Abstract
Background
There is a general concern that aged organs are more susceptible to ischaemia. In the light of recent proposals to change the liver allocation system by expanding regional sharing, it is feared that increased cold ischaemia time of grafts from older donors may reduce graft survival. The aim of this study was to correlate donor age and the patterns of ischaemia reperfusion injury and synthetic function early after liver transplantation.
Methods
We performed a retrospective study of first transplants using a single-centre electronic database. Patterns of liver injury (based on transaminases and post-reperfusion biopsy), synthetic function (international normalized ratio [INR]), and graft and patient survival in recipients receiving liver grafts from donors aged ≥65 years (group 1, n= 50) were compared with equivalent patterns in a matched cohort of recipients transplanted with grafts from donors aged <65 years (group 2, n= 50).
Results
There was no significant difference in transaminase levels from day 0 to day 6 after transplantation. When groups 1 and 2 were subdivided into two subgroups based on the duration of graft cold ischaemia time (<8 h and ≥8 h), there was no statistical difference in transaminase levels during the first 7 days. There were two cases (4%) of primary non-function in group 1 and one (2%) in group 2. Initial poor function did not differ significantly between the groups (26% vs. 24%; P= 0.81). In addition, there was no difference in histological changes in post-reperfusion biopsies (21% vs. 34%; P= 0.078) and rate of acute rejection episodes in the first year (30% vs. 32%; P= 0.99). There was no significant difference between groups 1 and 2 in 1-year patient and graft survivals (78% vs. 90% [P= 0.17]; 88% vs. 94% [P= 0.48], respectively).
Conclusions
Judiciously selected livers from aged donors are not associated with major increased susceptibility to ischaemia reperfusion injury.
Keywords: liver, transplantation, ischaemia reperfusion injury, outcomes, aging
Introduction
Because of a shortage of organs, livers from older donors have been increasingly used for transplantation. In the USA, the number of liver donors aged ≥65 years increased from 0.1% to 10.2% during 1988 to 2005.1 According to the Organ Procurement and Transplantation Network (OPTN), in 2009, liver donors aged ≥65 years represented 8.9% of all donors.
An old liver graft is considered an independent risk factor for mortality after transplantation.2 The age of the donor is also a predictive factor for the severity of recurrent liver hepatitis C virus (HCV)-related disease on the graft.3 In addition, there is a general concern that aged organs are more susceptible to ischaemia. In the light of recent proposals to change the liver allocation system by expanding regional sharing, it is feared that increased cold ischaemia time (CIT) of grafts from older donors might reduce graft survival. The aim of this study was to correlate donor age and the patterns of ischaemia reperfusion injury (IRI) and synthetic function early after liver transplantation.
Materials and methods
We performed a retrospective study of first liver transplants using a single-centre electronic database. Completeness of the database was above 95%. Retrospective analysis showed that the power of the study for survival analysis was 75–80%. This study analysed data for randomly selected non-consecutive liver transplants that met our inclusion criteria and were performed between January 2004 and December 2007 at Westchester Medical Center, New York. During this period our centre performed 324 liver transplants, 17% of which used organs from donors aged ≥65 years. A total of 76% of the grafts were procured by our team. Patterns of liver injury (based on transaminases), synthetic function (INR [international normalized ratio]) and cholestasis (bilirubin, gamma-glutamyl transferase [GGT], alkaline phosphatase [AP]) during the first week, and at days 30 and 60 post-transplant, as well as graft and patient survival at days 7, 30 and 60 in recipients receiving liver grafts from donors aged ≥65 years (group 1, n= 50) were compared with equivalent patterns in a matched cohort of recipients transplanted with grafts from donors aged <65 years (group 2, n= 50). We included all transplants during this period that used older donors and met our inclusion criteria (n= 50). By contrast, transplants from the younger group of donors were selected (n= 50). The selection procedure was blinded to recipient and donor demographics and outcomes.
We analysed the following graft and donor parameters: CIT; warm ischaemia time; cytomegalovirus (CMV) status; body mass index (BMI); cause of death; donor risk index (DRI); total bilirubin; GGT; ALT (alanine aminotransferase); AST (aspartate aminotransferase); creatinine, and blood urea nitrogen (BUN). Recipient parameters analysed were: age; sex; Model for End-stage Liver Disease (MELD) score; cause of end-stage liver disease (ESLD); creatinine, and pre-transplant transaminase and bilirubin levels. In a separate analysis we divided these groups in two subgroups based on graft CITs of ≥8 h and <8 h.
A recipient was classified as experiencing primary non-function (PNF) if he or she died or required retransplantation within the first 7 days in the absence of an identifiable cause (e.g. hepatic artery thrombosis, massive haemorrhagic necrosis), and as having initial poor function (IPF) if his or her AST level was >1500 U/l and prothrombin time was >20 s in the first postoperative week.4
Donor and recipient selection
At our institution, deceased donor livers are excluded from use according to the presence of: positive HIV or human T cell lymphotrophic virus serologies; most cases of donor malignancy, and poor organ quality based on the opinion of an experienced liver transplant surgeon on inspection of the organ or on histological findings in selected cases. Donors were selected based on biochemical, clinical and surgical (visualization/palpation of the liver during harvesting, quality of perfusion) factors. The subjective assessment of grafts from older donors is important in the decision-making process and grafts of questionable quality, including those with extensive atherosclerosis of the hepatic artery, are rejected. We also avoid transplanting grafts from older donors in HCV patients. Grafts that are positive for HCV are considered for HCV-positive recipients if the biopsy is normal or shows only stage I fibrosis. We try to minimize additional risk factors and CIT when using grafts from older donors. All cases involving organs from cardiac death donors, organs from live donors, partial grafts, paediatric recipients (<18 years), kidney and liver recipients, acute liver failure as indication and liver retransplants were excluded from analysis. Patients with hepatocellular carcinoma (HCC) were listed for transplant according to the Milan criteria.5 This analysis was performed after implementation of the MELD allocation system (February 2002). The MELD score utilized referred to the physiological or native MELD based on laboratory values obtained shortly prior to transplantation and not from adjustments for exception points (e.g. HCC points). Donor risk index was calculated according to standard formulae.6 About 76% of the liver grafts were procured by our team. All allografts were preserved in University of Wisconsin solution using established methods. The allocation of older grafts was decided by the senior author on a case-by-case basis (depending on ABO group, quality of life, HCC status, co-morbidities, etc.) and depending on the previous provision of informed consent to the transplantation of an extended criteria organ signed by the transplant candidate.
