Abstract
Polarization sensitivity is documented in a range of marine animals. The variety of tasks for which animals can use this sensitivity, and the range over which they do so, are confined by the visual systems of these animals and by the propagation of the polarization information in the aquatic environment. We examine the environmental physical constraints in an attempt to reveal the depth, range and other limitations to the use of polarization sensitivity by marine animals. In clear oceanic waters, navigation that is based on the polarization pattern of the sky appears to be limited to shallow waters, while solar-based navigation is possible down to 200–400 m. When combined with intensity difference, polarization sensitivity allows an increase in target detection range by 70–80% with an upper limit of 15 m for large-eyed animals. This distance will be significantly smaller for small animals, such as plankton, and in turbid waters. Polarization-contrast detection, which is relevant to object detection and communication, is strongly affected by water conditions and in clear waters its range limit may reach 15 m as well. We show that polarization sensitivity may also serve for target distance estimation, when examining point source bioluminescent objects in the photic mesopelagic depth range.
Keywords: bioluminescence, vision, communication, contrast
1. Introduction
Light under water is partially linearly polarized to as deep as the light penetrates [1]. This polarization is affected by a wide range of factors (figure 1) including the scattering and absorption properties of the water, the path length of the light and, therefore, water depth, the viewing direction, the air/water interface and the effects of waves on it and the properties of the light incoming to the water, i.e. position of the Sun/Moon in the sky, cloud coverage and other celestial conditions (reviewed by [2,3]). Each of these factors may change the light's polarization by reducing it (e.g. via multiple scattering), by inducing polarization (e.g. internal reflection from the water/air interface or Rayleigh scattering of downwelling light) or by changing the orientation (polarization angle/direction of propagation) or partial (%) polarization of the light (e.g. via refraction of skylight at the air/water interface). In addition, marine organisms may affect and even generate localized polarization via scattering, reflection and birefringence of their tissues.
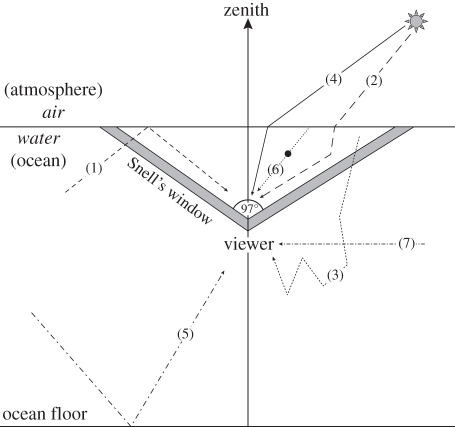
Figure 1.
Processes affecting underwater polarization of celestial illumination: (1) internal reflection from the sea surface, (2) single scattering, (3) multiple scattering, (4) refraction of direct light while travelling from air to water, (5) reflection from the sea floor, (6) forward scattering and (7) attenuation of polarized light. The thick grey lines delineate the boundaries of Snell's window with an acceptance angle of 97°. After Sabbah et al. [2].
Over 70 species of marine animals, mainly fishes, molluscs and arthropods, but also echinoderms, are currently known to be sensitive to the polarization of light (reviewed in [2,4]). These animals use the light's polarization for a range of functions such as body orientation and navigation, shore detection, object detection, prey identification and communication.
In this review, we examine these different functions and explore their physical constraints in marine waters. From these, one can estimate the limitations that animals encounter in their use of polarization sensitivity. We restrict our analysis to the epi- and mesopelagic zones, i.e. to areas that are illuminated by sunlight at least for part of the day (ca upper 1000 m). Although a few marine animals are known to be sensitive to circular polarization [5], only limited information about its distribution in sea water exists (but see [6]). Hence, we limit our discussion to the linear polarization state of the light.
