Abstract
Background
Irreversible electroporation (IRE) is a novel, non-thermal form of ablation. We studied the safety and efficacy of IRE for the ablation of liver tissue around the liver hilum. We also studied the ability of triphenyltetrazolium chloride staining (TTC) to predict the zone of ablation after IRE.
Methods
Eight swine underwent 20 ablations of the liver and liver hilum. Two monopolar probes were positioned 2 cm apart. IRE was performed using 90 pulses of 2500–3000 V/cm. IRE treatments were performed from 15 min to 14 days (n= 4) before sacrifice.
Results
All animals survived. No major complications were encountered. Ablation width ranged from 2.27 to 4.45 cm and ablation height ranged from 1.5 to 1.8 cm. TTC staining demonstrated the zone of ablation in all animals. Hepatocyte necrosis occurs immediately adjacent to large central veins without evidence of heat sink. Bile ducts, portal veins and hepatic arteries appear to be more resistant to the effects of IRE.
Conclusions
IRE appears to be safe and effective for liver tissue ablation in the liver hilum. The portal structures appear more resistant to the effects of IRE. TTC staining can predict the zone of IRE ablation as early as 15 min after treatment.
Keywords: liver, tumour, ablation, electroporation
Introduction
Thermal ablation is a proven and effective therapy for the treatment of selected primary and metastatic tumours of the liver. Thermal ablation can be achieved using radiofrequency ablation (RFA) or microwave ablation (MWA). Complete pathological response rates for liver tumours <3 cm in size are as high as 65% with RFA.1,2 Similar results can be achieved with MWA.3
Thermal ablation relies on the ability to heat the target tissue to 60°C for instantaneous cell death. Cell death is less reliable at lower temperatures and requires longer exposure at the target temperature.4 Heat sink represents a major limitation of thermal ablation.5 Heat sink refers to the loss of heat at the tissue level as a result of adjacent circulating blood of a cooler temperature. Tumours adjacent to the hepatic veins, large portal veins and inferior vena cava are at greatest risk for incomplete tumour necrosis and local recurrence because of heat sink. Another limitation of thermal ablation is the risk of collateral damage to healthy, vital structures adjacent to the tumour being ablated.
Irreversible electroporation (IRE) is a novel, non-thermal ablation technology that utilizes short pulses of high frequency electrical energy to ablate tissue.6 IRE is performed by placing electrodes into the tissue and delivering 90 pulses of 1000–3000 V/cm DC energy between the electrodes. Cell death is initiated via the creation of micropores in the cell membrane.
Previous pre-clinical studies in swine liver demonstrate the ability of IRE to ablate tissue immediately adjacent to large venous structures without evidence of heat sink.6,7 Because the location of IRE activity is the cell membrane, acellular structures are preserved.
Unlike thermal ablation, cell death after IRE is not instantaneous. Triphenyltetrazolium chloride (TTC) staining has been shown to predict the area of irreversible myocardial ischaemia before histological evidence of cell death using haematoxylin and eosin (H&E) staining.8
The aim of the present study was to evaluate the feasibility and safety of creating IRE ablations in liver tissue around the liver hilum and to compare liver hilum ablations to intra-hepatic liver ablations. Additionally, we studied the ability of TTC staining to predict the zone of IRE ablation.
Methods
This study was approved by our institutional animal care and use committee.
General anaesthesia was induced in four domestic female swine using glycopyrrolate 0.003 mg/kg intramuscularly (i.m.), telazol 5 mg/kg i.m., xylazine 2 mg/kg i.m. and sodium thiopental 20 mg/kg intravenously (i.v.) as needed. Animals were intubated to facilitate mechanical ventilation. General anaesthesia was maintained with 2–4% isoflurane in oxygen. Pre-emptive analgesia was administered using buprenorphine 0.03 mg/kg i.m. Adequacy of anaesthesia was determined using jaw tone and pedal reflex. Anaesthesia was increased if a response to stimuli was noted. A baseline-evoked motor response was obtained with a nerve stimulator positioned near the ulnar nerve. Animals were monitored intra-operatively using telemetry and pulse-oximetry.
