Abstract
Polarized light (PL) sensitivity is relatively well studied in a large number of invertebrates and some fish species, but in most other vertebrate classes, including birds, the behavioural and physiological mechanism of PL sensitivity remains one of the big mysteries in sensory biology. Many organisms use the skylight polarization pattern as part of a sun compass for orientation, navigation and in spatial orientation tasks. In birds, the available evidence for an involvement of the skylight polarization pattern in sun-compass orientation is very weak. Instead, cue-conflict and cue-calibration experiments have shown that the skylight polarization pattern near the horizon at sunrise and sunset provides birds with a seasonally and latitudinally independent compass calibration reference. Despite convincing evidence that birds use PL cues for orientation, direct experimental evidence for PL sensitivity is still lacking. Avian double cones have been proposed as putative PL receptors, but detailed anatomical and physiological evidence will be needed to conclusively describe the avian PL receptor. Intriguing parallels between the functional and physiological properties of PL reception and light-dependent magnetoreception could point to a common receptor system.
Keywords: polarized light, sun compass, magnetic compass, orientation, homing, birds
1. Introduction
The ability to perceive and use information from the skylight polarization pattern for orientation, navigation and in spatial orientation tasks is widespread among organisms, including many invertebrates and vertebrates (reviewed in [1]). In vertebrates, there is convincing evidence for the use of polarized light (PL) cues from all classes, including fishes (e.g. [2,3]), amphibians (e.g. [4,5]), reptiles (e.g. [6,7]) and birds (this review), apart from mammals. However, while PL sensitivity is relatively well studied in a large number of invertebrates and some fish species (for summary see [1]), the behavioural and physiological mechanism of PL sensitivity in most other vertebrate classes, including birds, remains one of the big mysteries in sensory biology.
Skylight polarization arises when unpolarized sunlight gets scattered when entering the atmosphere, reaching the Earth as partially polarized skylight [8]. The degree of polarization depends on the scattering angle, i.e. the angle between the unpolarized sunlight and the partially polarized skylight, with the highest degree of polarization found at 90° from the Sun, where the light is scattered maximally. The direction of vibration (electric vector or e-vector orientation) of the polarized skylight is always perpendicular to the plane of the scattering angle, resulting in a well-defined pattern across the sky, with the Sun as the centre [8]. Animals can use the information provided by the skylight polarization pattern as part of the sun compass for orientation, navigation and in spatial orientation tasks. It enables them to use the sun compass also on partly overcast days, when the Sun itself is obscured by clouds, but parts of the blue, partially polarized sky still visible (e.g. [6,9,10]; reviewed in [1]).
The aim of this review is to summarize and scrutinize the available literature on PL sensitivity in birds, both from a behavioural and physiological perspective. Birds are well known for using a sun compass as one of several compasses to determine their migratory direction and the direction towards home, or to find stored food caches (see reviews by [11–13]). In §2, I review the literature on sun-compass orientation in birds to examine the available evidence for an involvement of the skylight polarization pattern in avian sun-compass orientation. I also discuss the importance of the sunset polarization pattern for the decision-making process involved when migratory birds determine their departure direction (§3), and I summarize the available support for the proposed function of sunrise and sunset PL cues as compass calibration reference (§4). In §5, I compare and evaluate the direct experimental evidence for PL sensitivity in birds published to date and provide possible explanations for the many contradictory findings. Last, but not least, I discuss putative receptor systems for PL sensitivity in birds (§6) and highlight some intriguing parallels between the functional and physiological properties of PL reception and light-dependent magnetoreception, which could point to a common receptor system (§7).
2. Are polarized light cues part of the avian sun compass?
It is well established that birds can use directional information from the Sun during migration, homing and other spatial orientation tasks, like food caching (reviewed by [11–13]). Day-migrating birds are able to maintain their migratory direction throughout the day, when provided access to directional information from the sky and the Sun [14]. Measuring migratory restlessness in circular orientation cages with horizontal windows and artificially deflecting the position of the Sun with mirrors attached to the windows resulted in a corresponding change in the orientation direction as predicted by the sun compass, with the relative angle between the sun azimuth, and orientation direction changing over the day in a time-compensated manner (figure 1; [14,15]).
Figure 1.
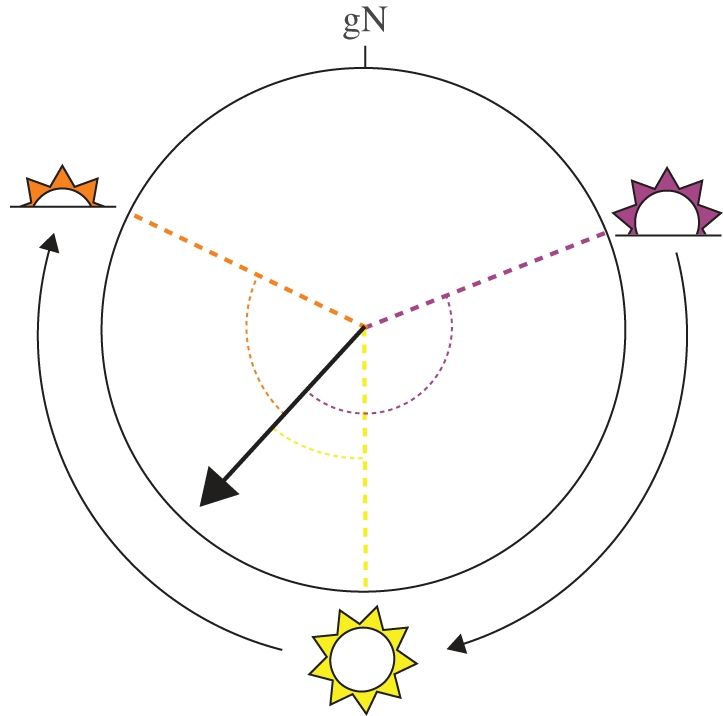
The avian sun compass is time compensated and takes into consideration the movement of the Sun across the sky during the day. Animals using such a time-compensated sun compass need to compensate for the azimuthal change of the Sun over the day when determining their goal direction (dark arrow). The example shows three sun positions over a day (sunrise in pink, noon in yellow and sunset in orange) and the changing relationships (angles in respective colours) between sun position and the goal direction. In order to be able to determine this angle correctly, the animals needs to be able to precisely measure local time. gN, geographical north.
