Abstract
The human eye is insensitive to the angular direction of the light e-vector, but several animal species have the ability to discriminate differently polarized lights. How the polarization is detected is often unclear, however. Egg-laying Papilio butterflies have been shown to see false colours when presented with differently polarized lights. Here we asked whether this also holds in foraging butterflies. After training individuals to feed on nectar in front of an unpolarized spectral light, we carried out three dual-choice tests, where the discrimination of (i) the spectral content, (ii) the light intensity, and (iii) the e-vector orientation were investigated. In the first test, the butterflies selected the trained spectrum irrespective of its intensity, and in the second test they chose the light with the higher intensity. The result of the e-vector discrimination test was very similar to that of the second test, suggesting that foraging butterflies discriminate differently polarized lights as differing in brightness rather than as differing in colour. Papilio butterflies are clearly able to use at least two modes of polarization vision depending on the behavioural context.
Keywords: butterfly, colour vision, brightness vision, photoreceptor, compound eye, ommatidium
1. Introduction
Humans visually discriminate objects basically depending on the wavelength (spectral content) and intensity (photon flux density) of the light. These physical qualities of light, respectively, produce the senses of colour and brightness. There is in fact another fundamental quality of light; the direction of the electric vector (e-vector). When the e-vector orientation of light is dominated by the direction parallel to the horizon, we call the light horizontally polarized. Humans cannot directly sense the direction of polarization, because the retinal photoreceptors equally respond to polarized lights with differing e-vector orientation. The eyes of many animals including insects are however equipped with rhabdomeral photoreceptors that are strongly sensitive to a particular e-vector orientation [1–3], thus providing the ability to discriminate differently polarized lights. Yet, how the eventual perception of polarization is achieved is often unclear.
There are at least three possibilities of how animals subjectively perceive polarized light [4]. The first possibility is via the colour discrimination system. Differently polarized lights would then be perceived as different colours. Indeed, perception of polarization-based false colours has been demonstrated in the female orchard swallowtail butterfly, Papilio aegeus [5,6]. Secondly, animals may have some e-vector-specific sense, which should be called true polarization vision, defined as the ability to discriminate visual stimuli solely based on e-vector orientation. The backswimmer water bug Notonecta glauca trying to find a body of water while flying in the air [7] and trained mantis shrimps Gonodactylus chiragra and Odontodactylus scyllarus [8] do discriminate differently polarized lights irrespective of their intensity. Thirdly, animals may sense differently polarized lights as differing in brightness. We can fairly easily imagine this case by viewing polarizing objects through a rotating polarizer. The brightness hypothesis, which assumes that polarization discrimination occurs via brightness discrimination [9], has so far not been extensively tested. Kelber et al. [6] tested the ability of polarization vision in egg-laying and foraging Papilio butterflies. The female butterflies appeared to have polarization-dependent colour vision when searching for leaves on which to deposit eggs. The situation remained unsettled in foraging individuals, where some intensity dependency was also observed, leaving the possibility that the butterflies have multiple modes of polarization vision, depending on the behavioural context. Here, we describe a series of experiments investigating whether foraging Papilio butterflies perceive differently polarized lights as differing in colour or in brightness.
2. Material and methods
(a). Animals
We used newly emerged females of the Japanese yellow swallowtail butterfly, Papilio xuthus Linnaeus, reared in the laboratory. The laboratory culture was derived from eggs laid by females caught in Kanagawa, Japan. The larvae were fed on fresh citrus leaves under a light regime of L : D = 10 L : 14 D or 14 L : 10 D at 28°C. The short-day condition produced diapausing pupae, which were stored at 4°C for at least three months and allowed to emerge at 25°C. The long-day condition produced non-diapausing pupae, from which adults emerged in about 10 days after pupation. The day of emergence was defined as the post-emergence day-1 (PED-1).
