Abstract
Background
Infected necrotizing pancreatitis is a major burden for both the patient and the health care system. Little is known about how hospital costs break down and how they may have shifted with the increasing use of minimally invasive techniques. The aim of this study was to analyse inpatient hospital costs associated with pancreatic necrosectomy.
Methods
A prospective database was used to identify all patients who underwent an intervention for necrotizing pancreatitis. Costs of treatment were calculated using detailed information from the Decision Support Department. Costs for open and minimally invasive surgical modalities were compared.
Results
Twelve open and 13 minimally invasive necrosectomies were performed in a cohort of 577 patients presenting over a 50-month period. One patient in each group died in hospital. Overall median stay was 3.8 days in the intensive care unit (ICU) and 44 days on the ward. The median overall treatment cost was US$56 674. The median largest contributors to this total were ward (26.3%), surgical personnel (22.3%) and ICU (17.0%) costs. These did not differ statistically between the two treatment modalities.
Conclusions
Pancreatic necrosectomy uses considerable health care resources. Minimally invasive techniques have not been shown to reduce costs. Any intervention that can reduce the length of hospital and, in particular, ICU stay by reducing the incidence of organ failure or by preventing secondary infection is likely to be cost-effective.
Keywords: surgery, pancreas, necrosectomy, retroperitoneal
Introduction
Acute pancreatitis has an incidence in the Western world of 40 cases per 100 000 population and would appear to be increasing.1,2 Approximately 20% of patients will develop severe disease, which, in 30–70% of cases, will require surgical intervention.3 Such an intervention is a life-changing event for most patients. Recent contemporary series have shown the median hospital stay for patients undergoing pancreatic necrosectomy is 3 months and mortality is in the order of 7–40%.4–7 Thus, necrotizing pancreatitis results in a significant health burden for the individual patient, the health care system and society as a whole.
There has been an increasing trend towards delaying surgical intervention. In addition, several minimally invasive techniques4,8,9 have been described in an effort to reduce the mortality rates associated with this condition. Although good evidence exists for the claim that late intervention is associated with lower mortality,10,11 no similar evidence yet exists to guide the optimal technique. A recent – and so far only – randomized controlled trial (the PANTER trial) has shown that a step-up approach via drainage to minimally invasive surgery reduced the amount of new onset organ failure and prevented the development of endocrine and exocrine pancreas insufficiency at 6 months follow-up in comparison with the open-surgery approach.5 However, the PANTER trial was neither designed to distinguish between different surgical techniques, nor powered to find a difference in mortality rates.
Previous patient series have indicated that minimally invasive techniques require more operations and a longer hospital stay following the intervention, yet seem to reduce the need for intensive care unit (ICU) usage.6,12 Thus, the effect of these techniques on overall cost is unclear. Other than the PANTER trial, no cost analysis of minimally invasive surgery has yet been performed and no confirmation of the trial's finding of a cost benefit for drainage or minimally invasive surgery5 can be found in the literature. The aim of this study was therefore to analyse the breakdown of the in-hospital costs associated with pancreatic necrosectomy.
Materials and methods
Since October 2005, an evidence-based pancreatitis pathway has been used in our institution for all patients admitted with acute pancreatitis.13 A prospective database records demographics, severity of illness, interventions and outcome for audit and quality assurance purposes. All patients who underwent pancreatic necrosectomy during the study period were subsequently identified using this database.
Our procedures for predicting the severity of pancreatitis and developing a treatment plan are undertaken according to the 2005 UK Guidelines for the Management of Acute Pancreatitis.2 The only exception is that we use antibiotics only in the presence of proven infection. Severe pancreatitis is defined according to the Atlanta criteria.14 Indications for necrosectomy are defined according to the International Association of Pancreatology guidelines15 and include the presence of infected necrosis or extensive necrosis in patients who have failed to progress and remain in hospital and unwell at 4 weeks. Infected necrosis was determined by the presence of extraluminal gas within the necrosis (on cross-sectional imaging) or a positive culture at fine needle aspiration (FNA). During the study period, the frequency of FNA decreased from routine use to selective application in patients with persistent systemic inflammatory response syndrome (SIRS) at 2 weeks.