Compelling evidence for sun-compass orientation has also been provided by homing experiments with pigeons, Columba livia domesticus (reviewed in [11–13]). Vanishing bearings of homing pigeons, whose clocks were reset either clockwise or anticlockwise, were deflected relative to control birds in accordance with the direction and magnitude predicted by the use of a time-compensated sun compass (e.g. [16,17]). Thus, the avian sun compass takes into consideration the movement of the Sun across the sky during the day, requiring the animals to measure time and to compensate for the azimuthal change of the Sun with a time-compensation mechanism (figure 1). The accuracy with which pigeons compensate for the apparent movement of the Sun across the sky over the course of the day appears to be quite high and closely tuned to the differential speed of the azimuthal changes of the Sun, and specific for the time of year and testing location [17].
Sun-compass orientation is used by birds not only during homing and migration, but also in spatial orientation tasks. Seed-caching corvids and chickadees [18–21], but also non-caching homing pigeons [22–24] and migratory passerines, including both day and night migrants [25–28], trained to relocate a food reward in a fixed geographical compass direction were generally able to solve these tasks using cues solely from either the real or an artificial Sun. Even when training and testing were carried out at different times of day, birds were able to relocate the food reward, indicating the use of a time-compensated compass and not a simple menotactic orientation in a constant angle relative to the orientation cue [27]. Clock-shifted birds compensate for the movement of the Sun across the sky over the day, further indicating that the avian sun compass is time-compensated [18,20,21,29].
The question of whether PL cues associated with the Sun also provide sun-compass information in birds, as is the case in other animals (see below), has not been conclusively answered. The majority of experiments suggest that the view of the disc of the Sun itself, or alternatively an artificial sun, is necessary to operate the sun compass. Under completely overcast conditions, birds become either disoriented or resort to other compass mechanisms, like the magnetic compass (e.g. [14,15,30]). In the laboratory, an artificial sun seems to be readily accepted as a replacement for the natural Sun, and in free-flying homing pigeons, the view of the Sun as a diffuse light point on the sky as seen through frosted lenses seems to suffice for sun-compass orientation [15,25,27,31]. Vanishing bearings of homing pigeons and directional choices in food relocation tasks of clock-shifted birds are generally deflected in agreement with time-compensated sun-compass orientation, as long as the birds can directly see the disc of the Sun (e.g. [14,30]; reviewed in [12]). Mirror experiments deflecting visual cues only in close vicinity of the Sun, leaving the skylight polarization pattern from the regions of sky opposite to the Sun unchanged, indicate that the Sun is the dominant cue and that the conflicting PL pattern is either ignored or over-ruled [14,15]. Only two studies suggest that visibility of the disc of the Sun may not necessarily be needed for time-compensated sun-compass orientation. In one study, jays (Aphelocoma coerulescens) had to cache seeds in sand-filled cups at a time of day when the aviary, but not the adjacent surroundings, lay in the shade [18]. Recovery results of this group were similar to groups allowed to cache and recover when the aviary was in the full Sun, questioning the need of a direct view of the Sun. While it is tempting to assume that the birds use PL cues to relocate the caches, it is equally probable that they used the distribution of shadows in the surrounding area learned during the habituation phase prior to the experiments, or alternatively a magnetic compass [18]. More convincing evidence that PL cues may be part of the avian sun compass was provided by orientation experiments with a day-migrating Australian honeyeater (Lichenostomus chrysops). When tested during the morning hours in orientation funnels surrounded by a magnetic coil that cancelled the horizontal component of the Earth's magnetic field, these birds were well oriented, as long as they could either see only the Sun, only the natural skylight polarization pattern, or both cues [32]. Birds tested under partially covered skies with the Sun hidden by clouds and the skylight polarization pattern depolarized by a filter were disoriented, indicating that the skylight polarization pattern provided directional compass information. However, the honeyeaters did not show any response to a deflection of the polarization axis with sheet polarizers, or to clock shift [32,33], suggesting that they primarily relied on magnetic compass information.
In summary, the available evidence for an involvement of the skylight polarization pattern in sun-compass orientation in birds is very weak, and it is questionable whether the skylight polarization pattern associated with the Sun is part of the time-compensated sun compass in birds. The majority of studies on time-compensated sun-compass orientation in birds did not further investigate whether a view of the disc of the Sun itself is required for a successful use of the sun compass, or whether the skylight polarization pattern associated with the Sun also contains sun-compass information. Additional experiments are necessary to specifically test whether birds can use time-compensated PL cues on a partly cloudy day, when the Sun is hidden behind clouds, but parts of the polarization pattern are visible in patches of blue sky.
3. The role of the sunset polarization pattern to determine the migratory direction
While there is no clear evidence that PL cues play any role in time-compensated sun-compass orientation in birds, cue-conflict and cue-calibration experiments with migratory passerines indicate that the skylight polarization pattern during sunrise and sunset provides the birds with important directional information (see figure 2a and text below). Specifically at sunset, the time when nocturnally migrating birds determine their departure direction, PL cues have been shown to play an important role in the decision-making process [34–36]. Orientation experiments with migratory songbirds during sunset demonstrated that birds shift their orientation when tested in orientation funnels covered with sheet polarizers aligned perpendicular to the natural polarization axis, with the observed shift corresponding with the relative shift between the artificial and natural polarization axis (e.g. [34,36–39]). Unfortunately, since the birds tended to align along the axis of the polarizers in the majority of the experiments, it is difficult to distinguish whether the observed responses were true compass responses or simply alignments along the polarization axis. During spring and autumn, the natural polarization axis at sunset is closely aligned along the north–south axis, and thereby coincides with the migratory directions of the majority of bird species tested. The expected alignments of the birds' orientation under a polarizer will therefore correspond with the axis of the polarizer, which makes it virtually impossible to distinguish whether the observed responses were the result of an alignment along the e-vector of the polarizer (polaritaxis) or a menotactic response, i.e. orientation at a certain angle relative to the polarization axis. Irrespective of the type of response, polaritaxis or menotaxis, the change in orientation is probably the result of the change in PL information perceived by the birds in the funnels. It has to be noted, however, that it can not be excluded that these positive findings are the result of light intensity artefacts created by the sheet polarizers.
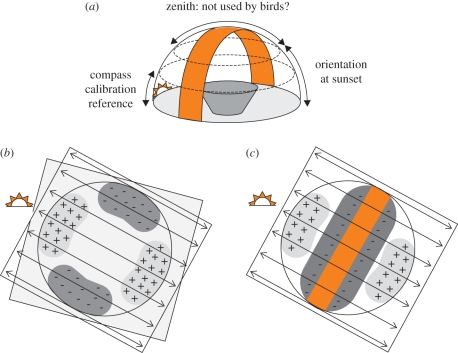
Figure 2.