Transplant technique and immunosuppression
Patients were transplanted using the classical technique (removal of the retrohepatic inferior vena cava). A veno–venous bypass was used in a minority of cases. Recipients received tacrolimus, mycophenolate mophetyl and methylprednisone (and, later, prednisone) as postoperative immunosuppression. If renal function was impaired pre-transplant, patients received induction with thymoglobulin and tacrolimus administration was postponed.
Histopathological analysis
Post-reperfusion biopsy specimens were obtained within 1 h after complete revascularization of the allograft in most of the patients. All biopsy specimens were fixed in 10% buffered formalin, processed, embedded in paraffin, and stained with haematoxylin and eosin before assessment. The severity of IRI was assessed by one pathologist qualitatively as significant or non-significant based on graft infiltration by acute inflammatory cells, ballooning degeneration, and the presence of coagulative necrosis. We did not conduct protocol biopsies. Biopsies were taken when transaminases and cholestatic parameters were suggestive of rejection. Assessment of acute cellular rejection in the first year post-transplant was carried out quantitatively (using a scale of 0–9), but, for the sake of statistical power, we divided our patients into only two groups: those with acute rejection and those with no acute rejection episodes.
Statistical analysis
Unless specified otherwise, data are presented as mean ± standard error of the mean (SEM). Normal distribution was assessed with the Kolmogorov–Smirnov test. Differences between groups were assessed with Student's t-test for normally distributed variables. Differences between groups were assessed with non-parametric Mann–Whitney–Wilcoxon tests for non-Gaussian distributed variables (DRI, creatinine, GGT, AST, ALT, bilirubin, INR). Comparisons of multiple groups were made using the Kruskal–Wallis test. Categorical variables (cause of death, race, gender, CMV status, aetiology of cirrhosis, HCC status, IRI, acute rejection, retransplantation rate) were assessed using Fisher's exact test. Survival analysis (1-year patient survival and rejection-free survival within the first year) was performed using the Kaplan–Meier method. Survival curves were compared using the log-rank (Mantel–Cox) test. Differences were considered statistically significant when the P-value was <0.05. The statistical software used was GraphPad Prism for Windows Version 5.00 (GraphPad Software, Inc., San Diego, CA, USA).
Results
There was no significant difference between the two recipient groups before transplant except in MELD scores (Table 1). Older grafts were more likely to be allocated to recipients with low MELD scores (18.02 ± 7.34 vs. 25.77 ± 10.55; P < 0.0001). There was no difference in the HCV status of recipients between the two groups because about 50% of our liver transplant candidates are HCV-positive and in the early phase of the study we were more liberal in allocating older organs to HCV patients. Over the study period there was no statistically significant difference in CIT despite the intention to reduce it to a minimum when allocating older grafts. Mean CIT was 7.7 h (range: 2–12 h) for younger grafts and 7.3 h (range: 2–11 h) for older grafts. The fact that there was no difference in CIT was partially associated with a more liberal approach in the earlier period of this study. The mean age of donors aged ≥65 years was 73.94 years (median: 72.5 years, range: 65–86 years); the mean age of donors aged <65 years was 41.9 years (median: 45.5 years, range: 18–64 years). The donor groups differed in: DRI; cause of death; gender; CMV status, and transaminases (Table 2). The fact that there were no differences between groups in CIT, BMI, cause of death or race allowed us to test the effect of donor age as the main determinant of IRI. Donors aged ≥65 years had a higher DRI (2.17 ± 0.44 vs. 1.46 ± 0.44; P= 0.0001), were less likely to have died of trauma (10% vs. 26%; P= 0.05) or anoxia (4% vs. 26%; P= 0.004), were more frequently female (70% vs. 42%; P= 0.0085), were more frequently CMV-positive (76% vs. 52%; P= 0.021), and had lower pre-procurement transaminase levels (AST: 66.39 ± 16.72 vs. 103.54 ± 19.25 [P= 0.006]; ALT: 47.68 ± 11.52 vs.130.06 ± 33.86 [P= 0.0004]). There were two cases (4%) of PNF in the group that received older grafts and one (2%) in the group that received younger grafts. The incidence of IPF did not differ significantly between the groups (26% vs. 24%; P= 0.81). There was no mortality in the first post-transplant week in either group.
Table 1.