When intervention is indicated, the preferred approach is a minimally invasive retroperitoneal pancreatic necrosectomy (MIRP), as previously described.16 Open necrosectomy is performed when MIRP is not possible.
Because New Zealand operates a publicly funded health system, it is impossible to generate direct billing data for an individual. However, fixed costs per diagnostic, intervention, treatment and bed day consumed can be generated and thus a virtual cost per patient can be calculated. Costs were divided into those pertaining to personnel, investigations, treatment and ward stay, respectively.
Personnel costs include the costs of the anaesthetist and surgeon. Both are reimbursed partly at a rate per hour or day and partly by fixed fees. In the New Zealand system, the fixed fees for the anaesthetist may be upgraded for higher ASA (American Society of Anesthesiologists) levels (>3), older patients (>70 years) and operations of longer duration (>3 h). In addition, hospital ICU patients who require ventilation for a procedure performed outside the ICU (radiology or gastroenterology) will be accompanied by the anaesthetist, for which a fixed fee applies. Surgical personnel costs include the costs of the consultant, registrars and house officers, both on the surgical ward and in theatre. Miscellaneous personnel costs include the costs of consultations in other departments and costs of personnel such as diabetes nurses, occupational therapists, speech and language therapists and social workers. The hours worked by each of these groups could not be isolated and therefore total costs were used.
Given the great heterogeneity in the costs of different tests or materials for endoscopy, nutrition, pathology, microbiology, chemistry, haematology and radiology, only the number of tests and total costs per patient were retrieved. Radiology tests were divided into interventional and diagnostic computed tomography (CT) and ‘other’.
Endoscopic procedures are performed by both gastroenterologists and surgeons in the gastroenterology department or the operating theatre. The extra personnel-related costs of surgeons performing endoscopic procedures were included under endoscopy costs. Endoscopies performed in theatre were reimbursed under theatre costs.
Theatre costs (including costs for recovery and excluding personnel costs for anaesthetists and surgeons) were calculated according to a rate per hour and a fixed fee per visit.
Intensive care unit costs included costs for all ICU provisions and costs of personnel, including the intensivist, and were calculated according to the actual time used. A medium-care surgical ward was defined as a ward with monitoring facilities and a higher nurse : patient ratio than a standard ward. In this setting, patients are cared for under the supervision of the surgical team. Only in-hospital costs were calculated. Extra costs for home nursing, convalescence or patient-based costs were not included in this study.
All costs including those quoted from literature were transferred into US dollars. The (historic) exchange rates as provided by the Reserve Bank of New Zealand were used.17 For the data presented in this study, the average exchange rate of September 2010 (NZ$1.00 = US$0.7259) was used. For quoted data, the rate at the month of publication was used. Correction for inflation or devaluation was not performed.
Statistical analysis was performed using StatView® 5.0.1 (SAS Institute, Inc., Cary, NC, USA). Statistical differences were computed using the Mann–Whitney U-test for continuous data and the chi-squared and Fisher's exact tests for categorized data. A P-value of <0.05 was considered significant.
Medical ethical approval was obtained from the Upper South A Regional Ethical Committee, Ministry of Health, New Zealand.
Results
Between 22 October 2005 and 31 December 2008, a total of 577 patients were admitted or referred to our institution with acute pancreatitis. Of these, 260 (45.1%) patients were predicted to have severe pancreatitis according to either an APACHE II (Acute Physiology and Chronic Health Evaluation) score ≥8 (30.0%) or a C-reactive protein (CRP) level ≥150 mg/l (37.2%). Twenty-five (4.3%) patients underwent a pancreatic necrosectomy and all others were managed non-operatively. Patient demographics and severity of illness are shown in Table 1. The rate of existing co-morbidity was low and referred to hypertension, mild asthma and atrial fibrillation in four patients equally divided over both groups and one case of obesity in the open-surgery group. The other patients had no relevant medical history.
Table 1.
