Abstract
All forager (or hunter–gatherer) societies construct niches, many of them actively by the concentration of wild plants into useful stands, small-scale cultivation, burning of natural vegetation to encourage useful species, and various forms of hunting, collectively termed ‘low-level food production’. Many such niches are stable and can continue indefinitely, because forager populations are usually stable. Some are unstable, but these usually transform into other foraging niches, not geographically expansive farming niches. The Epipalaeolithic (final hunter–gatherer) niche in the Near East was complex but stable, with a relatively high population density, until destabilized by an abrupt climatic change. The niche was unintentionally transformed into an agricultural one, due to chance genetic and behavioural attributes of some wild plant and animal species. The agricultural niche could be exported with modifications over much of the Old World. This was driven by massive population increase and had huge impacts on local people, animals and plants wherever the farming niche was carried. Farming niches in some areas may temporarily come close to stability, but the history of the last 11 000 years does not suggest that agriculture is an effective strategy for achieving demographic and political stability in the world's farming populations.
Keywords: hunter–gatherer, forager, farmer, niche construction, origins of agriculture, low-level food production
1. Introduction
Niche construction has been much discussed by anthropologists and archaeologists, albeit under a variety of terminologies. In this contribution, we propose to look at hunting, gathering and farming as forms of niche construction. In humans, the creation of new niches may lead to both genetic and behavioural modifications or culture change. The modern farming environment or ‘artificial steppe’ is perhaps the ultimate form of niche construction by humans. But hunter–gatherers also construct niches in a variety of ways. Some of these are stable: once created, they may continue indefinitely, without any need for subsequent changes in human behaviour. Others are however unstable: change is inherent in such niches, and this sooner or later precipitates human cultural change.
The outcome of instability is that the niche is reconstructed. Usually these reconstructed niches are altered forms of hunting and gathering. But in some cases the niche is transformed into what we conventionally term farming. In this paper we (i) explore a variety of niche types constructed by hunter–gatherers. Some of these involved small-scale cultivation that caused genetic changes in the plants—the most simple working definition of domestication [1]. We will ask why these and other activities did not take off but remained small-scale. We then (ii) consider why just a few niches did take off and were transformed into what we consider ‘farming’; and finally we (iii) examine the ways in which the early farming niches were exported to cover a wide geographical area. The processes identified point to the conclusion that farming originated not as a deliberate process of intensifying resource production, but as a series of small, accidental changes in the way that niches were constructed.
2. Hunter–gatherers: stable and unstable niches
All hunter–gatherers remove animals and plants from the wild gene pool, and thus modify their ecological niche. In this section, we examine instances where their practices have gone beyond this and have amounted to active niche construction. We consider stable hunter–gatherer niches under four headings: the concentration of useful wild plants into accessible stands; small-scale plant cultivation; the burning of vegetation to encourage useful animals and plants; and hunting practices that modify animal populations. Only the second is likely to cause genetic change in the exploited species and thus qualify as ‘domestication’ (see above), but all four can usefully be termed ‘low-level food production’ [2,3].
(a). Concentration of wild plants
The Nukak of the Columbian Amazon take fruit that needs to be processed before eating back to their camp. By discarding non-edible parts of fruit, including seeds, near camp sites, they unconsciously intensify fruit tree production. This practice creates what Politis [4] calls ‘wild orchards’ in abandoned camp sites. Although old camp sites are not reoccupied, the concentration of fruit trees creates small patches in the ecosystem, reducing subsequent travel time to harvest fruit. Politis points out that because the forest canopy is not cleared (as it is in horticulture and swidden cultivation), weed growth that would otherwise choke wild food plants is prevented. The more intensive husbandry of nut trees during the later Jomon in Japan (see below) may represent a development of such practices. In view of the previously argued difficulties of hunting and gathering in tropical forests, might such behaviour have been more widespread in such environments? Archaeologists are divided on the capacity of tropical forests to support hunting and gathering. Various authors [5,6] have persuasively argued that hunter–gatherers could only survive in tropical forest if they had access to cultivated plant resources obtained from neighbouring farmers. More recently, Froment (2000, personal communication) and Fairbairn et al. [7] have argued for more nuanced interpretations. Fromont reports that wild yams are sufficiently dense in some parts of the African tropical forest to support pure hunting and gathering, but not in other parts [8]. Fairbairn et al. [7] agree that the forests of the New Guinea highlands contain few plant foods, but argue that the first human colonists may have used selective burning as early as 30 000 BP to increase the productivity of fruit-bearing pandanus plants.
Altman [9] dispelled the myth created by McCarthy & McArthur [10] that hunter–gatherers in Arnhem Land (Northern Australia) need only work 4 to 5 h per day to obtain enough food. Altman lived with an Aboriginal band for an entire year, and discovered that in the days before purchased food was available the three wet season months would have been the most difficult to survive. Altman [8, pp. 80–1, 90–1] calculates that, if women had worked at gathering 63 h per week during the wet season (February–April), the highest productivity they could have achieved would have been 1600–1800 kcal d−1. Altman's findings are supported by those of Jones [11] that, during the critical month of April, women would have had to dig for yams 28 out of 30 days to provide their 50–60% contribution to the diet. Such seasonal bottlenecks may well explain why hunter–gatherers normally appear to live at below the carrying capacity of the land ([12] gives other examples).
Jones & Meehan [13] documented the practice, in Arnhem Land, northern Australia, of leaving the top of the tubers of harvested long yams (Dioscorea transversa) in the ground, and one of us (R.L.) was present when, during a 1974 fieldtrip to Cape York, a local Aboriginal man, Bill McGreen, described the same practice to David Harris. Aboriginal women told Jones & Meehan that ‘plants left in this condition will grow again the following year’. Given the vital importance of yams in the annual food gathering cycle, why is their husbandry not intensified? Jones [11, p. 139] speculates as to why yams were not more intensively husbanded in Arnhem Land. He concludes that the prolonged dry season precludes intensification, in contrast to wetter environments in New Guinea Highlands. As we describe below, however, yams were intensively husbanded in southwest Australia until colonial expropriation of the land.
(b). Small-scale plant cultivation
Various groups of humans conventionally regarded as hunter–gatherers have in fact cultivated plants on a small scale. In eastern North America before the arrival of maize cultivation from Mexico around AD 1000, several native species were cultivated. This is demonstrated by the genetic changes that cultivation caused; these species therefore qualify as ‘domestic’ under the definition put forward above. The seeds of squash (Cucurbita pepo ssp. ovifera) become larger from 2500 BC, testifying to human selection. Sumpweed (Iva annua) and sunflower (Helianthus annuus var. macrocarpus) provide similar evidence from 2000 BC. In goosefoot (Chenopodium berlandieri ssp. jonesianum) the domestic form has a thinner testa (seed coat) than the wild forms. A thinner testa means reduced dormancy, i.e. the seed germinates faster, so this suggests artificial selection for early germinating individuals. Cultivation of this plant started around 1500 BC and was recorded by Europeans until the eighteenth century; the cultivar is, however, now extinct and known only from the archaeological record. These genetic changes must have been engineered by the repeated planting of seeds from plants with the desired characteristics [3,14–16]. Maygrass (Phalaris caroliniana) and little barley (Hordeum pusillum) were not apparently genetically modified, but are found in archaeological contexts well outside their wild range, suggesting that their distributions were extended by cultivation [15]. Tobacco cultivation also has an antiquity of several millennia in this region, although it is not known whether this was of native Nicotiana attenuata or Nicotiana trigonophylla, or of domestic Nicotiana rustica introduced from Mexico, because the seeds are indistinguishable [17].
In Japan, rice cultivation arrived in the first millennium BC. Hunter–gatherers prior to this lived mainly on nuts and marine resources, but also made use of cultivated millets and/or their wild relatives. It is, however, impossible to distinguish between wild barnyard grass (Echinochloa crus-galli) and its domestic relative Japanese millet Echinochloa esculenta, and the same is true for species of Setaria (domesticated foxtail millet and its wild relatives). Other plants including Chenopodium spp. and the beefsteak plant (Perilla frutescens) are also found. Genetic change has not been suggested for these plants, but it is possible that they might have been cultivated. None of these ever rivalled nuts or fish in importance [18,19]. Reduced genetic diversity has however been detected in archaeological remains of chestnuts (Castanea crenata) dating to ca 4500–2500 BC. This suggests that chestnut trees were under prolonged human selection [20].
Early British explorers in Southwest Australia during the 1830s and 1840s reported that Native communities practised husbandry of wild Dioscorea. George Grey, quoted in Hallam [21, p. 139], described an extensive area perhaps measuring 5–6 km by 2 km covered in a ‘light fertile soil quite overrun with warran plants … a species of Dioscorea, a sort of yam like the sweet potatoe’, approached by permanent paths and watered by deliberately constructed dykes. He also recorded two native villages containing substantial huts that were apparently permanently occupied, with ‘well-marked roads, deeply sunk wells and extensive warran grounds’, These were not exceptional, and Hallam quotes numerous similar records from the same region, some of which indicate that production of wild flags was also intensified to enable permanent residence during the time of year that yams were regenerating. Hallam interprets such practices as an intensification of the wild yam harvesting recorded by Jones in Arnhem Land (see above), and concludes that archaeological evidence indicates Dioscorea cultivation had been practised for about 4000 years.