Liver donor characteristics
| Donors aged <65 years (n= 50) | Donors aged ≥65 years (n= 50) | P-value | |
|---|---|---|---|
| Age, mean ± SD, years | 41.9 ± 14.5 (median: 45.5) | 73.9 ± 7.0 (median: 72.5) | <0.0001 |
| Gender | 42% F, 52% M | 70% F, 30% M | 0.0085 |
| Race | 54% W, 18% AA, 16% H, 2% A | 44% W, 8% AA, 26% H, 2% A | >0.05 (NS) |
| Body mass index, mean ± SD | 28.7 ± 5.4 | 28.6 ± 5.9 | 0.89 (NS) |
| Cold ischaemia time, mean ± SD, min | 461.6 ± 168.8 | 439.0 ± 125.2 | 0.45 (NS) |
| Warm ischaemia time, mean ± SD, min | 45.9 ± 7.5 | 46.0 ± 8.2 | 0.93 (NS) |
| Cytomegalovirus+ | 42% | 76% | 0.021 |
| Cause of death | Trauma 25% | Trauma 10% | 0.05 (NS) |
| Stroke 26% | Stroke 86% | <0.001 | |
| Anoxia 26% | Anoxia 4% | 0.004 | |
| Donor risk index, mean ± SEM | 1.46 ± 0.04 | 2.17 ± 0.04 | 0.0001 |
| Creatinine, mean ± SEM | 1.6 ± 0.2 | 1.5 ± 0.2 | 0.67 (NS) |
| GGT, mean ± SEM | 78.2 ± 13.1 | 62.3 ± 12.5 | 0.97 (NS) |
| AST, mean ± SEM | 103.5 ± 19.2 | 66.4 ± 16.7 | 0.006 |
| ALT, mean ± SEM | 130.1 ± 33.9 | 47.7 ± 11.5 | 0.0004 |
| Total bilirubin, mean ± SEM | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.04 |
F, female; M, male; W, White; AA, African-American; H, Hispanic; A, Asian; SD, standard deviation; SEM, standard error of the mean; GGT, gamma-glutamyl transferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NS, not significant
Table 2.
Recipient characteristics
| Recipients of donor grafts aged <65 years (n= 50) | Recipients of donor grafts aged ≥65 years (n= 50) | P-value | |
|---|---|---|---|
| Age, mean ± SD, years | 53.3 ± 14.6 (median: 55) | 57.6 ± 11.2 (median: 61) | 0.082 (NS) |
| Gender | 44% F, 56% M | 38% F, 52% M | 0.47 (NS) |
| Race | 50% W, 10% AA, 26% H, 4% A | 44% W, 8% AA, 24% H, 4% A | >0.05 (NS) |
| Body mass index, mean ± SD | 27.8 ± 5.4 | 28.1 ± 3.6 | 0.81 (NS) |
| MELD, mean ± SD | 26.0 ± 10.6 | 19.8 ± 9.2 | 0.003a |
| Aetiology of cirrhosis | Alcohol 12% | Alcohol 20% | >0.05 (NS) |
| HCV 48% | HCV 42% | >0.05 (NS) | |
| HBV 8% | HBV 0% | >0.05 (NS) | |
| Other 32% | Other 38% | >0.05 (NS) | |
| Hepatocellular carcinoma | 10% | 10% | >0.05 (NS) |
| AST, mean ± SEM | 148.8 ± 9.7 | 88.4 ± 10.8 | 0.21 (NS) |
| ALT, mean ± SEM | 164.9 ± 102 | 74.5 ± 19.8 | 0.56 (NS) |
| Total bilirubin, mean ± SEM | 7.7 ± 1.1 | 6.3 ± 0.7 | 0.30 (NS) |
| INR, mean ± SEM | 2.3 ± 0.2 | 1.8 ± 0.1 | 0.06 (NS) |
The two groups differ only on MELD scores. Older grafts were allocated more frequently to recipients with lower MELD scores
F, female; M, male; W, White; AA, African-American; H, Hispanic; A, Asian; MELD, Model of End-stage Liver Disease; SD, standard deviation; SEM, standard error of the mean; HBV, hepatitis B virus; HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; NS, not significant
There was no significant difference in transaminase levels from day 0 to day 5 after transplantation, or at days 30 and 60 (Fig. 1). However, at day 6, recipients of older grafts presented higher AST (157.29 ± 43.56 vs. 86.04 ± 8.97; P= 0.01) Total bilirubin did not differ between groups in the first 5 days. On days 6 and 7, older grafts were associated with higher bilirubin levels (day 7: group 1, 7.32 ± 0.93; group 2, 4.14 ± 0.55; P= 0.004) (Fig. 1). Increased transaminases and bilirubin at this point indicate that aged grafts may facilitate activation of antigen-specific effector arms of the immune system. Interestingly, transaminases and cholestatic parameters (total bilirubin and GGT) were not significantly higher at 1 year in group 1 recipients that in group 2 recipients. In addition, there was no difference in synthetic function (based on INR and albumin values) during the first week, at days 30 and 60, and at 1 year (Table 3).
Figure 1.
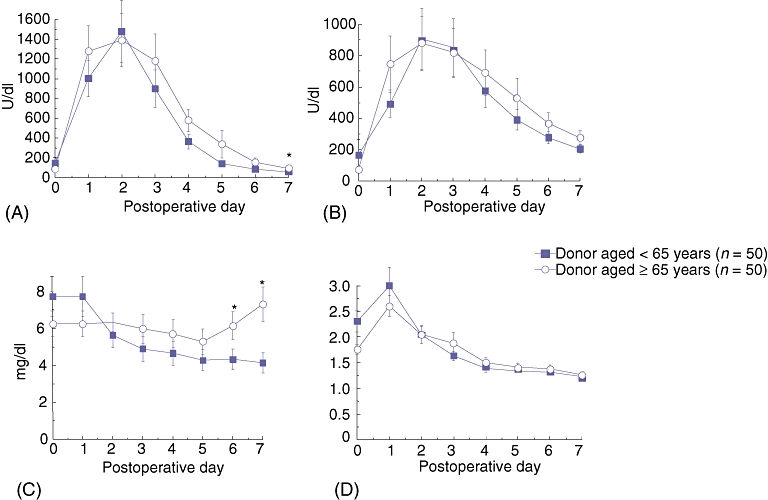
Seven-day curves showing (A) AST (aspartate aminotransferase), (B) ALT (alanine aminotransferase), (C) total bilirubin and (D) INR (international normalized ratio) in recipients of grafts aged ≥65 years vs. recipients of grafts aged <65 years (n= 50 per group). AST and bilirubin levels in recipients of grafts from older donors were significantly elevated only at days 6 and 7 (P= 0.01, P= 0.01 and P= 0.002, P= 0.004, respectively). ALT and INR levels did not differ significantly between the two groups in the first 7 days. Error bars represent the standard error of the mean
Table 3.