Demographics and severity of illness in the 25 patients who underwent pancreatic necrosectomy between October 2005 and December 2008
| Variable | Total | Open surgery | MIRP | P-value |
|---|---|---|---|---|
| Patients, n | 25 | 12 | 13 | |
| Median (range) age, years | 55 (20–81) | 55 (20–77) | 53.5 (21–81) | 0.892 |
| Male, n | 15 | 8 | 7 | 0.688 |
| Tertiary referral, n | 19 | 10 | 9 | 0.645 |
| Median (range) APACHE II score | 9.0 (3–17) | 7.5 (3–16) | 9.0 (5–17) | 0.525 |
| Median (range) CRP | 320 (124–446) | 295 (124–421) | 345 (226–446) | 0.204 |
| Necrosis >50%, n | 16 | 6 | 10 | 0.226 |
| Infected necrosis, n | 17 | 9 | 8 | 0.670 |
MIRP, minimally invasive retroperitoneal pancreatic necrosectomy; CRP, c-reactive protein
In the 25 patients who underwent pancreatic necrosectomy, pancreatitis was caused by cholelithiasis (n= 18), was idiopathic (n= 3), reflected pancreatic divisum (n= 1), occurred post-endoscopic retrograde cholangiopancreatography (ERCP) (n= 1), was caused by alcohol abuse (n= 1) and was familial (n= 1). The indication for necrosectomy was proven infection in 13 patients (nine had evidence of extraluminal gas on CT, four had positive FNA), persistent SIRS despite maximal conservative treatment in 11 patients and haemorrhage into the necrosis which was not controlled by interventional radiology in one patient.
Thirteen patients were treated with MIRP. An open approach was chosen in the remaining 12 patients because the necrosis was not reachable by MIRP (n= 6), co-existing bowel ischaemia of the transverse colon was shown on preoperative CT (n= 2), haemorrhage into necrosis occurred (n= 1), large intra-abdominal collections not amenable to percutaneous drainage were found (n= 1), the MIRP instruments were too short to reach the necrotic cavity in an obese patient (n= 1), and the surgeon was unskilled in MIRP (n= 1). The median time to surgery was significantly longer in the MIRP group compared with the open-surgery group, at 25 days (range: 8–44 days) vs. 13 days (range: 1–92 days), respectively (P= 0.032). Two of the 25 patients died while in hospital; one of these deaths occurred in each group (P= 1.00).
Costs of treatment are shown in Table 2. The median total cost was US$56 674 (range: US$11 259–223 177). There was no significant difference in cost between open necrosectomy (median cost: US$60 913; range: US$11 259–138 802) and MIRP (median cost: US$56 673; range: US$23 317–223 177) (P= 0.786). The median largest contributor to overall costs was represented by ward costs (26.3%), followed by surgery personnel costs (22.3%) and ICU costs (17.0%). Neither the actual costs nor the proportion of costs differed significantly between the groups. The only significant differences were seen in personnel costs for anaesthesia (P= 0.022), theatre costs (P= 0.009), which reflected the larger number of theatre visits for MIRP procedures, and pathology costs (P= 0.002), which mainly reflected the number of bowel resections conducted in the open-surgery group. None of these, however, represented a major contribution to the total costs.
Table 2.
