Abstract
The underlying mechanisms of polarization sensitivity (PS) have long remained elusive. For rhabdomeric photoreceptors, questions remain over the high levels of PS measured experimentally. In ciliary photoreceptors, and specifically cones, little direct evidence supports any type of mechanism. In order to promote a greater interest in these fundamental aspects of polarization vision, we examined a varied collection of studies linking membrane biochemistry, protein–protein interactions, molecular ordering and membrane phase behaviour. While initially these studies may seem unrelated to polarization vision, a common narrative emerges. A surprising amount of evidence exists demonstrating the importance of protein–protein interactions in both rhabdomeric and ciliary photoreceptors, indicating the possible long-range ordering of the opsin protein for increased PS. Moreover, we extend this direction by considering how such protein paracrystalline organization arises in all cell types from controlled membrane phase behaviour and propose a universal pathway for PS to occur in both rhabdomeric and cone photoreceptors.
Keywords: polarization sensitivity, polarization vision, protein interactions, molecular tethering, membrane composition, lipid rafts
1. Introduction
Polarization vision epitomizes sensory biology; it is a complex, integrated and plastic modality that detects, encodes and processes the polarization information contained within the visual light environment. It has been just over 60 years since Karl von Frisch discovered that bees (Apis mellifera) see and use polarized light [1], and we know now that sensitivity to linearly polarized light occurs widely throughout the animal kingdom (figure 1). Most recently we have seen how animals can even detect and discriminate the handedness of circularly polarized light [2]. However, while polarization vision has been recognized as an important sensory modality for many animals, the underlying mechanisms have yet to be understood.
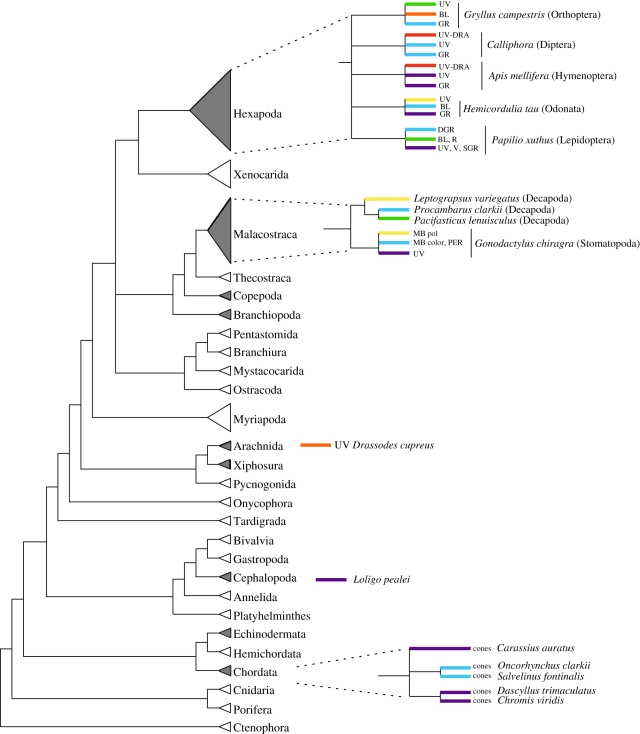
Figure 1.
Evolutionary distribution of polarization vision in animal visual systems. Relationships among major animal groups generalized from the phylogenetic studies of Regier et al. [87] and Dunn et al. [88]. Groups where there is evidence for polarization vision are shaded grey (based on information contained within Johnsen [89] and Horváth & Varjú [27]). Within each group containing polarization vision, representative species where polarization sensitivity (PS) has been quantified using either electrophysiology or axial dichroic ratio measurements are shown. Abbreviations for arthropod (Hexapoda and Malacostraca) receptor classes where PS was measured: UV, ultraviolet-sensitive; UV-DRA, ultraviolet-sensitive receptor in dorsal rim area of eye; V, violet-sensitive; BL, blue-sensitive; GR, green-sensitive; SGR, green-sensitive with single peak absorbance; DGR, green-sensitive with double peak absorbance; R, red-sensitive; MB pol, midband polarization specialist receptors; MB colour, midband colour specialist receptors; PER, peripheral eye receptors. See the following references for detailed methods: (i) vertebrate cones [14,41,90]; (ii) Hexapod receptors [22,25,91–94]; (iii) Malacostracan receptors [95–97]; (iv) Arachnida receptors [21]; (v) Cephalopoda receptors [98]. Maximum recorded polarization sensitivity: purple line, <2; blue line, 2.1–4; green line, 4.1–6; yellow line, 6.1–8; orange line, 8.1–10; red line, >10.
Like the spectral component of light (e.g. colour), the polarization of light provides visual information that can be used by animals. The information gained from discriminating between different electric field vectors of light augments normal visual functionality, providing visual contrast enhancement for object detection and recognition [3], a navigational compass [4–6], an aid for orientating within the environment [7] and a separate channel for communication and display [8,9]. In many animals, polarization vision is truly a multi-level pathway, leading from molecular properties defining a cellular response through several layers of neuronal processing and interpretation in higher brain areas. Recent works have clearly demonstrated the processing of polarization information downstream of the photoreceptor layer in both invertebrates [10–12] and vertebrates [13–15], and suggested how that information can inform and direct behaviour [3,6,16,17]. However, the mechanisms that begin the sequence by creating a polarized light detector at the level of the photoreceptors have received surprisingly little attention.
The foundation of animal polarization sensitivity (PS) is in the visual pigment molecules, which are inherently dichroic because the light-absorbing chromophore, retinal, is itself a dichroic molecule. Based on the inherent visual pigment dichroism alone, rhabdomeric photoreceptors can theoretically achieve PS values of 2 [18,19]. Much higher PS values of 5–15 in a plethora of animals have been discovered (figure 1; [20–25]), and such large sensitivities are only attainable if molecular ordering and alignment of the visual pigment elevate the overall dichroism of the photoreceptor. Molecular-level considerations have been discussed previously [26], and these discussions still represent the current state of knowledge. While oligomerization, cytoskeletal tethering and protein–protein and protein–lipid interactions present possible mechanisms for achieving the hypothesized order and alignment at the molecular level, these interactions have been little investigated in photoreceptors. This has left the problem of the molecular mechanisms underlying polarization vision unsolved and even uninvestigated.
The aim of this paper is to bring together a diverse range of literature on photoreceptor membrane biochemistry and biophysics in order to place the spotlight on a neglected area of polarization vision. While this paper may not provide the answers to these questions, it will hopefully inspire new research directions within the field to provide a more comprehensive understanding of the specific molecular mechanisms underlying the detection of polarized light in photoreceptor membranes.
2. Photoreceptors as polarization detectors: current knowledge of geometric mechanisms
Polarization vision was first described in bees [1], and has since been widely investigated in arthropod compound eyes, vertebrate and cephalopod camera-type eyes and in several extraocular photoreceptors. For an extensive review of behavioural and electrophysiological demonstrations of polarization vision, the reader is referred to Polarized Light in Animal Vision [27], which provides a very complete review of the literature. Also, Waterman's [28] classic review in the Handbook of Sensory Physiology is worth seeking out. What is important in the context of this paper is that underlying all such forms of polarization vision are photoreceptor detectors that respond differentially to different polarizations of light. However, it is clear throughout the literature, and as we will demonstrate below, that the mechanisms of detection have been primarily considered in terms of geometric arguments of photoreceptor structure.