The Southwest Australian case may have resembled that recorded ethnographically among the Nuaulu of Eastern Indonesia, who both collect wild sago from Metroxylon palms, and cultivate these palms in swiddens. The Nuaulu have a mixed economy, combining hunting and gathering with cultivation of coconut and sago. Ellen [22] calculates that non-domesticated foods contribute 41 per cent of kcalories in the diet, but at least 56 per cent of energy expended in subsistence goes on obtaining wild resources. Part of these costs arise through travel and transport. Cultivating sago palms around villages reduces such costs, but imposes the burden of cutting and burning the swiddens. It thus appears that the relative effort the Nuaulu choose to allocate to the two modes of subsistence is relatively finely balanced, and could be tipped either way by a change in ecological or social circumstances.
(c). Burning of vegetation
The Ju/'hoansi (!Kung) hunter–gatherers of the Kalahari used controlled burning in late winter and early spring to encourage the growth of new grass and hence to attract game [23]. While grass seeds appear to be a famine food in the Kalahari, they are, or were, more commonly eaten by native peoples in North America and Australia. Stewart [24] documented the widespread use of controlled burning in North America as a means to increase the yields of wild grass seeds and berry- and nut-bearing plants, as well as promoting the availability of forage for game animals (for a case study, see [25]). In Alberta, native people set controlled meadow fires in early spring, when grasses were dry enough to burn but the surrounding forest too damp to catch fire. This caused new grass to spring up two to three weeks earlier than would occur naturally, attracted game, and increased the yield of berries on sunlit forest margins. Reeds and grasses on lake margins were burned to improve the feeding and nesting areas of ducks and geese, and to improve the growth of the roots on which musk rats depended [26].
The Alawa, living in savannah woodland south of the Gulf of Carpentaria, northern Australia, gave R.L. two principal reasons for burning the bush: to make walking easier by burning dead wood lying on the ground, and to clear vegetation so that new grass would provide feed for kangaroo. Responsibility for controlled burning is rigorously allocated to the sister's sons of men born into the land-holding clan, since it is their responsibility to ensure that sacred trees are not damaged. Burning should take place at the start of the dry season.
Among the Anangu of the Western Desert, where residence is more important than descent in determining a person's local group affiliation, practising controlled burning is one of the traditional ways in which an individual demonstrated their commitment to ‘holding the country’ of a particular band, along with keeping waterholes clean and performing rites at sacred sites.
After the Federal Australian National Parks Service allowed the resumption of controlled burning by traditional owners in the Uluru National Park, the impact of patch burning was studied. The Parks Service noted that spinifex grass, while providing shelter for small birds, mammals and reptiles, had little food value. Patch burning cleared areas of spinifex and allowed food plants to regenerate, on which both animals sheltering in nearby spinifex and humans could feed [27].
These findings have been reiterated and extended by Bliege Bird et al. [28], through their work with the Martu, in a more westerly district of Australia's Western Desert. Bliege Bird et al. found that the majority of controlled burning takes place during women's foraging for monitor lizards and other small- to medium-sized prey. Mature spinifex grass is burnt to reveal lizards' burrows. The Martu know that burning allows food-bearing plants to regenerate. Aerial photographs show that human patch-burning creates a more fine-grained succession of vegetation types than do lightning-induced wild fires; the starker vegetational distribution caused by wildfires is most common furthest from Aboriginal camps [27,28]. The longer camps are occupied, the more visible the distinction becomes [28].
Stewart [24, p. 119] noted that Euro-American forestry practices had deprived the Klamath and Pomo peoples of the Western United States of much traditional hunting territory, by preventing seasonal burning and thus allowing the uncontrolled growth of trees and brushwood. Dods [29] has spelled out the disastrous consequences of such practices for wildlife. The Australian Northern Territory Government policy of discouraging Aboriginal residence at Uluru put a stop to the traditional practice of controlled burning, resulting in destructive wildfires during 1950 and 1976. The re-introduction of controlled burning in the Uluru National Park after it was returned to Aboriginal ownership similarly resulted in the return of the striated grasswren (Amytornis striatus), and protected the malgara, a rare carnivorous marsupial (Dasycersus cristicauda) [27]. Palynological evidence for increased frequency of fires in Australia, presumably the result of human activity, goes back to 38 000 BP [30], perhaps 60 000 BP [31].
Prehistoric hunter–gatherers elsewhere can sometimes be shown to have burnt vegetation in analogous ways. Mellars [32, p. 16] concludes that ‘the deliberate and systematic burning of vegetation was an almost universal practice among recent hunting and gathering populations occupying forested or shrubland environments’ (and see [7]). In Britain, palynology has revealed signs of clearance attributed to human activity going back to about 9000 BC, early in the Mesolithic [33]. Very detailed work has been carried out at North Gill in Yorkshire covering the period 5000–4000 BC. Eleven pollen profiles were examined over just 350 m of a shallow valley; high-resolution sampling enables changes to be examined over periods as short as three years. This has revealed short-term clearance, burning and regeneration, the clearings themselves being remarkably small: tens rather than hundreds of metres in diameter [34,35]. Such niche construction would benefit humans in two ways. First, the fire-resistant hazel would be encouraged, leading to an increase in the productivity of its highly nutritious nuts. Acorns have been widely eaten by recent hunter–gatherers, and Mesolithic people might also have used fire to encourage acorn productivity [36]. Second, ground vegetation such as grasses and herbs would be encouraged, and this would both attract game animals, such as red deer, and allow their overall numbers to increase. One estimate is that a systematic burning regime might cause deer populations to multiply as much as tenfold [32].
Jones [37] famously termed controlled burning ‘firestick farming’, and it is not unreasonable to regard swidden cultivation as an intensification of controlled burning.
(d). Hunting as niche construction
Hunting may amount to niche construction in a variety of ways. Here we consider three. Competitor removal involves the displacement of competitor species whose niches overlap with that of the human hunters. When modern humans entered Ice Age Europe over 30 000 years ago, the continent was dominated by several large carnivores. By 20 000 years ago the cave lion and cave hyena had become extinct, followed by the largely vegetarian cave bear [38]. Whether humans ever actively hunted these animals or merely out-competed them is unknown. Their population densities were probably low, so indirect competition could have been sufficient. Neanderthals were also resident, and were extinct by 28 000 years ago [39]. Stable isotope analysis of Neanderthal bones shows that they were apex carnivores [40]. Climatic change as a cause of extinction is unconvincing, so competition with modern humans is probable [41]. The archaeological record reveals little about the nature of this competition, although a 30 000 year old probable Neanderthal mandible from Les Rois suggests that it was sometimes direct. The mandible was found in a stratum containing bones of modern humans, artefacts and ornaments of modern human manufacture, and bones of hunted animals. The Neanderthal mandible had cut marks on it identical to those on the bones of the prey species. One possibility is that this Neanderthal was hunted and eaten by the modern humans [42]. Whatever the truth of this, modern humans arriving in Europe encountered a variety of native carnivore species that had survived several previous glacial cycles. When the subsequent cycle ended, modern humans were the only remaining large carnivore. This can hardly be coincidence.
Niche deterioration occurs when human hunting causes a hunted population to decrease or become extinct. According to Optimal Foraging Theory, resources may be ranked according to their energetic return per hour expended in their acquisition. Higher ranked resources should be exploited while lower ranked ones should not; the diet breadth model seeks to predict where on the rank scale this division should fall. One key point is that if a high ranked resource becomes less common, for example, through hunting reducing its numbers, it will be encountered less frequently. This may cause hunters to broaden their diet to include previously ignored prey species [43,44]. Particularly clear examples occur when hunter–gatherers colonize new habitats. The arrival of humans in the Americas led to the rapid disappearance around 11 000 BC of some 33 genera of animals weighing over 44 kg. The mammoth and mastodont were the largest of these, and may have been particularly important as ‘keystone species’ that maintained ecological diversity at patch level. Their extinction, and the consequent loss of this diversity, may have caused many of the other extinctions [45]. Faced with this utterly transformed niche, human behaviour altered radically as people began hunting bison and other species. Further north, Palaeoeskimo peoples spread across the American Arctic around 3000 BC. Population levels during the first few centuries appear to have exceeded those at any subsequent time. One probable explanation is that Palaeoeskimo people concentrated on the most easily available prey species, the musk ox, and enjoyed a population boom at its expense. When threatened, musk oxen do not flee but form a defensive phalanx. This deters wolves, but presents an easy target to missile-equipped humans. After a few centuries musk ox populations were much depleted, leading to a human population crash [46,47]. Faunal remains from Palaeoeskimo sites reveal a trend away from musk oxen and caribou, towards marine resources, with a concomitant development of the specialized technology needed to obtain these species [48]. Both these examples resulted in massive cultural change as humans adjusted to the changes they themselves had wrought.
Niche enhancement occurs when hunters act to increase the numbers and availability of prey species. Animals may be introduced onto islands to found populations that can be hunted. The earliest known example is the introduction of a marsupial, the cuscus (Phalanger orientalis), from New Guinea to New Ireland around 23 500 years ago; other animals and plants were moved later [49]. Wild boar have been introduced to various islands long before farming was established anywhere nearby: Ireland [50], Okinawa [51] and the Izu Islands [52] were all populated in this way. Such introductions are conscious and deliberate acts by hunter–gatherers to construct new niches for themselves.