Total bilirubin, GGT and INR values at 30 days, 60 days and 1 year after liver transplantation
| 30 days | P-value | 60 days | P-value | 1 year | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Graft aged <65 years | Graft aged ≥65 years | Graft aged <65 years | Graft aged ≥65 years | Graft aged <65 years | Graft aged ≥65 years | ||||
| Total bilirubin, mean ± SEM | 1.2 ± 0.1 | 2.9 ± 0.6 | 0.001 | 1.2 ± 0.4 | 1.7 ± 0.4 | 0.009 | 1.5 ± 0.5 | 1.5 ± 0.4 | 0.53 |
| GGT, mean ± SEM | 132.4 ± 19.0 | 244.4 ± 32.5 | 0.0004 | 133.7 ± 23.6 | 190.0 ± 38.8 | 0.09 | 170.2 ± 43.7 | 164.2 ± 35.3 | 0.62 |
| INR, mean ± SEM | 1.1 ± 0.03 | 1.1 ± 0.02 | 0.59 | 1.1 ± 0.14 | 1.1 ± 0.09 | 0.38 | 1.0 ± 0.04 | 1.2 ± 0.10 | 0.17 |
Bilirubin and GGT levels in recipients of grafts from older donors were significantly elevated at day 30 after transplant, but did not differ from those in recipients of younger grafts at day 60 and 1 year after transplantation
GGT, gamma-glutamyl transferase; INR, international normalized ratio; SEM, standard error of the mean
When the older and younger graft groups were each divided into two subgroups based on the duration of graft CIT (≥8 h and <8 h), we again observed no statistical difference in levels of AST, ALT, bilirubin and INR during the first 7 days (Fig. 2). These results show that there was no additive or synergistic association between advanced donor age and longer CIT.
Figure 2.
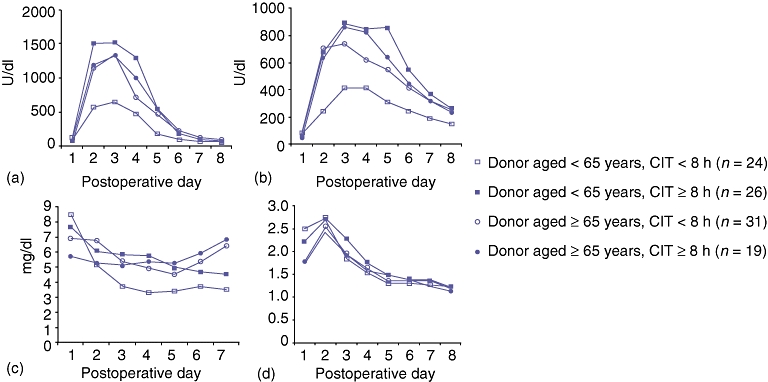
Seven-day curves showing (A) AST (aspartate aminotransferase), (B) ALT (alanine aminotransferase), (C) total bilirubin and (D) INR (international normalized ratio) in four groups of recipients of grafts aged ≥65 years or <65 years with cold ischaemia time of <8 h or ≥8 h. There was no statistical difference in any of these parameters among these groups. CIT, cold ischaemia time
Rates of retransplantation within 1 year were similar in both groups (group 1: n= 3, 6%; group 2: n= 2, 4%; P= 1.0). Mortality in the first 60 days and at 1 year was higher in recipients of older livers (group 1), but this difference did not reach statistical significance (12% vs. 6% [P= 0.48], 22% vs. 10% [P= 0.17], respectively). The most common cause of death in both groups was sepsis (Fig. 3).
Figure 3.
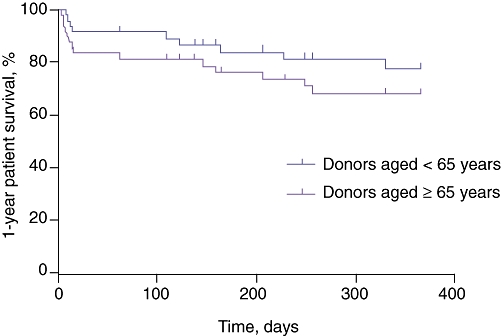
One-year patient survival curves after transplantation. Although 1-year survival was lower in recipients of livers from donors aged ≥65 years (78% vs. 90%), this difference did not reach statistical significance (n= 50 per group; P= 0.13). The most common cause of death in both groups was sepsis
Histologically, there was no difference in the occurrence of significant IRI or in the time and number of acute cellular rejection episodes between younger and older grafts (Table 4, Fig. 4).
Table 4.
Histopathological analysis
| Ischaemia reperfusion injury | P-value | Acute cellular rejection | P-value | ||||
|---|---|---|---|---|---|---|---|
| Significant | Not significant | Present | Absent | ||||
| Grafts aged <65 years | 11 (34%) | 21 (64%) | 16 (32%) | 34 (68%) | |||
| 0.078 | 0.99 | ||||||
| Grafts aged ≥65 years | 7 (21%) | 26 (79%) | 15 (30%) | 35 (70%) | |||
Post-reperfusion biopsy was assessed for the presence of significant ischaemia reperfusion injury (IRI). Acute cellular rejection within 1 year of liver transplantation was assessed here only qualitatively (presence or absence of rejection). The number of grafts that showed significant IRI and acute cellular rejection did not differ significantly between the groups
Figure 4.