(a). Rhabdomeric photoreceptors and microvillar geometry
Rhabdomeric photoreceptors are by far the best studied with respect to how geometric arrangements contribute to PS. In rhabdomeric photoreceptors, the dichroic visual pigments are found in microvilli, which extend in groups from one margin of the cell (figure 2a). De-Vries et al. [29] first suggested that if (i) microvilli were assembled in a parallel arrangement and (ii) the collective visual pigment displayed a degree of linear dichroism in an aligned manner, we would be able to explain overall photoreceptor PS. This prediction turned out to be broadly true and the subsequent 50 years of microscopic data have demonstrated how arthropod (in particular insect and crustacean) and cephalopod photoreceptors are superbly equipped both structurally (e.g. microvillar geometric structure) and genetically (e.g. constant cell numbers in ommatidia) for polarization vision [30].
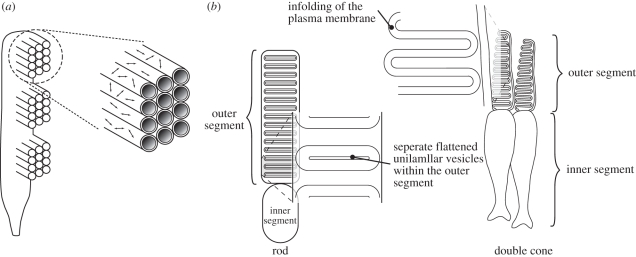
Figure 2.
Schematic of generalized photoreceptor cells. (a) Illustration of the arrangement of microvilli along the margin of an arthropod rhabdomeric photoreceptor cell and the previously conceived theoretical random arrangement of the visual pigment chromophore within microvillar membranes, with the double arrow lines representing the dipole axis. The random arrangement of the chromophores would result in a dichroic ratio of 2, which does not fit with current experimental evidence. (b) Illustration of membrane arrangements in ciliary rod and cone photoreceptors comparing the separate flattened discs within rod outer segments with the in-folding of the plasma membrane in cones.
An individual microvillus displays dichroism for several reasons. Since the foundation of all PS lies in the dichroic chromophore of the visual pigment, one could hypothesize that a degree of preferential alignment of chromophores between multiple visual pigments is required for an overall dichroism of the membrane. However, the cylindrical nature of the microvillus creates dichroism simply owing to geometric arguments; even when the dipoles are orientated randomly in the plane of the membrane, the cylindrical shape results in a dichroic ratio of 2 [18,19,31].
For a rhabdomeric structure with many microvilli, the dichroism of the cell is further prescribed by a contribution owing to the packing arrangement of the microvilli themselves, a factor called form dichroism [32]. Form dichroism occurs when the dimensions of individual elements of a periodic structure are less than the wavelength of the incident light, but the periodic structure itself extends to a greater than wavelength size. Even optically isotropic absorbing structures can exhibit form dichroism and it is important to note form contributions are not owing to a single microvillus, as suggested by Horváth & Varjú [27].
Studies often note that twisted rhabdomeres [22,33,34] result in measured polarization insensitivity. The commonly cited explanation derives from the simple fact that chromophores must thus be available to all incident polarizations. However, the reason that twisted rhabdoms are polarization-insensitive is not just that they are twisted, but twisted with low effective birefringence thanks to the offset between the intrinsic and the form birefringence components. Wehner et al. [34] elegantly showed that when the effective birefringence was higher, the photoreceptor would display a dichroic ratio (up to 3.5 in their calculations). Interestingly, the combination of twist and birefringence is how the most common liquid crystal devices, the twisted nematic type, guide linearly polarized light.
(b). Ciliary photoreceptors and disc membrane structure
For vertebrates, containing ciliary photoreceptors within the retina, the story is much less clear. Unlike rhabdomeric photoreceptors, the ultrastructure and organization of the membranes that contain the visual pigments in ciliary photoreceptors are not geometrically suited for polarized light detection (figure 2b). Consequently, important questions regarding the underlying mechanism that provides dichroism in typical vertebrate photoreceptors have remained unanswered for over 40 years. Anchovies (Anchoa mitchilli, Engraulis encrasicholus and Engraulis mordax, [35–37]) have provided the clearest evidence for one mechanism, owing to the unusual arrangement and the morphology of the cones in their retina. Fineran & Nicol [35] first demonstrated that the long, bi-lobed anchovy cones have the visual-pigment-containing membranes orientated axially, such that normally incident light illuminates the visual pigment transversely. As the chromophore lies closely parallel (approx. 17°) to the plane of the membrane [38–40] in all vertebrate photoreceptors that have been examined, the resulting transverse dichroism [37], coupled with the orthogonal arrangement of the long, bi-lobed cell types, could provide a dichroic detection system.
In more typical vertebrate photoreceptors, only one study [41] has provided direct experimental measurements of dichroism with the cell under physiological illumination. While light in the eye enters these cells ‘end-on’, most spectral absorbance experiments use ‘side-on’ microspectrophotometry. Roberts & Needham [41] used a laser tweezer system to control the photoreceptor cell orientation in three dimensions, and discovered that the mid-wavelength-sensitive members of double cones in goldfish (Carassius auratus) are axially dichroic and thus could display PS in vivo. In contrast, rods absorb all orientations of polarized light to the same degree when illuminated axially. This is consistent with earlier indirect evidence of axial dichroism in the cones of salmonids [42–44].
In the absence of a body of experimental evidence, several theoretical models have also been proposed to explain the mechanism underlying PS in vertebrates. Flamarique et al. [45] proposed that the curved limiting membrane between double cone inner segments could act as a directional dichroic reflector of incident light, thus transversely illuminating the outer segments of the corner ultraviolet-sensitive cones within a square cone mosaic. The resulting difference in the polarization of the transmitted light could then be analysed by the double cone outer segments. However, the theory used by Flamarique et al. [45] is based on macroscopic Fresnel equations that are not valid for sub-wavelength thicknesses of cell membranes. In a separate study, Cameron & Pugh [46] proposed that refractive index gradients in inner segments of twin cones could operate as anisotropic polarization waveguides. They supported this hypothesis with both optical modelling and an electrophysiological study discovering PS in the orthogonal array of twin cones in green sunfish [47]. However, Flamarique & Hawryshyn [48] could not verify the PS measurements in green sunfish, and Roberts & Needham [41] measured isotropic transmission through similar double cone inner segments.
(c). The limits of geometric considerations in the detection of polarized light
We described earlier how rhabdomeric photoreceptors are inherently polarization sensitive owing to the geometry of the microvilli and their arrangement in the cell. However, neither the cylindrical shape nor microvillar packing explain the levels of PS measured in many arthropod species. For example, Labhart [22] found PS values of 18 in honeybees (figure 1). It is worth noting that experimentally, PS is generally measured from electrophysiological recordings, however there is a direct equivalence to optical dichroic ratio measurements intrinsic to the photoreceptor cell (see eqns (14)–(18) in [43]) and not subject to any neural processing. Similarly, as described, the literature still lacks any broad base of experimental evidence for why typical vertebrate ciliary photoreceptors exhibit PS. Logically, PS can be increased through the ordering and alignment of the chromophores with the plane of the cell membranes in both rhabdomeric and ciliary cell types [26]. In the following sections, we discuss how protein–protein and protein–lipid interactions may underlie the dichroic alignment of visual pigments and how membrane composition lamellar ordered phases may bring about such interactions, thus explaining the high values of PS measured experimentally.