The abdominal wall was prepared using betadine scrub and solution. Before incision, animals received a single dose of cephazolin 40 mg/kg i.v. An 8-cm incision was made in the upper midline and the peritoneal cavity entered. Pancuronium 0.1 mg/kg was administered to achieve reversible paralysis as monitored by an evoked motor response.
A portal triad entering the liver hilum was identified and mono-polar IRE electrodes were positioned within the liver parenchyma, 2 cm apart, on either side of the portal triad. The active portion of the electrodes was exposed for a length of 2 cm. Probe positioning was confirmed by intra-operative ultrasound. Three thousand volts/cm was delivered between the electrodes in 100 microsecond pulses. Ninety pulses were delivered per ablation. The pulses are delivered in groups of ten with an intervening 250 mSec pause which allows the capacitor to recharge according to the manufacturer's treatment software (Nanoknife, Angiodynamics, Queensbury, NY). The polarity of the electrodes was then reversed without repositioning and the treatment was repeated using the same parameters in the same anatomic location with reverse polarity.
A remote segment of the left, lateral liver was then treated using the same technique and parameters in an intra-hepatic location remote from the liver hilum.
The muscle relaxation was reversed using neostigmine 0.044–0.066 mg/kg i.v. and atropine 0.005 mg/kg i.v. Animals were recovered. Post-operative pain control was achieved using a fentanyl transdermal patch 75 mcg/h and tramadol 150 mg by mouth twice daily for 3 days and as needed.
Phlebotomy for liver serum chemistries was performed pre-procedure while under anaesthesia and on post-operative days 2 and 5 for surviving animals.
Animals survived for 2 h (n= 1), 2 days (n= 1) and 14 days (n= 2). Once the target survival time was reached, animals were anaesthetized as previously described and euthanasia performed with pentobarbital 100 mg/kg. A laparotomy was performed and the liver and extra-hepatic portal structures were recovered.
The area of ablation was sectioned at 5-mm intervals for gross evaluation. The 5-mm section in the centre of the ablation cavity was stained with triphenyltetrazolium chloride. The remaining sections were fixed in formalin. Height and width of ablation cavities were measured. Histological analysis was performed after H&E staining of 6–8 micron sections.
An additional four female domestic swine were subjected to general anaesthesia and chemical paralysis as previously described. IRE of the pancreatic head was performed as part of a separate protocol and the results have already been reported.9 Animals were recovered as previously described and survived for 2 h (n= 1), 2 days (n= 1) and 2 weeks (n= 2). Before euthanasia, animals were subject to general anaesthesia as previously described. IRE was performed at three separate sites within the liver parenchyma 1 h, 30 min and 15 min before euthanasia. During this phase of the study the electrodes were positioned 2 cm apart with an exposed electrode length of 2.5 cm. One animal was treated with 3000 V/cm without reverse polarity and the remaining three animals were treated with 2500 V/cm and repeat ablation with reverse polarity as previously described. Animals were then euthanized and the liver and pancreas were recovered for analysis.
Results
All eight swine survived to the designated time. At the time of laparotomy for euthanasia, one animal designated for 2-day survival was noted to have a distended stomach consistent with gastroparesis. This was felt to be more likely related to the laparotomy than to the IRE directly. No other complications were encountered.
Using two mono-polar electrodes as previously described, an elliptical, dumbbell-shaped ablation zone was reliably created (Fig. 1). Tissue within the zone of ablation exhibited features of haemorrhagic necrosis (Fig. 2). Hepatocyte necrosis extended to the margin of large hepatic veins within the zone of ablation without evidence of heat sink (Fig. 1).
Figure 1.
