Abstract
Since most solid tumor growth depends on angiogenesis, non-invasive imaging of tumor angiogenesis can allow for much earlier diagnosis and better prognosis of cancer, as well as more accurate treatment monitoring, which will eventually lead to personalized molecular medicine. CD105, also known as endoglin, is required for endo-thelial cell proliferation. The currently accepted standard method for quantifying tumor angiogenesis is to assess microvessel density based on CD105 staining, which has been shown to be an independent prognostic factor for survival in patients of almost all solid tumor types. In this review, we will summarize the progress to date on multimo-dality molecular imaging of CD105 expression during tumor angiogenesis which includes targeted contrast-enhanced ultrasound, molecular magnetic resonance, near-infrared fluorescence, single-photon emission computed tomography, and positron emission tomography. Although molecular imaging of CD105 expression is surprisingly understudied, non-invasive imaging of CD105 expression has already been achieved with every single molecular imaging modality. In the future, significant research effort should be directed towards non-invasive visualization of CD105 expression, such as quantitative imaging, the use of long-lived isotopes for antibody-based imaging, development of peptide, small molecule, or antibody fragment-based imaging agents, multimodality imaging of CD105 expression with a single agent, the application of nanotechnology, among others.
Keywords: Tumor angiogenesis, CD105 (Endoglin), molecular imaging, positron emission tomography (PET), single-photon emission computed tomography (SPECT), monoclonal antibody (mAb), cancer, anti-angiogenic therapy
Introduction
Cancer is the second leading cause of death in the United States (http://www.cdc.gov). In 2010, a total of 1,529,560 new cancer cases and 569,490 deaths from cancer are projected to occur in the United States alone [1]. One of the key requirements during tumor development is angiogenesis, the formation of new blood vessels, without which the tumor cannot grow beyond a few millimeters in diameter [2, 3]. Tumor angiogenesis is regulated by a variety of proteins such as growth factors/growth factor receptors, G-protein-coupled receptors for an-giogenesis-modulating proteins, endogenous angiogenesis inhibitors, integrins, among others [3-5]. The fact that tumor progression is dependent on angiogenesis has inspired scientists to search for anti-angiogenic molecules and design anti-angiogenic strategies for cancer treatment and prevention of cancer recurrence/metastasis [6, 7].
Many traditional medical imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound, have been routinely used to monitor the therapeutic effects of cancer intervention [8, 9]. However, with the shift in drug discovery from conventional cytotoxic drugs to novel agents against specific molecular targets, these conventional imaging modalities are usually no longer adequate. Molecular imaging, “the visualization, characterization and measurement of biological processes at the molecular and cellular levels in humans and other living systems” [10], has evolved dramatically over the last decade and played an increasingly more important role in cancer diagnosis and patient management. In general, molecular imaging modalities include molecular MRI (mMRI), magnetic resonance spectroscopy (MRS), optical bioluminescence, optical fluorescence, targeted contrast-enhanced ultrasound, single photon emission computed tomography (SPECT), and positron emission tomography (PET) [11]. Many hybrid systems that combine two or more of these imaging modalities are also commercially available (both clinically and pre-clinically) and certain others are under active development [12-14].
Non-invasive molecular imaging of tumor angio-genesis can allow for much earlier diagnosis and better prognosis of cancer, as well as more accurate treatment monitoring, which will eventually lead to personalized molecular medicine. Over the last decade, many tumor angiogenesis-related targets have been explored for imaging and therapeutic applications to fight cancer. Among these, two most extensively studied targets are vascular endothelial growth factor receptors (VEGFRs) and integrin αvβ3, for which several excellent review articles are available [7, 15-22].
CD105, also known as endoglin, is a member of the TGF-β family of receptors that is required for endothelial cell proliferation [23, 24]. The currently accepted standard method for quantifying tumor angiogenesis is to assess microvessel density (MVD) based on CD105 immunohisto-chemistry (IHC). Not surprisingly, CD105-based MVD is an independent prognostic factor for survival in patients of almost all solid tumor types [25-27]. One key feature of CD105 is that it is selectively expressed on angiogenic endothelial cells at significantly higher levels (up to 3 × 106 copies per cell) than other angiogenesis-related targets such as the VEGFRs (< 0.2 × 106 copies per cell) [28, 29]. Therefore, non-invasive imaging of CD105 expression has the potential to accelerate drug development by providing a reliable measure of angiogenesis in the entire body of an intact system, thereby facilitating individualized treatment monitoring and dose optimization in animal models, clinical trials, and ultimately in the day-to-day management of cancer patients. In this review, we will summarize the progress to date on multimodal-ity molecular imaging of CD105 expression during tumor angiogenesis.