2. Navigation
Navigation is one of the most documented functions for polarization sensitivity in terrestrial animals [7]. It is based on calibrating a sun compass even when the Sun is obscured by clouds or is too bright to be viewed directly. However, in marine environments such navigation is reported in far fewer cases and it involves several mechanisms. Open-water visual navigation often involves examination of components that are related to the position of the Sun/Moon in the sky. Obviously, this can be achieved at depths where the light maintains its orientation component and does not appear to come from a single point source overhead. For practical reasons, this means depths of 400 m and shallower. However, the question can be asked, can polarization-based navigation exist in such deep waters? Sabbah et al. [8] showed that the sky's pattern of polarization penetrates into the water in a predictable way. However, this pattern is greatly affected by waves at the air–water interface [9]. Shashar et al. [10] examined the distance at which a linearly polarized pattern can be transmitted in water. They found strong correlation with water clarity, where in clear water a polarization pattern deteriorated at ca 15 m. Therefore, one may conclude that when the Sun/Moon is not seen (clouded, etc.) polarization-based navigation is limited to shallow waters (ca 15 m deep). Waterman & Westell [11] and Ivanoff & Waterman [12] found a correlation between the per cent polarization at a horizontal line of sight and the Sun's position down to 120 m, and Waterman [1,13] suggested such a correlation down to 200 m. Therefore, when the Sun is visible, polarization may serve as a proxy, yet possibly redundant, indicator of the Sun's position, at least down to 200 m and most probably deeper.
A different type of navigation that can be found in aquatic environments is shore detection and the attraction to or avoidance of open waters [14–16]. The ability to detect a distant shore, by examining a polarization difference between light coming from it and from the open waters, is based on downwelling light being side scattered in the water. Such scattering, especially when caused by small Rayleigh-size particles, induces linear polarization to the light. This scattering and, therefore, the strength of its linear polarization component, is related to the volume of the water scattering the light, hence to the optical path and the distance from shore. Light coming from open waters is scattered by a large volume of water and is therefore highly linearly polarized. Light coming from the shore's direction is less scattered and therefore less polarized; furthermore, the light reflected from the shore's bottom has a further depolarizing effect. At 10 m away from a coral reef, N. Shashar & E. Zarfati (2002, 2003, unpublished data) measured a 13 per cent difference between the onshore and offshore lines of sight, over the differences recorded in open waters. Detailed examination in different water types, including different scatterers and absorbers in the water, wavelength effects, types of shore, etc. is still missing. One may expect that in murky waters, where scattering by large Mie particles and multiple scattering are common, the extent of this difference between the on-shore and the open-water lines of sight will be noticeable at a shorter distance. On the other hand, near-shore sedimentation may reduce per cent polarization in both on shore and offshore lines of sights. This aspect, not known to be used by animals, means that as one approaches the shore, polarization is expected to be reduced with increased turbidity. Preliminary measurements in relatively clear coral-reef waters recorded a reduction of 25–30% of linear polarization at distances of up to 15 m from shore (N. Shashar & E. Zarfati 2004, unpublished data). Whether such changes in polarization can be used for indications of an approaching shore, and whether they have an advantage over other means of turbidity assessment, remains to be examined. One aspect is that scattering-induced differences in the polarized light field are not limited by the amount of light and should be noticeable at any depth to which light penetrates [17].
3. Contrast enhancement
Simple filtration of a given orientation of polarization, such as filtering out horizontally polarized light and with it veiling light, may enhance the contrast between an underwater object and its background, even without true polarization sensitivity. However, differential polarization sensitivity of the light detectors (i.e. polarization sensitivity) provides the basis for polarization discrimination and for detection of a polarization contrast. Such polarization-based contrast enhancement may serve for detection of objects (especially objects that do not emit their own light) or specific patterns in them. Marine animals use polarization sensitivity for detecting and identifying prey, ranging from fish to plankton, as well as for increasing their overall range of view [18–21]. The contrast examined can be between the object and its background, such as the case of plankton detection, or of patterns of polarization within an object. Several independent studies ([20,22,23]; see also values in [19]), using different set-ups and having different goals, found that such polarization-based contrast enhancement can increase detection range by 1.7–1.8 of intensity-based sensitivity. The actual distance where this effect is meaningful, depends on the viewing conditions and visual acuity of the animal. For example, Novales Flamarique & Browman [19] found in early-stage rainbow trout (Oncorhynchus mykiss) an increase in plankton detection range from ca 1.5 cm under depolarized illumination to ca 2.5 cm under fully polarized white light and somewhat more under short-wavelengths illumination. Visual interactions in zooplanktonic animals occur in the centimetre range [24], and adult planktivorous fishes detect zooplankton at tens of centimetres (e.g. [25,26], see also [27]). However, for larger objects, the polarization effect may reach several metres [23,28]. An upper limit can be obtained at 15 m [10], after which such a pattern gradually deteriorates. One should recall that, in shallow waters, where significant fluctuations of the downwelling polarization occur [9], detection of transparent objects, such as plankton, will be further limited.