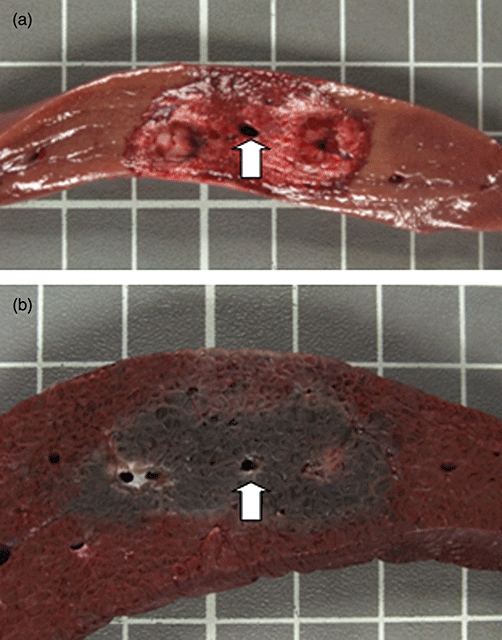
Liver irreversible electroporation (IRE) ablation using two monopolar probes, spaced 2 cm apart with a 2-cm exposure. Ablation was performed using 3000 V/cm and was repeated with reverse polarity. The tissue was harvested 2 h after IRE ablation. (a) Shows fresh tissue and (b) shows the tissue after fixation with triphenyltetrazolium chloride. The area of necrosis extends to the wall of the central vein (arrow) with preservation of the vein and no evidence of heat sink
Figure 2.
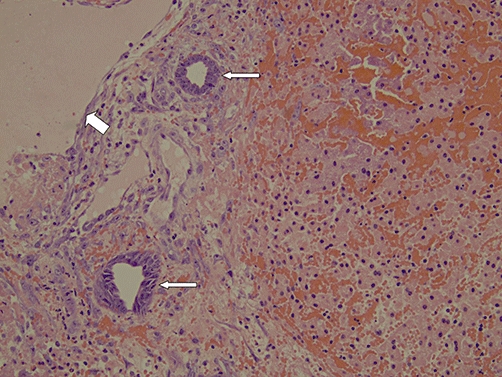
A 20× photomicrograph depicts haemorrhagic necrosis of the hepatocyte lobules after irreversible electroporation (IRE) ablation. Portal structures are more preserved. Preserved bile ducts are highlighted by the narrow arrows and the preserved portal vein is highlighted by the wide arrow
Ablation parameters and size are shown in Table 1. The ablation zone for liver hilum ablations is larger and more irregular than for intra-hepatic ablations using the same treatments parameters (Table 1+Fig. 3a). Large bile ducts, portal veins and hepatic arteries within the portal triads appear more resistant to the effects of IRE (Figs 2, 3b).
Table 1.
Irreversible electroporation (IRE) ablations created with two monopolar probes positioned 2 cm apart in swine liver
| Location | n | Exposurea | Voltage (V/cm) | Reverse polarityb | Ablation zone (cm)c |
|---|---|---|---|---|---|
| Intra-hepatic | 9 | 2.5 cm | 2500 | yes | 2.95 ± 0.31 × 1.5 ± 0.44 |
| Intra-hepatic | 3 | 2.5 cm | 3000 | No | 2.27 ± 0.23 × 1.5 ± 0.2 |
| Intra-hepatic | 4 | 2 cm | 3000 | yes | 3.25 ± 0.35 × 1.45 ± 0.21 |
| Liver hilum | 4 | 2 cm | 3000 | yes | 4.45 ± 0.07 × 1.8 ± 0 |
Exposure refers to the length of the electrode that is active during delivery of the treatment.
Reverse polarity refers to repeating the IRE treatment without repositioning the electrodes but after reversing the polarity of the electrodes.
Width and height of the ablation zones were measured after triphenyltetrazolium chloride staining and fixation.
Figure 3.