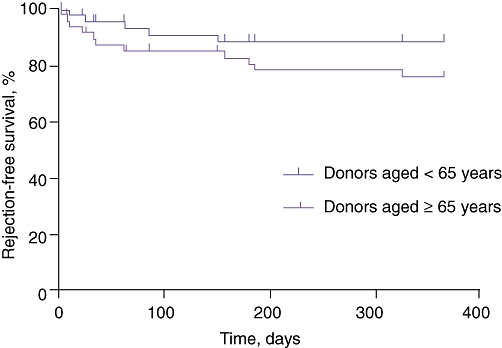
Rejection-free survival within 1 year after transplantation (time until first episode of biopsy-proven acute cellular rejection in the first year). There was no difference between the two groups in the timing and number of rejection episodes (n= 50 per group; P= 0.24)
Discussion
Liver ageing and ischaemia reperfusion injury
The ageing process causes important anatomic and functional changes in a number of systems that result in the reduction of functional reserve and inability to cope with stress. The ageing process is associated with changes in the intracellular redox balance that occur as a consequence of reduced capacity to produce active heat-shock proteins.7–9 It has been shown that there is a shift toward the production of pro-inflammatory cytokines (interleukin-6 [IL-6], IL-1, tumour necrosis factor-α, interferon-γ).10,11 Investigation in animal models showed that increasing age is associated with increased IRI.12,13 Previous clinical reports have also suggested that old age of the donor is associated with increased IRI and, consequently, PNF, delayed graft function and rejection.2,4,14–20 In an analysis of 7988 liver transplantations from the United Network for Organ Sharing (UNOS) registry, donor age >50 years was associated with a 7.1% decrease in 6-month retransplantation-free survival (69.0% vs. 76.1%).14 However, other factors, such as susceptibility to acute rejection and biliary complications, may be responsible for these poorer results. Several studies have shown that older grafts are more immunogenic and more susceptible to acute and chronic rejection (for review, see21). It has been postulated that IRI can potentiate the immunogenicity of the graft by releasing ‘danger signals’, leading to the recruitment of inflammatory cells that promote acute rejection.21,22 In kidney transplantation, but not in liver transplantation, there is a clear correlation between older organs and IRI (acute tubular necrosis). The use of kidneys from older donors is associated with an increased risk for delayed graft function and acute rejection.23 The difference between the liver and other organs may be related to the liver's regenerative and immunomodulatory capacities.24,25 It is also believed that the liver suffers little decrease in function with ageing.26–29 Rosen et al. demonstrated that rejection is not related to the extent of IRI in liver transplant patients.30 These authors showed that short-term graft survival is proportional to the extent of IRI only when it is extreme (ASTmax >5000 U/l), but grafts that are not lost to PNF have equivalent 1- and 2-year survival rates, irrespective of the magnitude of IRI. Rosen et al. also showed that 40% of grafts with extreme IRI are lost to PNF, but the same proportion also provide longterm function; in surviving grafts, longterm biochemical function and incidence of biliary complications and chronic rejection are unrelated to the extent of IRI.30 In another study, glutathione content, the main hepatic-free radical scavenger, measured at the time of early reperfusion, was similar in livers obtained from young and elderly (>60 years) cadaveric donors.31 In a small study involving 16 liver transplants from donors aged >50 years, Deschênes et al. showed the use of older grafts to be associated with more histology-proven extensive ischaemic damage immediately after reperfusion, but there was no statistically significant difference between the severity of the damage and the incidence of IPF.32 In a rat model of liver transplantation Sakai et al. showed that recipients of young and old livers after 30 h of cold preservation had identical survival rates (60%) and no difference in the peak of AST.33
Clinical outcomes of older grafts and impact of IRI
Until the late 1980s, organs from donors aged >50 years were rarely used.34 However, the discrepancy between demand and supply imposed the use of high-risk donors, including elderly donors. Extended criteria grafts are especially used in patients with poorer prognoses who cannot wait long for a graft (e.g. subjects with HCC) or who have poor quality of life and low MELD scores.
There are several reports showing inferior outcomes when using older donors.4,31,35–37 Data from the OPTN for all liver transplants performed in the USA between 1997 and 2004 showed that the survival rates for both recipients and grafts from donors aged ≥65 years are lower than those for recipients and grafts from donors aged 18–34 years at 1 year (80.6% vs. 87.7%, and 74.2% vs. 84.2%, respectively) and 5 years (66.1% vs. 76.5% and 51.6% vs. 70.4%, respectively).38 However, single-centre analyses have failed to demonstrate significant differences in short- and medium-term graft and patient survival using donors aged >60 years,39–49 especially in the absence of other donor risk factors and in carefully selected recipients.46–49 In a large cohort study, Hoofnagle and colleagues showed that the overall incidence of poor graft survival was more common among recipients of older livers, but that recipients of older liver grafts assessed as good by the procuring surgeon had a retransplant-free survival similar to that of recipients of younger livers (87% vs. 91% at 3 months). Thus, the utilization of selected older livers was not associated with a decrease in patient or graft survival.50 The same study found that although older donors were more likely to have died of central nervous system-related causes and to be CMV-positive, they also had some favourable characteristics such as lower occurrences of pre-harvest episodes of acidosis, hypoxemia and transfusions, as well as less chronic drug use and acute alcohol intoxication. We have also shown in our study that livers from older donors have lower transaminase and total bilirubin levels, and possibly have experienced less ischaemic insult before harvesting as the incidence of death associated with hypoxia in this group is significantly lower.