Targeted contrast-enhanced ultrasound
Ultrasonography is the most commonly used clinical imaging modality due to its safety, low cost, ease of use, and wide availability [30]. Ultrasound contrast agents have been used for a variety of applications such as blood pool enhancement, characterization of liver lesions, and perfusion imaging [31, 32]. These contrast agents are typically small acoustically active particles (e.g. microbubbles) ranging from several hundred nanometers to a few micrometers in diameter [33]. Targeting is achieved either through manipulatingthe chemical properties of the microbubble shell or through conjugation of target-specific ligands to the microbubble surface [30, 34]. Since microbubbles are too large to extravasate, the disease process must be characterized by molecular changes in the vascular compartment to be imaged, which makes CD105 an ideal target.
In one study, avidin (Av) was incorporated into the shell of perfluorocarbon-exposed sonicated dextrose albumin microbubbles (Av-MBs) to anchor biotinylated monoclonal antibodies (mAbs) [35]. A rat anti-mouse CD105 mAb (MJ7/18) and an isotype-matched control mAb were investigated for biotinylation, microbubble incorporation, and cellular studies. It was found that MJ7/18-conjuagted microbubbles bound specifically to endothelial cells but not fibroblasts, while the control mAb-conjugated Av-MBs did not exhibit CD105-specific targeting.
After demonstrating the proof-of-principle, a follow-up study was carried out to follow the vascular response of therapy in mouse models of subcutaneous and orthotopic pancreatic ade-nocarcinoma [36]. For comprehensive investigation of angiogenesis in tumor-bearing mice treated with anti-VEGF mAbs and/or gemcit-abine (a nucleoside analog with known activity against pancreatic adenocarcinoma [37]), the localization of microbubbles targeting CD105, VEGFR-2, or VEGF-activated blood vessels (the VEGF-VEGFR complex) was monitored by ultrasound. In the subcutaneous model, receptor-targeted microbubbles gave significantly better enhancement of tumor vasculature than the non-targeted or control mAb-conjugated micro-bubbles. In addition, video intensity from targeted microbubbles correlated with the level of target expression (CD105, VEGFR-2, or the VEGF-VEGFR complex), as well as with MVD in tumors under either anti-angiogenic or cytotoxic therapy. Together, these two studies demonstrated that microbubble-based targeted ultrasound represents an attractive non-invasive tool for imaging tumor angiogenesis and monitoring the therapeutic effect of various anti-cancer therapies.
Although exhibiting relatively high spatial resolution (50-500 μm), ultrasound has certain disadvantages such as relatively poor tissue penetration (usually a few centimeters depending on the frequency used) and limited sensitivity [11]. Further development of targeted ultrasound will involve the expansion of targeted disease states, improvements in technology for ligand attachment to microbubbles, better characterization of the acoustic behavior of targeted contrast agents, and development of more sensitive/accurate imaging methods. Acoustic destruction of “payload-bearing” microbubbles has been used to deliver drugs or to augment gene transfection [38]. Therefore, CD105-targeted microbubbles may also have future applications in tumor vasculature-targeted cancer therapy.
Molecular MRI
MRI is a non-invasive diagnostic technique based on the interaction of protons (or other nuclei) with each other and with surrounding molecules in a tissue of interest [39]. Different tissues have different relaxation times which can result in endogenous contrast for MRI. MRI has good spatial resolution (usually sub-millimeter level) with exquisite soft tissue contrast yet it suffers from inherent low sensitivity, which can be partially compensated for by working at higher magnetic fields (4.7 -14 T in small animal models), acquiring data for a much longer time period, and/or using exogenous contrast agents. Many exogenous agents can enhance the contrast in MRI by selectively shortening either the T1 (longitudinal) or T2 (transverse) relaxation time. Traditionally, gadolinium (Gd) chelates have been used to increase the T1 contrast [40] while iron oxide nanoparti-cles have been used to increase the T2 contrast [41].