3. Photoreceptors as polarization detectors: visual pigment dimers and higher order structural protein architecture contributing to polarization sensitivity
Snyder & Laughlin [26] first calculated how the alignment of the visual pigment chromophore within the microvilli membranes could increase the dichroic ratio to reach the measured levels of rhabdomeric PS. However, few studies have investigated the potential of protein–protein interactions and cytoskeletal coupling as mechanisms of visual pigment alignment. A number of potential molecular interaction mechanisms have been previously suggested to occur within photoreceptor membranes, including: (i) visual pigment dimerization and ordered protein–protein arrays [49,50]; (ii) direct anchoring of visual pigment molecules to a relatively fixed axial microvillar cytoskeleton [51–53]; and (iii) extracellular tethering of visual pigment molecules across microvillar membranes [53]. Studies of these mechanisms are in their infancy, and in many cases are still contentious. We will highlight the information suggesting that these ordering and anchoring mechanisms significantly contribute to the observed PS values in both rhabdomeric and ciliary photoreceptors.
(a). Visual pigment interactions in rhabdomeric photoreceptors
Cephalopods present some of the best-studied rhabdomeric photoreceptor systems in terms of microvillar structure within the retina and structure of the visual pigment. In fact, rhodopsin from the squid Todarodes pacificus is only the second opsin, and the single non-vertebrate opsin, to have been crystallized [50]. Early studies of cephalopod photoreceptor structure reveal that the microvilli contain a cytoplasmic core bundle of actin filaments with crossbridges to the membranes, as well as special membrane junctions linking adjoining microvilli [53]. Microvillar actin cores have also been characterized in Drosophila and crayfish rhabdoms [54,55]. The observed actin cores and the core-to-membrane crossbridges fit with the idea from studies of Drosophila phototransduction of a ‘signalplex’—a macromolecular complex linking the visual pigment and associated phototransduction proteins with the cytoskeleton (figure 3; for review see [56]). Although these protein cross-microvillar and cytoskeletal interactions have been studied in very few invertebrate species, together they suggest a highly structured mechanism for increasing the ordering of chromophore alignment.
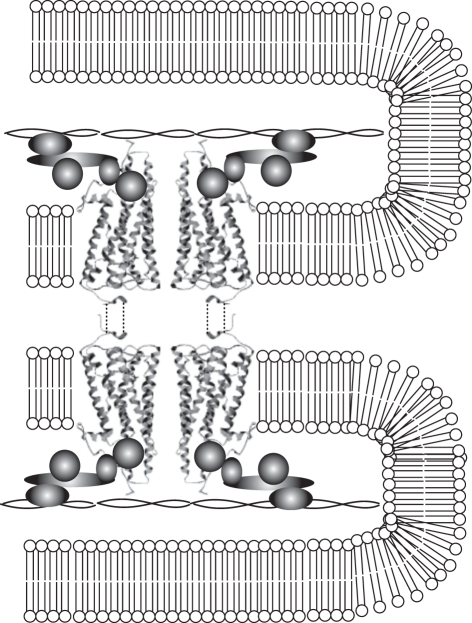
Figure 3.
Hypothetical arrangement of anchoring and tethering mechanisms within rhabdomeric receptors. Visual pigments are arranged in dimers, which are anchored to each other across microvillar membranes (represented by dashed lines), and tethered to cytoskeletal elements (actin core represented by double helix in the middle of the microvilli) by phototransduction molecules (represented by shaded forms).
In conjunction with these studies, evolutionary studies of expressed opsin genes in stomatopod crustaceans have provided some tantalizing evidence that higher order protein interactions may be a common feature of rhabdomeric photoreceptors. In most arthropod visual systems, the photoreceptors that detect polarized light express visual pigments that are also expressed in photoreceptors devoted to the task of colour discrimination. However, in some stomatopod crustacean visual systems, these two tasks have been de-coupled, with the photoreceptors specialized for polarized light detection expressing different opsin genes than those optimized for colour discrimination [57]. This allows for the comparison of opsin gene sequences from photoreceptors devoted to different tasks, with the goal of elucidating specific mechanisms within the opsin protein that may contribute to PS. Preliminary studies indicate that the genes expressed in polarization-sensitive photoreceptors are evolutionarily distinct from those in colour-sensitive photoreceptors (figure 4). Furthermore, comparative evolutionary analyses have identified a set of amino acids that are diversifying among stomatopod opsins that are likely to interact with machinery inside the cell, either in the phototransduction system or with cytoskeletal elements similar to the Drosophila signalplex [58].
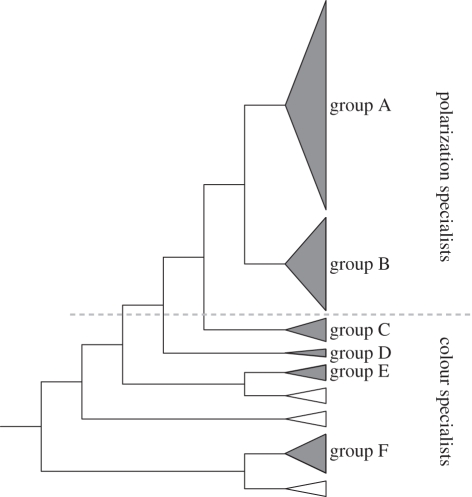
Figure 4.
Evolutionary history of stomatopod crustacean opsins, illustrating a separation between opsins involved in colour vision from those expressed in photoreceptors specialized for the detection of polarized light (for more detail, see [57]). Phylogeny is derived from Porter et al. [58]. Stomatopod opsins form six groups, labelled A—F, and the sequence groups (triangles) have been shaded grey. The sequence groups coloured white represent opsin sequences from other crustaceans.
In addition to these hypothetical protein/cytoskeleton interactions, Murakami & Kouyama's [50] crystallization study of squid rhodopsin suggested several protein–protein interactions among visual pigments. First, squid visual pigments are thought to form dimers in the membrane. Second, Murakami & Kouyama [50] also found a tight association across microvillar membranes between the amino-terminal polypeptides of neighbouring monomers, which is suggested to play a role in the hexagonal packing of the microvilli. In fact, these authors suggested that the across-membrane protein–protein interactions may be stronger than the dimer interactions within the membrane. This is one of the first indications that visual pigments could have protein–protein contacts across adjacent microvillar membranes. These proposed protein–protein interactions within and across membranes form a tetrameric structure in which four chromophores are oriented in a nearly parallel arrangement, and they may also play a role in the highly parallel ordering of microvilli needed for polarized light detection.
Functioning either individually or in combination, these visual pigment protein–protein and protein–cytoskeletal interactions observed in Drosophila and cephalopods, and suggested in stomatopods, may provide a rigid organization of visual pigment molecules that contributes to PS by ordering and aligning the chromophores parallel to the axis of the microvillus. However, these studies represent a very limited sampling of invertebrate diversity. Much more research on the interactions among visual pigments and cytoskeletal elements from animals containing rhabdomeric photoreceptors is needed.