It is feared that regional sharing of livers might increase CIT and reduce even further the survival of older grafts.51 In a retrospective analysis of UNOS data from 18 787 liver transplants carried out between 2002 and 2007 (post-MELD era), Cassuto et al. showed that grafts from donors aged >60 years with longer CIT have particularly reduced rates of survival.52 Compared with grafts from a reference group (donors aged <60 years, CIT < 6 h), grafts aged >60 years with CIT of >12 h had a 92.7% increased risk for overall graft loss, whereas that for grafts aged <60 years with CIT > 12 h was 57.0%.52 Reese and colleagues, in a retrospective study of the UNOS database of 44 742 liver transplants, demonstrated the effect of a negative interaction between donor age >45 years and CIT > 12 h on 90-day graft survival (17.3% vs. 11.1%; odds ratio [OR] 1.24, P < 0.01), but this difference did not reach statistical significance in the post-MELD era (OR 1.18, P= 0.38).19 Segev and colleagues also used post-MELD UNOS data to compare the characteristics and outcomes of 1357 liver transplants with prolonged CIT (>12 h) with those of 13 280 transplants with CIT of <12 h.53 They noted that donor age did not significantly amplify the risk for graft loss in grafts with prolonged CIT. The only donor-related risk factor for these grafts with increased CIT was African-American ethnicity.53 In a study of the UNOS data for 58 576 liver transplants, the rate of PNF increased exponentially from the year 1990 (<1%) to 2000 (>6%), reflecting the more frequent use of ECDs. This rate decreased after 2000 despite the increased use of older donors.42 Mangus et al. showed that outcomes of imported ECD grafts (with longer CITs) were similar to those of locally procured grafts.54 This would appear to be paradoxical, because a higher incidence of PNF might be expected to occur under the MELD allocation system as more morbid patients gain access to transplant. Exactly why the impact of prolonged CIT in older grafts is reduced in the MELD era is unclear and is probably multifactorial. It is likely that the decreased rate of PNF may reflect not the changes in the system of allocation, but, instead, increased experience in the selection and allocation of ECD grafts. In our study, we did not investigate the effect of regional allocation because <10% of all livers were imported from other regions and all of them had CITs of <12 h. In these circumstances, this study would have had very low power to investigate the effect of transport on IRI. However, we investigated the effect of different CITs (<8 h and ≥8 h) on graft and patient outcomes.
Older organs may have a lower threshold for injury; however, they have similar outcomes when transplanted within a certain range of CIT. Data from the Eurotransplant Registry for 5002 liver transplants using grafts from donors aged >65 years showed that the survival difference between grafts with CIT of >12 h and grafts with CIT of <12 h was only marginally significant (70% vs. 78% at 1 year, 59% vs. 60% at 5 years; P= 0.047) (Rene Adam, Eurotransplant Registry, personal communication, 2009). A meta-analysis by Stahl et al. showed that the relationship between CIT and PNF is not linear.55 Primary non-function and graft and patient survival were worse for both very short and long CITs (<5 h and >12.5 h). This probably represents a selection bias because the sickest patients tend to receive organs with short CITs.55 Tekin et al. also demonstrated an exponential rather than a linear increase in graft dysfunction of ECD grafts when the CIT was >12 h (45.5% vs. 15.0%).56 In our study, almost all grafts were transplanted within 12 h, which may explain why outcomes in younger and older grafts were similar.
The main limitations of our study concern its status as a retrospective single-centre study and the small number of patients (n= 50 per group). Retrospective studies are subject to selection bias and details of clinical management and other data can be incomplete or lacking. Although the magnitude of aminotransferase elevation in the early post-transplant period reflects the extent of hepatocellular injury and has historically been used to classify the severity of preservation injury,30 other measures of IRI (quantitative histological assessment, functional and genetic assays) were not used. Our study analysed only the effect of donor age on IRI based on the fact that the two recipient populations were similar. However, the recipient groups differed in MELD scores. Although recipient variables (MELD score, co-morbidities) probably play a role in the ultimate severity of IRI, donor- and preservation-related factors are likely to represent its major determinants.57 In our study, control of these variables was not possible. Randomization is particularly difficult in a study that measures the effect of age on IRI because we cannot randomly assign ECD organs in clinical transplantation. Patients with high MELD scores usually receive better organs, whereas older grafts are allocated to recipients with low MELD scores. In addition, older grafts are preferably not allocated to HCV-positive patients because HCV has been shown to have higher recurrence rates.3
Although some reports of experiments in animal models have suggested that aged organs are more susceptible to IRI, our analysis was not able to identify any significant impact of donor age on the incidence of IRI and short-term liver function. Ischaemia reperfusion injury is probably not linearly correlated with CIT. There may be a time-point at which the damage caused by cold ischaemia becomes irreversible and this may compromise graft function and survival. Although it is probable that older age of the liver donor increases susceptibility to IRI by reducing this threshold, these effects may not be clinically relevant when these donors are strictly selected (e.g. by eliminating additional risk factors) and CIT is minimized. Access to older donors will continue to represent an important way of expanding the liver donor pool. Large, prospective, observational multicentre studies are needed to confirm these findings.
Conflicts of interest
None declared.
References
- 1.Scientific Registry of Transplant Recipients. 2005 Annual Report of the US Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. 2005. Transplant data 1995–2004. http://www.ustransplant.org/annual_reports/current/. [Accessed June 2010.