(b). Setup and stimuli
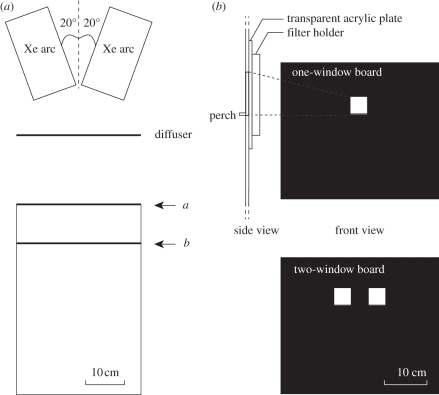
We carried out the behavioural experiments in a cage (W × D × H = 36 × 55 × 35 cm3, figure 1a) in a room at 28°C, illuminated with ceiling light (spectral distribution, see figure 2a) with luminosity about 3000 lx at the cage floor. A vertical, black plastic board at one end of the cage (a in figure 1a) contained one or two windows of 4 × 4 cm2 (figure 1b) for presenting stimuli. The lower side of each window had an acrylic perch (40 mm width × 5 mm depth) for butterflies to land on when taking a sucrose reward (figure 1b, side view). The windows were illuminated from the backside with two 100 W Xenon lamps (XC-100B, Seric, Tokyo, Japan), through a diffuser (figure 1a). Figure 2b shows the emission spectrum of the Xenon light after passing through the diffuser, measured inside the cage with a calibrated spectrometer (HSU-100S, Asahi Spectra, Tokyo Japan). We used the one-window board for training and the two-window board for dual-choice tests.
Figure 1.
(a) Top view of the experimental setup. Light stimuli were presented at a. When changing stimulus conditions, the scene (b) front and side views of the boards positioned at a for presenting stimuli. While changing a board to another, the end was blocked by inserting a black screen at b.
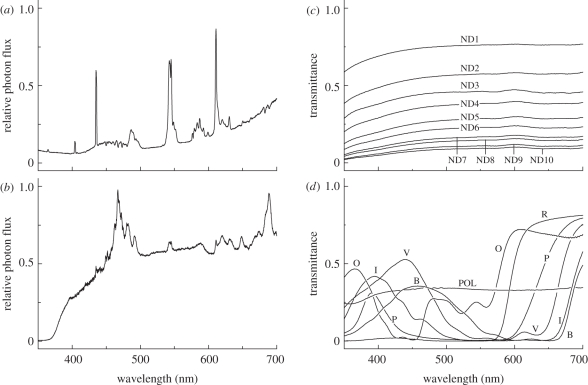
Figure 2.
(a) Spectral content of the ceiling light, measured as the reflection from MgO-coated surface using a calibrated spectrometer (HSU-100S, Asahi Spectra, Tokyo Japan). (b) Spectrum of Xe light measured from the inside of the cage after passing through the diffusing screen without having other filters shown in C and D. (c) Transmittance spectra of neutral density (ND) filters. (d) Transmittance spectra of colour and polarization filters: B, blue; I, indigo; O, orange; P, pink; R, red; V, violet: POL, polarization filter.
Different stimulus lights were produced by inserting neutral-density (Kodak WRATTEN ND Filter 96), spectral and/or polarization filters in various combinations in the filter holder behind the windows (figure 1b). For the transmittance spectra of the filters, see figure 2c,d: the resulting spectral lights will be indicated by their human-subjective colours: red, orange, pink, blue, violet and indigo. When changing the board and/or the filters, we hid the board from the butterfly in the cage with a removable screen, 10 cm in front of it (b in figure 1a).
For quantifying the intensity of the lights we calculated the total photon flux of light i, Pi, with
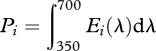 |
2.1 |
with Ei(λ) being the emission spectrum of light i. For comparing the intensity of lights with different spectral content, we calculated the Papilio-subjective brightness of light i, Bi, with:
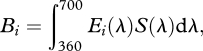 |
2.2 |
where S(λ) was the action spectrum of the foraging behaviour to monochromatic lights [10].
(c). Behavioural experiments
We carried out two series of dual-choice tests of foraging Papilio to investigate (i) the innate preference to polarized light, and (ii) how the butterflies discriminate differently polarized lights, by quantifying visiting behaviour to each stimulus. We defined a visit as an approach to one of the two windows followed by probing it with the extended proboscis.
The intensity of the stimuli, as defined above, was adjusted by inserting appropriate neutral density (ND) and/or polarization filters (figure 2c,d). We note here that the relative transmittance of these filters appeared to be not perfectly independent of wavelength, so that the resulting spectral distributions of the attenuated lights differed very slightly, but its effect on the results was negligible (see §3).