(a) Use of polarized light cues by birds; (b,c) potential artefacts produced by sheet polarizers on top of orientation funnels. (a) Three-dimensional illustration of the band of maximum polarization at sunset (orange band), running through the zenith and intersecting the horizon vertically, 90° from the Sun (cf. [8]). Birds are suggested to use information from the lowest 5°–10° of the polarization pattern near the horizon to recalibrate their compasses (left double arrow), and the lower 45° of the sky to determine their migratory direction (right double arrow). It is unclear whether birds use information from the skylight polarization for sun-compass orientation during daytime (double arrow over zenith). (b,c) Illustration of light intensity artefacts produced by sheet polarizers placed on top of an orientation funnel (large circle). The sheet polarizer is shown as a square, with the black double arrows illustrating the orientation of the e-vector. Light will be reflected on the white, sloping walls of the orientation funnels maximally on (light grey areas with plus signs) and minimally perpendicular to the axis of the polarizer (dark grey areas with minus signs). (b) Polarizing filter interacting with natural light first filtered by a depolarizer (light grey sheet on top of polarizing filter). (c) Polarizing filter interacting with the natural polarization pattern at sunset (orange band), and transmitted light reflecting on the funnel walls on the axis of the polarizer.
When birds are tested outdoors in orientation funnels covered with linear sheet polarizers, two potentially confounding artefacts can occur: (i) reflection of PL on the white, sloping walls of the orientation funnel, maximal along and minimal perpendicular to the axis of the polarizer (figure 2b). This artefact can occur when the light entering the orientation funnel is first depolarized, before being sent through a sheet polarizer, creating two lighter areas on the funnel walls on the axis of the polarizer and two darker areas perpendicular to it; (ii) interaction between the natural polarization of the sky and the artificial polarization of the sheet polarizer, reducing light intensity along the natural polarization axis. This effect will be additionally enhanced by the reflection of the PL on the funnel walls along the polarization axis of the sheet polarizer (figure 2c). It is unclear, however, whether these artefacts pose any significant biases in outdoor funnel experiments, where light intensity and spectral composition over the sky vary and change considerably during the hour around sunset [40]. The observation that orientation is abolished when the natural polarization pattern is eliminated by depolarizers on top of orientation funnels (e.g. [41,42]) indicates that birds indeed use PL cues and not simply variation in light intensity or colour to determine their migratory direction. Still, owing to the potentially biasing effects of polarizers during orientation experiments, the most convincing evidence for PL sensitivity in birds comes from cue-calibration experiments with migratory songbirds, which did not manipulate the PL information during the actual orientation experiments, but instead during the calibration phase prior to testing (see below).
4. Sunrise and sunset polarized light cues as compass calibration reference
Apart from the possible role of sunset PL cues for determining the departure direction, there is convincing evidence from short-term deflector loft experiments with homing pigeons [43–46] and cue-calibration experiments with migratory songbirds [47–53] that PL cues at sunrise and sunset play a key role in compass calibration. In the homing experiments with pigeons, non-resident birds were housed in lofts with an unobstructed view of the Sun overhead, but with specifically designed deflector panels installed on the side windows of the loft that deflected wind and light cues in opposite directions relative to each other and thereby making it possible to distinguish effects of celestial and olfactory cues. The deflector panels preferentially reflected vertically polarized light, resulting in the deflection of the PL cues most prominent at sunrise and sunset [43,44]. A direct effect of the deflectors on the sun compass could be excluded, because the deflector panels only deflected the light cues near the horizon, but not overhead, thus the mirrored false images of the Sun were only visible at times around sunrise and sunset, whereas the true position of the Sun was visible during all other times of the day. Thus, the sun compass (and possibly also the magnetic compass) was recalibrated by PL cues near the horizon, most prominently visible to the birds through the windows of the loft around sunrise and sunset, when the band of maximum polarization and the e-vector vertically intersect the horizon. Averaging sunrise and sunset calibration provides the birds with a seasonally and latitudinally independent calibration reference (figure 3; [43–46]). These findings do not exclude the possibility that PL cues are part of the time-compensated sun compass and used by birds throughout the day to complement or substitute sun-compass information derived from the disc of the Sun. To date, however, evidence for such a role in the time-compensated sun compass is largely lacking (see below).
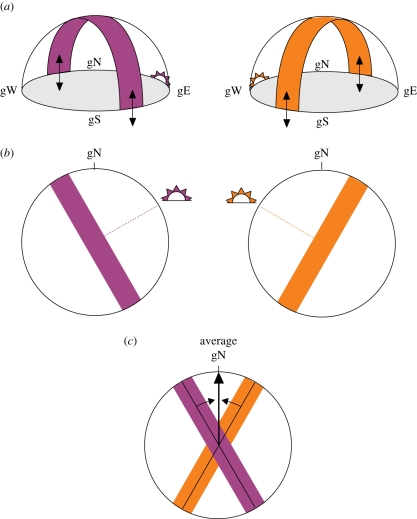
Figure 3.
Compass calibration by PL cues near the horizon at sunrise and sunset, when the band of maximum polarization and the e-vector vertically intersect the horizon. (a) Three-dimensional and (b) two-dimensional illustrations of the band of maximum polarization at sunrise (left) and sunset (right). Black arrows indicate the alignment of the e-vector intersecting the horizon vertically. (c) Averaging sunrise and sunset calibration provides the birds with a true geographical calibration reference, which is independent of season and latitude. gN, gS, gE, gW , geographical north, south, east and west, respectively.
Evidence for a key role of sunrise and sunset PL cues in compass calibration has also been provided by experiments with migratory songbirds, where PL information was not manipulated during the actual orientation experiment itself, but instead during a calibration period prior to testing [47–53]. In these studies, birds were exposed to conflicting information between magnetic and PL cues (one of the two cues being artificially deflected relative to the other by either shifting the horizontal component of the geomagnetic field relative to the natural skylight polarization pattern, or alternatively, by artificially shifting the band of maximum polarization relative to the natural magnetic field). When later tested for magnetic compass orientation without access to celestial cues, the birds showed recalibration of the magnetic compass reflecting the shifted relationship to the PL cues experienced during the cue-calibration period [47–53]. By contrast, birds exposed to a shifted magnetic field under depolarized natural skylight did not show a recalibration of their magnetic compass [48], and neither did birds exposed to a cue conflict at local noon [51,52].
