 | (0) | 21 | (3) | 0.50 |
| Primary intra-abdominal abscess | 1 | (8) | 25 | (4) | 0.52 |
| Wound infection | 1 | (8) | 55 | (9) | 0.87 |
| Other | 1 | (8) | 70 | (12) | 0.67 |
| Re-laparotomy, n (%) | 3 | (23) | 68 | (11) | 0.18 |
| Hospital mortality, n (%) | 1 | (8) | 13 | (2) | 0.18 |
| Median hospital stay (IQR), days | 12 | (14) | 14 | (11) | 0.68 |
RHA, right hepatic artery; IQR, interquartile range
Longterm survival
Figure 3 shows the Kaplan–Meier survival curves of patients with malignancy, with and without aRHA. Median survival times did not differ significantly between the groups and were 25.7 months in the aRHA group and 29.4 months in the normal RHA group (log-rank test, P= 0.67). If only patients with pancreatic cancer were taken into account, median survival times in the aRHA (n= 55) and normal RHA (n= 215) groups were also similar, at 17.9 months in the aRHA group and 19.1 months in the normal RHA group (log-rank test, P= 0.92).
Figure 3.
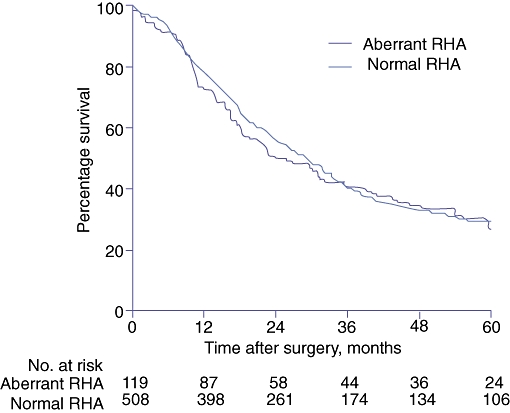
Kaplan–Meier survival curves for 627 patients who underwent pancreatoduodenectomy for a malignant lesion, with (n= 119) and without (n= 508) aberrant right hepatic artery. RHA, right hepatic artery
Discussion
Anatomic variations in the hepatic arterial supply are a common phenomenon. The vascular anomaly encountered most frequently during PD is the aRHA. Preservation of an aRHA during PD may lead to compromised cancer resections, whereas damage or ligation may lead to bleeding complications and liver or bile duct ischaemia and subsequent breakdown of the bilioenteric anastomosis.
In the present study, 18% of patients had an aRHA; an additional 1% had a replaced CHA arising from the SMA. The presence of an aRHA did not lead to more complications and did not influence longterm survival in patients with a malignancy.
The incidence of aRHA in the current series (17% incidence of Hiatt type III variations [either rRHA or accRHA]) accords well with rates reported in previous publications.7,15,17,24 The operation reports did not always distinguish between replaced and accessory anomalies (i.e. Michels type III or VI). However, in the 82 patients whose surgical reports clearly stated whether the aberrant vessel represented a replaced or accessory RHA, the majority (n= 60, 73%) were replaced, as might be expected according to previously published incidences of Michels type III and VI variations.8,9,13 Nine patients (1%) showed Hiatt type IV variations (aRHA and aLHA). In four of these, both aberrant vessels were replaced. This is, to our knowledge, the first surgical study on hepatic arterial variations to describe this variant.12 Seven patients (1%) had a replaced CHA arising from the SMA (Hiatt type V). This incidence is somewhat lower than those reported in previous series.8,9,24
In the vast majority of patients with aRHA, the aberrant vessel could be preserved. In eight patients it was willingly sacrificed, mainly for oncological reasons. Five aRHAs were accidentally damaged (n= 2) or ligated (n= 3), two of which were not reconstructed. Thus, the number of patients in whom the aRHA was permanently lost totalled 10 (7%). Lee et al. described a series of 103 PDs in which 15 aRHAs were encountered and all were preserved.14 Jah et al. found 28 aRHAs in 135 PDs; three of these were divided and one of the three was anastomosed to the gastroduodenal artery stump, leading to two permanently lost aRHAs (7%).15 Turrini et al. reported a series in which two of 47 aRHAs needed reconstruction and one was ligated.17
Morbidity in the aRHA group did not differ from that in the normal RHA group, which is similar to findings in the smaller series by Lee et al.,14 Jah et al.15 and Turrini et al.17 Even in the current series of more than 750 PDs, which included a large aRHA group of 143 patients, no differences were found in rates of surgical complications, not even for those complications that might be expected to occur more frequently in patients with an aRHA, such as hepaticojejunostomy leakage, bleeding complications and intra-abdominal abscess.
Morbidity in the subgroup of patients in whom aRHAs were sacrificed, accidentally damaged or ligated was comparable with that in the non-aRHA group. The numbers in this subgroup were probably too small to detect possible differences in morbidity, although complications that may be related did occur in two patients in whom the aRHA was sacrificed.