4. Polarization and detection of bioluminescent sources
Linear polarization of light emanating from bioluminescence has not been reported (but see [29]), although several types of photophores possess light-reflecting platelets that help broadcast the light from such organs [30]. Since reflected light generally becomes partially linearly polarized (depending upon platelet structure or any depolarizing structures that may be associated with photophores), the possibility exists that some bioluminescence is partly polarized. Thus, polarization sensitivity may play a role in detecting bioluminescent sources, yet this possibility awaits empirical examination.
In dim waters, detecting a flash of light at near horizontal lines of sight is mainly a question of contrast detection ([31–33], reviewed in [34,35]). However, estimating the distance to this point source is much more challenging, especially if no other distance markers are available. Although binocular vision is the predominant way for depth perception, such light flashes can often be detected only by a single eye or they appear at an orientation where binocular vision is not reliable. Polarization sensitivity may play a role in estimating the range to bioluminescing objects. In the open mesopelagic region, where light is coming down in a fairly regular and predictable manner [36], it is possible to estimate the distance to an unpolarized source by examining the polarization characteristics of the light arriving from it. Veiling light, meaning downwelling light that is scattered between the object and the viewer, will be partially polarized. The amount of this partial polarization is positively correlated with the amount of veiling light, i.e. the distance between the object and the viewer. This relationship holds up to a given maximum of polarization that is characteristic of the water properties and the orientation of the line of sight. This maximal value can be seen at the open waters near by, yet unobscured by the examined light source [10]. In a terrestrial setting, Namer et al. [37] have been able to use such levels of linear polarization to estimate distances to objects located several kilometres away from a camera. In an aquatic setting, this range is naturally much shorter.
Assuming a non-polarized bioluminescing source, one can calculate the distance to it (d) by examining the level of polarization arriving in the beam.
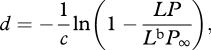 |
where c is the beam attenuation coefficient of the water, L is the radiance of the bioluminescence viewed at distance d, Lb the radiance of the background, P∞ the degree of background polarization and P the degree of polarization of light arriving from the bioluminescent source (see appendix A for derivation). Since c is relatively stable at these depths [36], one can assume that animals have a sense of the attenuation of light in their environment.
Several species of mesopelagic squid possess polarization sensitivity [38]. Such range-detection capabilities can be an important function of this sensitivity. It is unlikely that squid perform such calculations in each case, but it can be expected that a distance estimation system is hard-wired in their nervous system or acquired over time and experience. Such a neurological approach for polarization calculations can be found in compact nervous systems such as those of insects [39,40].
Behavioural experiments as well as physical modelling are required to examine the actual use of this polarization-based target ranging. Anatomical studies of photophore structure correlated with polarization measurements from individual photophores are also needed to determine if/how certain types of photophores produce polarized light.
5. Communication
Several animals, including cephalopods and crustaceans, possess patterns reflecting or transmitting light that can be partially to nearly fully polarized [17]. In many cases, these patterns can be modulated by the animal. One possible advantage of polarization signalling in the marine environment is that they are less affected by spectral changes in downwelling illumination that occur with water depth [17]. The distances by which such polarization patterns can travel depend mostly on their initial polarization and radiance contrasts. In most aspects, the essences of the signal's propagation are similar to those reported in §3. However, one should note that in the case of communication, the polarization difference is often the only signal available. Here, there can be a difference between the information transmitted in the polarization domain—a conspicuous signal versus the information in the intensity and spectral domains—which may be used at the same time as part of the animals camouflage system. Therefore, one should examine the propagation of information in each domain separately (one cannot simply add ca 80% to the distance of intensity-based detection). Further studies are needed to understand how animals interpret and map the visual information obtained in each of these domains.