(i). Experiment 1: innate polarization preference
We tested the innate preference to polarized light using butterflies of PED-3 without training. The butterflies were not fed before the test. Using the two-window board, we presented a polarized light either vertically (V-pol) or horizontally polarized (H-pol), in one window together with an unpolarized (U-pol) light of the same colour and photon flux Pi in the other window. We provided no sucrose reward during the experiments. We released one butterfly into the cage at a time, and recorded the very first stimulus visited by the butterfly as the innately preferred stimulus for the individual: one individual provided only one result. Immediately after the first visit, we took the butterfly out of the cage and tested the next individual. If the butterfly did not visit any stimulus in 5 min, we finished the test. We first used red, because female Papilio innately prefers red [11]. As a confirmation, we also used blue, which has little spectral overlap with red light (figure 2d).
(ii). Experiment 2: spectral, intensity and polarization discrimination
To elucidate how the butterflies discriminate differently polarized lights, we carried out three dual-choice tests: spectral discrimination, intensity (photon flux) discrimination, and e-vector discrimination. In all three tests, we systematically controlled the relative photon flux of two presented stimuli.
Training: In all of these tests, we first trained the butterflies. We released one butterfly of PED-2 into the cage at a time, and let it take 8 per cent sucrose solution put on the perch of the single-window board with U-pol red light or U-pol blue light. The training session lasted about 10 min, during which we let the butterfly visit the window about 10 times so as to feed the butterfly until satiated. In the case that a butterfly did not spontaneously visit the window at the beginning of the training session, we captured it, uncoiled the proboscis and forced it to take sucrose solution on the perch. We did the training once a day and repeated it for six days. When a butterfly became able to visit the window at least 10 times within 10 min, we concluded that the butterfly was successfully trained.
Dual-choice tests: The trained butterflies were subjected to three dual-choice tests. All the following tests were carried out using the same procedure. We released a trained butterfly, always PED-8, in the cage and fed it with only a few microlitres of 12 per cent sucrose solution to stimulate its feeding motivation: the amount of the ‘starter’ was insufficient to satiate a starved Papilio. No sucrose reward was provided during the test except the starter. We then presented the training U-pol light, red or blue depending on the individual, together with a test light that differed either in spectral content, intensity or e-vector direction, depending on the test. We then let the butterfly visit any window for eight times in total and recorded the eight visited stimuli, which represented the first session of the test. The position of the two lights was interchanged every two visits. After the first session was completed, we fed the butterfly with 12 per cent sucrose solution under the condition of the training (see above) until satiated. We performed only one session in one day for an individual. On the next day, the same individual was again tested with the same test light presented together with the training light the intensity of which was changed. By repeating these tests for several days, we obtained the intensity dependency of the choices. When a butterfly did not visit eight times within 10 min in a particular session, we did not incorporate the data of that session in the final analysis.
Spectral discrimination: The butterflies trained to U-pol red (blue) light were tested for their preference between the training light and U-pol orange or pink (violet or indigo), presented simultaneously on the two-window board. The subjective brightness, Bi, of the two stimuli were adjusted to equal value on the first day of the test. From the next day on, we reduced the subjective brightness of the training light, while retaining the subjective brightness of the test light constant.
Intensity discrimination: To the butterflies trained to U-pol red (blue), we presented two U-pol red (blue) lights of different intensities, and recorded the visited lights. The total photon flux Pi of the two stimuli was adjusted to equal on the first day of the test. From the next day on, we changed the Pi of one stimulus, while retaining the intensity of the other stimulus constant.
e-vector discrimination: To the butterflies trained to U-pol red (blue), we presented U-pol red (blue) and red (blue) of V-pol or H-pol to measure their preference. The total photon flux of the stimuli, Pi, was equal on the first day. From the next day on, we changed the Pi of the training U-pol light, while retaining the Pi of the polarized test light constant.
(d). Data analysis
We evaluated the number of visits of the individuals to each stimulus (experiment 1) or the visit ratios between two stimuli simultaneously presented (experiment 2) using the Mann-Whitney's U-test.
3. Results
(a). Innate preference
We presented two lights of the same spectrum (red or blue), one unpolarized (U-pol) and another vertically or horizontally polarized (V- or H-pol) to untrained naive butterflies to check their innate preference to polarized light.