(b). Visual pigment interactions in ciliary photoreceptors
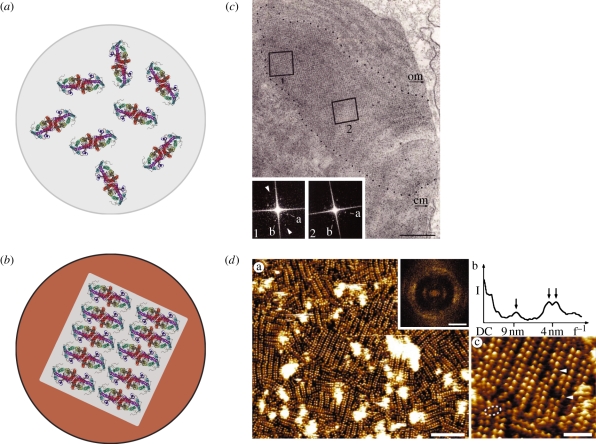
Over the last 20 years, numerous studies have detailed how oligomerization via receptor–receptor interaction is a universal aspect of G-protein coupled receptor (GPCR) biology [59]. While there is still no conclusive answer to whether a particular order of oligomerization is prevalent (from dimers to large complexes), oligomerization appears to be a pivotal component of the structure and function of GPCRs. Opsin proteins, as members of subfamily A and some of the best-studied GPCRs, also exhibit oligomerization. Two atomic force microscopy (AFM) studies of native rod outer segments (ROS) discs clearly show that rhodopsin dimerizes and forms long-range-ordered paracrystalline protein arrays of those dimers (figure 5) [49,60]. Further X-ray powder diffraction results confirmed the double row and axial repeat periodicities of the rhodopsin dimers to be 8.4 and 4.2 nm−1, respectively [49]. To exclude the possibility that the absorption of the disc membrane onto mica promotes the formation of the arrays, disc membranes were also absorbed onto carbon-coated electron microscopy (EM) grids. The power spectra from the EM images of these discs confirmed the unit size and array order seen in the AFM image.
Figure 5.
Potential lipid and protein interactions in ciliary photoreceptors. Schematics of (a) rod discs with opsin dimers still free to rotate versus (b) cone discs with phase separation into cholesterol-rich and more ordered (Lo, blue) and cholesterol-poor and more fluid (Lα, grey) domains, with dimers ordered into rows. Evidence for membrane phase separation from (c) cone transmission electron microscopy studies (adapted from [61]) and (d) rod AFM and x-ray diffraction studies (adapted from [49]).
Other levels of order in rod and cone outer segments have also been consistently revealed by EM. Coreless et al. [61,62] showed extensive areas of order within the membranes—not only in the membrane plane but also between bilayers, where the inter-discal space seemed expanded, with processes linking across the extra- and inter-faces of the membranes. These studies show remarkable similarities with the work described above by Saibil [53] of the linkages between microvilli.
Further evidence of ordered arrays of rhodopsin has been obtained using recombinant membranes via saturation-transfer spin-label electron spin resonance [63]. The key findings of Ryba & Marsh [63], using different synthetic membranes, were that the level of oligomerization was correlated with a reduction of rotational diffusion and was principally dependent on the lipid membrane composition. They found that greater levels of protein aggregation take place with longer lipid chain lengths in these artificial membranes. Botelho et al. [64] concurred with this finding, providing direct fluorescence resonance energy transfer (FRET) evidence that the membrane lipid composition causes oligomerization of rhodopsin.
(c). Implications of visual pigment interactions and common themes in both rhabdomeric and ciliary photoreceptors
Long-range spatial organization in both rhabdomeric and ciliary photoreceptors provides an ideal mechanism for cooperatively ordering the visual pigment to produce the experimentally measured levels of dichroism seen in all cell types. In ciliary cells, such organization would seem to be incompatible with several studies [65,66] that have concluded that the visual pigment rotates in the membrane resulting in isotropic absorbance. Note, however, that both Brown's [65] and Cone's [66] studies were conducted on rods of Rana pipiens, which is not a known polarization-sensitive animal. In general, rods are known not to be polarization sensitive, and recent studies have shown that rotational diffusion may be inhibited in cone outer segment membranes [41]. Furthermore, a very recent study by Govardovskii et al. [67] has demonstrated that similar lateral diffusion measurements made at about the same time as these earlier studies [68] may have over-estimated levels of fluidity in ciliary membranes by an order of magnitude and that protein–protein interactions are physiologically relevant in vertebrate photoreceptors.
Therefore, in the remainder of this paper, we consider how the protein–protein interactions may occur in both rhabdomeric and ciliary cell types and how these may be linked by a common theme. As the current understanding is that both rhabdomeric and cone photoreceptors mediate polarization information, we ask the question: are there reasons to suspect that such protein–protein interactions and protein oligomerization as discussed above occurs with more prevalence in rhabdomeric cells and cones than in rods?
4. Photoreceptors as polarization detectors: the composition and phase behaviour of cell membranes contributing to polarization sensitivity
The local lipid environment within a membrane is crucial for correct protein function and therefore, in photoreceptors, visual function. The effects of the lipid composition on membrane structure have wide reaching implications. In tertiary model systems, particular lipid compositions create lipid tubes or discs (analogous to microvilli or outer segment structures) with prescribed spatial order in the plane of the membrane [69]. These lamellar-ordered (Lo) phases [70] exhibit higher degrees of spatial order compared with the fluid lamellar (Lα) and are currently an extremely exciting area of research.
(a). The effect of membrane lipid composition on visual pigment function in rhabdomeric photoreceptors
Lipid analysis of rhabdomeric membranes indicates a high concentration of cholesterol (Limulus [71]; squid [72]; insects [73]), which was originally believed to imply less-fluid homogeneous membranes and correspondingly slower visual pigment diffusion than in ciliary types [52]. Studies of arthropod and cephalopod photoreceptor fatty acid compositions also found large variations among species [74]. The effect of membrane lipid composition on visual pigment function is indicated by recent in vitro expression studies of honeybee opsins [75]. Historically, invertebrate visual pigments have been difficult to express in cultured cells, and most attempts at the functional expression of arthropod visual pigments have been unsuccessful. While it is possible to express invertebrate opsin genes and make protein in mammalian cell lines, once solubilized from the membrane, the reconstituted visual pigments are not normally functional. However, honeybee UV and blue-sensitive visual pigments have been successfully expressed in a mammalian cell line, solubilized and functionally characterized using spectrophotometry. To functionally characterize these visual pigments, the purified pigments were reconstituted in lipid vesicles, suggesting that lipid composition plays an important role in insect visual pigment function. It is also worthy of noting that, using these same techniques, Terakita et al. [75] were unable to functionally express the honeybee long-wavelength-sensitive opsin, suggesting that membrane lipid composition affects visual pigment spectral classes differently.
(b). The effect of membrane lipid composition on visual pigment function in ciliary photoreceptors
We currently understand that ROS discs and the surrounding outer cell membrane differ significantly in lipid composition. Work by Boesze-Battaglia et al. [76,77] has shown that the photoreceptor plasma membranes have considerably higher levels of cholesterol and a markedly different ratio of saturated to unsaturated fatty acids compared with the inter-cellular discs. In this regard, altering the lipid and cholesterol composition has been shown to significantly affect the function of rhodopsin, particularly the way the protein forms its enzymatically active state of metarhodopsin II. Work by Brown et al. [78–81] continues to illustrate the complex effects of different lipid compositions in model vertebrate membranes.
(c). Implications for polarization sensitivity as membrane compositions of rhabdomeric and ciliary cells drive the formation of dichroic rhodopsin oligomers
The above studies make it clear that lipid composition is important because it both controls membrane phase behaviour and influences visual pigment function; however, the impact of membrane compositional variations in the context of polarization vision in either ciliary or rhabdomeric photoreceptors is unknown. Several studies have previously suggested that simply a restricted orientation and decreased mobility of the visual pigment within photoreceptor membranes is the mechanism for increased PS, particularly in rhabdomeric photoreceptors. The suggested mechanisms include decreased microvillar membrane fluidity [32,52] and channelling of opsin molecules by membrane-associated barriers, such as integral membrane glycoproteins, to reduce translational diffusion [51,55,82]. However, on its own, a simple change in fluidity, i.e. an increase in rotational and translational viscosity, and reduction in mobility will not bring about any increase in chromophore alignment and PS.