- 2.Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation – a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer M. Risk of extended criteria donors in hepatitis C virus-positive recipients. Liver Transpl. 2008;14(Suppl):45–50. doi: 10.1002/lt.21617. [DOI] [PubMed] [Google Scholar]
- 4.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 5.Mazzafero V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 7.Verbeke P, Fonager J, Clark BF, Rattan SI. Heat shock response and ageing: mechanisms and applications. Cell Biol Int. 2001;25:845–857. doi: 10.1006/cbir.2001.0789. [DOI] [PubMed] [Google Scholar]
- 8.Lee YK, Manalo D, Liu AK. Heat shock response, heat shock transcription factor and cell aging. Biol Signals. 1996;5:180–190. doi: 10.1159/000109187. [DOI] [PubMed] [Google Scholar]
- 9.Liu AY, Lee YK, Manalo D, Huang LE. Attenuated heat shock transcriptional response in ageing: molecular mechanism and implication in the biology of ageing. EXS. 1996;77:393–408. doi: 10.1007/978-3-0348-9088-5_26. [DOI] [PubMed] [Google Scholar]
- 10.Cossarizza A, Monti D, Cantini M, Bersani F, Paganelli R, Montagnani G, et al. Extremely low frequency pulsed electromagnetic fields increase interleukin-2 (IL-2) utilization and IL-2 receptor expression in lymphocytes from old subjects. FEBS Lett. 1989;248:141–144. doi: 10.1016/0014-5793(89)80449-1. [DOI] [PubMed] [Google Scholar]
- 11.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–521. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 12.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, et al. Age-dependent responses to hepatic ischaemia/reperfusion injury. Shock. 2005;24:421–427. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Hirose R, Coatney JL, Ferrell L, Behrends M, Roberts JP, et al. Ischaemia-reperfusion injury is more severe in older versus young rat livers. J Surg Res. 2007;137:96–102. doi: 10.1016/j.jss.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Detre KM, Lombardero M, Belle S, Beringer K, Breen T, Daily OP, et al. Influence of donor age on graft survival after liver transplantation – United Network for Organ Sharing Registry. Liver Transpl Surg. 1995;1:311–319. doi: 10.1002/lt.500010507. [DOI] [PubMed] [Google Scholar]
- 15.Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, et al. Liver retransplantation for primary non-function: analysis of a 20-year single-centre experience. Liver Transpl. 2007;13:227–233. doi: 10.1002/lt.20992. [DOI] [PubMed] [Google Scholar]
- 16.Buckel E, Sanchez-Urdazpal L, Steers J, Sterioff S, Wiesner R, Krom RA. Impaired initial function in liver grafts from donors >50 years of age. Transplant Proc. 1993;25:1558–1559. [PubMed] [Google Scholar]
- 17.Alexander JW, Vaughn WK. The use of marginal donors for organ transplantation. The influence of donor age on outcome. Transplantation. 1991;51:135–141. doi: 10.1097/00007890-199101000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Adam R, Sanchez C, Astarcioglu I, Bismuth H. Deleterious effect of extended cold ischaemia time on the post-transplant outcome of aged livers. Transplant Proc. 1995;27:1181–1183. [PubMed] [Google Scholar]
- 19.Reese PP, Sonawane SB, Thomasson A, Yeh H, Markmann JF. Donor age and cold ischaemia interact to produce inferior 90-day liver allograft survival. Transplantation. 2008;85:1737–1744. doi: 10.1097/TP.0b013e3181722f75. [DOI] [PubMed] [Google Scholar]
- 20.Tullius SG, Reuzel-Selke A, Ergmann F, Nieminen-Kelha M, Jonas S, Bechstein WO, et al. Contribution of prolonged ischaemia and donor age to chronic allograft dysfunction. J Am Soc Nephrol. 2000;11:1317–1324. doi: 10.1681/ASN.V1171317. [DOI] [PubMed] [Google Scholar]
- 21.Martins PN, Pratschke J, Pascher A, Fritsche L, Frei U, Neuhaus P, et al. Age and immune response in organ transplantation. Transplantation. 2005;79:127–132. doi: 10.1097/01.tp.0000146258.79425.04. [DOI] [PubMed] [Google Scholar]
- 22.Martins PN, Chandraker A, Tullius SG. Modifying graft immunogenicity and immune response prior to transplantation: potential clinical applications of donor and graft treatment. Transpl Int. 2007;19:351–359. doi: 10.1111/j.1432-2277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 23.Terasaki PI, Gertson DW, Cecka JM, Cho YW. Significance of the donor age effect on kidney transplants. Clin Transplant. 1997;11:366–372. [PubMed] [Google Scholar]
- 24.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(Suppl):45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 25.Calne RY. Immunological tolerance – the liver effect. Immunol Rev. 2000;174:280–282. doi: 10.1034/j.1600-0528.2002.017419.x. [DOI] [PubMed] [Google Scholar]
- 26.Popper H. Ageing and the liver. Prog Liver Dis. 1986;8:659–683. [PubMed] [Google Scholar]
- 27.Woodhouse KW, James OFW. Hepatic drug metabolism and ageing. Br Med Bull. 1990;46:22–35. doi: 10.1093/oxfordjournals.bmb.a072387. [DOI] [PubMed] [Google Scholar]
- 28.Schnegg M, Lauterburg BH. Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation DH. J Hepatol. 1986;3:164–171. doi: 10.1016/s0168-8278(86)80022-8. [DOI] [PubMed] [Google Scholar]
- 29.Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975;55:347–359. doi: 10.1172/JCI107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen HR, Martin P, Goss J, Donovan J, Melinek J, Rudich S, et al. Significance of early aminotransferase elevation after liver transplantation. Transplantation. 1998;65:68–72. doi: 10.1097/00007890-199801150-00013. [DOI] [PubMed] [Google Scholar]
- 31.Santori G, Andorno E, Morelli N, Gianelli A, Castiglione A, Casaccia M, et al. Impact of ischaemia/reperfusion on transplanted livers procured from elderly cadaveric donors. Transplant Proc. 2004;36:2909–2913. doi: 10.1016/j.transproceed.2004.10.069. [DOI] [PubMed] [Google Scholar]
- 32.Deschênes M, Forbes C, Tchervenkov J, Barkun J, Metrakos P, Tector J, et al. Use of older donor livers is associated with more extensive ischaemic damage on intraoperative biopsies during liver transplantation. Liver Transpl Surg. 1999;5:357–361. doi: 10.1002/lt.500050501. [DOI] [PubMed] [Google Scholar]
- 33.Sakai Y, Zhong R, Garcia B, Wall WJ. Tolerance by old livers of prolonged periods of preservation in the rat. Transplantation. 1993;55:18–23. doi: 10.1097/00007890-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Makowka L, Gordon RD, Todo S, Ohkohci N, Marsh JW, Tzakis AG, et al. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19(1 Pt 3):2378–2382. [PMC free article] [PubMed] [Google Scholar]
- 35.Greig PD, Woolf H, Sinclair SB, Abecassis M, Strasberg SM, Taylor BR, et al. Treatment of primary liver graft non-function with prostaglandin E1. Transplantation. 1989;48:447–453. doi: 10.1097/00007890-198909000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Moore DE, Feurer ID, Speroff T, Gorden DL, Wright JK, Chari RS, et al. Impact of donor, technical, and recipient risk factors on survival and quality of life after liver transplantation. Arch Surg. 2005;140:273–277. doi: 10.1001/archsurg.140.3.273. [DOI] [PubMed] [Google Scholar]
- 37.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of longterm outcomes of 3200 liver transplantations over two decades: a single-centre experience. Ann Surg. 2005;241:905–916. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organ Procurement and Transplantation Network (OPTN) OPTN home page. 2010. http://optn.transplant.hrsa.gov/. [Accessed June 2010.