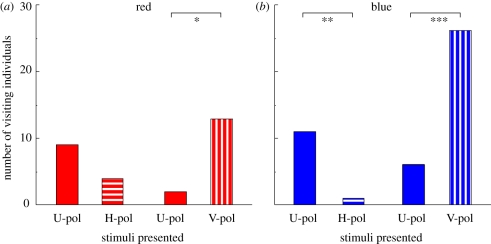
Among 140 butterflies tested with red, 28 individuals (20%) approached one of the stimuli and touched around the stimulus with the extended proboscis: the behaviour was defined as a ‘visit’ (see §2). We recorded the very first stimulus that an individual visited as the innate preference of the individual, and plotted the number of visiting individuals for each stimulus (figure 3). When presented U-pol red and H-pol red, more individuals visited U-pol red, but there was no statistical significance between the visit numbers. In the selection between U-pol red and V-pol red, statistically more individuals selected V-pol red (figure 3a). For blue lights, 44 out of 52 tested individuals (85%) visited any one of the stimuli. U-pol blue was selected significantly more than H-pol blue, and V-pol blue was selected significantly more than U-pol blue (figure 3b). When presented V-pol and H-pol of the same intensity at the same time, the butterflies of course selected V-pol significantly more (data not shown).
Figure 3.
Innate preference of naive butterflies tested with (a) red or (b) blue light. U-pol, unpolarized light; H-pol, horizontally polarized light; V-pol, vertically polarized light. Asterisks indicate statistical difference between the number of visited individuals (Mann–Whitney U-test, *p < 0.001, **p < 0.01, ***p < 0.05).
(b). Spectrum-based discrimination of unpolarized light
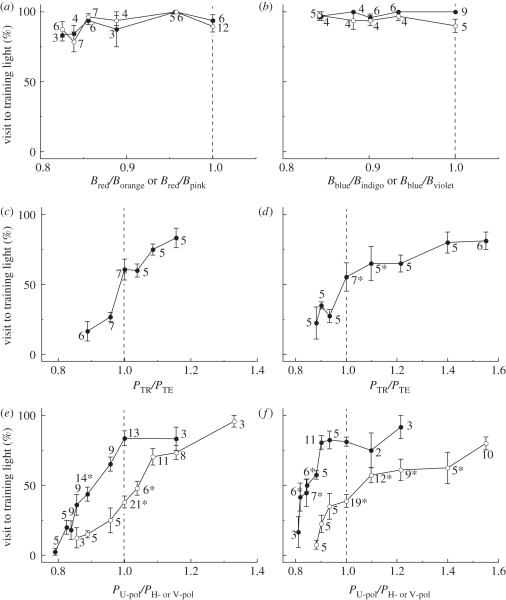
Figure 4a shows the results of two series of tests of red-trained butterflies, where the response to red versus orange and of red versus pink was tested. We systematically reduced the subjective brightness of the red stimulus, Bred, while keeping that of the orange stimulus, Borange, or pink, Bpink, constant, resulting in a decrease of the relative Papilio-subjective brightness of the red light, Bred/Borange or Bred/Bpink (see the abscissa of figure 4a). The red-trained butterflies kept selecting red even when the relative subjective brightness of the red light was close to 0.8. Blue-trained butterflies responded similarly in the tests of blue versus indigo and blue versus violet (figure 4b). The results indicate that in the present tests the butterflies used their colour vision, i.e. the ability to discriminate visual stimuli based on their chromatic contents independent of the intensity.
Figure 4.
Results of the colour discrimination (a) U-pol red versus U-pol orange (filled circles)/pink (open circles), (b) U-pol blue versus U-pol indigo (filled circles)/violet (open circles); brightness discrimination (c) U-pol red (TR) versus U-pol red (TE), (d) U-pol blue (TR) versus U-pol blue (TE); polarization discrimination (e) U-pol red versus H- (filled circle) or V-pol red (open circle), (f) U-pol blue versus H-(filled circles) or V-pol (open circles) blue tests with red-trained (a,c,e) and blue-trained (b,d,f) butterflies. The vertical axes indicate the % visit ratio to the training light. The horizontal axes are the relative subjective brightness Bi (a,b) or relative photon flux Pi (c–f) of the training light. The number at each data point (mean ± s.e.m.) indicates the number of individuals used for the data. The individuals may have been repeatedly used, but only in the particular set of experiments shown in a single panel. Asterisks indicate choice distributions that were NOT significantly different from the chance level (Mann–Whitney U-test, p < 0.001).