Thirty-five years ago it was discovered that differing lipid compositions de-mixed rhodopsin in model membranes. Hubbell [83] showed that cholesterol played a central role when incorporated into model synthetic membranes containing rhodopsin (3 : 1 molar ratio of phospholipid–cholesterol). Freeze-fracture analysis illustrated large domains with no rhodopsin in the fracture plane. In contrast, controls without cholesterol resulted in a random rhodopsin distribution in the plane. Hubbell [83] concluded that rhodopsin associates with the more fluid regions in the membrane while being excluded from the less fluid, more-ordered cholesterol-containing regions, just as it is excluded from the rigid, ordered gel phase in phospholipid bilayers in the solid state. More recent work has presented further evidence for similar micro-domain formation (commonly called lipid rafts) in native photoreceptor membranes [84–86]. In the context of polarization vision, it is relevant that rhodopsin associates within particular micro-domains. Botelho et al. [64] proposed that the minimization of the spontaneous membrane curvature drives the micro-phase separation, and thus protein oligomerization follows as the rhodopsin associates with particular micro-domains.
5. Summary
Despite the fact that polarization vision in animals has been studied for over 50 years, the specific molecular mechanisms responsible for the detection of polarized light are still unknown. In this paper, we have highlighted a diverse set of potential mechanisms that may increase photoreceptor PS beyond the limits set by geometric mechanisms alone. Although any one of the mechanisms discussed—oligomerization, protein–protein or protein–cytoskeletal interactions—has the possibility of increasing the photoreceptor PS, even more intriguing is the hypothesis that these mechanisms act in concert to achieve the highly ordered chromophore arrangement needed to produce the PS observed in some animals. The evidence suggests it is possible that a carefully controlled membrane composition, with a higher cholesterol content in rhabdomeric and cone ciliary photoreceptors, may be responsible for driving the formation of liquid-ordered Lo and Lα micro-domains in the membranes. Opsin proteins then affiliate with the Lα micro-domains enabling protein–protein and protein–cytoskeletal (in rhabdomeric cases) interactions. This association allows rhodopsin to oligomerize and thus aligns the chromophores into a cellular-scale polarization detector, producing the higher levels of dichroism measured experimentally than predicted theoretically. This would be a common mechanism used by both rhabdomeric and ciliary photoreceptors, a possibility up until now not considered.
Acknowledgements
The authors gratefully acknowledge the support from the National Science Foundation (IOS 0721608), the Air Force Office of Scientific Research (FA9550-09-1-0149) and from the Biotechnology and Biological Sciences Research Council (BB/G022917/1).
Footnotes
One contribution of 20 to a Theme Issue ‘New directions in biological research on polarized light’.
References
- 1.von Frisch K. 1949. Die Polarisation des Himmelslichts als orientierender Faktor bei den Tanzen der Bienen. Experientia 5, 142–148 10.1007/BF02174424 (doi:10.1007/BF02174424) [DOI] [PubMed] [Google Scholar]
- 2.Chiou T.-H., Kleinlogel S., Cronin T., Caldwell R., Loeffler B., Siddiqi A., Goldizen A., Marshall J. 2008. Circular polarization vision in a stomatopod crustacean. Curr. Biol. 18, 429–434 10.1016/j.cub.2008.02.066 (doi:10.1016/j.cub.2008.02.066) [DOI] [PubMed] [Google Scholar]
- 3.Shashar N., Cronin T. W. 1996. Polarization contrast vision in octopus. J. Exp. Biol. 199, 999–1004 [DOI] [PubMed] [Google Scholar]
- 4.Goddard S., Forward J. R. B. 1991. The role of the underwater polarized light pattern, in sun compass navigation of the grass shrimp, Palaemonetes vulgaris. J. Comp. Physiol. A 169, 479–491 10.1007/BF00197660 (doi:10.1007/BF00197660) [DOI] [Google Scholar]
- 5.Wehner R. 1976. Polarized-light navigation by insects. Sci. Am. 235, 106–115 10.1038/scientificamerican0776-106 (doi:10.1038/scientificamerican0776-106) [DOI] [PubMed] [Google Scholar]
- 6.Wehner R. 2001. Polarization vision–a uniform sensory capacity? J. Exp. Biol. 204, 2589–2596 [DOI] [PubMed] [Google Scholar]
- 7.Waterman T. 2006. Reviving a neglected celestial underwater polarization compass for aquatic animals. Biol. Rev. 81, 111–115 10.1017/S1464793105006883 (doi:10.1017/S1464793105006883) [DOI] [PubMed] [Google Scholar]
- 8.Cronin T., Shashar N., Caldwell R., Cheroske A., Chiou T. 2002. Polarization vision and its role in underwater signaling. Integr. Comp. Biol. 42, 1215–1215 [DOI] [PubMed] [Google Scholar]
- 9.Shashar N., Rutledge P., Cronin T. 1996. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 199, 2077–2084 [DOI] [PubMed] [Google Scholar]
- 10.Labhart T. 1988. Polarization-opponent interneurons in the insect visual-system. Nature 331, 435–437 10.1038/331435a0 (doi:10.1038/331435a0) [DOI] [Google Scholar]
- 11.Sakura M., Lambrinos D., Labhart T. 2006. Polarization-sensitive neurons in the central complex of crickets—how does the CNS code orientation? Comp. Biochem. Phys. B 145, 406–406 10.1016/j.cbpb.2006.10.018 (doi:10.1016/j.cbpb.2006.10.018) [DOI] [Google Scholar]
- 12.Sakura M., Lambrinos D., Labhart T. 2008. Polarized skylight navigation in insects: model and electrophysiology of e-vector coding by neurons in the central complex. J. Neurophysiol. 99, 667–682 10.1152/jn.00784.2007 (doi:10.1152/jn.00784.2007) [DOI] [PubMed] [Google Scholar]
- 13.Coughlin D. J., Hawryshyn C. W. 1994. Ultraviolet sensitivity in the torus semicircularis of juvenile rainbow-trout (Oncorhynchus mykiss). Vis. Res. 34, 1407–1413 10.1016/0042-6989(94)90140-6 (doi:10.1016/0042-6989(94)90140-6) [DOI] [PubMed] [Google Scholar]
- 14.Parkyn D., Hawryshyn C. 2000. Spectral and ultraviolet-polarisation sensitivity in juvenile salmonids: a comparative analysis using electrophysiology. J. Exp. Biol. 203, 1173–1191 [DOI] [PubMed] [Google Scholar]
- 15.Ramsden S. D., Anderson L., Mussi M., Kamermans M., Hawryshyn C. W. 2008. Retinal processing and opponent mechanisms mediating ultraviolet polarization sensitivity in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 211, 1376–1385 10.1242/jeb.015941 (doi:10.1242/jeb.015941) [DOI] [PubMed] [Google Scholar]