Hunting may enhance a niche in another way. Biologists distinguish between r-selected species, with high rates of reproduction, catastrophic mortality and fluctuating population numbers; and K-selected species, with lower reproductive rates, density-dependent mortality and steadier population numbers. There is a continuum between the two, and behaviour may vary along the continuum depending on circumstances [53,54]. If hunting needs to be intensified, the deliberate targetting of many young can cause the adults to behave in a more r-selected manner and produce more young. In beaver, for example, if juveniles are culled the adults are ‘tricked’ into producing more young the next year [29]. Many Native American groups in the nineteenth century hunted beaver intensively to obtain pelts for sale, and knew exactly what they were doing. As one Ojibwa informant stated: ‘we would only kill the small beaver and leave the old ones to keep breeding. Then when they got too old, they too would be killed, just as a farmer kills his pigs, preserving the stock for his supply of young’ [55, p. 294].
Several aspects of this discussion come together in the Epipalaeolithic of the Near East, around 19–12 000 years ago. This was a period of increasing warmth and moisture after the Last Glacial Maximum. Previous oscillations of this kind had been accompanied by an increase in fallow deer, because this species is suited to the expansion of woodland that occurred. This time, however, fallow deer decreased in frequency through time. Over-predation is the most probable cause of this, because human populations were increasing at this time [56]. The archaeological record reveals that people were living in larger and more sedentary groups, and this is likely to account for the increasing pressure on the fallow deer [57]. Two things reveal increased population and hunting intensity. First, diet broadened to include several smaller, less energetically productive resources. Previous such episodes had concentrated on slow, easy to catch prey such as tortoise. In the later Epipalaeolithic, however, these were replaced by faster, more elusive prey such as hare, partridge and fox [58], and wild grasses and legumes—the much-discussed ‘Broad Spectrum Revolution’. This fits with the prediction of the diet breadth model (see above) that human diets should broaden when high-ranked species become rare [59].
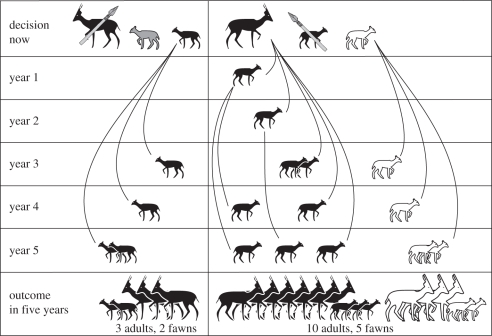
Second, young animals formed an increasing part of the kill of the remaining large mammal, the gazelle [56,57]. It is probable that the hunting of juveniles was a conscious strategy, as it was with the Ojibwa beaver hunter quoted above. The way it might operate is shown in figure 1. Mountain gazelle (Gazella gazella gazella) was the main species exploited in the Epipalaeolithic Levant. These animals rarely produce twins, but in better-watered areas usually produce two offspring per year, births occurring all year round [60, table II]. In the moister conditions of the Epipalaeolithic many, perhaps most, gazelle populations would have achieved this. Young male gazelle remain with their mothers until the age of 15–18 months, young females even longer [61]. Thus most females would be accompanied by two fawns of different ages. Since juvenile females in well-watered areas usually give birth at the age of 12 months [60,61], the older juvenile in figure 1 might herself be accompanied by another fawn. Encountering a female and two juveniles, a hunter could choose to shoot the mother. Figure 1 assumes that, without her protection, only one fawn will survive and breed. All other things being equal, after five years this will result in three adults (the surviving juvenile and her first two offspring) and two fawns. Alternatively, the hunter could choose to kill one of the juveniles. The other will probably survive to reproduce—and the adult will also continue to breed, resulting in many more gazelle after 5 years. If a gazelle fawn is lost, the mother generally becomes oestrous [61, p. 732], which suggests that gazelle might be ‘tricked’ into increased reproduction in the same way as the beavers described above. This strategy clearly enhances the hunter–gatherer niche. It runs counter to modern European notions of hunting and sportsmanship, but it conforms to our notions of farming and profit—as the Ojibwa informant (see above) was fully aware.
Figure 1.
The choices facing a gazelle hunter upon encountering an adult female with two young of different ages. Left: the outcome if he kills the adult. Right: the outcome if he kills one of the juveniles.
(e). Why did these activities not ‘take off’?
We have shown that hunter–gatherers construct niches in a wide variety of ways, far more often than our common understanding of the label ‘hunter–gatherer’ would suggest. Hunter–gatherers are not merely passive recipients of environmental bounty, but are just as aware of the potential of niche construction as farmers.
And yet for tens of millennia people continued to exist as what we conventionally term hunter–gatherers. Niche constructions of the types discussed above, and no doubt very many others unknown to us, did not turn into geographically expanding agricultural systems. Many of the niches were stable, bringing about no subsequent human cultural change. Plant domestication in eastern North America was just a small adjunct to the overall hunter–gatherer system. Tobacco provided no food value at all, but was cultivated for its narcotic effects. Trees like chestnut or hazel were not annuals, and must grow for a couple of decades before producing many nuts. Trees are not flexible enough to form the primary basis of an expanding agricultural system. There seems to have been no inherent instability in such niches. Unstable niches by definition cause human cultural change, but commonly result in new hunter–gatherer niches, not agricultural ones. The over-hunting of mammoths or musk oxen led not to agriculture but to transformed hunter–gatherer niches.
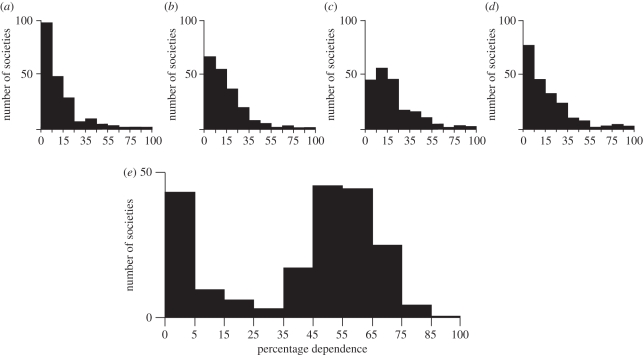
While hunter–gatherers may thus practise ‘low-level food production’ [2,3], it may however be very difficult to go from here to ‘high-level food production’, i.e. to construct a predominantly agricultural niche. This is suggested by a survey of the economic attributes of over 200 recent ethnographically known societies (figure 2). Gathering contributes little or nothing to farming societies, who make up a large percentage of the sample. It makes a progressively larger contribution to an ever smaller number of societies, so that very few depend on it for most of their livelihood. The result is a fairly regular fall-off curve. The same goes for hunting, fishing and herding. Agriculture is however very different. Many societies depend on it for 5 per cent or less of their subsistence—these are the hunter–gatherers. Most of the rest depend upon it for over about 50 per cent of their food. But there are remarkably few who depend upon it for between 5 and 50 per cent [62]. This suggests that hunter–gatherers rarely expand their minor cultivation activities. The 5–50% farming dependence zone is one that societies do not often venture into; it appears to be an unstable intermediate zone that the earliest farmers would have crossed rapidly.
Figure 2.
Percentage dependence on five major economic activities (redrawn after [62, fig. 3]). (a) Gathering; (b) hunting; (c) fishing; (d) herding; and (e) agriculture.
So how do hunter–gatherers become farmers? With hindsight, we know that the Epipalaeolithic of the Near East ended with the origins of agriculture, which endows the niche created by Epipalaeolithic intensification with a particular interest. Intensification does not inevitably lead to agriculture, however, and many local intensification trajectories never did so—because when agriculture did emerge in the Near East it did not involve the gazelle or hares or most plant species that had been the targets of local intensification, but a few of the minor ones, and for the most unpredictable of reasons.
3. Unstable niches and the origins of agriculture
The major agricultural systems are classic examples of niche construction. Major agricultural systems appeared in various places around the world. Each involved human control of a restricted range of species; the integration of these species into a mutually supporting working system; and their genetic modification. Agricultural communities expanded geographically, spreading around the globe, and modifying as they encountered new environmental constraints and opportunities. Human cultures changed massively as a result of both (i) the development, and (ii) the geographical expansion of agriculture. We concentrate largely on the Near Eastern agricultural system, and stress that the developmental trajectories of other major systems were very different [63].
Agriculture is not just a further intensification of the hunter–gatherer niche but is a new and transformed niche of its own. We argue this in two ways: first, major long-term exploitation of wild cereals and animals does not necessarily lead to agriculture; and second, Near Eastern agriculture was based on species that played relatively minor roles in the Epipalaeolithic economy.