Breakdown of costs by component of care and by type of necrosectomy (open or MIRP) in US$
| Cost per unit, US$ | Total Median (range) | % of total cost Median (range) | Open surgery; (n= 12) Median (range) | % of total cost Median (range) | MIRP (n= 13) Median (range) | % of total cost Median (range) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Personnel | |||||||||
| Anaesthesia | $340/h + fixed fees | Hours | 6.0 (2.0–16.0) | 3.0 (2.0–10.0) | 6.0 (4.0–16.0) | ||||
| Costs | $2601 (1102–8961) | 4.6 (1.8–11.3) | $1470 (1102–5564) | 3.2 (1.8–11.0) | $3599 (1617–8961) | 5.1 (2.0–11.3) | 0.022 | ||
| General surgery | $188/day + fixed fees | Days | 44.4 (5.0–109.0) | 32.4 (11.8–109.0) | 48.7 (5.0–91.5) | ||||
| Costs | $10 066 (2679–22108) | 22.3 (2.2–30.7) | $7505 (2679–22 108) | 17.7 (5.3–29.1) | $10 705 (2822–19 678) | 22.7 (2.2–30.7) | 0.513 | ||
| Physiotherapy | $65/h | Hours | 6.7 (0.0–50.0) | 7.7 (0.5–50.0) | 5.5 (0.0–40.8) | ||||
| Costs | $430 (0–3229) | 0.8 (0.0–3.8) | $495 (33–3228) | 1.1 (0.2–3.8) | $355 (0–2636) | 0.7 (0.0–1.2) | 0.384 | ||
| Miscellaneous | Costs | $458 (0–1507) | 0.8 (0.0–2.0) | $438 (0–1031) | 0.8 (0.0–2.0) | $669 (41–1507) | 0.8 (0.2–1.8) | 0.446 | |
| Intervention | |||||||||
| Operating theatre | Visits | 3 (1–8) | 1 (1–4) | 4 (1–8) | |||||
| $218/h + fixed fees | Theatre hours | 5.6 (2.1–16.1) | 3.1 (2.1–10.0) | 6.6 (3.7–16.1) | |||||
| $73/h + fixed fees | Recovery hours | 2.1 (0.0–13.2) | 0.0 (0.0–3.8) | 5.0 (0.0–13.2) | |||||
| Costs | $2120 (875–6935) | 2.3 (1.3–9.9) | $1227 (913–3374) | 2.3 (1.3–9.9) | $2878 (875–6935) | 5.0 (1.7–10.1) | 0.009 | ||
| Endoscopy | Costs | $0 (0–5486) | 0.0 (0.0–11.1) | $0 (0–3002) | 0.0 (0.0–11.1) | $0 (0–5486) | 0.0 (0.0–4.9) | 0.514 | |
| Nutrition | Costs | $332 (0–1860) | 0.8 (0.0–2.4) | $639 (0–1860) | 1.1 (0–2.4) | $281 (0–1379) | 0.3 (0.0–1.8) | 0.102 | |
| Blood products | $1459/unit | Units FFP | 0 (0–4) | 0 (0–4) | 0 (0–0) | ||||
| $176/unit | Units RBC | 4 (0–14) | 4 (0–14) | 4 (0–12) | |||||
| Costs | $707 (0–3051) | 1.0 (0.0–3.1) | $707 (0–3051) | 1.1 (0–2.4) | $706 (0–2120) | 1.0 (0.0–3.1) | 0.999 | ||
| Investigations | |||||||||
| Pathology | Specimens, n | 1 (0–10) | 2 (1–10) | 1 (0–3) | |||||
| Costs | $42 (0–666) | 0.1 (0.0–1.6) | $76 (38–666) | 0.2 (0.1–1.6) | $19 (0–78) | 0.0 (0.0–0.3) | 0.002 | ||
| Microbiology | Tests, n | 24 (2–81) | 25 (3–57) | 24 (2–81) | |||||
| Costs | $507 (69–1683) | 0.9 (0.3–1.8) | $516 (78–1318) | 0.8 (0.4–1.6) | $507 (69–1683) | 0.9 (0.3–1.8) | 0.384 | ||
| Chemistry and Haematology | Tests, n | 591 (106–1763) | 750 (209–1763) | 531 (106–1211) | |||||