6. Discussion
In this short report, we aimed to examine the constraints that the physical environment sets on some of the functions of linear polarization sensitivity as it is used by marine animals. Other functions for this sensitivity will emerge with the expansion of our knowledge of the ways polarization signals are detected and processed in the visual systems of animals. These will require detailed examination to understand their capacities and limitations under different marine conditions, and to continue to develop better instruments for more convenient in situ measurements of polarization under conditions in which marine animals use their polarization sensitivity. Typically, we aim at examining the upper (maximal) range of the various functions of polarization sensitivity. However, sea waters are not always clear, sea surfaces not always calm, and the sky is sometimes covered with clouds. Such changes in the physical environment are likely to reduce the range at which different functions of polarization sensitivity operate. For example, it is probable that polarization-based food detection by cuttlefish [41] has a much shorter range in the turbid English/La Manche Channel than in the clear east Mediterranean. This difference may not mean much to a small cuttlefish hatchling hunting at a short range but may be more significant to an adult [42] searching for larger shrimps and fishes [21].
The processes described in this paper focus on constraints that are related to the medium and the physical environment of the sensing animal. Additional constraints are set by the visual system of the observer. These include, among others, resolution and sensitivity limitations, contrast detection ability and spatial and temporal integration processes. The interplay of the two classes of constraints will set the actual limitations for linear polarization used by each species. One important question is: what is the minimal polarization signal that can be detected by the animal? In many cases this translates to polarization-contrast detection. Three types of polarization contrast may be meaningful for an animal: (i) the polarization contrast between an object and its background, as in object detection, (ii) the polarization contrast between parts of the object or animal, as in polarization signalling, and (iii) the polarization contrast between different parts of the background, as in shore detection and polarization-based navigation. Neurological examination suggested that squid can detect polarization orientation (angle) differences of up to 4° and a contrast in polarization orientation of 9.6° [43]. In a behavioural study, octopus was found to be able to detect a contrast in polarization orientation as little as 20° [44]. Mussi et al. [45] showed that a damselfish could detect polarization orientation differences of 10°–15° when coming from a single light source; while Degner & Hawryshyn [46] have shown that rainbow trout discriminate between two linearly polarized light patches when the e-vector orientation differed between them by more than 45°. Polarization contrast can occur not only in e-vector orientation but also in per cent polarization. Indeed such contrast is the key component in shore detection tasks. Previous studies have indicated that the minimum per cent polarization detected by salmonids ranges between 60 and 70 per cent under laboratory conditions [19,47,48] and 45 per cent in field conditions (reviewed by [49]); however, when considering fish UV polarization sensitivity the value could be lower (N. S. Hawryshyn 2008, personal communication). However, the small planktonic crustacean Daphnia pulex detects a polarization signal that is only 37 per cent polarized [16]. Owing to technical reasons, studies are lacking in estimating the minimal per cent polarization difference that can be detected by animals. The interplay between orientation of polarization and per cent polarization, and the effects of light intensity on them, will set further limits to the ability of an animal to detect a polarization difference.
Circular polarization has been poorly studied in marine environments (but note [6]). However, it is known that some animals do sense and use this light modality [5]. In a scattering medium, circular polarization may well allow better propagation of a signal when compared with depolarized or linearly polarized light, and the sensitivity to both linear and circular polarization can provide information as to the nature of the scatterers in the water [50,51]. Better theoretical and empirical understanding of the distribution and propagation of circular or perhaps elliptical polarization should be the focus of future studies.
Acknowledgements
We are grateful for the opportunity of knowing and working with the late Errol Zarfati (1947–2009), whose ingenuity and technical skills produced many of the instruments and data described in this paper. Comments by two anonymous reviewers greatly improved this manuscript. We thank the AFOSR who sponsored the polarization conference. R.H. is grateful for partial funding from ONR grant N0001406-1-0202, and N.S. for support by the ISF and BSF.
Appendix A
(a). Relationship between polarization and distance of an unpolarized bioluminescent source viewed horizontally in water with background horizontal polarization
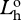
radiance of horizontal polarization component of source at distance zero.
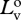
radiance of vertical polarization component of source at distance zero.