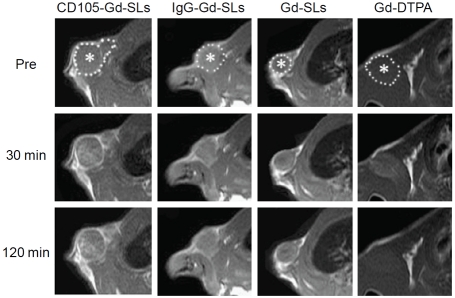
Recently, molecular MRI of CD105 expression in tumor-bearing rats was achieved with Gd-DTPA-containing stabilized liposomes (Gd-SLs) [42]. A series of targeted and non-targeted MRI contrast agents were compared in glioma-bearing rats: Gd-DTPA, Gd-SLs, Gd-SLs conjugated to anti-CD105 mAbs (CD105-Gd-SLs), and Gd-SLs conjugated to control mAbs (IgG-Gd-SLs). Serial T1-weighed MRI before and after contrast agent administration revealed that the area with enhanced MRI contrast was restricted for CD105-Gd-SLs but not for the other three groups (i.e. the MRI contrast enhancement were more diffused; Figure 1). In addition, the degree of contrast enhancement over time also varied between different groups. For example, Gd-DTPA gave an early contrast enhancement which peaked at 30 min and declined to baseline values at 60 min, while the signal intensity for CD105-Gd-SLs continued to increase over a period of 120 min. The signal intensity of IgG-Gd -SLs and Gd-SLs both peaked at 60 min followed by a decline, yet the rate of decease was quite different. Ex vivo histology further revealed that the enhancement in the CD105-Gd-SLs group resulted mainly from new microvessels. However, both mature microvessels and new microvasculature were responsible for contrast enhancement in the other three groups.
Figure 1.
Molecular MRI of CD105 expression. T1-weighted images at different time points post-injection of various contrasts agents are shown. *: subcutaneous tumor. Adapted from reference [42].
To date, this is the only report available in the literature for molecular MRI of CD105 expression. Although the proof-of-principle has been demonstrated for mMRI of CD105 expression [42], whether mMRI can significantly improve cancer patient management remains unclear. Since the major disadvantage of MRI is its inherent low sensitivity and Gd-based MRI can only be reliably detected at millimolar concentration, superparamagnetic iron oxide (SPIO) nanoparticles, which can be detected at much lower levels because of its high magnetism [41], deserve to be investigated for mMRI of CD105 in the future.
Near-infrared fluorescence (NIRF) imaging
Optical imaging is a relatively low-cost method suitable primarily for small animal studies. In fluorescence imaging, excitation light illuminates the subject and the emission light is collected at a shifted wavelength [43]. The major types of contrast agents used for fluorescence imaging include organic dyes [44], fluorescent proteins [45], and quantum dots [46]. The main drawback of fluorescence imaging is that it is typically not quantitative and the image information is surface-weighted due to tissue absorption. In most cases, significant background signal is also observed because of tissue autofluo-rescence. For in vivo applications, imaging in the near-infrared region (NIR; 700 - 900 nm), where the absorbance spectra for all bio-molecules reach minima thus providing a clear optical window [47], can provide better opportunities for visualizing tumor angiogenesis in both small animal models and various clinical scenarios.
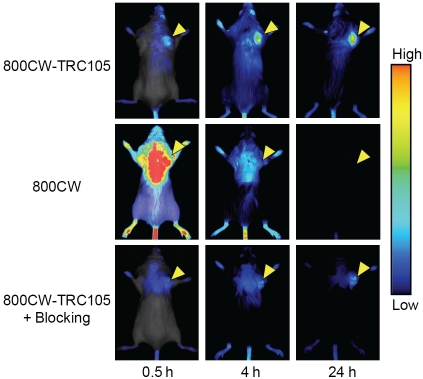
One study investigated the applicability of a human umbilical vein endothelial cell (HUVEC)-based in vitro model to mimic physiological and angiogenic vasculature [48]. High fluorescence signal was observed in proliferating HUVECs due to CD105 expression. We recently investigated tumor angiogenesis in a mouse tumor model through NIRF imaging of CD105 expression [49]. TRC105, a human/murine chimeric mAb which binds to both human and murine CD105, was conjugated to a NIRF dye (800CW). After confirming the CD105-binding affinity/specificity of 800CW-TRC105 in vitro, in vivo/ex vivo NIRF imaging, blocking studies, and ex vivo histology were performed on 4T1 murine breast tumor-bearing mice to evaluate the ability of 800CW-TRC105 to target tumor angiogenesis. Serial NIRF imaging after intravenous injection of 800CW-TRC105 revealed that the 4T1 tumor could be clearly visualized as early as 30 minutes post-injection (Figure 2). Tumor uptake of 800CW-TRC105 plateaued at about 24 h post-injection with excellent tumor contrast. In vivo target specificity of 800CW-TRC105 was further confirmed with several control studies (e.g. blocking studies and imaging with unconjugated 800CW dye).