(a). The development of the agricultural niche: cereal cultivation
Certain cereal harvesting practices induced a cycle of positive feedback leading to full-scale cultivation, while others did not. In Western Asia and the Yangzhe Basin, cereals were harvested with a sickle, selectively favouring non-shattering seed heads that had to be replanted artificially (see below). In the Darling Basin of western New South Wales, Australia, Aboriginal people harvested wild grasses before the seeds had ripened, to prevent seed loss, building hayricks that were burnt when dry to separate seeds from stems, thus avoiding the unconscious selection of non-shattering heads. In 1839 the explorer Mitchell [64, p. 313] described ‘ricks or haycocks’ extending for miles. In sub-Saharan Africa, Jack Harlan has documented the ‘swinging basket’ technique of grass seed production, which favours the selection of shattering seed heads. Of these three harvesting techniques only one, harvesting with a sickle, creates a (unintended) process of positive feedback leading to full-scale cultivation once seeds are replanted. On Cooper's Creek, in southwest Queensland, the explorer Gregory in 1887 described ‘fields of 1,000 acres’ of Panicum. He wrote that ‘The natives cut it down by means of stone knives, cutting down the stalk half way, beat out the seed, leaving the straw which is often met with in large heaps’ [64, p. 314]. There is however no record that seeds were replanted.
Archaeological evidence for grass seed collection in Australia has been found at 40 000 BP [65]. Grindstones definitely used on plants go back to 30 000 BP [66], although Smith [67] points out the need to distinguish seed grinding from grindstones used more generally for plant processing. In arid Australia, grass seeds were a fall-back food, exploited when more easily harvested plants had been locally exhausted. Brokensha [68], who observed women collecting and processing wild millet, recording that it took three women 3 h to harvest 2 kg of seed and a further 2 h to process the seeds and cook them as damper (unleavened bread). In the Western Desert, grindstones with the distinctive polish created by the silica in grass seeds are only found during the last four thousand years, which Smith [67] interprets as the consequence of people living at a higher population density than in previous periods of higher rainfall, adapting to a new period of increasing aridity.
In the Near East, the agricultural niche was completely different from its hunter–gatherer predecessor. The literature dealing with the phenomenon is huge, and we can do no more than allude to some relevant aspects. After millennia of ameliorating climate following the Last Glacial Maximum, the abrupt Younger Dryas oscillation at ca 10 500–9500 BC marked a major reversal to colder and drier conditions. Many have argued that this destabilized the Epipalaeolithic way of life [69,70].
Epipalaeolithic plant exploitation involved a wide array of species. At Abu Hureyra in Syria, over 250 species were probably consumed, some 120 of these being seed foods. Wild einkorn wheat and rye were among these, but the most important were club-rush (Bolboschoenus [=Scirpus] maritimus), Euphrates knotgrass (Polygonum corrigioloides) and feather grasses (Stipa spp.) [71]. Species frequencies at other sites vary but wild wheats and barley were at best of modest importance [72]. Club-rush produces not just seeds but also tubers; relatively complex processing is required to turn these into edible flour, but the technology was available in the Epipalaeolithic [73].
Near the end of the Epipalaeolithic, when the Younger Dryas was exerting pressure, some plants appear to have been cultivated: einkorn wheat, rye and lentil are all found outside their natural habitats, accompanied by the weedy species that would thrive in cultivated fields; but apart from the enlargement of some seeds, domestication (defined as genetic modification) had not taken place [74]. Cereals were domesticated independently in several different regions of the Near East [63]. The genetic change taken as the definition of this is the development of a non-shattering seed head: wild grass seed heads shatter naturally but domestic ones do not, and cannot therefore reproduce unless they are resown by humans.
How the change to intensive cereal cultivation in some areas came about is unclear. A switch from swinging-basket to sickle harvesting has been suggested: if done as the heads were beginning to ripen, sickling would dislodge and lose some seeds from wild-type shattering heads, while collecting all of the non-shattering form (this occurs as a rare mutant in wild cereal stands). The non-shattering form would thus be slightly more common in the collected seeds than in the wild stand. If people replanted part of what they had collected, the non-shattering mutant form would be increased. In theory this could lead to 100 per cent non-shattering forms in as little as 25–200 years [75]. In reality it took somewhere between one and two millennia, as frequencies of the non-shattering form gradually increased on sites over this period [63,76,77]. The precise nature of the selective pressures that caused changes on such time scales needs further elucidation. But the final outcome was that minor Epipalaeolithic wild resources were transformed into major domestic ones that have had a huge influence on subsequent history.
(b). The development of the agricultural niche: animal domestication
Epipalaeolithic animal exploitation in Western Asia was based on gazelle and (further east) onager. Wild sheep, goat and cattle were relatively minor hunted resources. Yet it was these species that the early cereal farmers used as close-herded domesticates. The intensively hunted gazelle might have seemed a more logical choice—but in common with almost all antelope and deer species they do not form fixed-membership herd units suitable for domestication. Males become territorial during the rut, and frequently fight each other; subdominant males form loose groups moving around the territorial peripheries; and females with their young move fairly freely between territories despite the efforts of the dominant males to constrain them. This is true for almost all species of sub-Saharan ungulates [78], all species of gazelle [79], and all deer [80]. This loose social structure makes close-herding by humans an impossibility. One study of impala as a possible domesticate concluded that:
‘The behaviour of impala does not seem to be compatible with their domestication, for which animals with fixed-membership herds, like buffalo or eland, are more appropriate. The problems experienced by a territorial male impala trying to restrict the movements of a female herd should be observed by anyone who wishes to put himself in the male's position’. [81, p. 880].
Various domestication experiments have shown that the culling of most males, and investment in fencing and pens, does not solve the problem. Penned males of various sub-Saharan species exhibit aggressive behaviour in seasons when they would be territorial, and attack females and young [82, p. 847]. Gazelle males, in the absence of rivals of their own sex, vent their aggression on females, young, inanimate objects and humans [79, pp. 214–215]. Penned red deer are similarly unpredictable and potentially dangerous to humans during the rut [83, pp. 57–59].
Rather few wild species have fixed-membership herds. Among them are sheep, goat and cattle. A few sub-Saharan antelope species are similar, but most of these are solitary; only the gregarious eland forms larger herds. Eland, like sheep, goat and cattle, do not become territorial during the rut, but maintain a male hierarchy within the herd. Consequently the males do not become territorial during the rut, and herds do not fission and scatter. As a result, ‘the eland, as every Masai herdsman knows, is more like an ox than an antelope’ [78, p. 194]. Experiments have shown that eland is effectively the only antelope species that is amenable to domestication and close human control—they can be managed and milked in exactly the same way as domestic cattle [82,84–87]. Animal domestication has thus moved along very narrow taxonomic pathways. The key animal behavioural attribute is the fixed-membership herd based on a male hierarchy within the herd. This behavioural trait renders these species amenable to close herding, because the herd units can be controlled and moved much more easily (figure 3).
Figure 3.
Sheep and goat, demonstrating their amenability to close domestic control. The sheep follow the shepherd (right) or lead sheep, lining up nose to tail, while the goats are in open order formation. Jebel Oustani, Syria, 1983 (photo PR-C).
In the Near East shortly after the domestication of cereals, domesticated herds of sheep and goat rapidly became important, signalled by the presence on archaeological settlements of not just the young males that intensification produces, but also of the elderly females who had reached the end of their reproductive lives [88,89]. As these high-ranked resources became ubiquitous, diet narrowed and the small animals of the ‘Broad Spectrum Revolution’ all but disappeared [90].
Thus the genetics of certain wild grasses, and the social behaviour of certain wild ungulates, were what gave them the potential to be intensified to the point of domestication when the Younger Dryas climatic change brought the intensive Epipalaeolithic exploitation of wild resources to an end. They had not previously been major resources, and an observer would probably not have predicted that these particular species would form the basis of an agricultural system that would transform the face of the globe. Had they not been domesticated, human populations would have decreased and become more mobile—reverted, in other words, to the adaptations of their ancestors at the Last Glacial Maximum.
4. The exporting of the farming niche
A small number of agricultural systems have spread to dominate food production almost everywhere on the planet. In the region where the Near Eastern agricultural system developed, agriculture can support more people per unit area than hunting and gathering. But agriculture as an integrated system closely controlled on a day to day basis by people has a further advantage: it is a niche that can be exported to areas outside the original heartland. Within a few millennia, the Near Eastern system extended from Ireland to northern China (where it encountered and was integrated with the Chinese agricultural system), and from the Urals to the Sudan. No hunter–gatherer niche could match this.
The niche was not just transported, it was modified to be able to cope with new environments. Its early spread westward through the Mediterranean required few modifications, because the environment was largely similar to the Near East. The earliest cultivation in eastern Spain used the same four cereals and five pulses as the Near East [91]. But when agriculture spread into temperate Europe the range diminished to just three cereals and the occasional pea and lentil [92]. When it spread further north into Scotland, some farmers initially cultivated a substantial proportion of emmer wheat. Within a few centuries this had been replaced by barley, a crop much better suited to the Scottish environment [93]. The frequency of animals was also adjusted to suit local conditions: in temperate European forests sheep and goat, dominant in the Mediterranean, gave way to cattle and pigs [94]. In a pioneering study, Clark [95] argued that the subsequent increase in sheep was a result of forest clearance and the creation of open grassland by farmers, a further modification of the farming niche.