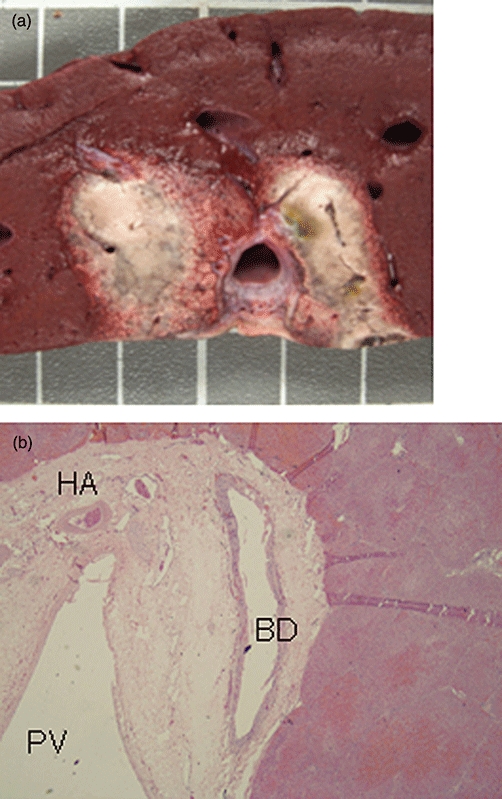
Liver irreversible electroporation (IRE) ablation with monopolar electrodes positioned on either side of the portal triad, 2 cm apart in the liver hilum. Ablation was performed with 3000 V/cm and reverse polarity. The zone of ablation is larger and more irregular when compared with intra-hepatic liver IRE ablations (3a). Hepatocyte necrosis occurs immediately up to the fibrous capsule of the portal triad with histological preservation of the bile duct (BD), hepatic artery (HA) and portal vein (PV) (3b; 4× photomicrograph)
TTC staining can predict the zone of ablation after IRE. The surrounding, viable liver tissue retains the red dye. Tissue that is not viable does not retain the dye, and therefore, does not stain red. The ablation zone is predictable at 15 min after IRE using TTC staining and becomes more apparent by 1 h (Fig. 4).
Figure 4.
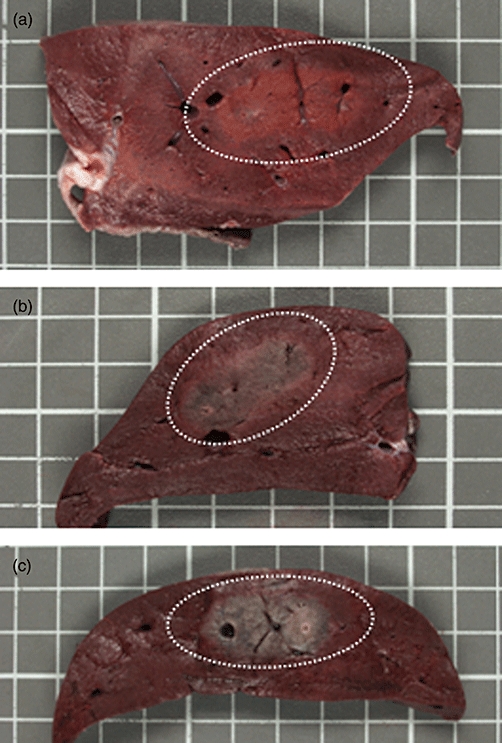
Triphenyltetrazolium chloride (TTC) staining can predict the zone of ablation after irreversible electroporation (IRE). TTC fixed liver tissue is shown 15 min (4a), 30 min (4b) and 1 h (4c) after IRE. The surrounding, viable liver tissue retains the red, triphenyltetrazolium chloride dye while dye washes out of the necrotic, ablated liver over time. The ablation zone is predictable at 15 min and becomes more apparent by 1 h. The zone of ablation is highlighted by the dotted white line
Two weeks after ablation by IRE, the liver is grossly and histologically normal.
Mean total bilirubin was 0.1 mg/dL at baseline and on post-ablation day 2. Mean total bilirubin peaked at 0.25 mg/dL on post-ablation day 5 and normalized to 0.1 mg/dL by post-ablation day 14. No identifiable pattern of change was noted for either aspartate aminotransferase (AST) or alanine transaminase (ALT) on post-ablation days 2, 5 and 14.
Discussion
Our results demonstrate for the first time that IRE can be performed safely in the liver hilum for the ablation of liver tissue around a major portal pedicle without collateral damage to the bile duct, hepatic artery and portal vein and with no evidence of incomplete ablation from heat sink. These findings suggest that IRE may prove to be superior to conventional, thermal ablation (e.g. RFA and MWA) for the treatment of small, unresectable liver tumours abutting a major vascular or portal structure.