| Costs | $1441 (370–3205) | 2.6 (0.6–6.3) | $1625 (397–3205) | 2.7 (1.4–4.1) | $1441 (370–2740) | 2.5 (0.6–6.3) | 0.999 | ||
| Radiology | $208/CT | Diagnostic CT | 4 (1–10) | 5 (1–8) | 4 (1–10) | ||||
| $352/CT | Interventional CT | 1 (0–3) | 0 (0–3) | 1 (0–3) | |||||
| Other procedures | 25 (3–60) | 24 (3–60) | 33 (4–58) | ||||||
| Total costs | $5592 (751–11 765) | 7.7 (2.1–20.6) | $5467 (751–9327) | 7.4 (3.6–15.4) | $5592 (1368–11 765) | 7.7 (2.1–20.6) | 0.514 | ||
| Ward | |||||||||
| Intensive care unit | $2864/day | Days | 3.8 (0.0–61.6) | 5.0 (0.0–34.0) | 0.8 (0.0–61.6) | ||||
| Costs | $10 025 (0–176 511) | 17.0 (0.0–87.2) | $14 380 (0–97 266) | 29.9 (0.0–72.2) | $2267 (0–176 511) | 4.0 (0.0–87.2) | 0.532 | ||
| Medium care unit | $372/day | Days | 4.8 (0.0–11.4) | 5.9 (1.2–9.0) | 2.7 (0.0–11.4) | ||||
| Costs | $1768 (0–4250) | 3.1 (0.0–21.1) | $2194 (450–3366) | 3.4 (0.4–21.1) | $1008 (0–4250) | 2.6 (0.0–7.3) | 0.289 | ||
| Normal ward | $301/day | Days | 44.0 (5.0–109.3) | 39.8 (5.3–101.2) | 44.0 (5.0–109.3) | ||||
| Costs | $13 232 (1505–32 901) | 26.3 (1.2–50.2) | $11 966 (1593–30 465) | 22.4 (3.6–50.2) | $13 232 (1505–32 901) | 29.6 (1.2–45.2) | 0.827 | ||
| Total costs | $56 674 (11 259–223 177) | $60 913 (11 259–138 801) | $56 674 (23 317–223 177) | 0.786 | |||||
MIRP, minimally invasive retroperitoneal pancreatic necrosectomy; FFP, fresh frozen plasma; RBC, red blood cells; CT, computed tomography
At a rate of US$2864/bed/day, the costs of ICU care consumed a median of 29.9% (range: 0.0–72.2%) of overall costs in the open-surgery group and a median of 4.0% (range: 0.0–87.2%) of overall costs in the MIRP group for median stays of 5.0 days (range: 0.0–34.0 days) and 0.8 days (range: 0.0–61.6 days), respectively (Table 2). Although the MIRP group had a lower median need for ICU, it included the three most expensive patients, who remained in the ICU longest (Fig. 1). A more detailed breakdown of ICU need and length of stay by treatment type is shown in Table 3. No difference in preoperative, postoperative or total ICU stay between the two groups (open surgery vs. MIRP) was noted. The most costly patient remained in the ICU for 61.6 days, which accounted for US$176 511 (79.1%) of a total treatment cost of US$223 177.
Figure 1.
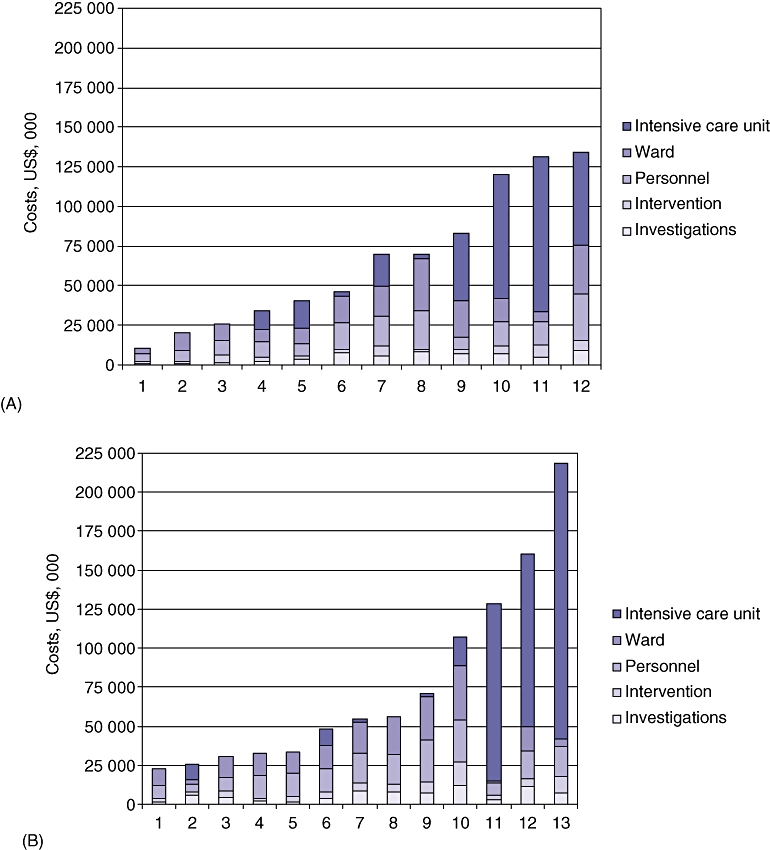
Breakdown of hospital costs by treatment modality per patient ranked by total costs; the same scale is used for both groups. (A) Open necrosectomy patients. (B) Minimally invasive necrosectomy patients
Table 3.