Forest clearance was a major outcome of the arrival of the Near Eastern farming niche almost everywhere. The transformation of local ecosystems, which continues to this day, involved the reduction of native plant and animal communities, and the destruction or absorption of local hunter–gatherers. The effects naturally varied depending on local circumstances. Some species such as roe deer have accommodated themselves reasonably well to the peripheries of the agricultural landscape. In Europe, wild oats and rye were initially native weeds that spread into wheat and barley fields, but eventually they did so well that they were taken into cultivation in their own right [96]. For many other species the arrival of agriculture spelt disaster; for example, when Polynesian agriculturalists arrived in New Zealand they eradicated the giant flightless moa in about three centuries [97]. Agricultural immigrants would face their biggest problem in their first months in a new area, before their newly planted crops could provide food. They coped in various ways. In Europe, the transport of lactating cattle may have enabled people to use dairy products in this interval [98]—dairying has recently been identified among the earliest agriculturalists in Anatolia [99]. This option was not available in the Pacific, so local wild resources bore more of the brunt. A wide variety of native species including large flightless birds and terrestrial crocodilians inhabited the various islands. One estimate is that as many as 8000 taxa became extinct across the Pacific as humans arrived [100].
(a). Can farming form a stable niche?
Probably the biggest single process tending to destabilize farming as a form of niche construction is population increase. Contrary to many theorists (e.g. [101,102]), we do not regard the origin of farming as a response to population pressure. We have cited seasonal shortfalls of wild foods as one factor limiting hunter–gatherer population growth. Other factors include the difficulty of carrying children, and the low body fat levels sustained by mobile women living on wild resources. Richard Lee calculated that an adult Ju/'hoansi woman walked about 2400 km yr−1. For the first two years of life, a child was carried on the mother's back. From three years of age, children could be left at times in camp with a babysitter, but children were still carried long distances up to the age of four. These data enabled Lee to calculate that a Ju/'hoansi woman giving birth once every four years would carry an average child load of 9.2 kg d−1, but one giving birth every two years would carry an average child load of 17.0 kg d−1 [103, p. 325]. Wilmsen found that foraging !Kung suffered double the weight loss pastoral !Kung experienced during lean months. He concurred with other authors that foraging !Kung women averaged only 4.5 live births in their lifetime, while women who had adopted agro-pastoralism and sedentary village life had an average of 7 live births [104]. Wilmsen's findings also agree with Jones' conclusion that birth spacing among the contemporary Australian Gidjingali is half the 4–5 years it appears to have been before contact [11, pp. 134–135].
We do however consider population increase to be the main motive force behind the geographical spread of farming. Demographic expansion can be matched by emigration and the export of the farming niche—not an option available to most hunter–gatherers. Farming has however not always been sustainably implemented. It crossed Europe in a series of rapid moves punctuated by lengthy pauses [98]. On the Vistula River, farming reached to within 200 km of the Baltic coast by 5400 BC. The first farming settlements near the coast were established by 5000 BC—but this penetration failed and hunter–gatherers reoccupied the area [105]. Farming was only permanently established on the coast around 4000 BC, in another major spread. The spread at this time reached across the Baltic into Sweden, extending rapidly to north of Stockholm—but once again the advance was not sustained, and the farmers were replaced by hunter–gatherers shown by ancient DNA extracted from the human skeletons to come from the Northeast Baltic [106]. The northern edge of farming retreated to the southernmost part of Sweden for several centuries.
Instability of the farming niche may also occur in more mature agricultural regimes. The Norse occupation of the Faroes, Iceland and Greenland from the ninth century AD introduced agriculture into pristine but simple environments. Considerable environmental damage ensued. As agriculture moved west, it was progressively less suited to the environments it encountered. In Greenland a combination of circumstances including environmental degradation and climate change led to the extinction of farming after some 400 years [107]. Britain provides a prehistoric analogue: farmers moving into the northern and western uplands cleared the oak and hazel woodland, exposing the soils to the increasing rainfall. This led to podsolization, acidity and the growth of peat over what had previously been agricultural land. Dartmoor provides a clear example: farming expanded and the woodland was cleared around 1600 BC, but after some centuries of animal grazing peat began to grow around 800 BC and the area was abandoned [108]. Much of Britain's moorland is in fact not a ‘natural’ landscape at all, but was created by the self-destruction of the farming niche.
Malthus [109] is famous for identifying the population cycles that accompanied European farming and recognized that the stability of farming depends crucially on the stabilising of the population practising it. After developing his hypothesis that populations increased faster than their food supply, Malthus travelled through Europe in search of supporting data. He was surprised to discover that Alpine populations appeared to have stabilized, and proposed a homeostatic regulatory mechanism to account for this. Malthus argued that the peak demand for labour occurred at harvest time, to collect sufficient hay to feed stabled livestock through the long winter. Since the productivity of meadows was determined by supply of manure, the available hay and livestock each limited the other. Food production, determined by the number of livestock, in turn limited the number of people who could survive and hence the available labour force (figure 4). A peasant to whom Malthus spoke near the Lac de Joux explained that, even though he himself had married young, late marriage was needed to prevent over-population and bring birth and death rates into equilibrium. Malthus noted that where cottage industry had provided alternative income, age at marriage fell and the population increased [109, pp. 210–212, 110]. Viazzo adds that the optimum population level is that at which average output per head is maximized. If it falls below a lower threshold, crucial communal activities cannot be performed; if it rises above a higher threshold the available labour will exceed productive capacity [110].
Figure 4.
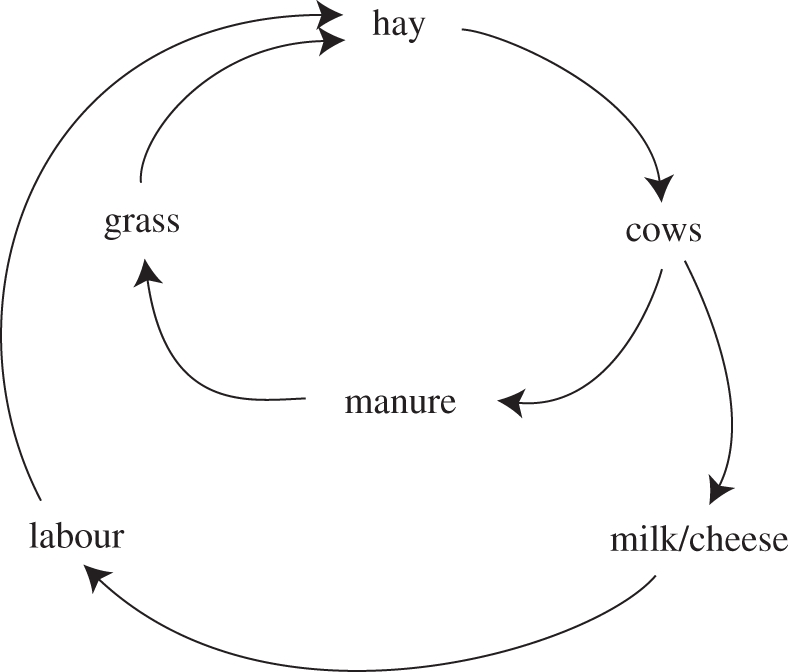
Diagram showing Malthus' view of the relationship between resources, labour and productivity in Alpine regions.
The U.S. anthropologist Netting studied records of birth, marriage and death over 300 years in a Swiss Alpine village, Törbel, which practises partible inheritance. Although the villagers never quite stabilized their population, and always relied on some outmigration, Netting found that each time the village had suffered a higher than usual death rate from an epidemic, the age at marriage fell, then gradually rose again as the population level was restored [111,112]. Franche-Comté, on the French border with Switzerland, also practised late marriage and high celibacy during the nineteenth century [113]. Between 1650 and 1850 the population had been rising steadily. Although the French industrial revolution enabled those at the bottom of the social hierarchy (farm labourers and domestic servants) to escape to the cities and relieve population pressure, there were still some households in the mid twentieth century whose older members were celibate. Villagers some 20 km from the Lac de Joux told Layton that adult celibacy was a deliberate strategy to prevent the division of family land holdings under the local principle of partible inheritance. Division of the land could be averted by forming a joint holding in which all children have equal shares, although only one son was allowed to marry. The cadastral surveys of village fields show the average size of parcelles (strips) did not diminish between 1834 and 1965, despite the rule of partible inheritance. An area of land divided into 304 parcelles in 1834 was divided into 254 parcelles in 1965 [113, pp. 151–152].
We conclude that, viewed dispassionately, increasing food production does not appear to be an effective strategy for achieving demographic and political stability in the world's farming populations. Whether farming will provide a stable solution to human subsistence, it is, as Zhou Enlai said of the supposed benefits of the French Revolution, ‘too early to say’.
Acknowledgements
We would like to thank Gary Crawford, Gayle Fritz, Sandra Knapp, Natalie Munro and Jim Savelle for their assistance. Any imperfections remain our own.
Footnotes
One contribution of 13 to a Theme Issue ‘Human niche construction’.