The estimated incidence of colorectal cancer was approximately 147 000 cases in the United States in 2009.10 It is estimated that 40% of patients will develop liver metastasis at some time in their disease course. Unfortunately, only about 10–25% of patients with colorectal cancer metastatic to the liver will be candidates for liver resection.11–14
Hepatocellular carcinoma is one of the most common tumours worldwide with an estimated incidence of 564 000 new cases in 2000.15 Unfortunately, only about 10% of patients with hepatocellular carcinoma are candidates for surgical resection at the time of diagnosis.16
Limitations in surgical treatment may be due either to the extent of extra-hepatic disease, the extent of intra-hepatic disease or limited functional hepatic reserve in the planned future liver remnant. Liver tumour ablation has become a highly successful tool in the treatment of unresectable liver tumours. Radiofrequency ablation has a 65% complete pathological response rate for the treatment of small (<3 cm) hepatocellular carcinomas. Results with microwave ablation appear similar.1–3
As with all therapies, thermal ablation of liver tumours has limitations. The first limitation for tumour ablation is tumour size. When tumour size exceeds 3 cm the complete pathological response rate falls to between 10 and 25%.1,2 Recent data suggests that microwave ablation may be more effective for tumours up to 4.5 cm; however, these data have not been corroborated in a prospective trial with pathological correlation.17
Thermal ablation by either microwave or radiofrequency is also limited by collateral damage to surrounding structures. Liver tumours abutting a major portal triad or central bile duct may not be amenable to ablation for this reason.
Finally, heat sink refers to the loss of heat at the tissue level as a result of adjacent circulating blood of a cooler temperature. Tumours abutting a large hepatic vein or the inferior vena cava are particularly vulnerable to local recurrence because of incomplete ablation from heat sink.
Electroporation has been used in a reversible manner to improve the delivery of bleomycin in the treatment of squamous cell carcinoma of the head and neck.18–22 IRE is a novel technique of tissue ablation which utilizes short pulses of high frequency electrical energy to kill cells via the creation of micropores in the lipid bilayer of the cell membrane.
IRE has previously been shown to be an effective means of ablating hepatocytes in swine liver with preservation of the portal structures. IRE can induce hepatocyte necrosis immediately adjacent to large veins within the ablation zone, unaffected by heat sink.6,7
The experience using IRE to treat cancer remains limited. Miller et al. reported the ability of IRE to ablate human hepatocarcinoma cells in vitro. They showed that multiple pulses appeared to be more effective in cancer cell ablation than delivery of the same energy in a single pulse.23 Al-Sekere et al. reported complete regression in 12 of 13 cutaneous tumours implanted in mice using IRE. Their work confirmed improved results with cancer cell death using multiple short pulses of electrical energy.24
We are the first to show that IRE is safe and effective for liver tissue ablation in the liver hilum with a major portal pedicle included within the zone of ablation. The bile duct, portal vein and hepatic artery appear more resistant to the effects of IRE. These findings suggest that IRE may prove to be superior to conventional forms of thermal ablation for the treatment of small, unresectable liver tumours abutting a major portal or vascular structure. Additional studies will be needed to test this theory.
While the effects of thermal ablation are evident immediately after treatment, the mechanism of cell death after irreversible electroporation is a slower process. Gross inspection of liver tissue immediately after IRE reveals oedema and haemorrhage; however, the zone of ablation is not obvious either grossly or using H&E staining.
TTC staining relies on the ability of viable cells to retain the dye while non-viable cells do not. Fishbein et al. reported the ability of triphenyltetrazolium chloride staining to reliably quantify myocardial necrosis as early as 3 h after coronary artery occlusion before histological changes are clearly diagnostic.8
Our results show that TTC staining accurately predicts the zone of IRE ablation as early as 15 min after IRE treatment. This is an important advance that will open the door for future clinical trials of IRE using an ablation followed by resection study design.
IRE requires the insertion of electrodes into the target tissue for the delivery of the treatment. As with other forms of ablation (e.g. radiofrequency ablation and microwave ablation), bleeding can occur at the puncture site. The high voltage current needed for ablation is unique to IRE. This necessitates reversible chemical paralysis to prevent muscle contractions. Although cardiac arrhythmias have been reported, the risk of arrhythmia may be minimized with cardiac synchronization.25 No IRE-related complications were encountered in our study.