Comparison of specified intensive care unit (ICU) use between treatment modalities
| ICU need | Open surgery (n= 12) | MIRP (n= 13) | P-value |
|---|---|---|---|
| Median (range) time to surgery, days | 13 (1–92) | 25 (8–44) | 0.032 |
| Preoperative ICU need, n | 3/12 | 4/13 | 0.471 |
| Median (range) preoperative ICU stay if needed, days | 6.0 (1–7) | 4.0 (1–18) | 0.361 |
| Postoperative ICU need, n | 9/12 | 6/13 | 0.141 |
| Median (range) postoperative ICU stay if needed, days | 6.0 (1–33) | 13.0 (1–58) | 0.773 |
| Overall ICU need, n | 9/12 | 8/13 | 0.471 |
| Median (range) overall ICU stay if needed, days | 6.0 (1–34) | 5.5 (1–62) | 0.597 |
MIRP, minimally invasive retroperitoneal pancreatic necrosectomy
Discussion
As the understanding of the pathophysiology of pancreatitis has increased, indications for intervention have changed. Since it emerged that delaying surgery for pancreatitis leads to a survival benefit,10 it has become recognized that the disease ‘acts’ in two stages.3,18 The first phase concerns a sterile SIRS response to the decay of pancreatic tissue, which is followed by an infection of the necrotic pancreatic tissue in 40–70% of patients.3,18,19 This leads to a second episode of sepsis. The probability of secondary infection increases with the amount of necrosis3,19 and previously established bacteraemia.19 Secondary infection occurs during weeks 2–7 and peaks in incidence during weeks 3–4.3,18–20 It is now generally agreed that medical support represents the treatment of choice in the first phase of the disease and surgical intervention is kept for the second phase.3,18 A recent review showed that postponed surgery was associated with lower mortality rates and most centres delayed surgical intervention for a median of 26 days.11 However, most patients admitted for pancreatitis will have mild and self-limiting disease; only 10–20% need ICU treatment1,3,21 and fewer will develop secondary infection. The current series confirms this: 260 (45.1%) of our 577 patients were predicted to have severe pancreatitis according to the UK Guidelines,2 but only 25 (4.3%) patients eventually required surgical intervention. Both the APACHE II score and the CRP are considered to overestimate the incidence of actual severe pancreatitis.22,23
Two previous cost analyses in patients undergoing pancreatic necrosectomy were identified. Fenton-Lee and Imrie performed a cost analysis in 1992 on 10 patients treated with open necrosectomy and related the outcome to a quality of life scale resulting in a cost of US$3214/life-year saved for an average of 8.5 years saved.24 However, the calculations assumed a 100% mortality rate in the absence of surgery and a life expectancy of 75 years. These assumptions were justly challenged because four of the 10 patients did not have infected necrosis and one was a 29-year-old alcoholic for whom a life expectancy of 75 years might prove something of a challenge.25
The other cost analysis was conducted as part of the PANTER trial.5 Although this trial found a significant reduction in new ICU admissions after first treatment in favour of the step-up approach, it failed to find any significant reduction in overall ICU use or hospital stay.5 It may well be that longer stays in the ICU before first treatment in the step-up approach group counteracted the advantage conferred by the lower number of new admissions afterwards. In the current series, patients in the open-surgery group were operated on significantly earlier. The PANTER trial used the same timescale for the first intervention of drainage vs. open surgery. In its drainage group, 60% of subjects ultimately required further (minimally invasive) surgical intervention, which was performed at a median of 10 days (range: 1–52 days) later.5
The current series did not show a difference in costs between surgical approaches, despite the increased number of complex patients in the open-surgery group (ischaemic colon, large intra-abdominal collection, active bleeding). However, there exists a significant risk for type II error. Although the PANTER trial showed a cost benefit of US$14 558 (95% confidence interval [CI]−32 071 to 61 188) in favour of the step-up approach,5 only US$6533 of this was saved on in-hospital costs. The other main savings referred to nursing home costs US$2318 (95% CI −2218 to 6349) and the costs of absence from work during the 6 months follow-up US$3690 (95% CI −2722 to 9321). Ward and ICU costs accounted for 33.7–37.2% and 45.2–48.1% of in-hospital costs, respectively. Both had a huge 95% CI ranging into both negative and positive values and indicating that there was no significant difference between the two groups. All the other costs accounted for 18% of the total in-hospital costs.