- 39.Grande L, Matus D, Rimola A, Manyalic M, Cabrer C, García-Valdecasas JC, et al. Expanded liver donor age over 60 years for hepatic transplantation. Clin Transpl. 1998:297–301. [PubMed] [Google Scholar]
- 40.Zhao Y, Lo CM, Liu CL, Fan ST. Use of elderly donors (60 years) for liver transplantation. Asian J Surg. 2004;27:114–119. doi: 10.1016/S1015-9584(09)60323-7. [DOI] [PubMed] [Google Scholar]
- 41.Gastaca M, Valdivieso A, Pijoan J, Errazti G, Hernandez M, Gonzalez J, et al. Donors older than 70 years in liver transplantation. Transplant Proc. 2005;37:3851–3854. doi: 10.1016/j.transproceed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Zapletal C, Faust D, Wullstein C, Woeste G, Caspary WF, Golling M, et al. Does the liver ever age? Results of liver transplantation with donors above 80 years of age. Transplant Proc. 2005;37:1182–1185. doi: 10.1016/j.transproceed.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 43.Mangus RS, Fridell JA, Vianna RM, Kwo PY, Chestovich P, Milgrom ML, et al. No difference in clinical transplant outcomes for local and imported liver allografts. Liver Transpl. 2009;15:640–647. doi: 10.1002/lt.21726. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CD, Vachharajani N, Doyle M, Lowell JA, Wellen JR, Shenoy S, et al. Advanced donor age alone does not affect patient or graft survival after liver transplantation. J Am Coll Surg. 2008;207:847–852. doi: 10.1016/j.jamcollsurg.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Cescon M, Grazi GL, Cucchetti A, Ravaioli M, Ercolani G, Vivarelli M, et al. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14:672–679. doi: 10.1002/lt.21433. [DOI] [PubMed] [Google Scholar]
- 46.Segev DL, Maley WR, Simpkins CE, Locke JE, Nguyen GC, Montgomery RA, et al. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatology. 2007;46:1907–1918. doi: 10.1002/hep.21888. [DOI] [PubMed] [Google Scholar]
- 47.Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, et al. Optimal utilization of donor grafts with extended criteria: a single-centre experience in over 1000 liver transplants. Ann Surg. 2006;243:748–753. doi: 10.1097/01.sla.0000219669.84192.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruttadauria S, Vizzini G, Biondo D, Mandala L, Volpes R, Palazzo U, et al. Critical use of extended criteria donor liver grafts in adult-to-adult whole liver transplantation: a single-centre experience. Liver Transpl. 2008;14:220–227. doi: 10.1002/lt.21359. [DOI] [PubMed] [Google Scholar]
- 49.Neipp M, Bektas H, Lueck R, Ceylan D, Becker T, Klempnauer J, et al. Liver transplantation using organs from donors older than 60 years. Transpl Int. 2004;17:416–423. doi: 10.1007/s00147-004-0735-2. [DOI] [PubMed] [Google Scholar]
- 50.Hoofnagle JH, Lombardero M, Zetterman RK, Lake J, Porayko M, Everhart J, et al. Donor age and outcome of liver transplantation. Hepatology. 1996;24:89–96. doi: 10.1002/hep.510240116. [DOI] [PubMed] [Google Scholar]
- 51.Brown RS, Jr, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant. 2005;5:203–204. doi: 10.1111/j.1600-6143.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 52.Cassuto JR, Patel SA, Tsoulfas G, Orloff MS, Abt PL. The cumulative effects of cold ischaemic time and older donor age on liver graft survival. J Surg Res. 2008;148:38–44. doi: 10.1016/j.jss.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Segev DL, Kucirka LM, Nguyen GC, Cameron AM, Locke JE, Simpkins CE, et al. Effect modification in liver allografts with prolonged cold ischaemic time. Am J Transplant. 2008;8:658–666. doi: 10.1111/j.1600-6143.2007.02108.x. [DOI] [PubMed] [Google Scholar]
- 54.Kemmer N, Secic M, Zacharias V, Kaiser T, Neff GW. Longterm analysis of primary non-function in liver transplant recipients. Transpl Proc. 2007;39:1477–1480. doi: 10.1016/j.transproceed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold ischaemia time on primary non-function and patient and graft survival in liver transplantation: a meta-analysis. PLoS ONE. 2008;3:e2468. doi: 10.1371/journal.pone.0002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tekin K, Imber CJ, Atli M, Gunson BK, Bramhall SR, Mayer D, et al. A simple scoring system to evaluate the effects of cold ischaemia on marginal liver donors. Transplantation. 2004;77:411–416. doi: 10.1097/01.TP.0000110318.70879.20. [DOI] [PubMed] [Google Scholar]
- 57.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]