The only published report to date regarding treatment of human tumours in vivo with IRE is from Ball et al. from Australia.26 They reported primarily on the safety and complications encountered while performing 28 IRE ablations in 21 patients. Eight procedures treated renal tumours, 17 liver tumours and 3 lung tumours. In their experience, complications included transient systolic hypertension in all patients, muscular contractions in inadequately paralyzed patients, post-operative pain after 13 procedures (46%), acid-base disturbances after 4 procedures (14%) and pneumothorax related to electrode placement in three procedures (11%). Of greatest significance, ventricular tachycardia occurred during seven procedures (25%). In 4 of these 7 cases the arrhythmia was associated with ‘markedly decreased blood pressure.’ They report that all arrhythmias resolved immediately after cessation of treatment. This study does not include any data regarding tumour response or oncologic outcomes.
In conclusion, IRE appears to be safe and effective for ablation of liver tissue in the liver hilum with preservation of the bile ducts, hepatic arteries and portal veins within the zone of ablation. Triphenyltetrazolium chloride staining accurately predicts the zone of ablation as early as 15 min after treatment with IRE.
Conflict of interest
This study was partially funded by a grant from AngioDynamics, Inc., Queensbury, NY.
References
- 1.Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Dupuy D. Image-guided radiofrequency tumor ablation: challenges and opportunities-Part I. J Vasc Interv Radiol. 2001;12:1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 6.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality-clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 7.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6:287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- 8.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier KP, Wolf F, Resnick M, Noble L, Winn B, Dupuy D. Irreversible electroporation of the pancreas in swine: a pilot study. HPB. 2010;12:348–351. doi: 10.1111/j.1477-2574.2010.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975-2006/index.html, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 11.Scheele J, Stangl R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastasis. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 12.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Okuno A, et al. Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am J Surg. 1999;177:294–298. doi: 10.1016/s0002-9610(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 14.Boring CC, Squire TS, Tong T, Montgomery S. Cancer statistics. CA Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Di Bisceglie AM. Epidemiology and clinical presentation of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S169–S171. doi: 10.1016/s1051-0443(07)61783-7. [DOI] [PubMed] [Google Scholar]
- 17.Wolf FJ, Beland MD, Grand DJ, Iannitti D, Hass RA, Charpentier KP, et al. Microwave Ablation of Hepatic Malignancies: Effectiveness and Safety in 70 Patients. Chicago, IL: Radiology Society of North America; 2009. [Google Scholar]
- 18.Mir LM, Orlowski S, Belehradek J, Paoletti C. Electrochemotherapy potentiation of antitumour effects of bleomycin by local electrical pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 19.Panje WR, Hier MP, Garman GR, Harrell E, Goldman A, Bloch I. Electroporation therapy of head and neck cancer. Ann Otol Rhinol Laryngol. 1998;107:779–785. doi: 10.1177/000348949810700908. [DOI] [PubMed] [Google Scholar]
- 20.Allegretti JP, Panje WR. Electroporation therapy for head and neck cancer including carotid artery involvement. Laryngoscope. 2001;111:52–56. doi: 10.1097/00005537-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Rabussay DP, Nanda GS, Goldfarb PM. Enhancing the effectiveness of drug-based cancer therapy by electroporation (electropermeabilization) Technol Cancer Res Treat. 2002;1:71–82. doi: 10.1177/153303460200100110. [DOI] [PubMed] [Google Scholar]
- 22.Bloom DC, Goldfarb PM. The role of intratumour therapy with electroporation and bleomycin in the management of advanced Squamous cell carcinoma of the head and neck. Eur J Surg Oncol. 2005;31:1029–1035. doi: 10.1016/j.ejso.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4:699–705. doi: 10.1177/153303460500400615. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sakere B, Andre F, Bernat C, Connault E, Opolon P, Davalos R, et al. Tumor ablation with irreversible electroporation. PLoS ONE. 2007;11:1–8. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mali B, Jarm T, Corovic S, Paulin-Kosir MS, Cemazar M, Sersa G, et al. The effect of electroporation pulses on functioning of the heart. Med Biol Eng Comput. 2008;46:745–757. doi: 10.1007/s11517-008-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in ‘out of operating theater’ anesthesia. Anesth Analg. 2010;110:1305–1309. doi: 10.1213/ANE.0b013e3181d27b30. [DOI] [PubMed] [Google Scholar]