In a review of the available cue-conflict literature, Muheim et al. [54] concluded that a view of the polarization pattern at sunrise and sunset near the horizon is crucial for compass calibration in migratory songbirds: only birds that had access to the lower parts of the morning/evening sky recalibrated their magnetic compass, whereas birds exposed to the cue conflict in orientation funnels or cages blocking the view of the horizon did not (figure 2a). Cue-calibration experiments specifically testing this hypothesis by shielding the lower part of the sky from view during the cue-calibration phase confirmed these findings [51,52]. It has to be noted, however, that birds do not always recalibrate their magnetic compass, even when given full access to PL cues near the horizon [55,56]. Reasons for the failure to update their compasses may be motivational and/or may reflect problems with the experimental procedure (cf. [51]).
It has also been demonstrated that a view of orientation cues near the horizon is important for migratory birds to determine their departure direction. Birds tested in orientation experiments where the field of view was artificially restricted to a 90° sector around the zenith showed phototactic orientation towards the direction of the setting Sun, whereas birds tested in funnels allowing a view of 160° around the zenith were oriented in the seasonally appropriate migratory direction [57]. Thus, the use of PL cues in birds is restricted to the times around sunrise and sunset, and to the region of sky close to the horizon.
5. Direct experimental demonstration of polarized light sensitivity in birds
Despite of the convincing evidence that birds use PL cues for orientation and navigation, unequivocal experimental evidence for PL sensitivity is still largely lacking. Two early conditioning experiments successfully demonstrated PL sensitivity in homing pigeons [58,59], but other studies reported negative findings [60–63]. Also, recent discrimination experiments with Japanese quails, Coturnix coturnix japonica, and European starlings, Sturnus vulgaris, were unsuccessful in demonstrating PL sensitivity in birds [64]. There are at least two reasons for the mixed success: (i) reflectance artefacts can be a significant problem in indoor set-ups (cf. [61,62]) and (ii) birds may need to be tested in a behavioural context closely simulating natural situations, which might not have been the case in some of the failed studies (e.g. [64]).
The experimental set-ups of the two studies reporting positive findings differ from the other studies in a number of aspects. The behavioural assay used by Delius et al. [58] simulated most closely an orientation task, where pigeons were rewarded along the axis of an overhead linear PL source in a visually symmetric, octagonal Skinner box. The birds had to move and choose between four keys, situated on the side walls of the box on two orthogonal axes, to receive a food reward provided along the two directions parallel to the polarization axis, but not perpendicular to it. This set-up differed from other Skinner box experiments in which the birds had to distinguish between a rewarded (overhead PL cues aligned along one axis) and an unrewarded stimulus (overhead PL cues aligned along the perpendicular axis), by responding to only one key [60,61]. Kreithen & Keeton [59] also used a Skinner box with only one key, but in contrast to other studies the birds had to distinguish between a rotating and a stationary PL source. The rotation was produced by rotating a linear polarizer in the light path reaching the box [59]. It is difficult to judge in retrospect whether the rotation just amplified light intensity artefacts produced by the linear polarizer or whether it made it easier for the birds to perceive the actual PL cue. Owing to the small aperture in the testing box, the risk of light intensity artefacts is expected to be minimal, but can not be excluded.
In conclusion, experimental evidence for PL sensitivity in birds is to date restricted to cue-calibration experiments with migratory birds. None of the experiments directly testing PL sensitivity has been repeatable and/or free of potential light intensity artefacts. An experimental assay allowing direct tests for PL vision is therefore crucial to further examine and characterize PL sensitivity in birds. Future experiments should take meticulous care in eliminating any potential biases created by light intensity artefacts resulting from differential reflectance.
6. Putative polarized light receptors
There is no generally accepted mechanism for PL reception in birds, to a large extent because of the failures to demonstrate PL sensitivity in controlled and repeatable experiments. A role of extraocular photoreceptors, like the avian pineal, can almost completely be ruled out, because the feathers are expected to depolarize any light reaching the skull. Since extracranial structures homologous to the parietal eye of reptilians or frontal organ of amphibians are lacking in birds, PL information can not be perceived other than through the eyes. Retinal photoreceptors are therefore the most likely candidates for PL receptors. Of these, double cones have been proposed to be involved in PL reception [65,66]. It is well established that internal reflections in the double cones in the fish retina form the basis of the PL sensors in fishes [65,67–70]. Avian double cones, like the ones in fish, consist of a principal cone with an oil droplet and an accessory cone [71]. They are the most numerous cone types in the avian retina and have been found to form mosaic patterns, either with four or six double cones surrounding one or two single cones [72–74]. More importantly, the orientation of the double cones forms a cross pattern, with two opposite double cones facing each other and the other two pointing away from each other. Young & Martin [66] argued that the presence of oil droplets in the principal cone and the lack of screening pigment between the outer segments of the double cones could be the basis for PL vision in birds. Polarization-dependent light scattering by the oil droplets of the principal cone to the outer segment of the accessory cone could in theory provide a measure of the e-vector orientation of incident light [66]. Still, more detailed anatomical and physiological studies of the avian retina are necessary to conclusively determine the type and the location of putative PL receptors.
7. Parallels between polarized light reception and light-dependent magnetoreception
There are some intriguing parallels between the functional and physiological properties of PL reception and light-dependent magnetoreception (cf. [75]). Besides using celestial cues like the Sun or stars to derive compass information, birds also have a magnetic compass, providing them with directional information from the Earth's magnetic field [76]. The avian magnetic compass has been shown to depend on the presence of light and its functional properties to vary with both the wavelength and intensity of light (e.g. [77–79]). Magnetic field sensitivity of the light-dependent magnetic compass is assumed to be mediated by a radical-pair mechanism taking place in specialized photoreceptor molecules in the avian retina (cf. [80]). In this process, absorption of light leads to the formation of radical-pair intermediates whose lifetime is modulated depending on their alignments to an Earth-strength magnetic field. Cryptochromes have been proposed to be the photoreceptor molecules involved in magnetoreception, because of their unique ability to form persistent, spin-correlated radical pairs (for review, see [81]). As in PL receptors, the receptor molecules that underlie the light-dependent magnetic compass need to be fixed in an ordered array, making it intrinsically polarization sensitive. Thus, the receptors of the light-dependent magnetic compass and PL sense could theoretically be based on similar receptor types ([75]; see also below).