(c). Brightness-based discrimination of unpolarized light
When we presented two lights with the same spectral content but with different intensity (photon flux density), the butterflies always exhibited a higher preference to the more intense light, independent of the training spectrum.
We presented U-pol red-trained butterflies two red lights, one identical to the training U-pol red (TR), and another U-pol red for comparison (TE). The photon flux of test light TE, PTE, was changed in six steps while the photon flux of the training light, PTR, was kept equal to what was used during training. When the photon flux of two lights was identical (relative photon flux PTE/PTR = 1.0), the choice frequency for the test light was 60.2 ± 7.4 per cent, which was not significantly different from the chance level (figure 4c). However, the frequencies significantly changed when the relative photon flux changed only 4 per cent: when PTE/PTR = 1.16 and 0.89, the choice frequencies for the test light were 83.3 and 16.6 per cent, respectively, (figure 4c). In blue-trained butterflies, the choice frequency for the test light reached 80 per cent when the relative amplitude (PTE/PTR) was 1.4, whereas it decreased to 22.5 per cent when PTE/PTR = 0.88 (figure 4d).
As stated in §2, the relative transmittance spectra of the ND filters were not wavelength independent. The difference in the transmittance spectra might cause a difference in the colour appearance for Papilio. For example, the training U-pol red light was produced by inserting the red filter and an ND10 filter (T = 10%, see figure 2), whereas the test lights had filters in different combinations to control their intensities. Strictly speaking therefore, the spectral distributions of the test lights were different from that of the training light, which could be perceived as different colours. When the butterflies see the training light and a training light with additional ND filters, i.e. PTE/PTR < 1.0, they selected the training light. In this case, we cannot exclude the possibility that the test lights appeared as different in colour and that they were therefore not selected. However, when PTE/PTR > 1.0, the butterflies selected the test light but not the training light (figure 4c,d). If the test light looked different in colour, the butterflies should have selected the training light even though it contained less photons, as expected from the results of spectral discrimination (figure 4a,b). This was not the case, so the spectral changes due to subtle differences in the transmittance spectra of ND filters were negligible in the present experimental condition.
(d). Effect of relative intensity on polarization discrimination
We presented red- or blue-training U-pol light together with V- or H-pol light with the same colour and recorded the visit frequency. The frequencies for the U-pol light were plotted against the relative intensities PU-pol/PH-pol or PU-pol/PV-pol (figure 4e,f). Clearly the choice frequency for U-pol light changed with its relative intensity.
From U-pol red and H-pol red of the same intensity, PU-pol/PH-pol =1, the red-trained butterflies selected U-pol red with a frequency of 83.6 per cent, significantly above the chance level (figure 4e). The innate preference to U-pol of untrained butterflies was about 63 per cent (figure 3a), so the preference to U-pol was significantly enhanced by the training to U-pol red. The difference in visit frequencies between U-pol and H-pol became insignificant when the relative intensity, PU-pol/PH-pol was 0.89. The visit to U-pol red further decreased and became almost zero at the relative intensity around 0.8 (figure 4e, filled circles). In the case of U-pol red versus V-pol red at the same intensity, the trained butterflies visited to U-pol with a frequency of 38.1 per cent, which was not different from the chance level. In non-trained butterflies, only 13 per cent of individuals selected U-pol over V-pol (figure 3a). The visit frequencies became significantly different when the relative intensity of U-pol red was 1.1 or 0.96. The choice of U-pol increased to 95.8 per cent at the relative intensity of 1.33, while it decreased to 12.5 per cent at the relative intensity of 0.86 (figure 4e, open circles).
We carried out the same series of tests with blue-trained butterflies. The results were similar to those in the case of the red-trained butterflies: when presented at the same intensity, the choice frequency was U-pol blue < H-pol blue and U-pol blue > V-pol blue (figure 4f).
4. Discussion
(a). Polarization-based intensity vision
In order to determine how the foraging Papilio butterflies recognize differently polarized lights, we first carried out two experiments to establish the characteristics of colour discrimination and intensity discrimination. In the test series of colour vision, both red- and blue-trained butterflies selected the training spectral light over a different spectral light even when its intensity was distinctly lower (figure 4a,b). The series of brightness discrimination indicated that the butterflies preferentially selected from two spectrally identical stimuli the one with the higher intensity (figure 4c,d). Based on these results, we can reliably evaluate the outcomes of the polarization-involved dual-choice tests.