- Lo
total radiance of source at distance zero.
- L
total radiance of source at distance d, including path radiance.
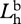
radiance of horizontal polarization component of background.
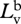
radiance of vertical polarization component of background.
- Lb
total radiance of background.
- p∞
degree of polarization of background.
- c
beam attenuation coefficient of water.
Suppose an unpolarized, bioluminescent signal is being viewed horizontally against a water background in a pelagic environment. Its apparent radiance is composed of two parts: the inherent radiance of signal and the radiance of the light scattered into the path between the animal and the viewer (referred to as path radiance). From Duntley [52]:
| A 1 |
Suppose that the background light is horizontally polarized (a reasonable assumption in the asymptotic mesopelagic realm). Then the degree of linear polarization of the bioluminescent signal is
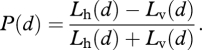 |
A 2 |
Because the signal is unpolarized and the path radiance has a constant degree of polarization [53], we can substitute equation (A 1) into equation (A 2) and get
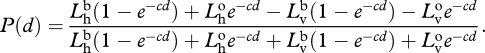 |
A 3 |
Again, since the bioluminescence is unpolarized, 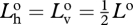 . Also,
. Also, 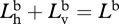 , so
, so
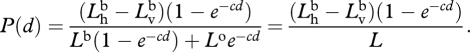 |
A 4 |
Dividing top and bottom by 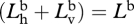 gives
gives
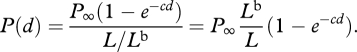 |
A 5 |
So, the polarization is the polarization of the background multiplied by the fraction of the apparent radiance of the source that is path radiance. The same arguments hold for polarization at 45° and circular polarization.
Solving equation (A 5) for d gives
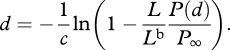 |
A 6 |
Thus, if given the radiance and polarization of both observed signal and the background, one can determine its distance. Alternatively, using equation (A 2), one can rewrite equation (A 6) as
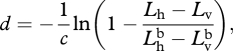 |
A 7 |
which, given that it only involves measuring the difference in the two polarization channels, rather than computing the more abstract degree of polarization, may be more physiologically plausible.
Footnotes
One contribution of 20 to a Theme Issue ‘New directions in biological research on polarized light’.
References
- 1.Waterman T. H. 1955. Polarization of scattered sunlight in deep water. Deep-Sea Res. 3(suppl.), 426–434 [Google Scholar]
- 2.Sabbah S., Lerner A., Erlick C., Shashar N. 2005. Under water polarization vision—a physical examination. Recent Res. Dev. Exp. Theor. Biol. 1, 123–176 [Google Scholar]
- 3.Waterman T. H. 1981. Polarization sensitivity. In Comparative physiology and evolution of vision in invertebrates. (ed. Autrum H.), pp. 281–463 Berlin, Germany: Springer [Google Scholar]
- 4.Horváth G., Varjú D. 2004. Polarized light in animal vision: polarization patterns in nature. Heidelberg, Germany: Springer [Google Scholar]
- 5.Chiou T. H., Kleinlogel S., Cronin T. W., Caldwell R., Loeffler B., Siddiqi A., Goldizen A., Marshall N. J. 2008. Circular polarization vision in a stomatopod crustacean. Curr. Biol. 18, 429–434 10.1016/j.cub.2008.02.066 (doi:10.1016/j.cub.2008.02.066) [DOI] [PubMed] [Google Scholar]
- 6.Ivanoff A., Waterman T. H. 1958. Elliptical polarization of submarine illumination. J. Mar. Res. 16, 255–282 [Google Scholar]