Figure 2.
Optical imaging of CD105 expression. The 4T1 murine breast cancer tumors (arrowheads) in Balb/c mice can be clearly visualized in a small animal optical scanner after intravenous injection of 800CW-TRC105. The CD105 specificity was confirmed by the much lower tumor uptake of 800CW or 800CW-TRC105 when co-injected with TRC105 (denoted as “800CW-TRC105 + Blocking”).
The abovementioned study represents the first successful example of NIRF imaging of CD105 expression in vivo. Due to the poor tissue penetration and intense scattering of light, optical imaging (even in the NIRF region) will only be possible in the clinical setting in limited sites such as the tissues and lesions close to the skin surface, tissues accessible by endoscopy, and during intra-operative visualization. In future studies, spectral imaging techniques (where fluorescence signals can be separated based on the emission spectra of different fluorophores [50]) and fluorescence-mediated tomography [51, 52] may also facilitate accurate interpretation of the fluorescence imaging data.
Single-photon emission computed tomography
Radionuclide-based imaging techniques (i.e. SPECT and PET) have been routinely used in the clinic over the last decade. Because of the wider availability of gamma cameras and SPECT scanners in the past, CD105 imaging was achieved with SPECT earlier than with PET. Due to the use of collimators to define the angle of incidence of emitted gamma rays [53], SPECT imaging has a very low detection efficiency (< 10−4). Common radioisotopes used for SPECT imaging are 99mTc (t1/2: 6.0 h), 111In (t1/2: 2.8 d), 123I (t1/2: 13.2 h), and 131I(t1/2: 8.0 d).
One pioneering study investigated an 111In-labeled anti-CD105 mAb (MJ7/18) and compared its neovascular binding, tumor accumulation, and in vivo behavior to an isotype-matched control mAb [54]. In a B16 melanoma model, the tumors in animals receiving 111In-labeled MJ7/18 were more easily identified than animals receiving the radiolabeled control mAb. However, the tumor contrast was only modest. Ex vivo autoradiography and histology experiments of the tumor sections corroborated the different patterns of in vivo tumoral accumulation for the two antibodies. MJ7/18 exhibited intense activity in the peripheral region of the tumor, where the highest concentration of vessels was found, and much lower activity in the tumor centre. On the other hand, little accumulation of activity could be found in the tumors of mice that had been injected with 111In-labeled control mAb. It was suggested that imaging of abundantly expressed endothelial targets could circumvent delivery barriers normally associated with other tumor targeting strategies and could potentially be used to quantitate molecular an-giogenic markers.
At about the same time, a 125I-labeled anti-CD105 mAb (MAEND3) was tested in a canine mammary carcinoma model [55]. After demonstrating differential expression of CD105 on human breast cancer and endothelial cells, two dogs with spontaneous mammary tumors were intravenously injected with 125I-labeled MAEND3 and imaged eight hours later. Rapid and intense uptake of the radiolabeled mAb with excellent tumor-to-background ratio was observed in the tumor areas of both dogs, which were confirmed as ductal mammary adenocarcinomas after surgical excision ten days later. Another study also explored the use of a 125I-labeled anti-CD105 mAb for radioimmunotherapy applications in mouse tumor models [56]. Significant growth suppression of the tumors was observed while a 125I-labeled control mAb did not show any significant anti-tumor efficacy.
A few years after these studies in preclinical models (mice and dogs respectively), a 99mTc-labeled anti-CD105 mAb (E9) was investigated in freshly excised kidneys from renal carcinoma patients [57]. Since E9 does not cross-react with animal tissues, this strategy can serve as clinically relevant ex vivo model. After perfusion of 99mTc-E9 through the renal artery, immunos-cintigraphy revealed the presence of well-defined radioactive hot spots, which matched the positions of the tumors as identified by pre-surgery MRI and subsequent histology. Gamma-counting revealed that the median values of radioactivity uptake per gram of wet weight were > 10 fold higher in the tumors than in normal kidney tissues. Not only was CD105 specificity of the tracer confirmed by blocking studies, immunescintigraphy with 99mTc-E9 was also able to identify tumors that were not detected during pre-surgery MRI. These findings warranted future investigation of the in vivo phar-macokinetics of 99mTc-E9 (preferably after hu-manization) in renal cancer patients.