In the e-vector discrimination test, the visit frequencies by the foraging Papilio changed with the relative intensity values (figure 4e,f). Clearly, the results resembled those of the intensity discrimination tests (figure 4c,d) rather than those of the colour discrimination tests (figure 4a,b). Based on the generally accepted view that animals perceive a light with a high intensity (photon flux) as brighter than a light of low intensity, we conclude that the foraging butterflies discriminate differently polarized lights as lights of different brightness rather than as differently coloured lights. Because butterflies innately prefer light of higher intensity, it appears that V-pol light basically looks brighter than H-pol light of the same intensity for foraging butterflies.
Papilio butterflies are now shown to have at least two modes of polarization vision, depending on the behavioural context. The first mode, applied in oviposition, is by colour [6], and the second mode, used in foraging, is by brightness, as demonstrated in the present study. Of course this does not exclude the third mode, true polarization vision. The backswimmer, Notonecta glauca, uses true polarization vision to find a body of water from the air. As soon as a backswimmer recognizes a patch reflecting horizontally polarized light on the ground, the insect dives into it irrespective of its intensity [7]. Papilio butterflies also quite often land on a puddle to take water there. Similar to the backswimmers, the butterflies may detect the polarization glare when searching for a puddle.
(b). Innate preference and learning of polarized light
Foraging female Papilio butterflies innately prefer V-pol, irrespective of the colour (figure 3). They also have an innate preference to red [11], which can be easily changed to different colours by training. Unlike in colours however, the preference to V-pol is difficult to change: after extensive training to H-pol, the butterflies still preferred V-pol [6].
Here the U-pol-trained butterflies became unselective between U-pol and V-pol (figure 4e,f, white circle at 1.0), indicating that the training was not ineffective. Here, they most probably learned the absolute brightness of the training light, but we cannot exclude the possibility that they used the presumptive true polarization vision, the existence of which remains an open question.
Interestingly, the learning of brightness difference seems as difficult as the learning of polarization in Papilio (Kinoshita & Takahashi 2005, unpublished data), and is also true in other lepidopteran species (a diurnal hawkmoth Macroglossum [12]; Heliconius charitonius [13]). The slow process of learning seems a general property of brightness learning in butterflies, which gives further support to the hypothesis that the polarization is perceived as brightness. Or in reverse, the training effects should be much stronger if the butterflies recognize the polarization as colour.
(c). Possible mechanism underlying polarization discrimination in Papilio
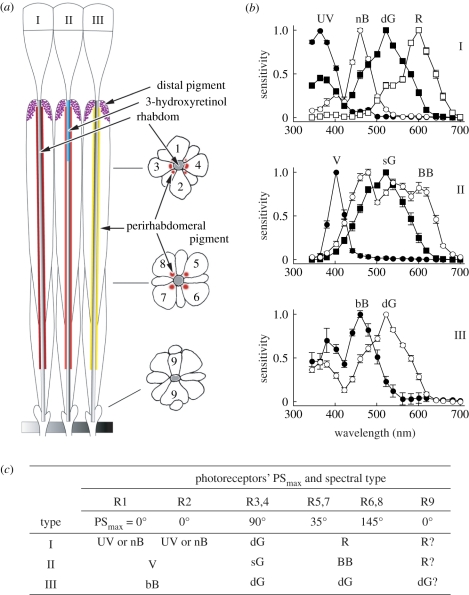
The physiological basis of polarization vision is attributed to the e-vector sensitivity of individual photoreceptors. A compound eye of Papilio consists of about 12 000 ommatidia, each containing nine photoreceptor cells, R1–9 (figure 5a). All of them bear microvilli together forming a rhabdom along the central axis of the ommatidium. Four out of nine photoreceptors, R1–4, contribute the microvilli to the distal two-thirds of the rhabdom, and therefore are called the distal photoreceptors. The proximal one-third is constructed by four proximal photoreceptors, R5–8, and the R9 photoreceptor adds a few microvilli at the base of the rhabdom. The microvilli of a given photoreceptor are parallel and straight in most cases, so the photoreceptors are most sensitive to polarized light the e-vector orientation of which is parallel to the longitudinal axis of the microvilli [1,14,15].
Figure 5.