In several species of fish, responses to PL show a wavelength-dependent antagonistic mechanism that closely resembles antagonistic, wavelength-dependent effects on magnetic compass orientation in eastern red-spotted newts, Nothophthalmus viridescens [75,81]. These fish have a UV-sensitive mechanism with polarization sensitivity to a vertically aligned e-vector and a green- and red-sensitive receptor mechanism with maximum polarization sensitivity to a horizontally aligned e-vector [2,67,68,82,83]. The intermediate blue-sensitive receptor mechanism is insensitive to PL. Likewise, light-dependent magnetic compass orientation in newts is mediated by an antagonistically interacting spectral mechanism, with a short-wavelength sensitive receptor mediating shoreward orientation and a long-wavelength sensitive mechanism mediating bimodal orientation along an axis perpendicular to shore [84]. Like fishes, newts are disoriented at intermediate wavelengths, which can be explained by an equal excitation of both mechanisms [84].
In birds, the wavelength dependence of light-dependent magnetic compass orientation shows a more complex pattern [78,79]. There is experimental evidence for an antagonistic mechanism, with a short-wavelength mechanism mediating orientation towards the innate migratory direction, a long-wavelength mechanism mediating perpendicular orientation and disorientation at an intermediate wavelength [77]. However, a more detailed and systematic examination of this wavelength dependence with a behavioural assay allowing training and testing under different light conditions is necessary to conclusively identify the wavelength dependence in magnetic compass orientation in birds.
8. Conclusions
The review of the available literature on sun-compass orientation in birds suggests that they ignore information from the skylight polarization pattern during time-compensated sun-compass orientation, but instead use it as a non-time-compensated calibration reference at sunrise and sunset. If the available evidence holds and it can be confirmed that birds ignore, or alternatively, are unable to perceive information from the skylight polarization pattern during times of day other than sunrise and sunset, the functional role of PL cues in avian orientation and navigation, and more specifically in avian sun-compass orientation, would be distinct from most other animals. Reasons why birds may not use the skylight polarization pattern as part of their sun compass may be of functional and/or physiological nature.
Sunrise and sunset mark those two times of day when both the e-vector and the band of maximum polarization are vertically aligned on the horizon, and intersect the sky at a 90° angle to the Sun through the zenith ([8]; figure 2). At other times of the day, the relationship between the skylight polarization pattern and the compass directions is more complex, and the pattern is less pronounced, and the e-vector intersects the horizon at various angles. Thus, in contrast to other times of the day, the skylight polarization pattern at sunrise and sunset is relatively simple and the signal intensity maximal. Animals with a relatively poorly developed PL sense would therefore be able to use it only at this time of day. A PL receptor based on a very simple matching filter [85], triggering only a response when the e-vector is vertically aligned, would also reduce the use of PL cues to sunrise and sunset times. However, if the PL receptor and the magnetoreceptor are based on the same photoreceptor system, we would expect the response to PL at these two times of day to over-ride or be superimposed on the magnetic compass response. According to current theory, birds may perceive the magnetic field as a three-dimensional pattern superimposed on their visual field (e.g. [80,81,86]). If PL reception takes place in parallel to magnetoreception, we would therefore expect an equally high resolution also for this system. Future behavioural and physiological experiments are needed to test whether and to what degree these two receptor systems are interconnected.
Acknowledgements
I am grateful to Almut Kelber and John Phillips for valuable discussions on PL sensitivity and magnetoreception. I would like to thank them, Marie Dacke and an anonymous referee for valuable comments on this paper. This work was supported by the Swedish Research Council.
Footnotes
One contribution of 20 to a Theme Issue ‘New directions in biological research on polarized light’.
References
- 1.Horváth G., Varjú D. 2004. Polarized light in animal vision. Berlin, Germany: Springer [Google Scholar]
- 2.Parkyn D. C., Austin J., Hawryshyn C. W. 2003. Acquisition of polarized light orientation in salmonids under laboratory conditions. Anim. Behav. 65, 893–904 10.1006/anbe.2003.2136 (doi:10.1006/anbe.2003.2136) [DOI] [Google Scholar]
- 3.Waterman T. H., Forward R. B. 1970. Field evidence for polarized light sensitivity in the fish Zenarchopterus. Nature 228, 85–87 10.1038/228085a0 (doi:10.1038/228085a0) [DOI] [PubMed] [Google Scholar]
- 4.Adler K., Taylor D. H. 1973. Extraocular perception of polarized light by orienting salamanders. J. Comp. Physiol. A 87, 203–212 10.1007/BF00696042 (doi:10.1007/BF00696042) [DOI] [Google Scholar]
- 5.Auburn J. S., Taylor D. H. 1979. Polarized light perception and orientation in larval bullfrogs, Rana catesbeiana. Anim. Behav. 27, 658–668 10.1016/0003-3472(79)90003-4 (doi:10.1016/0003-3472(79)90003-4) [DOI] [Google Scholar]
- 6.Adler K., Phillips J. B. 1985. Orientation in a desert lizard, Uma notata: time-compensated compass movement and polarotaxis. J. Comp. Physiol. A 156, 547–552 10.1007/BF00613978 (doi:10.1007/BF00613978) [DOI] [Google Scholar]