- 7.von Frisch K. 1949. Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tänzen der Bienen. Experiemtia 5, 142–148 10.1007/BF02174424 (doi:10.1007/BF02174424) [DOI] [PubMed] [Google Scholar]
- 8.Sabbah S., Barta A., Gál J., Horváth G., Shashar N. 2006. Experimental and theoretical study of skylight polarization transmitted through Snell's window of a flat water surface. J. Opt. Soc. Am. A 28, 1978–1988 10.1364/JOSAA.23.001978 (doi:10.1364/JOSAA.23.001978) [DOI] [PubMed] [Google Scholar]
- 9.Sabbah S., Shashar N. 2006. Underwater light polarization and radiance fluctuations induced by surface waves. Appl. Opt. 45, 4726–4739 10.1364/AO.45.004726 (doi:10.1364/AO.45.004726) [DOI] [PubMed] [Google Scholar]
- 10.Shashar N., Sabbah S., Cronin T. W. 2004. Transmission of linearly polarized light in sea water—implications for polarization signaling. J. Exp. Biol. 207, 3619–3628 10.1242/jeb.01187 (doi:10.1242/jeb.01187) [DOI] [PubMed] [Google Scholar]
- 11.Waterman T. H., Westell W. E. 1956. Quantitative effect of the sun's position on submarine light polarization. J. Mar. Res. 15, 149–169 [Google Scholar]
- 12.Ivanoff A., Waterman T. H. 1958. Factors, mainly depth and wavelength, affecting the degree of underwater light polarization. J. Mar. Res. 16, 283–307 [Google Scholar]
- 13.Waterman T. H. 2006. Reviving a neglected celestial underwater polarization compass for aquatic animals. Biol. Rev. 81, 111–115 10.1017/S1464793105006883 (doi:10.1017/S1464793105006883) [DOI] [PubMed] [Google Scholar]
- 14.Goddard S. M., Forward R. B. 1991. The role of underwater polarized light pattern in sun compass navigation of the grass shrimp, Palaemonetes vulgaris. J. Comp. Physiol. A 169, 479–491 10.1007/BF00197660 (doi:10.1007/BF00197660) [DOI] [Google Scholar]
- 15.Ritz D. A. 1991. Polarized light responses in the shrimp Palaemonetes vulgaris (Say). J. Exp. Mar. Biol. Ecol 154, 245–250 10.1016/0022-0981(91)90167-U (doi:10.1016/0022-0981(91)90167-U) [DOI] [Google Scholar]
- 16.Schwind R. 1999. Daphnia pulex swims towards the most strongly polarized light—a response that leads to ‘shore flight’. J. Exp. Biol. 202, 3631–3635 [DOI] [PubMed] [Google Scholar]
- 17.Cronin T. W., Shashar N., Caldwell R. L., Marshall N. J., Cheroske A. G., Chiou H. 2003. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 43, 549–558 10.1093/icb/43.4.549 (doi:10.1093/icb/43.4.549) [DOI] [PubMed] [Google Scholar]
- 18.Browman H. I., Novales Flamarique I., Hawryshyn C. W. 1994. Ultraviolet photoreception contributes to prey search behavior in 2 species of zooplanktivorous fishes. J. Exp. Biol. 186, 187–198 [DOI] [PubMed] [Google Scholar]
- 19.Novales Flamarique I., Browman H. I. 2001. Foraging and prey-search behaviour of small juvenile rainbow trout (Oncorhynchus mykiss) under polarized light. J. Exp. Biol. 204, 2415–2422 [DOI] [PubMed] [Google Scholar]
- 20.Shashar N., Hanlon R. T., Petz A. D. 1998. Polarization vision helps detect transparent prey. Nature 393, 222–223 10.1038/30380 (doi:10.1038/30380)9607759 [DOI] [Google Scholar]
- 21.Shashar N., Hagan R., Boal J. G., Hanlon R. T. 2000. Cuttlefish use polarization sensitivity in predation on silvery fish. Vis. Res. 40, 71–75 10.1016/S0042-6989(99)00158-3 (doi:10.1016/S0042-6989(99)00158-3) [DOI] [PubMed] [Google Scholar]