References
- 1.Panter-Brick C., Layton R., Rowley-Conwy P. 2001. Lines of enquiry. In Hunter–gatherers: an interdisciplinary perspective (eds Panter-Brick C., Layton R., Rowley-Conwy P.), pp. 1–11 Biosocial Society Symposium 13. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Smith B. D. 2001. Low-level food production. J. Archaeol. Res. 9, 1–43 10.1023/A:1009436110049 (doi:10.1023/A:1009436110049) [DOI] [Google Scholar]
- 3.Smith B. D. 2011. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Phil. Trans. R. Soc. B 366, 836–848 10.1098/rstb.2010.0253 (doi:10.1098/rstb.2010.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politis G. 2007. Nukak: ethnoarchaeology of an Amazonian people. Walnut Creek, CA: Left Coast Press [Google Scholar]
- 5.Bailey R. C., Head G., Jenike M., Owen B., Rechtman R., Zechenter E. 1989. Hunting and gathering in tropical rain forest: is it possible? Am. Anthropol. 91, 59–82 10.1525/aa.1989.91.1.02a00040 (doi:10.1525/aa.1989.91.1.02a00040) [DOI] [Google Scholar]
- 6.Headland T., Reid L. 1989. Hunter-gatherers and their neighbours from prehistory to the present. Curr. Anthropol. 30, 43–66 10.1086/203710 (doi:10.1086/203710) [DOI] [Google Scholar]
- 7.Fairbairn A., Hope G., Summerhayes G. 2006. Pleistocene occupation of New Guinea's highland and subalpine environments. World Archaeol. 38, 371–386 10.1080/00438240600813293 (doi:10.1080/00438240600813293) [DOI] [Google Scholar]
- 8.Froment A. 2001. Evolutionary biology and health of hunter-gatherer populations. In Hunter–gatherers: an interdisciplinary perspective (eds Panter-Brick C., Layton R., Rowley-Conwy P.), pp. 239–266 Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Altman J. C. 1987. Hunters and gatherers today: an Aboriginal economy in North Australia. Canberra, Australia: Australian Institute of Aboriginal Studies [Google Scholar]
- 10.McCarthy F., McArthur M. 1960. The food quest and the time factor in Aboriginal economic life. In Records of the Australian–American scientific expedition to Arnhem Land, vol 2: Anthropology and nutrition (ed. Mountford C. P.), pp. 145–194 Melbourne, Australia: Melbourne University Press [Google Scholar]
- 11.Jones R. 1981. Hunters in the Australian coastal savannah. In Human ecology in savanna environments (ed. Harris D.), pp. 107–146 London, UK: Academic Press [Google Scholar]
- 12.Jenike M. 2001. Nutritional ecology: diet, physical activity and body size. In Hunter–gatherers: an interdisciplinary perspective (eds Panter-Brick C., Layton R., Rowley-Conwy P.), pp. 205–238 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Jones R., Meehan B. 1989. Plant foods of the Gidjingali: ethnographic and archaeological perspectives from northern Australia on tuber and seed exploitation. In Foraging and farming (eds Harris D. R., Hillman G. C.), pp. 120–135 London, UK: Unwin Hyman [Google Scholar]
- 14.Fritz G. J., Smith B. D. 1988. Old collections and new technology: documenting the domestication of Chenopodium in eastern North America Midcontinental. J. Archaeol. 13, 3–27 [Google Scholar]
- 15.Scarry C. M. 2008. Crop husbandry practices in North America's Eastern Woodlands. In Case studies in environmental archaeology (eds Reitz E. J., Scudder S. J., Scarry C. M.), pp. 391–404, 2nd edn Interdisciplinary Contributions to Archaeology. New York, NY: Springer [Google Scholar]
- 16.Smith B. D. 1995. The emergence of agriculture. New York, NY: Scientific American Library [Google Scholar]
- 17.Fritz G. J. 2006. Introduction and spread of Mexican crops. In Handbook of North American Indians vol. 3. Environment, origins, and population (eds Ubelaker D., Smith B. D.), pp. 437–446 Washington, DC: Government Printing Office [Google Scholar]
- 18.Matsui A., Kanehara M. 2006. The question of prehistoric plant husbandry during the Jomon Period in Japan. World Archaeol. 38, 259–273 10.1080/00438240600708295 (doi:10.1080/00438240600708295) [DOI] [Google Scholar]
- 19.Crawford G. W. In press Advances in understanding early agriculture in Japan. Curr. Anthropol. [Google Scholar]
- 20.Sato Y.-I., Yamanaka S., Takahashi M. 2003. Evidence for Jomon plant cultivation based on DNA analysis of chestnut remains. In Hunter–gatherers of the North Pacific rim (eds Habu J., Savelle J. M., Koyama S., Hongo H.), pp. 187–197 Senri Ethnological Studies 63. Osaka, Japan: National Museum of Ethnology [Google Scholar]
- 21.Hallam S. 1989. Plant usage and management in Southwest Australian Aboriginal societies. In Foraging and farming: the evolution of plant exploitation (eds Harris D., Hillman G.), pp. 136–151 London, UK: Unwin [Google Scholar]
- 22.Ellen R. 1982. Environment, subsistence and system: the ecology of small-scale formations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 23.Yellen J., Lee R. 1976. ‘The Dobe-/Du/da environment: background to a hunting and gathering way of life.’ In Kalahari hunter–gatherers: studies of the !Kung San and their neighbours (eds lee R., de Vore I.), pp. 28–46 Cambridge, MA: Harvard University Press [Google Scholar]
- 24.Stewart O. C. 1956. Fire as the first great force employed by man. In Man's role in changing the face of the Earth (ed. Thomas W. L.), pp. 115–133 Chicago, IL: Chicago University Press [Google Scholar]
- 25.Shipek F. 1989. An example of intensive plant husbandry: the Kumeyaay of southern California. In Foraging and farming: the evolution of plant exploitation (eds Harris D., Hillman G.), pp. 159–170 London, UK: Unwin [Google Scholar]
- 26.Lewis H. T. 1982. A time for burning. Occasional Publication 17. Alberta, Canada: University of Alberta, Boreal Institute for Northern Studies [Google Scholar]
- 27.Commonwealth of Australia 1994. Renomination of Uluru—Kata Tjuta National Park by the Government of Australia for inscription on the World Heritage List. Canberra, Australia: Department of the Environment, Sport and Territories, Commonwealth of Australia. [R.L. was senior author of this document; passages on fire use in the text of our paper are taken from his contributions to the document.] [Google Scholar]
- 28.Bliege Bird R., Bird D. W., Codding B. F., Parker C. H., Jones J. H. 2008. The ‘fire stick farming’ hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc. Natl Acad. Sci. USA 105, 14 796–14 801 10.1073/pnas.0804757105 (doi:10.1073/pnas.0804757105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dods R. R. 2002. The death of Smokey Bear: the ecodisaster myth and forest management practices in prehistoric North America. World Archaeol. 33, 475–487 10.1080/00438240120107486 (doi:10.1080/00438240120107486) [DOI] [Google Scholar]
- 30.Kershaw A. P. 1994. Pleistocene vegetation of the humid tropics of northeastern Queensland, Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 109, 399–412 10.1016/0031-0182(94)90188-0 (doi:10.1016/0031-0182(94)90188-0) [DOI] [Google Scholar]
- 31.Wright R. V. S. 1986. How old is zone F at Lake George? Archaeology in Oceania 21, 138–139 [Google Scholar]
- 32.Mellars P. 1976. Fire, ecology, animal populations and man; a study of some ecological relationships in prehistory. Proc. Prehistoric Soc. 42, 15–45 [Google Scholar]
- 33.Bush M. B. 1988. Early Mesolithic disturbance: a force on the landscape. J. Archaeol. Sci. 15, 453–462 10.1016/0305-4403(88)90042-8 (doi:10.1016/0305-4403(88)90042-8) [DOI] [Google Scholar]
- 34.Innes J., Simmons I. G. 2000. Mid-Holocene charcoal stratigraphy, fire history and palaeoecology at North Gill, North York Moors, UK. Palaeogeogr. Palaeoclimatol. Palaeoecol. 164, 151–165 10.1016/S0031-0182(00)00184-X (doi:10.1016/S0031-0182(00)00184-X) [DOI] [Google Scholar]
- 35.Simmons I. G. 1996. The environmental impact of Later Mesolithic cultures. Edinburgh, UK: Edinburgh University Press [Google Scholar]
- 36.Mason S. L. R. 2000. Fire and Mesolithic subsistence- managing oaks for acorns in northwest Europe? Palaeogeogr. Palaeoclimatol. Palaeoecol. 164, 139–150 10.1016/S0031-0182(00)00181-4 (doi:10.1016/S0031-0182(00)00181-4) [DOI] [Google Scholar]
- 37.Jones R. 1969. Firestick farming. Austral. Nat. History 16, 224–228 [Google Scholar]
- 38.Estévez J. 2004. Vanishing carnivores: what can the dissappearance of large carnivores tell us about the Neanderthal world? Int. J. Osteoarchaeol. 14, 190–200 10.1002/oa.755 (doi:10.1002/oa.755) [DOI] [Google Scholar]