- 7.Freake M. 1999. Evidence for orientation using the e-vector direction of polarized light in the sleepy lizard, Tiliqua rugosa. J. Exp. Biol. 202, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 8.Brines M. L., Gould J. L. 1982. Skylight polarization patterns and animal orientation. J. Exp. Biol. 96, 69–91 [Google Scholar]
- 9.von Frisch K. 1949. Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tänzen der Bienen. Experientia 5, 142–148 10.1007/BF02174424 (doi:10.1007/BF02174424) [DOI] [PubMed] [Google Scholar]
- 10.Wehner R. 1976. Polarized-light navigation by insects. Sci. Am. 235, 106–115 10.1038/scientificamerican0776-106 (doi:10.1038/scientificamerican0776-106) [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Koenig K. 1990. The sun compass. Experientia 46, 336–342 10.1007/BF01952166 (doi:10.1007/BF01952166) [DOI] [Google Scholar]
- 12.Wiltschko R. 1980. Die Sonnenorientierung der Vögel. I. Die Rolle der Sonne im Orientierungssystem und die Funktionsweise des Sonnenkompass. J. Ornithol. 121, 121–143 10.1007/BF01642927 (doi:10.1007/BF01642927) [DOI] [Google Scholar]
- 13.Wiltschko R. 1981. Die Sonnenorientierung der Vögel. II. Entwicklung des Sonnenkompass und sein Stellenwert im Orientierungssystem. J. Ornithol. 122, 1–22 10.1007/BF01643440 (doi:10.1007/BF01643440) [DOI] [Google Scholar]
- 14.Kramer G. 1951. Eine neue Methode zur Erforschung der Zugorientierung und die bisher damit erzielten Ergebnisse. Proc. Int. Ornithol. Congr. 10, 269–280 [Google Scholar]
- 15.Kramer G. 1952. Experiments on bird orientation. Ibis 94, 265–285 10.1111/j.1474-919X.1952.tb01817.x (doi:10.1111/j.1474-919X.1952.tb01817.x) [DOI] [Google Scholar]
- 16.Schmidt-Koenig K. 1958. Der Einfluss experimentell veränderter Zeitschätzung auf das Heimfindevermögen der Brieftauben. Naturwissenschaften 45, 47. 10.1007/BF00635030 (doi:10.1007/BF00635030) [DOI] [Google Scholar]
- 17.Wiltschko R., Walker M. M., Wiltschko W. 2000. Sun-compass orientation in homing pigeons: compensation for different rates of change in azimuth? J. Exp. Biol. 203, 889–894 [DOI] [PubMed] [Google Scholar]
- 18.Balda R. P., Wiltschko W. 1991. Caching and recovery in scrub jays: transfer of sun-compass direction from shaded to sunny areas. Condor 93, 1020–1023 10.2307/3247740 (doi:10.2307/3247740) [DOI] [Google Scholar]
- 19.Duff S. J., Brownlie L. A., Sherry D. F., Sangster M. 1998. Sun compass and landmark orientation by black-capped chickadees, Parus atricapillus. J. Exp. Psychol. Anim. B 24, 243–253 10.1037/0097-7403.24.3.243 (doi:10.1037/0097-7403.24.3.243) [DOI] [Google Scholar]
- 20.Wiltschko W., Balda R. P. 1989. Sun compass orientation in seed-caching scrub jays, Aphelocoma coerulescens. J. Comp. Physiol. A 164, 717–721 10.1007/BF00616744 (doi:10.1007/BF00616744) [DOI] [Google Scholar]
- 21.Wiltschko W., Balda R. P., Jahnel M., Wiltschko R. 1999. Sun compass orientation in seed-caching corvids: its role in spatial memory. Anim. Cogn. 2, 215–221 10.1007/s100710050042 (doi:10.1007/s100710050042) [DOI] [Google Scholar]
- 22.Budzynski C. A., Dyer F. C., Bingman V. P. 2000. Partial experience with the arc of the sun is sufficient for all-day sun compass orientation in homing pigeons, Columba livia. J. Exp. Biol. 203, 2341–2348 [DOI] [PubMed] [Google Scholar]
- 23.Chappell J., Guilford T. 1995. Homing pigeons primarily use the sun compass rather than fixed directional visual cues in an open-field arena food-searching task. Proc. R. Soc. Lond. B 260, 59–63 10.1098/rspb.1995.0059 (doi:10.1098/rspb.1995.0059) [DOI] [Google Scholar]
- 24.Kramer G., Riese E. 1952. Die Dressur von Brieftauben auf Kompaßrichtung im Wahlkäfig. Z. Tierpsychol. 9, 245–251 10.1111/j.1439-0310.1952.tb01852.x (doi:10.1111/j.1439-0310.1952.tb01852.x) [DOI] [Google Scholar]
- 25.Kramer G., von Saint Paul U. 1950. Stare, Sturnus vulgaris, lassen sich auf Himmelsrichtungen dressieren. Naturwissenschaften 37, 526–527 10.1007/BF01184829 (doi:10.1007/BF01184829) [DOI] [Google Scholar]
- 26.von Saint Paul U. 1953. Nachweis der Sonnenorientierung bei nächtlich ziehenden Vögeln. Behaviour 6, 1–7 10.1163/156853954X00013 (doi:10.1163/156853954X00013) [DOI] [Google Scholar]
- 27.Kramer G., von Saint Paul U. 1954. Das Heimkehrvermögen gekäfigter Brieftauben. J. Ornithol. 51, 3–12 [Google Scholar]
- 28.von Saint Paul U. 1956. Compass directional training of western meadowlarks, Sturnella neglecta. Auk 73, 203–210 [Google Scholar]
- 29.Hoffmann K. 1954. Versuche zu der im Richtungsfinden der Vögel enthaltenen Zeitschätzung. Z. Tierpsychol. 11, 453–475 10.1111/j.1439-0310.1954.tb02169.x (doi:10.1111/j.1439-0310.1954.tb02169.x) [DOI] [Google Scholar]
- 30.Keeton W. T. 1969. Orientation by pigeons: is the Sun necessary? Science 165, 922–928 10.1126/science.165.3896.922 (doi:10.1126/science.165.3896.922) [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Koenig K., Keeton W. T. 1977. Sun compass utilization by pigeons wearing frosted contact lenses. Auk 94, 143–145 [Google Scholar]
- 32.Munro U., Wiltschko R. 1995. The role of skylight polarization in the orientation of a day-migrating bird species. J. Comp. Physiol. A 177, 357–362 10.1007/BF00192424 (doi:10.1007/BF00192424) [DOI] [Google Scholar]
- 33.Munro U., Wiltschko R. 1993. Clock-shift experiments with migratory yellow-faced honeyeaters, Lichenostomus chrysops (Meliphagidae), an Australian day-migrating bird. J. Exp. Biol. 181, 233–244 [Google Scholar]
- 34.Able K. P. 1982. Skylight polarization patterns at dusk influence migratory orientation in birds. Nature 299, 550–551 10.1038/299550a0 (doi:10.1038/299550a0) [DOI] [Google Scholar]
- 35.Moore F. R. 1978. Sunset and the orientation of a nocturnal migrant bird. Nature 274, 154–156 10.1038/274154a0 (doi:10.1038/274154a0) [DOI] [Google Scholar]
- 36.Moore F. R. 1986. Sunrise, skylight polarization, and the early morning orientation of night-migrating warblers. Condor 88, 493–498 10.2307/1368277 (doi:10.2307/1368277) [DOI] [Google Scholar]