- 22.Shashar N., Adessi L., Cronin T. W. 1995. Polarization vision as a mechanism for detection of transparent objects. In Ultraviolet radiation and coral reefs (eds Gulko D., Jokiel P. L.), pp. 207–211 (HIMB and UNIHI- Sea Grant). Honolulu, HI: University of Hawaii [Google Scholar]
- 23.Schechner Y. Y., Karpel N. 2005. Recovery of underwater visibility and structure by polarization analysis. IEEE J. Oceanic Eng. 30, 570–587 10.1109/JOE.2005.850871 (doi:10.1109/JOE.2005.850871) [DOI] [Google Scholar]
- 24.Buskey E. J. 2000. Role of vision in the aggregative behavior of the planktonic mysid Mysidium columbiae. Mar. Biol. 137, 257–265 10.1007/s002270000361 (doi:10.1007/s002270000361) [DOI] [Google Scholar]
- 25.Gerking S. D. 1994. Feeding ecology of fish. San Diego, CA: Academic Press [Google Scholar]
- 26.Rickel S., Genin A. 2005. Twilight transitions in coral reef fishes: the input of light-induced changes in foraging behaviour. Anim. Behav. 70, 133–144 10.1016/j.anbehav.2004.10.014 (doi:10.1016/j.anbehav.2004.10.014) [DOI] [Google Scholar]
- 27.Hairston N. G., Li K. T., Easter S. S. 1982. Fish vision and the detection of planktonic prey. Science 218, 1240–1242 10.1126/science.7146908 (doi:10.1126/science.7146908) [DOI] [PubMed] [Google Scholar]
- 28.Schechner Y. Y., Karpel N. 2004. Clear underwater vision. In IEEE Computer Society Conf. on Computer Vision and Pattern Recognition, vol. 1, pp. 536–554 Piscataway, NJ: IEEE 10.1109/CVPR.2004.59 (doi:10.1109/CVPR.2004.59) [DOI] [Google Scholar]
- 29.Wynberg H., Meijer E. W., Hummelen J. C., Dekkers H. P. J. M., Schippers P. H., Carlson A. D. 1980. Circular polarization observed in bioluminescence. Nature 286, 641–642 10.1038/286641a0 (doi:10.1038/286641a0) [DOI] [Google Scholar]
- 30.Herring P. J. 2000. Bioluminescent signals and the role of reflectors. J. Opt. A Pure Appl. Opt. 2, R29–R38 10.1088/1464-4258/2/6/202 (doi:10.1088/1464-4258/2/6/202) [DOI] [Google Scholar]
- 31.Denton E. J. 1990. Light and vision at depths greater than 200 meters. In Light and life in the sea. (eds Herring P. J., Campbell A. K., Whitefield M., Maddock L.), pp. 127–148 Cambridge, UK: Cambridge University Press [Google Scholar]
- 32.Johnsen S., Widder E. A., Mobley C. D. 2004. Propagation and perception of bioluminescence: factors affecting counterillumination as cryptic strategy. Biol. Bull. 207, 1–16 10.2307/1543624 (doi:10.2307/1543624) [DOI] [PubMed] [Google Scholar]
- 33.Widder E. A. 2002. Bioluminescence and the pelagic visual environment. Mar. Freshw. Behav. Physiol. 35, 1–26 10.1080/10236240290025581 (doi:10.1080/10236240290025581) [DOI] [Google Scholar]
- 34.Haddock S. H. D., Moline M. A., Case J. F. 2010. Bioluminescence in the sea. Ann. Rev. Mar. Sci. 2, 443–493 10.1146/annurev-marine-120308-081028 (doi:10.1146/annurev-marine-120308-081028) [DOI] [PubMed] [Google Scholar]
- 35.Warrant E. J., Locket A. 2004. Vision in the deep sea. Biol. Rev. 79, 671–712 10.1017/S1464793103006420 (doi:10.1017/S1464793103006420) [DOI] [PubMed] [Google Scholar]
- 36.Jerlov N. G. 1976. Marine optics. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 37.Namer E., Shwartz S., Schechner Y. Y. 2009. Skyless polarimetric calibration and visibility enhancement. Opt. Expr. 17, 472–493 10.1364/OE.17.000472 (doi:10.1364/OE.17.000472) [DOI] [PubMed] [Google Scholar]
- 38.Shashar N., Milbury C. A., Hanlon R. T. 2002. Polarization vision in cephalopods: neuroanatomical and behavioral features that illustrate aspects of form and function. Mar. Freshw. Behav. Physiol. 35, 57–68 10.1080/10236240290025617 (doi:10.1080/10236240290025617) [DOI] [Google Scholar]