Longterm survival was not compromised in patients operated for a malignancy. These results confirm the findings of Lee et al.,14 Jah et al.15 and Turrini et al.17 and show that preservation of aRHA is feasible in the vast majority of patients and does not decrease overall longterm survival in patients with a malignancy.
A limitation of the current study is its retrospective identification of aRHA, which may have led to an underestimation of the incidence of aRHA. It was not possible to distinguish between replaced and accessory variants in a substantial number of reports; this distinction would have been possible if the aRHA had been recorded prospectively. However, a comparison between the incidence of aRHA in the current study and those in the current literature does not suggest an underestimation; we believe an aRHA is such an important intraoperative finding that surgeons will always mention it in their reports. Another limitation of the retrospective collection of aRHA data is that preoperative knowledge of the presence of the aRHA could not be analysed. In the local multidisciplinary hepatopancreatobiliary meeting, aRHAs are usually identified and discussed during the review of preoperative imaging, but the finding is not routinely mentioned in radiologists' imaging reports, a fact also noted by Turrini et al.17
In conclusion, the current study describes the incidence and consequences of aRHA in the largest surgical series to be used for this purpose to date. It is the first surgical report to describe patients with both rRHA and rLHA. It shows that aRHA is a common phenomenon and that preservation of the aRHA is generally feasible and does not compromise longterm survival in patients with a malignancy. Sacrifice or accidental damage or ligation of aRHAs may raise the risk for complications such as liver dysfunction or hepaticojejunostomy leakage, however, the number of patients in this subgroup in this series was too small to detect eventual differences. It is our belief that an aRHA can nearly always be preserved, without negatively impacting surgical outcomes and longterm survival in patients with a malignancy. Given the high incidence of aRHA, it is suggested that radiologists should include hepatic arterial anatomy in their standard preoperative imaging reports.
Conflicts of interest
None declared.
References
- 1.Pancreatic Section of the British Society of Gastroenterology, Pancreatic Society of Great Britain and Ireland, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, Royal College of Pathologists, Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut. 2005;54(Suppl 5):1–16. doi: 10.1136/gut.2004.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high-volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457. doi: 10.1159/000050193. [DOI] [PubMed] [Google Scholar]
- 3.de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117–1123. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- 4.de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Lameris JS, van Gulik TM, et al. Delayed massive haemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg. 2005;241:85–91. doi: 10.1097/01.sla.0000150169.22834.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. 788–790. doi: 10.1097/01.sla.0000188462.00249.36. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamani MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective coeliac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–244. doi: 10.1007/s00276-004-0229-z. [DOI] [PubMed] [Google Scholar]
- 9.Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 10.Volpe CM, Peterson S, Hoover EL, Doerr RJ. Justification for visceral angiography prior to pancreaticoduodenectomy. Am Surg. 1998;64:758–761. [PubMed] [Google Scholar]
- 11.Traverso LW, Freeny PC. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 1989;55:421–426. [PubMed] [Google Scholar]
- 12.Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186–193. doi: 10.1245/s10434-009-0757-1. [DOI] [PubMed] [Google Scholar]
- 13.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Lee YJ, Kim CW, Moon KM, Kim MW. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Surg. 2009;33:1727–1732. doi: 10.1007/s00268-009-0063-x. [DOI] [PubMed] [Google Scholar]
- 15.Jah A, Jamieson N, Huguet E, Praseedom R. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today. 2009;39:669–674. doi: 10.1007/s00595-009-3947-3. [DOI] [PubMed] [Google Scholar]
- 16.Makisalo H, Chaib E, Krokos N, Calne R. Hepatic arterial variations and liver-related diseases of 100 consecutive donors. Transpl Int. 1993;6:325–329. doi: 10.1007/BF00335969. [DOI] [PubMed] [Google Scholar]
- 17.Turrini O, Wiebke EA, Delpero JR, Viret F, Lillemoe KD, Schmidt CM. Preservation of replaced or accessory right hepatic artery during pancreaticoduodenectomy for adenocarcinoma: impact on margin status and survival. J Gastrointest Surg. 2010;14:1813–1819. doi: 10.1007/s11605-010-1272-1. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Post-pancreatectomy haemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Gouma DJ, Nieveen van Dijkum EJ, Obertop H. The standard diagnostic workup and surgical treatment of pancreatic head tumours. Eur J Surg Oncol. 1999;25:113–123. doi: 10.1053/ejso.1998.0612. [DOI] [PubMed] [Google Scholar]
- 22.van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158–163. doi: 10.1067/msy.2001.110221. [DOI] [PubMed] [Google Scholar]
- 23.Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, et al. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567–577. doi: 10.1097/00000658-199710000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemeny MM, Hogan JM, Goldberg DA, Lieu C, Beatty JD, Kokal WA, et al. Continuous hepatic artery infusion with an implantable pump: problems with hepatic artery anomalies. Surgery. 1986;99:501–504. [PubMed] [Google Scholar]
- 25.Blumgart LH. Surgery of the Liver, Biliary Tract and Pancreas, 4th edn. Oxford: Elsevier; 2007. p. 21. [Google Scholar]