- 39.Finlayson C., et al. 2006. Late survival of Neanderthals at the southernmost extreme of Europe. Nature 443, 850–853 10.1038/nature05195 (doi:10.1038/nature05195) [DOI] [PubMed] [Google Scholar]
- 40.Richards M. P., Pettitt P., Trinkhaus E., Smith F. P., Paunovic M., Karavanic I. 2000. Neanderthal diet at Vindija and Neanderthal predation: the evidence from stable isotopes. Proc. Natl Acad. Sci. USA 97, 7663–7666 10.1073/pnas.120178997 (doi:10.1073/pnas.120178997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks W. E., d'Errico F., Peterson A. T., Kageyama M., Sima A., Sánchez-Goñi F. 2008. Neanderthal extinction by competitive exclusion. PLoS ONE 31, e3972. 10.1371/journal.pone.0003972 (doi:10.1371/journal.pone.0003972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez Rozzi F. V., d'Errico F., Vanhaeren M., Grootes P. M., Kerautret B., Dujardin V. 2009. Cutmarked human remains bearing Neanderthal features and modern human remains associated with the Aurignacian at Les Rois. J. Anthropol. Sci. 87, 153–185 [PubMed] [Google Scholar]
- 43.Winterhalder B. 1981. Optimal foraging strategies and hunter-gatherer research in anthropology: theory and methods. In Hunter–gatherer foraging strategies (eds Winterhalder B., Smith E. A.), pp. 13–35 Chicago, IL: University of Chicago Press [Google Scholar]
- 44.Winterhalder B. 2001. The behavioural ecology of hunter-gatherers. In Hunter–gatherers: an interdisciplinary perspective (eds Panter-Brick C., Layton R. H., Rowley-Conwy P.), pp. 12–38 Biosocial Society Symposium Series 13. Cambridge, UK: Cambridge University Press [Google Scholar]
- 45.Haynes G. 2002. The catastrophic extinction of North American mammoths and mastodonts. World Archaeol. 33, 391–416 10.1080/00438240120107440 (doi:10.1080/00438240120107440) [DOI] [Google Scholar]
- 46.Savelle J. M., Dyke A. S. 2002. Variability in Palaeoeskimo occupation on south-western Victoria Island, Arctic Canada: causes and consequences. World Archaeol. 33, 508–522 10.1080/00438240120107503 (doi:10.1080/00438240120107503) [DOI] [Google Scholar]
- 47.Savelle J. M., Dyke A. S. 2009. Paleoeskimo demography on western Boothia Peninsula, Arctic Canada. J. Field Archaeol. 34, 267–283 [Google Scholar]
- 48.Darwent C. M. 2004. The highs and lows of High Arctic mammals: temporal change and regional variability in Paleoeskimo subsistence. In Colonisation, migration and marginal areas: a zooarchaeological approach (eds Mondini M., Muñoz S., Wickler S.), pp. 62–73 Oxford, UK: Oxbow Books [Google Scholar]
- 49.Swadling P., Hide R. 2005. Changing landscape and social interaction: looking at agricultural history from a Sepik-Ramu perspective. In Papuan pasts: cultural, linguistic and biological histories of Papuan-speaking peoples (eds Pawley A., Attenborough R., Golson J., Hide R.), pp. 289–327 Canberra, Australia: Australian National University, Research School of Pacific & Asian Studies [Google Scholar]
- 50.McCormick F., Murray E. 2007. Knowth and the zooarchaeology of Early Christian Ireland. Excavations at Knowth 3. Dublin, Ireland: Royal Irish Academy [Google Scholar]
- 51.Matsui A., Ishiguro N., Hongo H., Minagawa M. 2005. Wild pig? Or domesticated boar? An archaeological view on the domestication of Sus scrofa in Japan. In The first steps of animal domestication: new archaeological approaches (eds Vigne J.-D., Peters J., Helmer D.), pp. 148–159 Oxford, UK: Oxbow Books [Google Scholar]
- 52.Yamazaki K., Takahashi O., Sugawara H., Ishiguro N., Endo H. 2005. Wild boar remains from the Neolithic (Jomon Period) sites on the Izu Islands and in Hokkaido Island, Japan. In The first steps of animal domestication: new archaeological approaches (eds Vigne J.-D., Peters J., Helmer D.), pp. 160–176 Oxford, UK: Oxbow Books [Google Scholar]
- 53.Peters R. H. 1991. A critique for ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 54.Pianka E. R. 1970. On r- and K-selection. Am. Nat. 104, 592–597 10.1086/282697 (doi:10.1086/282697) [DOI] [Google Scholar]
- 55.Speck F. G. 1915. The family hunting band as the basis of Algonkian social organization. Am. Anthropol. New Series 17, 289–305 10.1525/aa.1915.17.2.02a00070 (doi:10.1525/aa.1915.17.2.02a00070) [DOI] [Google Scholar]
- 56.Munro N. 2009. Epipalaeolithic subsistence intensification in the southern Levant: the faunal evidence. In The evolution of hominin diets (eds Hublin J.-J., Richards M. P.), pp. 141–155 New York, NY: Springer [Google Scholar]
- 57.Davis S. J. M. 2005. Why domesticate food animals? Some zoo-archaeological evidence from the Levant. J. Archaeol. Sci. 32, 1408–1416 10.1016/j.jas.2005.03.018 (doi:10.1016/j.jas.2005.03.018) [DOI] [Google Scholar]
- 58.Stiner M., Munro N., Surovell T. A., Tchernov E., Bar-Yosef O. 1999. Paleolithic population growth pulses evidenced by small animal exploitation. Science 283, 190–194 10.1126/science.283.5399.190 (doi:10.1126/science.283.5399.190) [DOI] [PubMed] [Google Scholar]
- 59.Layton R., Foley R., Williams E. 1991. The transition between hunting and gathering and the specialised husbandry of resources. Curr. Anthropol. 32, 255–274 10.1086/203953 (doi:10.1086/203953) [DOI] [Google Scholar]
- 60.Baharav D. 1983. Reproductive strategies in female Mountain and Dorcas gazelles (Gazella gazella gazella and Gazella dorcas). J. Zool. (London) 200, 445–453 10.1111/j.1469-7998.1983.tb02808.x (doi:10.1111/j.1469-7998.1983.tb02808.x) [DOI] [Google Scholar]
- 61.Mendelssohn H. 1974. The development of the populations of gazelles in Israel and their behavioural adaptations. In The behaviour of ungulates and its relation to management (eds Geist V., Walther F.), pp. 722–743 Morges, Switzerland: IUCN [Google Scholar]
- 62.Hunn E. S., Williams N. M. 1982. Introduction. In Resource managers: North American and Australian hunter-gatherers (eds Williams N. M., Hunn E. S.), pp. 1–16 Canberra, Australia: Australian Institute of Aboriginal Studies [Google Scholar]
- 63.Fuller D. Q. 2007. Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World. Ann. Botany 100, 903–924 10.1093/aob/mcm048 (doi:10.1093/aob/mcm048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen H. 1977. The Bagunji of the Darling basin: cereal gatherers in an uncertain environment. World Archaeol. 5, 309–322 10.1080/00438243.1974.9979576 (doi:10.1080/00438243.1974.9979576) [DOI] [Google Scholar]
- 65.McConnell K., O'Connor S. 1997. 40,000 year record of food plants in the Southern Kimberley Ranges, Western Australia. Austral. Archaeol. 45, 20–31 [Google Scholar]
- 66.Fullagar R., Field J. 1997. Pleistocene seed-grinding implements from the Australian arid zone. Antiquity 71, 300–307 [Google Scholar]
- 67.Smith M. A. 1986. The antiquity of seedgrinding in arid Australia. Archaeology in Oceania 21, 29–39 [Google Scholar]
- 68.Brokensha P. 1975. The Pitjantjatjara and their crafts. Sydney, Australia: The Aboriginal Arts Board of the Australia Council [Google Scholar]
- 69.Hillman G., Hedges J. W., Moore A., Colledge S., Pettitt P. 2001. New evidence of Lateglacial cereal cultivation at Abu Hureyra on the Euphrates. The Holocene 11, 383–393 10.1191/095968301678302823 (doi:10.1191/095968301678302823) [DOI] [Google Scholar]
- 70.Bar-Yosef O. In press Climatic fluctuations and early farming—West and East Asia. Curr. Anthropol. [Google Scholar]
- 71.Hillman G. C. 2000. Abu Hureyra 1: the epipalaeolithic. In Village on the Euphrates. From foraging to farming at Abu Hureyra (eds Moore A. M. T., Hillman G. C., Legge A. J.), pp. 327–399 Oxford, UK: Oxford University Press [Google Scholar]
- 72.Savard M., Nesbitt M., Jones M. K. 2006. The role of wild grasses in subsistence and sedentism: new evidence from the northern Fertile Crescent. World Archaeol. 38, 179–196 10.1080/00438240600689016 (doi:10.1080/00438240600689016) [DOI] [Google Scholar]
- 73.Wollstonecroft M. M., Ellis P. R., Hillman G., Fuller D. Q. 2008. Advances in plant food processing in the Near Eastern Epipalaeolithic and implications for improved edibility and nutrient bioaccessibility: an experimental assessment of Bolboschoenus maritimus (L.) Palla (sea club-rush). Vegetation History and Archaeobotany 17(Suppl.), S19–S27 10.1007/s00334-008-0162-x (doi:10.1007/s00334-008-0162-x) [DOI] [Google Scholar]