Although the major advantage of SPECT imaging is that it can be used for simultaneous imaging of multiple radionuclides, since the gamma rays emitted from different radioisotopes can be differentiated based on the energy [58], thereby allowing simultaneous detection of multiple biological events with multiple isotopes, such strategy has rarely been adopted. Another imaging modality, PET, offers many advantages over SPECT (e.g. much higher sensitivity) and the increasing popularity of clinical PET and PET/CT scanners can facilitate clinical translation of promising new PET tracers.
Positron emission tomography
PET was first developed in the mid-1970s [59]. The most widely used PET isotopes include 11C (t1/2: 20 min), 18F (t1/2: 110 min), and 64Cu (t1/2: 12.8 h). Over the last several years, PET imaging with 64Cu has become increasingly more popular and significant research effort has been devoted to the development of ligands that can stably chelate 64Cu [60]. Since the targeting ligands used for CD105 imaging to date are exclusively mAbs which have relatively long circulations half-lives (hours to days), long-lived PET isotopes (e.g. 64Cu) are desirable for CD105 -targeted radioimmunoPET imaging.
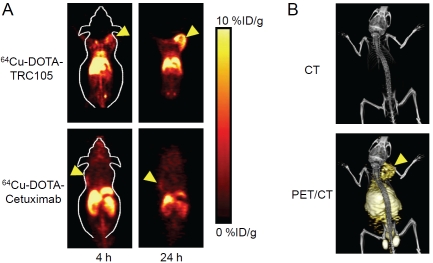
The abovementioned TRC105, with a very high avidity for human CD105 (KD: 2 ng/mL), is currently in a multicenter Phase 1 first-in-human dose-escalation trial at multiple centers in the United States [61]. Multiple Phase 2 therapy trials are planned or underway in patients with various solid cancer types. To investigate the in vivo pharmacokinetics and tumor targeting efficacy, we recently labeled TRC105 with 64Cu for PET imaging of CD105 expression [62]. In vitro, in vivo, and ex vivo studies were performed on 4T1 murine breast tumor-bearing mice to evaluate the ability of 64Cu-DOTA-TRC105 (DOTA denotes 1,4,7,10-tetraazacyclododecane-1,4,7,10 -tetraacetic acid) to target tumor angiogenesis. In vitro, FACS analysis and fluorescence microscopy revealed no difference in CD105 binding affinity between TRC105 and DOTA-TRC105. In vivo, serial PET imaging revealed that the 4T1 tumor uptake of the tracer was fast, prominent, persistent, and CD105-specific (Figure 3), which was further validated by several control experiments (e.g. with 64Cu-labeled cetuximab, an isotype matched mAb which binds to the human epidermal growth factor receptor [63]). At late time points, the tumor uptake was higher than most organs which provided excellent tumor contrast.
Figure 3.
PET imaging of CD105 expression. A. Coronal PET images of 4T1 tumor-bearing mice at 4 and 24 h post-injection of 64Cu-DOTA-TRC105 or 64Cu -DOTA-cetuximab. B. CT and PET/CT images of 64Cu -DOTA-TRC105 in 4T1 tumor-bearing mice at 24 h post-injection. Arrowheads indicate the 4T1 tumors.
This study represents the first successful example of PET imaging of CD105 expression. Since TRC105 is already in clinical investigation and therapeutic efficacy has been shown in various animal tumor models [28, 64, 65] and certain cancer patients, this study identifies a new perspective for tumor angiogenesis-related research and warrants future clinical translation of 64Cu-DOTA-TRC105, where it may be used to evaluate the pharmacokinetics, tumor targeting efficacy, dose optimization, and dose interval of TRC105 and TRC105-based anti-cancer agents in the clinic.
Conclusion and future perspectives
Given the pivotal role CD105 plays during tumor angiogenesis, molecular imaging of CD105 expression is surprisingly understudied. Nonetheless, non-invasive imaging of CD105 has been achieved with every single molecular imaging modality (Table 1). Non-invasive imaging of CD105 expression during tumor angiogenesis has clinical applications in many aspects: lesion detection, patient stratification, new drug development/validation, treatment monitoring, and dose optimization. Quantitative correlation of tracer uptake with CD105 expression level would be highly desirable for future treatment monitoring applications, as it would be ideal to non-invasively measure the changes of CD105 expression quantitatively, rather than qualitatively, in each individual patient upon anti-angiogenic therapies. Much further effort should be directed towards the development of clinically translatable CD105-targeted imaging agents, which can be widely used in anti-cancer clinical trials thereby paving the road to personalized medicine.