The large spread in both series of ICU use, hospital stay and equivalent treatment costs, of which 65%24 to 82%5 was spent on ward costs including ICU costs, is mirrored in the current study. Other non-cost studies have shown the same huge spread in lengths of ICU and hospital stay.18,20 Although the current study found the median ICU stay in the MIRP group to be shorter, this group also contained the three patients who remained longest in the ICU and therefore the difference in costs between the open-surgery and MIRP groups was not statistically significant (P= 0.532).
The various studies cite average daily costs of an ICU bed in the range of US$1047–4000, according to the country of study and the time of research.18,26,27 New Zealand's rate of US$2864 lies in the middle of that range. The lowest estimate, of US$1047, is cited by Moerer et al., of Germany, in whose study the most expensive patient group included patients who underwent acute surgery, were maintained on ventilating machines and were septic, which increased the cost of an ICU bed to US$1947 ± 407.27 All of these conditions apply to patients with infected necrotizing pancreatitis. Given the high daily cost of ICU care, it is highly likely that any measure that can shorten the first episode or prevent the second episode of sepsis and therefore reduce the ICU stay will be cost-effective. However, neither probiotics,28 antibiotics,29,30 early ERCP31,32 or (par)enteral feeding33 has yet made a significant contribution to improving the outcome of necrotizing pancreatitis.
Finally, the costs of longterm complications in this study were not included. The overall costs of treatment of pancreatitis are further increased by the fact that over 60% of survivors can be expected to develop longterm complications: 16% are likely to need a re-operation;4 7–33% will develop exocrine insufficiency, and 16–38% will become endocrine-insufficient.4,8
Conclusions
It is clear from this study that the major costs associated with treating necrotizing pancreatitis are ward, surgical personnel and ICU costs. However, there is significant variation amongst individual patients, reflecting the heterogeneity of this patient group. The current study did not demonstrate a difference in costs between the open-surgery and minimally invasive treatment modalities, although the small sample size may have precluded the detection of a true difference. Any measure that can reduce hospital and, especially, ICU stay either by shortening the first episode of sepsis or by preventing secondary infection will, in addition to improving the quality of patient care, very likely be cost-effective.
Conflicts of interest
None declared.