- 37.Helbig A., Wiltschko W. 1989. The skylight polarization pattern at dusk affects the orientation behaviour of blackcaps, Sylvia atricapilla. Naturwissenschaften 76, 227–229 10.1007/BF00627697 (doi:10.1007/BF00627697) [DOI] [Google Scholar]
- 38.Moore F. R., Phillips J. B. 1988. Sunset, skylight polarization and the migratory orientation of yellow-rumped warblers, Dendroica coronata. Anim. Behav. 36, 1770–1778 10.1016/S0003-3472(88)80116-7 (doi:10.1016/S0003-3472(88)80116-7) [DOI] [Google Scholar]
- 39.Phillips J. B., Moore F. R. 1992. Calibration of the sun compass by sunset polarized light patterns in a migratory bird. Behav. Ecol. Sociobiol. 31, 189–193 10.1007/BF00168646 (doi:10.1007/BF00168646) [DOI] [Google Scholar]
- 40.McFarland W. N., Munz F. W. 1975. The visible spectrum during twilight and its implications to vision. In Light as an ecological factor: II (eds Evans G., Bainbridge R., Rackham O.), pp. 249–270 Oxford, UK: Blackwell Scientific Publication [Google Scholar]
- 41.Helbig A. 1990. Depolarization of natural skylight disrupts orientation of an avian nocturnal migrant. Experientia 46, 755–758 10.1007/BF01939958 (doi:10.1007/BF01939958) [DOI] [Google Scholar]
- 42.Helbig A. 1991. Dusk orientation of migratory European robins, Erithacus rubecula: the role of sun-related directional information. Anim. Behav. 41, 313–322 10.1016/S0003-3472(05)80483-X (doi:10.1016/S0003-3472(05)80483-X) [DOI] [Google Scholar]
- 43.Phillips J. B., Waldvogel J. A. 1982. Reflected light cues generate the short-term deflector-loft effect. In Avian navigation (eds Papi F., Wallraff E.), pp. 190–202 Berlin, Germany: Springer [Google Scholar]
- 44.Phillips J. B., Waldvogel J. A. 1988. Celestial polarized patterns as a calibration reference for sun compass of homing pigeons. J. Theor. Biol. 131, 55–67 10.1016/S0022-5193(88)80120-6 (doi:10.1016/S0022-5193(88)80120-6) [DOI] [Google Scholar]
- 45.Waldvogel J. A., Phillips J. B. 1991. Olfactory cues perceived at the home loft are not essential for the formation of a navigational map in pigeons. J. Exp. Biol. 155, 643–660 [DOI] [PubMed] [Google Scholar]
- 46.Waldvogel J. A., Phillips J. B., Brown A. I. 1988. Changes in the short-term deflector loft effect are linked to the sun compass of homing pigeons. Anim. Behav. 36, 150–158 10.1016/S0003-3472(88)80258-6 (doi:10.1016/S0003-3472(88)80258-6) [DOI] [Google Scholar]
- 47.Able K. P., Able M. A. 1990. Calibration of the magnetic compass of a migratory bird by celestial rotation. Nature 347, 378–380 10.1038/347378a0 (doi:10.1038/347378a0) [DOI] [Google Scholar]
- 48.Able K. P., Able M. A. 1993. Daytime calibration of magnetic orientation in a migratory bird requires a view of skylight polarization. Nature 364, 523–525 10.1038/364523a0 (doi:10.1038/364523a0) [DOI] [Google Scholar]
- 49.Able K. P., Able M. A. 1995. Manipulations of polarized skylight calibrate magnetic orientation in a migratory bird. J. Comp. Physiol. A 177, 351–356 10.1007/BF00192423 (doi:10.1007/BF00192423) [DOI] [Google Scholar]
- 50.Cochran W. W., Mouritsen H., Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408 10.1126/science.1095844 (doi:10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- 51.Muheim R., Åkesson S., Phillips J. B. 2007. Magnetic compass of migratory savannah sparrows is calibrated by skylight polarization at sunrise and sunset. J. Ornithol. 148, 485–494 10.1007/s10336-007-0187-4 (doi:10.1007/s10336-007-0187-4) [DOI] [Google Scholar]
- 52.Muheim R., Phillips J. B., Åkesson S. 2006. Polarized light cues underlie compass calibration in migratory songbirds. Science 313, 837–839 10.1126/science.1129709 (doi:10.1126/science.1129709) [DOI] [PubMed] [Google Scholar]
- 53.Muheim R., Phillips J. B., Deutschlander M. E. 2009. White-throated sparrows calibrate their magnetic compass by polarized light cues during both autumn and spring migration. J. Exp. Biol. 212, 3466–3472 10.1242/jeb.032771 (doi:10.1242/jeb.032771) [DOI] [PubMed] [Google Scholar]
- 54.Muheim R., Moore F. R., Phillips J. B. 2006. Calibration of magnetic and celestial compass cues in migratory birds—a review of cue-conflict experiments. J. Exp. Biol. 209, 2–17 10.1242/jeb.01960 (doi:10.1242/jeb.01960) [DOI] [PubMed] [Google Scholar]
- 55.Gaggini V., Baldaccini N., Spina F., Giunchi D. 2010. Orientation of the pied flycatcher Ficedula hypoleuca: cue-conflict experiments during spring migration. Behav. Ecol. Sociobiol 64, 1333–1342. (doi:10.1007/s00265-010-0948-6) [Google Scholar]
- 56.Wiltschko R., Munro U., Ford H., Wiltschko W. 2008. Contradictory results on the role of polarized light in compass calibration in migratory songbirds. J. Ornithol. 149, 607–614 10.1007/s10336-008-0324-8 (doi:10.1007/s10336-008-0324-8) [DOI] [Google Scholar]
- 57.Sandberg R. 1991. Sunset orientation of robins, Erithacus rubecula, with different fields of sky vision. Behav. Ecol. Sociobiol. 28, 77–83 10.1007/BF00180983 (doi:10.1007/BF00180983) [DOI] [Google Scholar]
- 58.Delius J. D., Perchard R. J., Emmerton J. 1976. Polarized light discrimination by pigeons and an electroretinographic correlate. J. Comp. Physiol. Psychol. 90, 560–571 10.1037/h0077223 (doi:10.1037/h0077223) [DOI] [PubMed] [Google Scholar]
- 59.Kreithen M. L., Keeton W. T. 1974. Detection of polarized light by the homing pigeon, Columba livia. J. Comp. Physiol. A 89, 83–92 10.1007/BF00696165 (doi:10.1007/BF00696165) [DOI] [Google Scholar]
- 60.Coemans M. A. J. M., Vos Hzn J. J., Nuboer J. F. W. 1990. No evidence for polarization sensitivity in the pigeon. Naturwissenschaften 77, 138–142 10.1007/BF01134480 (doi:10.1007/BF01134480) [DOI] [PubMed] [Google Scholar]
- 61.Coemans M. A. J. M., Vos Hzn J. J., Nuboer J. F. W. 1994. The orientation of the e-vector of linearly polarized light does not affect the behaviour of the pigeon, Columba livia. J. Exp. Biol. 191, 107–123 [DOI] [PubMed] [Google Scholar]