Table 1.
CD105 expression has been imaged with every single molecular imaging modality
| Modality | mAb | Image Label | Model | References |
|---|---|---|---|---|
| Ultrasound | MJ7/18 | microbubble | pancreatic cancer | [35, 36] |
| MRI | not identified | Gd-DTPA | glioma | [42] |
| Fluorescence | TRC105 | IRDye 800CW | breast cancer | [48, 49] |
| SPECT | MJ7/18, E9, MAEND3 | 111In, 125I, 99mTc | melanoma, canine mammary carcinoma, renal carcinoma | [54, 55, 57] |
| PET | TRC105 | 64Cu | breast cancer | [62] |
Due to excellent sensitivity and tissue penetration, radionuclide-based imaging techniques (i.e. SPECT and PET) possess significantly higher potential than non-radionuclide-based techniques. To date, most of the CD105-targeted agents are based on mAbs. To provide more insight about the long-term behavior of TRC105 in vivo, other longer lived isotopes (e.g. 89Zr, 74As, etc.) may be explored in future studies. The advantages of antibody-based tracers are that they are quite antigen-specific and have high binding affinity and absolute tumor uptake, which makes them suitable for internal radiotherapy applications (e.g. after labeling with 90Y or 177Lu [66]) and/or targeted delivery of drugs.
The major limitations of antibody-based imaging are slow tumor accumulation and high background signal in the reticuloendothelial system, which may be overcome by peptide, small molecule, or antibody fragment-based tracers since they typically exhibit fast blood clearance thereby can be labeled with 11C or 18F for PET to confer higher throughput. High-affinity CD105-binding peptides could be generated from various high-throughput screening strategies such as phage display. Anti-CD105 antibody fragments, both human and murine [67, 68], as well as certain bi-specific antibodies [69, 70], may also be investigated in the future for imaging and potential therapeutic applications.
Multimodality imaging of CD105 expression, where the same probe can be simultaneously detected by two or more imaging modalities, should be developed in the future. By combining the advantages of various imaging modalities, quantitative and more accurate information can be obtained which no single modality alone can offer. Dualmodality probes that combine radionuclide-based imaging (very sensitive and highly quantitative) and non-radionuclide based approaches, for example optical imaging which can significantly facilitate ex vivo validation of the in vivo data and MRI probe which can provide high resolution anatomical information, are of particular interest.
Nanotechnology may also have many applications in CD105-targeted imaging and therapy in the future. Since many nanoparticles suffer from poor extravasation [22, 71, 72], CD105 is an ideal target for cancer nanomedicine as extravasation is not required to achieve tumor contrast or uptake. Lastly, the anti-cancer agents developed for CD105 targeting can also have broad applications in many other angio-genesis-related diseases [73].
Acknowledgments
The authors acknowledge financial support from the UW School of Medicine and Public Health's Medical Education and Research Committee through the Wisconsin Partnership Program, the University of Wisconsin Carbone Cancer Center, NCRR 1UL1RR025011, a Susan G. Komen Postdoctoral Fellowship, and a DOD PCRP IDEA Awa rd.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 7.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin avb3 antagonism. Anti-Cancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 8.Gwyther SJ. New imaging techniques in cancer management. Ann Oncol. 2005;16(Suppl 2):ii63–70. doi: 10.1093/annonc/mdi727. [DOI] [PubMed] [Google Scholar]
- 9.Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 10.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18N–21N. [PubMed] [Google Scholar]
- 11.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 12.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 13.Even-Sapir E, Lerman H, Lievshitz G, Khafif A, Fliss DM, Schwartz A, Gur E, Skornick Y, Schneebaum S. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003;44:1413–1420. [PubMed] [Google Scholar]
- 14.Catana C, Wu Y, Judenhofer MS, Qi J, Pichler BJ, Cherry SR. Simultaneous acquisition of multislice PET and MR images: initial results with a MR-compatible PET scanner. J Nucl Med. 2006;47:1968–1976. [PubMed] [Google Scholar]
- 15.Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front Biosci. 2007;12:4267–4279. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- 16.Haubner R. avb3-integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging. 2006;33(Suppl 13):54–63. doi: 10.1007/s00259-006-0136-0. [DOI] [PubMed] [Google Scholar]