- 16.Lythgoe J., Hemmings C. 1967. Polarized light and underwater vision. Nature 213, 893–894 10.1038/213893a0 (doi:10.1038/213893a0) [DOI] [PubMed] [Google Scholar]
- 17.Neville A., Luke B. 1971. Form optical activity in crustacean cuticle. J. Insect. Physiol. 17, 519–523 10.1016/0022-1910(71)90030-8 (doi:10.1016/0022-1910(71)90030-8) [DOI] [Google Scholar]
- 18.Land M. F. 1991. Vision—polarizing the world of fish. Nature 353, 118–119 10.1038/353118d0 (doi:10.1038/353118d0) [DOI] [Google Scholar]
- 19.Moody M., Parriss J. 1961. The discrimination of polarized light by octopus—a behavioural and morphological study. Z. Vergl. Physiol. 44, 268–291 10.1007/BF00298356 (doi:10.1007/BF00298356) [DOI] [Google Scholar]
- 20.Blum M., Labhart T. 2000. Photoreceptor visual fields, ommatidial array, and receptor axon projections in the polarisation-sensitive dorsal rim area of the cricket compound eye. J. Comp. Physiol. A 186, 119–128 10.1007/s003590050012 (doi:10.1007/s003590050012) [DOI] [PubMed] [Google Scholar]
- 21.Dacke M., Nilsson D., Warrant E., Blest A., Land M., O'Carroll D. 1999. Built-in polarizers form part of a compass organ in spiders. Nature 401, 470–473 10.1038/46773 (doi:10.1038/46773) [DOI] [Google Scholar]
- 22.Labhart T. 1980. Specialized photoreceptors at the dorsal rim of the honeybees compound eye—polarizational and angular sensitivity. J. Comp. Physiol. 141, 19–30 10.1007/BF00611874 (doi:10.1007/BF00611874) [DOI] [Google Scholar]
- 23.Labhart T. 1986. The electrophysiology of photoreceptors in different eye regions of the desert ant, Cataglyphis bicolor. J. Comp. Physiol. A 158, 1–7 10.1007/BF00614514 (doi:10.1007/BF00614514) [DOI] [Google Scholar]
- 24.Labhart T., Meyer E., Schenker L. 1992. Specialized ommatidia for polarization vision in the compound eye of cockchafers, Melolontha melolontha (Coleoptera, Scarabaeidae). Cell Tiss. Res. 268, 419–429 10.1007/BF00319148 (doi:10.1007/BF00319148) [DOI] [PubMed] [Google Scholar]
- 25.Laughlin S. 1976. Sensitivities of dragonfly photoreceptors and voltage gain of transduction. J. Comp. Physiol. 111, 221–247 10.1007/BF00606466 (doi:10.1007/BF00606466) [DOI] [Google Scholar]
- 26.Snyder A., Laughlin S. 1975. Dichroism and absorption by photoreceptors. J. Comp. Physiol. 100, 101–116 10.1007/BF00613963 (doi:10.1007/BF00613963) [DOI] [Google Scholar]
- 27.Horváth G., Varjú D. 2004. Polarized light in animal vision: polarization patterns in nature, p. 447 Berlin, Germany: Springer [Google Scholar]
- 28.Waterman T. H. 1981. Polarisation sensitivity. In Vision of invertebrates: Handbook of sensory physiology, vol. VII/B (ed. Autrum H.), pp. 281–469 Berlin, Germany: Springer [Google Scholar]
- 29.DeVries H., Spoor A., Jielof R. 1953. Properties of the eye with respect to polarized light. Physica 19, 419–432 10.1016/S0031-8914(53)80048-0 (doi:10.1016/S0031-8914(53)80048-0) [DOI] [Google Scholar]
- 30.Nilsson D.-E., Kelber A. 2007. A functional analysis of compound eye evolution. Arthropod Struct. Dev. 36, 373–385 10.1016/j.asd.2007.07.003 (doi:10.1016/j.asd.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 31.Stockhammer K. 1956. Zur Wahrnehmung der Schwingungsrichtung linear polarisierten Lichtes bei Insekten. Z. Vergl. Physiol. 38, 30–83 10.1007/BF00338622 (doi:10.1007/BF00338622) [DOI] [Google Scholar]
- 32.Israelachvili J., Wilson M. 1976. Absorption characteristics of oriented photopigments in microvilli. Biol. Cybern. 21, 9–15 10.1007/BF00326667 (doi:10.1007/BF00326667) [DOI] [PubMed] [Google Scholar]
- 33.Wehner R., Bernard G. 1993. Photoreceptor twist—a solution to the false-color problem. Proc. Natl Acad. Sci. USA 90, 4132–4135 10.1073/pnas.90.9.4132 (doi:10.1073/pnas.90.9.4132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wehner R., Bernard G., Geiger E. 1975. Twisted and non-twisted rhabdoms and their significance for polarization detection in bee. J. Comp. Physiol. 104, 225–245 10.1007/BF01379050 (doi:10.1007/BF01379050) [DOI] [Google Scholar]
- 35.Fineran B., Nicol J. 1978. Studies on photoreceptors of Anchoa mitchilli and Anchoa hepsetus (Engraulidae) with particular reference to cones. Phil. Trans. R. Soc. Lond. B 283, 25–60 10.1098/rstb.1978.0017 (doi:10.1098/rstb.1978.0017) [DOI] [PubMed] [Google Scholar]
- 36.Flamarique I., Hawryshyn C. 1998. Photoreceptor types and their relation to the spectral and polarization sensitivities of clupeid fishes. J. Comp. Physiol. A 182, 793–803 10.1007/s003590050224 (doi:10.1007/s003590050224) [DOI] [Google Scholar]
- 37.Flamarique I. N., Harosi F. I. 2002. Visual pigments and dichroism of anchovy cones: a model system for polarization detection. Vis. Neurosci. 19, 467–473 10.1017/S0952523802194089 (doi:10.1017/S0952523802194089) [DOI] [PubMed] [Google Scholar]
- 38.Grobner G., Burnett I., Glaubitz C., Choi G., Mason A., Watts A. 2000. Observations of light-induced structural changes of retinal within rhodopsin. Nature 405, 810–813 10.1038/35015604 (doi:10.1038/35015604) [DOI] [PubMed] [Google Scholar]
- 39.Harosi F., Malerba F. 1975. Plane-polarized light in microspectrophotometry. Vis. Res. 15, 379–388 10.1016/0042-6989(75)90086-3 (doi:10.1016/0042-6989(75)90086-3) [DOI] [PubMed] [Google Scholar]
- 40.Liebman P. 1962. In situ microspectrophotometric studies on pigments of single retinal rods. Biophys. J. 2, 161–178 10.1016/S0006-3495(62)86847-7 (doi:10.1016/S0006-3495(62)86847-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts N., Needham M. 2007. A mechanism of polarized light sensitivity in cone photoreceptors of the goldfish Carassius auratus. Biophys. J. 93, 3241–3248 10.1529/biophysj.107.112292 (doi:10.1529/biophysj.107.112292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts N., Gleeson H., Temple S., Haimberger T., Hawryshyn C. 2004. Differences in the optical properties of vertebrate photoreceptor classes leading to axial polarization sensitivity. J. Opt. Soc. Am. A-Opt. Image Sci. Vis. 21, 335–345 10.1364/JOSAA.21.000335 (doi:10.1364/JOSAA.21.000335) [DOI] [PubMed] [Google Scholar]
- 43.Roberts N. W. 2006. The optics of vertebrate photoreceptors: anisotropy and form birefringence. Vis. Res. 46, 3259–3266 10.1016/j.visres.2006.03.019 (doi:10.1016/j.visres.2006.03.019) [DOI] [PubMed] [Google Scholar]