- 74.Willcox G., Fornite S., Herveux L. 2008. Early Holocene cultivation before domestication in northern Syria. Vegetation History and Archaeobotany 17, 313–325 10.1007/s00334-007-0121-y (doi:10.1007/s00334-007-0121-y) [DOI] [Google Scholar]
- 75.Hillman G. C., Davis M. S. 1990. Measured domestication rates in wild wheats and barley under primitive cultivation, and their archaeological implications. J. World Prehistory 4, 157–222 10.1007/BF00974763 (doi:10.1007/BF00974763) [DOI] [Google Scholar]
- 76.Tanno K. I., Willcox G. 2006. How fast was wild wheat domesticated? Science 311, 1886. 10.1126/science.1124635 (doi:10.1126/science.1124635) [DOI] [PubMed] [Google Scholar]
- 77.Allaby R. G., Fuller D. Q., Brown T. A. 2008. The genetic expectations of a protracted model for the origins of domesticated crops. Proc. Natl Acad. Sci. USA 105, 13 982–13 986 10.1073/pnas.0803780105 (doi:10.1073/pnas.0803780105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Estes R. D. 1974. Social organization of the African Bovidae. In The behaviour of ungulates and its relation to management (eds Geist V., Walther F.), pp. 166–205 IUCN Publications new series 24. Morges, Switzerland: International Union for Conservation of Nature and Natural Resources [Google Scholar]
- 79.Walther F., Mungall E. C., Grau G. A. 1983. Gazelles and their relatives: a study in territorial behavior. Park Ridge, NJ: Noyes [Google Scholar]
- 80.Clutton-Brock T., Guinness F. E., Albon S. D. 1982. Red deer. Behaviour and ecology of two sexes. Edinburgh, UK: Edinburgh University Press [Google Scholar]
- 81.Jarman P. J., Jarman M. V. 1974. Impala behaviour and its relevance to management. In The behaviour of ungulates and its relation to management (eds Geist V., Walther F.), pp. 871–881 IUCN Publications new series 24. Morges, Switzerland: International Union for Conservation of Nature and Natural Resources [Google Scholar]
- 82.Bigalke R. C. 1974. Ungulate behaviour and management, with special reference to husbandry of wild ungulates on South African ranches. In The behaviour of ungulates and its relation to management (eds Geist V., Walther F.), pp. 830–852 IUCN Publications new series 24. Morges, Switzerland: International Union for Conservation of Nature and Natural Resources [Google Scholar]
- 83.Blaxter K. L., Kay R. N. B., Sharman G. A. M., Cunningham J. M. M., Hamilton W. J. 1974. Farming the red deer. Edinburgh, UK: HMSO [Google Scholar]
- 84.Lightfoot C. J., Posselt J. 1977. Eland (Taurotragus oryx) as a ranching animal complementary to cattle in Rhodesia. Rhodesia Agric. J. 74, 47–120 [Google Scholar]
- 85.Posselt J. 1963. The domestication of the eland. Rhodesian J. Agric. Res. 1, 81–87 [Google Scholar]
- 86.Treus V., Kravchenko D. 1968. Methods of rearing and economic utilization of eland in the Askaniya-Nova Zoological Park. Symp. Zool. Soc. London 21, 395–411 [Google Scholar]
- 87.Treus V., Lobanov N. V. 2007. Acclimatisation and domestication of the eland at Askanya-Nova Zoo. Int. Zoo Yearb. 11, 147–156 10.1111/j.1748-1090.1971.tb01892.x (doi:10.1111/j.1748-1090.1971.tb01892.x) [DOI] [Google Scholar]
- 88.Zeder M. A. 2006. A critical assessment of markers of initial domestication in goats (Capra hircus). In Documenting domestication: new genetic and archaeological paradigms (eds Zeder M. A., Bradley D. G., Emshwiller E., Smith B. D.), pp. 181–208 Berkeley, CA: University of California Press [Google Scholar]
- 89.Zeder M. A., Hesse B. 2000. The initial domestication of goats (Capra hircus) in the Zagros Mountains 10,000 years ago. Science 287, 2254–2257 10.1126/science.287.5461.2254 (doi:10.1126/science.287.5461.2254) [DOI] [PubMed] [Google Scholar]
- 90.Munro N. 2004. Small game indicators of human foraging efficiency and early herd management at the transition to agriculture in Southwest Asia. In Petits animaux et sociétés humaines, du complément alimentaire aux ressources utilitaires (eds Brugal J.-P., Desse J.), pp. 515–531 Antibes, France: Éditions APDCA [Google Scholar]
- 91.Zapata L., Peña-Chocarro L., Pérez-Jordá G., Stika P. 2004. Early Neolithic agriculture in the Iberian Peninsula. J. World Prehistory 18, 283–325 10.1007/s10963-004-5621-4 (doi:10.1007/s10963-004-5621-4) [DOI] [Google Scholar]
- 92.Lüning J. 2000. Steinzeitliche Bauern in Deutschland. Bonn, Germany: Rudolf Habelt [Google Scholar]
- 93.Bishop R., Church M., Rowley-Conwy P. 2010. Cereals, fruits and nuts in the Scottish Neolithic. Proc. of the Society of Antiquaries of Scotland. 139, 47–103 [Google Scholar]
- 94.Döhle J. 1997. Husbandry and hunting in the Neolithic of Central Germany. In Proceedings of the 7th ICAZ conference (eds Kokabi M., Wahl J.), pp. 441–448 Anthropozoologica 25/26 [Google Scholar]
- 95.Clark J. G. D. 1947. Sheep and swine in the husbandry of prehistoric Europe. Antiquity 21, 122–136 [Google Scholar]
- 96.Zohary D., Hopf M. 2000. Domestication of plants in the Old World, 3rd edn Oxford, UK: Clarendon [Google Scholar]
- 97.Anderson A. J. 1989. Prodigious birds. Moas and moa hunting in prehistoric New Zealand. Cambridge, UK: Cambridge University Press [Google Scholar]
- 98.Rowley-Conwy P. In press Westward Ho! The spread of agriculture from Central Europe to the Atlantic. Curr. Anthropol. [Google Scholar]
- 99.Evershed P., et al. 2008. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 455, 528–531 10.1038/nature07180 (doi:10.1038/nature07180) [DOI] [PubMed] [Google Scholar]
- 100.Anderson A. 2002. Faunal collapse, landscape change and settlement history in Remote Oceania. World Archaeol. 33, 375–390 10.1080/00438240120107431 (doi:10.1080/00438240120107431) [DOI] [Google Scholar]
- 101.Cohen M. N. 1977. The food crisis in prehistory. Overpopulation and the origins of agriculture. Yale, UK: Yale University Press [Google Scholar]
- 102.Smith P. E. L., Young T. C. 1983. The force of numbers: population pressure in the Central Western Zagros 12,000–4500 B.C. In The Hilly Flanks. Essays on the prehistory of southwestern Asia (eds Young T. C., Smith P. E. L., Mortensen P.), pp. 141–161 Studies in Ancient Oriental Civilization 36. Chicago, IL: Oriental Institute of the University of Chicago [Google Scholar]
- 103.Lee R. 1980. Lactation, ovulation, infanticide, and women's work: a study of hunter-gatherer population regulation. In Biosocial mechanisms of population regulation (eds Cohen N. M., Malpass R., Klein H.), pp. 321–348 New Haven, CT: Yale University Press [Google Scholar]
- 104.Wilmsen E. N. 1989. Land filled with flies: a political economy of the Kalahari. Chicago, IL: Chicago University Press [Google Scholar]
- 105.Czerniak L. 2007. The North-East frontier of the post-LBK cultures. In The Lengyel, Polgár and related cultures in the Middle/Late Neolithic in Central Europe (eds Kozlowski J. K., Raczky P.), pp. 233–248 Krakow, Russia: Polish Academy of Arts and Sciences [Google Scholar]
- 106.Malmström H., et al. 2009. Ancient DNA reveals lack of continuity between Neolithic hunter-gatherers and contemporary Scandinavians. Curr. Biol. 19, 1758–1762 10.1016/j.cub.2009.09.017 (doi:10.1016/j.cub.2009.09.017) [DOI] [PubMed] [Google Scholar]
- 107.Dugmore A. J., et al. 2005. The Norse landnám on the North Atlantic islands: an environmental impact assessment. Polar Rec. 41, 21–37 10.1017/S0032247404003985 (doi:10.1017/S0032247404003985) [DOI] [Google Scholar]
- 108.Fleming A. 1988. The Dartmoor Reaves. London, UK: Batsford [Google Scholar]
- 109.Malthus T. 1973 [1803]. An essay on the principle of population, books I and II. London, UK: Dent [Google Scholar]
- 110.Viazzo P. P. 1989. Upland communities. Cambridge, UK: Cambridge University Press [Google Scholar]
- 111.Netting R. McC. 1981. Balancing on an Alp: ecological change and continuity in a Swiss mountain community. Cambridge, UK: Cambridge University Press [Google Scholar]
- 112.McGuire R., Netting R. McC. 1982. Leveling peasants? The maintenance of equality in a Swiss Alpine community. Am. Ethnol. 9, 269–290 10.1525/ae.1982.9.2.02a00040 (doi:10.1525/ae.1982.9.2.02a00040) [DOI] [Google Scholar]
- 113.Layton R. 2000. Anthropology and history in Franche-Comté: a critique of social theory. Oxford, UK: Oxford University Press [Google Scholar]