Spectral organization of the eye of Papilio xuthus. (a) Schematic of the Papilio ommatidia, which are divided into three tpes (I–III) by the characteristic pigmentation. An ommatidium contains nine photoreceptors (1–9). 3-hyroxyretinal in type II functions as an UV-absorbing filter. (b) Photoreceptor spectral sensitivities of the photoreceptors contained in type I (top), II (middle) and III (bottom) ommatidium. UV, ultraviolet; V, violet; bB, broad-blue; nB, narrow-blue; dG, double-peaked green; sG, single-peaked green; R, red; BB, broad-band [14,15,18,19]. (c) Spectral sensitivity and the maximal angle of polarization sensitivity of photoreceptors, R1–9.
The photoreceptors have specific spectral sensitivities as well. We have identified at least six classes of receptors, which are of the ultraviolet (UV), violet (V), blue (B), green (G), red (R) and broad-band (BB) classes: the B class and the G class both contain two subclasses [16–18]. The receptors are embedded in the ommatidia in three fixed combinations (figure 5b), making the compound eye a mesh of three spectrally heterogeneous types of ommatidia [19]. Note the close relationship between the polarization and spectral sensitivities: for example, all the UV, V and B receptors have the PSmax at 0° (figure 5c). The relationship directly leads to the perception of polarization as false colour [6]. Note also that in all types of ommatidia, R3 and R4 photoreceptors are always green sensitive.
One would assume that the complete set of spectral receptors is used for colour vision, but this is not necessarily the case. We measured the wavelength discrimination ability in foraging Papilio and demonstrated that the G receptors in the position of R3 and R4 (G3–4), V receptors and BB receptors were not used in this behavioural context. In other words, the colour vision of foraging Papilio is tetrachromatic based on the UV, B, G5–8 and R receptors [20], and the V, BB and G3–4 receptors are for visual functions other than colour vision. In fact, the anatomy of G3–4 receptors is peculiar. First, the existence of a G receptor in all ommatidia in the position of R3 and R4 suggests that the G3–4 system provides the best spatial resolution, used in pattern vision, for example. Second, the axons of G3–4 receptors are thick and smooth, making contacts only with second-order neurons in the lamina, which suggests that information processing is fast, best for motion vision. Other randomly distributed photoreceptors extend fine axonal branches making an elaborate inter-photoreceptor network, which may be crucial for colour vision [21]. Third, the G3–4 receptors all have their PSmax at 90°, so the input of these receptors is strongly affected by the e-vector angle of light. Maybe, the egg-laying butterflies, which innately prefer H-pol and also brighter stimuli [6], rely on the input from the G3–4 system. The G3–4 receptors do not feed information to the colour vision system upon feeding, and therefore H-pol light may appear darker for foraging butterflies.
Acknowledgements
We thank Drs Thomas Cronin and Doekele Stavenga for constructively criticizing the manuscript. The work was supported by the Grants-in-Aid for Scientific Research from the JSPS to M.K. and K.A.
Footnotes
One contribution of 20 to a Theme Issue ‘New directions in biological research on polarized light’.
References
- 1.Moody M. F., Parriss J. R. 1961. The discrimination of polarized light by Octopus: a behavioral and morphological study. Z. vergl. Physiol. 44, 268–291 10.1007/BF00298356 (doi:10.1007/BF00298356) [DOI] [Google Scholar]
- 2.Rossel S. 1989. Polarization sensitivity in compound eyes. In Facets of vision (eds Stavenga D. G., Hardie R. C.), pp. 298–316 Berlin, Germany: Springer-Verlag [Google Scholar]
- 3.Waterman T. H. 1981. Polarization sensitivity. In Handbook of sensory physiology vol. VII/6B (ed. Autrum H.), pp. 281–469 Berlin, Germany: Springer-Verlag [Google Scholar]