- 44.Roberts N. W., Gleeson H. F. 2004. The absorption of polarized light by vertebrate photoreceptors. Vis. Res. 44, 2643–2652 10.1016/j.visres.2004.06.001 (doi:10.1016/j.visres.2004.06.001) [DOI] [PubMed] [Google Scholar]
- 45.Flamarique I., Hawryshyn C., Harosi F. 1998. Double-cone internal reflection as a basis for polarization detection in fish. J. Opt. Soc. Am. A-Opt. Image Sci. Vis. 15, 349–358 10.1364/JOSAA.15.000349 (doi:10.1364/JOSAA.15.000349) [DOI] [PubMed] [Google Scholar]
- 46.Cameron D. A., Pugh E. N. 1991. Double cones as a basis for a new type of polarization vision in vertebrates. Nature 353, 161–164 10.1038/353161a0 (doi:10.1038/353161a0) [DOI] [PubMed] [Google Scholar]
- 47.Rowe M., Engheta N., Easter S., Pugh E. 1994. Graded-index model of a fish double cone exhibits differential polarization sensitivity. J. Opt. Soc. Am. A-Opt. Image Sci. Vis. 11, 55–70 10.1364/JOSAA.11.000055 (doi:10.1364/JOSAA.11.000055) [DOI] [PubMed] [Google Scholar]
- 48.Flamarique I. N., Hawryshyn C. W. 1997. No evidence of polarization sensitivity in freshwater sunfish from multi-unit optic nerve recordings. Vis. Res. 37, 967–973 10.1016/S0042-6989(96)00243-X (doi:10.1016/S0042-6989(96)00243-X) [DOI] [PubMed] [Google Scholar]
- 49.Fotiadis D., Liang Y., Filipek S., Saperstein D., Engel A., Palczewski K. 2003. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature 421, 127–128 10.1038/421127a (doi:10.1038/421127a) [DOI] [PubMed] [Google Scholar]
- 50.Murakami M., Kouyama T. 2008. Crystal structure of squid rhodopsin. Nature 453, 363–368 10.1038/nature06925 (doi:10.1038/nature06925) [DOI] [PubMed] [Google Scholar]
- 51.Blest A., Eddey W. 1984. The extrarhabdomeral cytoskeleton in photoreceptors of diptera. II. Plasmalemmal undercoats. Proc. R. Soc. Lond. B 220, 353–359 10.1098/rspb.1984.0005 (doi:10.1098/rspb.1984.0005) [DOI] [Google Scholar]
- 52.Goldsmith T., Wehner R. 1977. Restrictions on rotational and translational diffusion of pigment in membranes of a rhabdomeric photoreceptor. J. Gen. Phys. 70, 453–490 10.1085/jgp.70.4.453 (doi:10.1085/jgp.70.4.453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saibil H. R. 1982. An ordered membrane-cytoskeleton network in squid photoreceptor microvilli. J. Mol. Biol. 158, 435–456 10.1016/0022-2836(82)90208-X (doi:10.1016/0022-2836(82)90208-X) [DOI] [PubMed] [Google Scholar]
- 54.Arikawa K., Hicks J., Williams D. 1990. Identification of actin-filaments in the rhabdomeral microvilli of Drosophila photoreceptors. J. Cell Biol. 110, 1993–1998 10.1083/jcb.110.6.1993 (doi:10.1083/jcb.110.6.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decouet H., Stowe S., Blest A. 1984. Membrane-associated actin in the rhabdomeral microvilli of crayfish photoreceptors. J. Cell Biol. 98, 834–846 10.1083/jcb.98.3.834 (doi:10.1083/jcb.98.3.834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montell C. 1999. Visual transduction in Drosophila. Annu. Rev. Cell Dev. Biol. 15, 231–268 10.1146/annurev.cellbio.15.1.231 (doi:10.1146/annurev.cellbio.15.1.231) [DOI] [PubMed] [Google Scholar]
- 57.Cronin T. W., Porter M. L., Bok M. J., Wolf J. B., Robinson P. R. 2010. The molecular genetics of colour and polarisation vision in stomatopod crustaceans. Opthalmic Physiol. Opt. 29, 1–10 [DOI] [PubMed] [Google Scholar]
- 58.Porter M., Bok M., Robinson P., Cronin T. W. 2009. Molecular diversity of visual pigments in Stomatopoda (Crustacea). Vis. Neurosci. 26, 255–265 10.1017/S0952523809090129 (doi:10.1017/S0952523809090129) [DOI] [PubMed] [Google Scholar]
- 59.George S., O'Dowd B., Lee S. 2002. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1, 808–820 10.1038/nrd913 (doi:10.1038/nrd913) [DOI] [PubMed] [Google Scholar]
- 60.Liang Y. 2003. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 278, 21 655–21 662 10.1074/jbc.M302536200 (doi:10.1074/jbc.M302536200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corless J. M., Worniallo E., Fetter R. D. 1994. 3-Dimensional membrane crystals in amphibian cone outer segments. I. Light-dependent crystal-formation in frog retinas. J. Struct. Biol. 113, 64–86 10.1006/jsbi.1994.1033 (doi:10.1006/jsbi.1994.1033) [DOI] [PubMed] [Google Scholar]
- 62.Corless J., Worniallo E., Schneider T. 1995. 3-Dimensional membrane crystals in amphibian cone outer segments. II. Crystal type associated with the saddle-point regions of cone disks. Exp. Eye Res. 61, 335–349 10.1016/S0014-4835(05)80128-9 (doi:10.1016/S0014-4835(05)80128-9) [DOI] [PubMed] [Google Scholar]
- 63.Ryba N., Marsh D. 1992. Protein rotational diffusion and lipid protein interactions in recombinants of bovine rhodopsin with saturated diacylphosphatidylcholines of different chain lengths studied by conventional and saturation-transfer electron-spin-resonance. Biochemistry 31, 7511–7518 10.1021/bi00148a011 (doi:10.1021/bi00148a011) [DOI] [PubMed] [Google Scholar]
- 64.Botelho A. V., Huber T., Sakmar T. P., Brown M. F. 2006. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys. J. 91, 4464–4477 10.1529/biophysj.106.082776 (doi:10.1529/biophysj.106.082776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown P. K. 1972. Rhodopsin rotates in visual receptor membrane. Nat. New Biol. 236, 35–38 [DOI] [PubMed] [Google Scholar]
- 66.Cone R. A. 1972. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat. New Biol. 236, 39–43 [DOI] [PubMed] [Google Scholar]
- 67.Govardovskii V. I., Korenyak D. A., Shukolyukov S. A., Zueva L. V. 2009. Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Mol. Vis. 15, 1717–1729 [PMC free article] [PubMed] [Google Scholar]
- 68.Poo M. M., Cone R. A. 1974. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature 247, 438–441 10.1038/247438a0 (doi:10.1038/247438a0) [DOI] [PubMed] [Google Scholar]
- 69.Yuan J., Hira S. M., Strouse G. F., Hirst L. S. 2008. Lipid bilayer discs and banded tubules: photoinduced lipid sorting in ternary mixtures. J. Am. Chem. Soc. 130, 2067–2072 10.1021/ja710305c (doi:10.1021/ja710305c) [DOI] [PubMed] [Google Scholar]