- 62.Montgomery K. C., Heinemann E. G. 1952. Concerning the ability of homing pigeons to discriminate patterns of polarized light. Science 116, 454–456 10.1126/science.116.3017.454 (doi:10.1126/science.116.3017.454) [DOI] [PubMed] [Google Scholar]
- 63.Vos Hzn J., Coemans M., Nuboer J. 1995. No evidence for polarization sensitivity in the pigeon electroretinogram. J. Exp. Biol. 198, 325–335 [DOI] [PubMed] [Google Scholar]
- 64.Greenwood V. J., Smith E. L., Church S. C., Partridge J. C. 2003. Behavioural investigation of polarisation sensitivity in the Japanese quail, Coturnix coturnix japonica, and the European starling, Sturnus vulgaris. J. Exp. Biol. 206, 3201–3210 10.1242/jeb.00537 (doi:10.1242/jeb.00537) [DOI] [PubMed] [Google Scholar]
- 65.Cameron D. A., Pugh E. N. 1991. Double cones as a basis for a new type of polarization vision in vertebrates. Nature 353, 161–164 10.1038/353161a0 (doi:10.1038/353161a0) [DOI] [PubMed] [Google Scholar]
- 66.Young S. R., Martin G. R. 1984. Optics of retinal oil droplets: a model of light collection and polarization detection in the avian retina. Vis. Res. 24, 129–137 10.1016/0042-6989(84)90098-1 (doi:10.1016/0042-6989(84)90098-1) [DOI] [PubMed] [Google Scholar]
- 67.Coughlin D. J., Hawryshyn C. W. 1995. A cellular basis for polarized-light vision in rainbow trout. J. Comp. Physiol. A 176, 261–272 10.1007/BF00239928 (doi:10.1007/BF00239928) [DOI] [Google Scholar]
- 68.Hawryshyn C. W., McFarland W. N. 1987. Cone photoreceptor mechanisms and the detection of polarized light in fish. J. Comp. Physiol. A 160, 459–465 10.1007/BF00615079 (doi:10.1007/BF00615079) [DOI] [Google Scholar]
- 69.Roberts N. W., Needham M. 2007. A mechanism of polarized light sensitivity in cone photoreceptors of the goldfish, Carassius auratus. Biophys. J. 93, 3241–3248 10.1529/biophysj.107.112292 (doi:10.1529/biophysj.107.112292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flamarique I. N., Hawryshyn C. W., Hárosi F. I. 1998. Double-cone internal reflection as a basis for polarization detection in fish. J. Opt. Soc. Am. A 15, 349–358 10.1364/JOSAA.15.000349 (doi:10.1364/JOSAA.15.000349) [DOI] [PubMed] [Google Scholar]
- 71.Hart N. S., Hunt D. M. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, S7–S26 10.1086/510141 (doi:10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 72.Engström K. 1958. On the cone mosaic in the retina of Parus major. Acta Zool. 39, 65–69 10.1111/j.1463-6395.1958.tb00523.x (doi:10.1111/j.1463-6395.1958.tb00523.x) [DOI] [Google Scholar]
- 73.Kram Y. A., Mantey S., Corbo J. C. 2010. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 5, e8992. 10.1371/journal.pone.0008992 (doi:10.1371/journal.pone.0008992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris V. B. 1970. Symmetry in a receptor mosaic demonstrated in the chick from the frequencies, spacing and arrangement of the types of retinal receptor. J. Comp. Neurol. 140, 359–397 10.1002/cne.901400308 (doi:10.1002/cne.901400308) [DOI] [PubMed] [Google Scholar]
- 75.Phillips J. B., Deutschlander M. E., Freake M. J., Borland S. C. 2001. The role of extraocular photoreceptors in newt magnetic compass orientation: parallels between light-dependent magnetoreception and polarized light detection. J. Exp. Biol. 204, 2543–2552 [DOI] [PubMed] [Google Scholar]
- 76.Wiltschko W., Wiltschko R. 2002. Magnetic orientation in birds and its physiological basis. Naturwissenschaften 89, 445–452 10.1007/s00114-002-0356-5 (doi:10.1007/s00114-002-0356-5) [DOI] [PubMed] [Google Scholar]
- 77.Muheim R., Bäckman J., Åkesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856 [DOI] [PubMed] [Google Scholar]
- 78.Wiltschko R., Stapput K., Thalau P., Wiltschko W. 2010. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interface 7, S163–S177 10.1098/rsif.2009.0367.focus (doi:10.1098/rsif.2009.0367.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiltschko W., Munro U., Ford H., Wiltschko R. 2003. Magnetic orientation in birds: non-compass responses under monochromatic light of increased intensity. Proc. R. Soc. Lond. B 270, 2133–2140 10.1098/rspb.2003.2476 (doi:10.1098/rspb.2003.2476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 10.1016/S0006-3495(00)76629-X (doi:10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips J. B., Jorge P. E., Muheim R. 2010. Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J. R. Soc. Interface 7, S241–S256 10.1098/rsif.2009.0459.focus (doi:10.1098/rsif.2009.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parkyn D. C., Hawryshyn C. W. 1993. Polarized-light sensitivity in rainbow trout, Oncorhynchus mykiss: characterization from multi-unit responses in the optic nerve. J. Comp. Physiol. A 172, 493–500 10.1007/BF00213531 (doi:10.1007/BF00213531) [DOI] [Google Scholar]
- 83.Ramsden S. D., Anderson L., Mussi M., Kamermans M., Hawryshyn C. W. 2008. Retinal processing and opponent mechanisms mediating ultraviolet polarization sensitivity in rainbow trout, Oncorhynchus mykiss. J. Exp. Biol. 211, 1376–1385 10.1242/jeb.015941 (doi:10.1242/jeb.015941) [DOI] [PubMed] [Google Scholar]
- 84.Phillips J. B., Borland S. C. 1992. Behavioural evidence for use of light-dependent magnetoreception mechanism by a vertebrate. Nature 359, 142–144 10.1038/359142a0 (doi:10.1038/359142a0) [DOI] [Google Scholar]
- 85.Wehner R. 1987. ‘Matched filters’—neural models of the external world. J. Comp. Physiol. A 161, 511–531 10.1007/BF00603659 (doi:10.1007/BF00603659)3316619 [DOI] [Google Scholar]
- 86.Phillips J. B., Muheim R., Jorge P. E. 2010. A behavioral perspective on the biophysics of the light-dependent magnetic compass: a link between directional and spatial perception? J. Exp. Biol. 213, 3247–3255 10.1242/jeb.020772 (doi:10.1242/jeb.020772) [DOI] [PubMed] [Google Scholar]