- 4.Nilsson D. E., Warrant E. J. 1999. Visual discrimination: seeing the third quality of light. Curr. Biol. 9, R535–R537 10.1016/S0960-9822(99)80330-3 (doi:10.1016/S0960-9822(99)80330-3) [DOI] [PubMed] [Google Scholar]
- 5.Kelber A. 1999. Why ‘false’ colours are seen by butterflies. Nature 402, 251. 10.1038/46204 (doi:10.1038/46204) [DOI] [PubMed] [Google Scholar]
- 6.Kelber A., Thunell C., Arikawa K. 2001. Polarisation-dependent colour vision in Papilio butterflies. J. Exp. Biol. 204, 2469–2480 [DOI] [PubMed] [Google Scholar]
- 7.Schwind R. 1984. Evidence for true polarization vision based on a two-channel analyzer system in the eye of the water bug, Notonecta glauca. J. Comp. Physiol. A 154, 53–57 10.1007/BF00605390 (doi:10.1007/BF00605390) [DOI] [Google Scholar]
- 8.Marshall J., Cronin T. W., Shashar N., Land M. 1999. Behavioural evidence for polarisation vision in stomatopods reveals a potential channel for communication. Curr. Biol. 9, 755–758 10.1016/S0960-9822(99)80336-4 (doi:10.1016/S0960-9822(99)80336-4) [DOI] [PubMed] [Google Scholar]
- 9.Rossel S., Wehner R. 1984. How bees analyse the polarization patterns in the sky. J. Comp. Physiol. A 154, 607–615 10.1007/BF01350213 (doi:10.1007/BF01350213) [DOI] [Google Scholar]
- 10.Koshitaka H., Kinoshita M., Arikawa K. 2004. Action spectrm of foraging behavior of the Japanese yellow swallowtail butterfly, Papilio xuthus. Acta Biol. Hung. 55, 71–79 10.1556/ABiol.55.2004.1-4.9 (doi:10.1556/ABiol.55.2004.1-4.9) [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M., Shimada N., Arikawa K. 1999. Colour vision of the foraging swallow tail butterfly Papilio xuthus. J. Exp. Biol 202, 95–102 [DOI] [PubMed] [Google Scholar]
- 12.Kelber A. 2005. Alternative use of chromatic and achromatic cues in a hawkmoth. Proc. R. Soc. B 272, 2143–2147 10.1098/rspb.2005.3207 (doi:10.1098/rspb.2005.3207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swihart C. A. 1971. Colour discrimination by the butterfly, Heliconius charitonius Linn. Anim. Behav. 19, 156–164 10.1016/S0003-3472(71)80151-3 (doi:10.1016/S0003-3472(71)80151-3) [DOI] [Google Scholar]
- 14.Arikawa K., Uchiyama H. 1996. Red receptors dominate the proximal tier of the retina in the butterfly Papilio xuthus. J. Comp. Physiol. A 178, 55–61 10.1007/BF00189590 (doi:10.1007/BF00189590) [DOI] [Google Scholar]
- 15.Bandai K., Arikawa K., Eguchi E. 1992. Localization of spectral receptors in the ommatidium of butterfly compound eye determined by polarization sensitivity. J. Comp. Physiol. A 171, 289–297 10.1007/BF00223959 (doi:10.1007/BF00223959) [DOI] [Google Scholar]
- 16.Arikawa K., Inokuma K., Eguchi E. 1987. Pentachromatic visual system in a butterfly. Naturwissenschaften 74, 297–298 10.1007/BF00366422 (doi:10.1007/BF00366422) [DOI] [Google Scholar]
- 17.Arikawa K., Mizuno S., Kinoshita M., Stavenga D. G. 2003. Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of a butterfly, Papilio xuthus. J. Neurosci. 23, 4527–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita M., Kurihara D., Tsutaya A., Arikawa K. 2006. Blue and double-peaked green receptors depend on ommatidial type in the eye of the Japanese yellow swallowtail Papilio xuthus. Zool. Sci. 23, 199–204 10.2108/zsj.23.199 (doi:10.2108/zsj.23.199) [DOI] [PubMed] [Google Scholar]
- 19.Arikawa K. 2003. Spectral organization of the eye of a butterfly, Papilio. J. Comp. Physiol. A 189, 791–800 10.1007/s00359-003-0454-7 (doi:10.1007/s00359-003-0454-7) [DOI] [PubMed] [Google Scholar]
- 20.Koshitaka H., Kinoshita M., Vorobyev M., Arikawa K. 2008. Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc. R. Soc. B 275, 947–954 10.1098/rspb.2007.1614 (doi:10.1098/rspb.2007.1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takemura S. Y., Arikawa K. 2006. Ommatidial type-specific interphotoreceptor connections in the lamina of the swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 494, 663–672 10.1002/cne.20830 (doi:10.1002/cne.20830) [DOI] [PubMed] [Google Scholar]