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain and Ireland, Pancreatic Society of Great Britain and Ireland, Association of Upper GI Surgeons of Great Britain and Ireland. UK Guidelines for the Management of Acute Pancreatitis. Gut. 2005;54(Suppl)(3):1–9. doi: 10.1136/gut.2004.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426–436. doi: 10.1136/gut.2003.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Van Santvoort HC, Besselink MG, Bakker OJ, Hofker S, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 6.Raraty MGT, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, et al. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787–793. doi: 10.1097/SLA.0b013e3181d96c53. [DOI] [PubMed] [Google Scholar]
- 7.Parikh PY, Pitt HA, Kilbane M, Howard TJ, Nakeeb A, Schmidt CM, et al. Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg. 2009;209:712–719. doi: 10.1016/j.jamcollsurg.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuijs VB, Besselink MG, van Minnen LP, Gooszen HG. Surgical management of acute necrotizing pancreatitis: a 13-year experience and a systematic review. Scand J Gastroenterol Suppl. 2003;239:111–116. doi: 10.1080/00855920310002799. [DOI] [PubMed] [Google Scholar]
- 9.Besselink MG, de Bruijn MT, Rutten JP, Boermeester MA, Hofker HS, Gooszen HG, Dutch Acute Pancreatitis Study Group Surgical intervention in patients with necrotizing pancreatitis. Br J Surg. 2006;93:593–599. doi: 10.1002/bjs.5287. [DOI] [PubMed] [Google Scholar]
- 10.Mier J, León EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–75. doi: 10.1016/S0002-9610(96)00425-4. [DOI] [PubMed] [Google Scholar]
- 11.Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194–1201. doi: 10.1001/archsurg.142.12.1194. [DOI] [PubMed] [Google Scholar]
- 12.Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–180. doi: 10.1097/00000658-200008000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor SJ, Lienert AR, Brown LA, Bagshaw PF. Closing the audit loop is necessary to achieve compliance with evidence-based guidelines in the management of acute pancreatitis. N Z Med J. 2008;121:19–25. [PubMed] [Google Scholar]
- 14.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 15.Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, et al. International Association of Pancreatology IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565–573. doi: 10.1159/000071269. [DOI] [PubMed] [Google Scholar]
- 16.Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, et al. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–277. doi: 10.1159/000071184. [DOI] [PubMed] [Google Scholar]
- 17.Reserve Bank of New Zealand. B1 Exchange Rates. 2010. http://www.rbnz.govt.nz/statistics/exandint/b1/. [Accessed 1 December 2010]
- 18.Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, et al. Dutch Acute Pancreatitis Study Group Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267–273. doi: 10.1002/bjs.6447. [DOI] [PubMed] [Google Scholar]
- 20.Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- 22.Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, et al. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9:601–614. doi: 10.1159/000212097. [DOI] [PubMed] [Google Scholar]
- 23.Lankisch PG, Warnecke B, Bruns D, Werner HM, Grossmann F, Struckmann K, et al. The APACHE II score is unreliable to diagnose necrotizing pancreatitis on admission to hospital. Pancreas. 2002;24:217–222. doi: 10.1097/00006676-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fenton-Lee D, Imrie CW. Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg. 1993;80:1579–1582. doi: 10.1002/bjs.1800801228. [DOI] [PubMed] [Google Scholar]
- 25.Carter DC. Acute pancreatitis: the value of life. Br J Surg. 1993;80:1499–1500. doi: 10.1002/bjs.1800801202. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin AM, Hardt J, Canavan JB, Donnelly MB. Determining the economic cost of ICU treatment: a prospective ‘micro-costing’ study. Intensive Care Med. 2009;35:2135–2140. doi: 10.1007/s00134-009-1622-1. [DOI] [PubMed] [Google Scholar]
- 27.Moerer O, Plock E, Mgbor U, Schmid A, Schneider H, Wischnewsky MB, et al. A German national prevalence study on the cost of intensive care: an evaluation from 51 intensive care units. Crit Care. 2007;11:R69. doi: 10.1186/cc5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomized, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 29.Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2006;(4):CD002941. doi: 10.1002/14651858.CD002941.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674–683. doi: 10.1097/01.sla.0000250414.09255.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oría A, Cimmino D, Ocampo C, Silva W, Kohan G, Zandalazini H, et al. Early endoscopic intervention versus early conservative management in patients with acute gallstone pancreatitis and biliopancreatic obstruction: a randomized clinical trial. Ann Surg. 2007;245:10–17. doi: 10.1097/01.sla.0000232539.88254.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayub K, Imada R, Slavin J. Endoscopic retrograde cholangiopancreatography in gallstone-associated acute pancreatitis. Cochrane Database Syst Rev. 2004;(4) doi: 10.1002/14651858.CD003630.pub2. CD003630. [DOI] [PubMed] [Google Scholar]
- 33.Al-Omran M, Groof A, Wilke D. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2003;(1):CD002837. doi: 10.1002/14651858.CD002837. [DOI] [PubMed] [Google Scholar]