- 70.Clarke J., Heron A., Seddon J., Law R. 2006. The diversity of the liquid ordered (L-o) phase of phosphatidylcholine/cholesterol membranes: a variable temperature multinuclear solid-state NMR and X-ray diffraction study. Biophys. J. 90, 2383–2393 10.1529/biophysj.104.056499 (doi:10.1529/biophysj.104.056499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benolken R., Anderson R., Maude M. 1975. Lipid-composition of Limulus photoreceptor membranes. Biochim. Biophys. Acta 413, 234–242 10.1016/0005-2736(75)90107-8 (doi:10.1016/0005-2736(75)90107-8) [DOI] [PubMed] [Google Scholar]
- 72.Mason W., Fager R., Abrahams E. W. 1973. Characterization of lipid composition of squid rhabdome outer segments. Biochim. Biophys. Acta 306, 67–73 [DOI] [PubMed] [Google Scholar]
- 73.Zinkler D. 1975. Zum Lipidmuster der Photorezeptoren von Insekten. Verh. Dtsch. Zool. Ges. 32, 28–32 [Google Scholar]
- 74.Eguchi E., Ogawa Y., Okamoto K., Mochizuki K. 1994. Fatty-acid compositions of arthropod and cephalopod photoreceptors—interspecific, seasonal and developmental studies. J. Comp. Physiol. B 164, 94–102 10.1007/BF00301649 (doi:10.1007/BF00301649) [DOI] [Google Scholar]
- 75.Terakita A., Tsukamoto H., Koyanagi M., Sugahara M., Yamashita T., Shichida Y. 2008. Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J. Neurochem. 105, 883–890 10.1111/j.1471-4159.2007.05184.x (doi:10.1111/j.1471-4159.2007.05184.x) [DOI] [PubMed] [Google Scholar]
- 76.Boesze-Battaglia K., Albert A. D. 1989. Fatty acid composition of bovine rod outer segment plasma membrane. Exp. Eye Res. 49, 699–701 10.1016/S0014-4835(89)80064-8 (doi:10.1016/S0014-4835(89)80064-8) [DOI] [PubMed] [Google Scholar]
- 77.Boesze-Battaglia K., Schimmel R. J. 1997. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol. 200, 2927–2936 [DOI] [PubMed] [Google Scholar]
- 78.Alves I., Salgado G., Salamon Z., Brown M., Tollin G., Hruby V. 2006. Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys. J. 90, 709–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Botelho A., Wang Y., Gibson N., Brown M. 2000. Membrane bilayer properties influence photoactivation of rhodopsin. Biophys. J. 78, 198–210 [Google Scholar]
- 80.Brown M. F. 1994. Modulation of rhodopsin function by properties of the membrane bilayer. Chem. Phys. Lipids 73, 159–180 10.1016/0009-3084(94)90180-5 (doi:10.1016/0009-3084(94)90180-5) [DOI] [PubMed] [Google Scholar]
- 81.Gibson N., Brown M. 1990. Influence of pH on the MI-MII equilibrium of rhodopsin in recombinant membranes. Biochem. Biophys. Res. Commun. 169, 1028–1034 10.1016/0006-291X(90)91997-7 (doi:10.1016/0006-291X(90)91997-7) [DOI] [PubMed] [Google Scholar]
- 82.Koppel D., Sheetz M., Schindler M. 1981. Matrix control of protein diffusion in biological membranes. Proc. Natl Acad. Sci. USA 78, 3576–3580 10.1073/pnas.78.6.3576 (doi:10.1073/pnas.78.6.3576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hubbell W. 1975. Characterization of rhodopsin in synthetic systems. Acc. Chem. Res. 8, 85–91 10.1021/ar50087a002 (doi:10.1021/ar50087a002) [DOI] [Google Scholar]
- 84.Elliott M. H., Nash Z. A., Takemori N., Fliesler S. J., McClellan M. E., Naash M. I. 2008. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J. Neurochem. 104, 336–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nair K., Balasubramanian N., Slepak V. 2002. Signal-dependent translocation of transducin, RGS9-1-G beta 5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr. Biol. 12, 421–425 10.1016/S0960-9822(02)00691-7 (doi:10.1016/S0960-9822(02)00691-7) [DOI] [PubMed] [Google Scholar]
- 86.Seno K., Kishimoto M., Abe M., Higuchi Y., Mieda M., Owada Y., Yoshiyama W., Liu H., Hayashi F. 2001. Light- and guanosine 5′-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J. Biol. Chem. 276, 20 813–20 816 10.1074/jbc.C100032200 (doi:10.1074/jbc.C100032200) [DOI] [PubMed] [Google Scholar]
- 87.Regier J. C., Shultz J. W., Zwick A., Hussey A., Ball B., Wetzer R., Martin J. W., Cunningham C. W. 2010. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463, 1079–1084 10.1038/nature08742 (doi:10.1038/nature08742) [DOI] [PubMed] [Google Scholar]
- 88.Dunn C. W., et al. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 10.1038/nature06614 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- 89.Johnsen S. 1994. Extraocular sensitivity to polarized light in an echinoderm. J. Exp. Biol. 195, 281–291 [DOI] [PubMed] [Google Scholar]
- 90.Hawryshyn C. W., Moyer H. D., Allison W. T., Haimberger T. J., McFarland W. N. 2003. Multidimensional polarization sensitivity in damselfishes. J. Comp. Physiol. A 189, 213–220 [DOI] [PubMed] [Google Scholar]
- 91.Bandai K., Arikawa K., Eguchi E. 1992. Localization of spectral receptors in the ommatidium of butterfly compound eye determined by polarization sensitivity. J. Comp. Physiol. A 171, 289–297 10.1007/BF00223959 (doi:10.1007/BF00223959) [DOI] [Google Scholar]
- 92.Hardie R. C. 1979. Electrophysiological analysis of fly retina. I. Comparative properties of R1-6 and R7 and 8. J. Comp. Physiol. 129, 19–33 10.1007/BF00679908 (doi:10.1007/BF00679908) [DOI] [Google Scholar]
- 93.Hardie R. C. 1984. Properties of photoreceptors R7 and R8 in dorsal marinal ommatidia in the compound eyes of Musca and Calliphora. J. Comp. Physiol. A 154, 157–165 10.1007/BF00604981 (doi:10.1007/BF00604981) [DOI] [Google Scholar]
- 94.Labhart T., Hodel B., Valenzuela I. 1984. The physiology of the cricket's compound eye with particular reference to the anatomically specialized dorsal rim area. J. Comp. Physiol. A 155, 289–296 10.1007/BF00610582 (doi:10.1007/BF00610582) [DOI] [Google Scholar]
- 95.Doujak F. E. 1984. Electrophysiological measurement of photoreceptor membrane dichoism and polarization sensitivity in a grapsid crab. J. Comp. Physiol. A 154, 597–605 10.1007/BF00610173 (doi:10.1007/BF00610173) [DOI] [Google Scholar]
- 96.Glantz R. M. 1996. Polarization sensitivity in the crayfish lamina monopolar neurons. J. Comp. Physiol. A 178, 412–425 10.1007/BF00193978 (doi:10.1007/BF00193978) [DOI] [Google Scholar]
- 97.Kleinlogel S., Marshall N. J. 2006. Electrophysiological evidence for linear polarization sensitivity in the compound eyes of the stomatopod crustacean Gonodactylus chiragra. J. Exp. Biol. 209, 4262–4272 10.1242/jeb.02499 (doi:10.1242/jeb.02499) [DOI] [PubMed] [Google Scholar]
- 98.Saidel W. M., Lettvin J. Y., MacNichol E. F., Jr 1983. Processing of polarized light by squid photoreceptors. Nature 304, 534–536 10.1038/304534a0 (doi:10.1038/304534a0) [DOI] [PubMed] [Google Scholar]