- 39.Labhart T., Meyer E. P. 2002. Neural mechanisms in insect navigation: polarization compass and odometer. Curr. Opin. Neurobiol. 12, 707–714 10.1016/S0959-4388(02)00384-7 (doi:10.1016/S0959-4388(02)00384-7) [DOI] [PubMed] [Google Scholar]
- 40.Wehner R., Martin M. 2006. The significance of direct sunlight and polarized skylight in the ant's celestial system of navigation. Proc. Natl Acad. Sci. USA 103, 12 575–12 579 10.1073/pnas.0604430103 (doi:10.1073/pnas.0604430103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darmaillacq A. S., Chichery R., Shashar N., Dickel L. 2006. Early familiarization overrides innate prey preference in newly-hatched Sepia officinalis cuttlefish. Anim. Behav. 71, 511–514 10.1016/j.anbehav.2005.04.019 (doi:10.1016/j.anbehav.2005.04.019) [DOI] [Google Scholar]
- 42.Loew E. R., McFarland W. N. 1990. The underwater visual environment. In The visual system of fishes (eds Douglas R. H., Djamgoz M. B. A.), pp. 1–43 London, UK: Chapman and Hall [Google Scholar]
- 43.Saidel W. M., Shashar N., Schmolesky M. T., Hanlon R. T. 2005. Discriminative responses of squid (Loligo pealeii) photoreceptors to polarized light. Comp. Biochem. Physiol. A 142, 340–346 10.1016/j.cbpa.2005.08.003 (doi:10.1016/j.cbpa.2005.08.003) [DOI] [PubMed] [Google Scholar]
- 44.Shashar N., Cronin T. W. 1996. Polarization contrast vision in octopus. J. Exp. Biol. 199, 999–1004 [DOI] [PubMed] [Google Scholar]
- 45.Mussi M., Haimberger T. J., Hawryshyn C. W. 2005. Behavioural discrimination of polarized light in the damselfish Chromis viridis (family Pomacentridae). J. Exp. Biol. 208, 3037–3046 10.1242/jeb.01750 (doi:10.1242/jeb.01750) [DOI] [PubMed] [Google Scholar]
- 46.Degner S. L., Hawryshyn C. W. 2001. Orientation of rainbow trout (Oncorhynchus mykiss) to multiple patches of linearly polarized light. Can. J. Zool. 79, 407–415 10.1139/cjz-79-3-407 (doi:10.1139/cjz-79-3-407) [DOI] [Google Scholar]
- 47.Hawryshyn C. W., Bolger A. 1991. Spatial orientation of rainbow trout: effects of the degree of polarization of the polarised light field. J. Comp. Physiol. A 167, 691–697 [Google Scholar]
- 48.Novales Flamarique I., Hawryshyn C. W. 1997. Is the use of underwater polarized light by fish restricted to crepuscular time periods? Vis. Res. 37, 975–989 10.1016/S0042-6989(96)00236-2 (doi:10.1016/S0042-6989(96)00236-2) [DOI] [PubMed] [Google Scholar]
- 49.Hawryshyn C. W. 2003. Mechanisms of ultraviolet polarization vision in fishes. In Sensory processing in aquatic environments (eds Collin S. P., Marshall N. J.), pp. 252–265 New York, NY: Springer [Google Scholar]
- 50.Antar Y. M. M., Allan L. E., Mishra R. 1988. Determination of radar targets scattering properties using circular and linear polarization technique. Antennas Propag. Soc. Int. Symp. 2, 526–529 10.1109/APS.1988.94124 (doi:10.1109/APS.1988.94124) [DOI] [Google Scholar]
- 51.Born M., Wolf E. Principles of optics, electromagnetic theory of propagation, interference and diffraction of light, 7th edn. Cambridge, UK: Cambridge University Press: 2002. [Google Scholar]
- 52.Duntley S. Q. The visibility of submerged objects. 1952 Final Report to Office of Naval Research. Visibility Lab., Massachusetts Institute of Technology. [Google Scholar]
- 53.Schechner Y. Y., Narasimhan S. G., Nayar S. K. 2003. Polarization-based vision through haze. Appl. Opt. 42, 511–525 10.1364/AO.42.000511 (doi:10.1364/AO.42.000511) [DOI] [PubMed] [Google Scholar]