- 17.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 18.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 19.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin avb3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 20.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 21.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up anti-angiogenic drug development. Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 22.Hong H, Zhang Y, Sun J, Cai W. Molecular imaging and therapy of cancer with radio-labeled nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. (PMC2753977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 24.Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010;86:12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 25.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 26.Fonsatti E, Sigalotti L, Arslan P, Altomonte M, Maio M. Emerging role of endoglin (CD105) as a marker of angiogenesis with clinical potential in human malignancies. Curr Cancer Drug Targets. 2003;3:427–432. doi: 10.2174/1568009033481741. [DOI] [PubMed] [Google Scholar]
- 27.Rubatt JM, Darcy KM, Hutson A, Bean SM, Havrilesky LJ, Grace LA, Berchuck A, Secord AA. Independent prognostic relevance of mi-crovessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:469–474. doi: 10.1016/j.ygyno.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophos-phamide. Cancer Res. 2001;61:7846–7854. [PubMed] [Google Scholar]
- 29.Lee S, Mandic J, Van Vliet KJ. Chemome-chanical mapping of ligand-receptor binding kinetics on cells. Proc Natl Acad Sci U S A. 2007;104:9609–9614. doi: 10.1073/pnas.0702668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Progess and opportunities for clinical and research applications. IEEE Eng Med Biol Mag. 2004;23:18–29. doi: 10.1109/memb.2004.1360405. [DOI] [PubMed] [Google Scholar]
- 31.Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Jakobsen JA. Ultrasound contrast agents: clinical applications. Eur Radiol. 2001;11:1329–1337. doi: 10.1007/s003300100964. [DOI] [PubMed] [Google Scholar]
- 33.Stride E, Saffari N. Microbubble ultrasound contrast agents: a review. Proc Inst Mech Eng [H] 2003;217:429–447. doi: 10.1243/09544110360729072. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18:11–16. doi: 10.1016/j.copbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Korpanty G, Grayburn PA, Shohet RV, Brekken RA. Targeting vascular endothelium with avidin microbubbles. Ultrasound Med Biol. 2005;31:1279–1283. doi: 10.1016/j.ultrasmedbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 37.Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. doi: 10.3892/or_00000749. [DOI] [PubMed] [Google Scholar]
- 38.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardio-graphic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 39.Pathak AP, Gimi B, Glunde K, Ackerstaff E, Artemov D, Bhujwalla ZM. Molecular and functional imaging of cancer: Advances in MRI and MRS. Methods Enzymol. 2004;386:3–60. doi: 10.1016/S0076-6879(04)86001-4. [DOI] [PubMed] [Google Scholar]
- 40.de Roos A, Doornbos J, Baleriaux D, Bloem HL, Falke TH. Clinical applications of gadolin-ium-DTPA in MRI. Magn Reson Annu. 1988:113–145. [PubMed] [Google Scholar]
- 41.Thorek DL, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Feng XY, Henning TD, Wen L, Lu WY, Pan H, Wu X, Zou LG. MR imaging of tumor angiogenesis using sterically stabilized Gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2009;70:180–189. doi: 10.1016/j.ejrad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 44.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 45.van Roessel P, Brand AH. Imaging into the future: visualizing gene expression and protein interactions with fluorescent proteins. Nat Cell Biol. 2002;4:E15–20. doi: 10.1038/ncb0102-e15. [DOI] [PubMed] [Google Scholar]
- 46.Cai W, Hsu AR, Li ZB, Chen X. Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res Lett. 2007;2:265–281. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Vag T, Schramm T, Kaiser WA, Hilger I. Proliferating and quiescent human umbilical vein endothelial cells (HUVECs): a potential in vitro model to evaluate contrast agents for molecular imaging of angiogenesis. Contrast Media Mol Imaging. 2009;4:192–198. doi: 10.1002/cmmi.280. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Hong H, Zhang Y, Leigh BR, Cai W. In vivo near-infrared fluorescence imaging of CD105 expression. Mol Imaging Biol submitted. doi: 10.1007/s00259-011-1886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansfield JR, Gossage KW, Hoyt CC, Levenson RM. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J Biomed Opt. 2005;10:41207. doi: 10.1117/1.2032458. [DOI] [PubMed] [Google Scholar]
- 51.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 52.Montet X, Ntziachristos V, Grimm J, Weissleder R. Tomographic fluorescence mapping of tumor targets. Cancer Res. 2005;65:6330–6336. doi: 10.1158/0008-5472.CAN-05-0382. [DOI] [PubMed] [Google Scholar]
- 53.Peremans K, Cornelissen B, Van Den Bossche B, Audenaert K, Van de Wiele C. A review of small animal imaging planar and pinhole spect Gamma camera imaging. Vet Radiol Ultrasound. 2005;46:162–170. doi: 10.1111/j.1740-8261.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- 54.Bredow S, Lewin M, Hofmann B, Marecos E, Weissleder R. Imaging of tumour neovascula-ture by targeting the TGF-beta binding receptor endoglin. Eur J Cancer. 2000;36:675–681. doi: 10.1016/s0959-8049(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 55.Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6:2037–2043. [PubMed] [Google Scholar]
- 56.Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999;82:737–742. doi: 10.1002/(sici)1097-0215(19990827)82:5<737::aid-ijc18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Costello B, Li C, Duff S, Butterworth D, Khan A, Perkins M, Owens S, Al-Mowallad AF, O'Dwyer S, Kumar S. Perfusion of 99Tcm-labeled CD105 Mab into kidneys from patients with renal carcinoma suggests that CD105 is a promising vascular target. Int J Cancer. 2004;109:436–441. doi: 10.1002/ijc.11699. [DOI] [PubMed] [Google Scholar]
- 58.Berman DS, Kiat H, Van Train K, Friedman JD, Wang FP, Germano G. Dual-isotope myocar-dial perfusion SPECT with rest thallium-201 and stress Tc-99m sestamibi. Cardiol Clin. 1994;12:261–270. [PubMed] [Google Scholar]
- 59.Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM. Application of annihilation coincidence detection to transaxial reconstruction tomography. J Nucl Med. 1975;16:210–224. [PubMed] [Google Scholar]
- 60.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr Pharm Des. 2007;13:3–16. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- 61.Mendelson DS, Gordon MS, Rosen LS, Hurwitz H, Wong MK, Adams BJ, Alvarez D, Seon BK, Theuer CP, Leigh BR. Phase I study of TRC105 (anti-CD105 [endoglin] antibody) therapy in patients with advanced refractory cancer. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 62.Hong H, Yang Y, Zhang Y, Engle JW, Barnhart TE, Nickles RJ, Leigh BR, Cai W. Positron emission tomography imaging of CD105 expression during tumour angiogenesis. Eur J Nucl Med Mol Imaging submitted. doi: 10.1007/s00259-011-1765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xeno-graft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850–858. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 64.Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, Harada N, Uneda S, Tsujie T, Toi H, Tsai H, Haruta Y. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8:135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsujie M, Uneda S, Tsai H, Seon BK. Effective anti-angiogenic therapy of established tumors in mice by naked anti-human endoglin (CD105) antibody: differences in growth rate and therapeutic response between tumors growing at different sites. Int J Oncol. 2006;29:1087–1094. [PubMed] [Google Scholar]
- 66.Lee SY, Hong YD, Felipe PM, Pyun MS, Choi SJ. Radiolabeling of monoclonal anti-CD105 with 177Lu for potential use in radioimmunotherapy. Appl Radiat Isot. 2009;67:1366–1369. doi: 10.1016/j.apradiso.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 67.Volkel T, Holig P, Merdan T, Muller R, Kontermann RE. Targeting of immunoliposomes to endothelial cells using a single-chain Fv fragment directed against human endoglin (CD105) Biochim Biophys Acta. 2004;1663:158–166. doi: 10.1016/j.bbamem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Muller D, Trunk G, Sichelstiel A, Zettlitz KA, Quintanilla M, Kontermann RE. Murine endoglin-specific single-chain Fv fragments for the analysis of vascular targeting strategies in mice. J Immunol Methods. 2008;339:90–98. doi: 10.1016/j.jim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Nettelbeck DM, Miller DW, Jerome V, Zuzarte M, Watkins SJ, Hawkins RE, Muller R, Kontermann RE. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105) Mol Ther. 2001;3:882–891. doi: 10.1006/mthe.2001.0342. [DOI] [PubMed] [Google Scholar]
- 70.Korn T, Muller R, Kontermann RE. Bispecific single-chain diabody-mediated killing of en-doglin-positive endothelial cells by cytotoxic T lymphocytes. J Immunother. 2004;27:99–106. doi: 10.1097/00002371-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 72.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptidelabeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2010;299:H959–974. doi: 10.1152/ajpheart.01251.2009. [DOI] [PubMed] [Google Scholar]